Abstract
Introduction:
Although many studies show an inverse association between operator procedural volume and short-term adverse outcomes after percutaneous coronary intervention (PCI), the association between procedural volume and longer term outcomes is unknown.
Methods:
Using the National Cardiovascular Data Registry (NCDR) CathPCI registry data linked with Medicare claims data, we examined the association between operator PCI volume and long-term outcomes among patients aged ≥ 65 years. Operators were stratified by average annual PCI volume (counting PCIs performed in patients of all ages): low- (< 50 PCIs), intermediate- (50–100), and high-volume operators (> 100). One-year unadjusted rates of death and major adverse coronary events (MACE, defined as death, readmission for myocardial infarction [MI], or unplanned coronary revascularization) were calculated using Kaplan-Meier methods. The proportional hazards assumption was not met and risk-adjusted associations between operator volume and outcomes were calculated separately from the time of PCI to hospital discharge and from hospital discharge to 1-year follow-up.
Results:
Between July 1, 2009 and December 31, 2014, 723,644 PCI procedures were performed by 8,936 operators: 2,553 high-, 2,878 intermediate-, and 3,505 low-volume. Compared with high- and intermediate-volume operators, low-volume operators more often performed emergency PCI and their patients had fewer cardiovascular comorbidities. Over 1 year follow-up, 15.9% of patients treated by low-volume operators had a MACE event compared with 16.9% of patients treated by high-volume operators (p = 0.004). After multivariable adjustment, intermediate- and high-volume operators had a significantly lower rate of in-hospital death than low-volume operators (OR 0.91, 95% CI 0.86–0.96 for intermediate vs. low; OR 0.79, 95% CI 0.75–0.83 for high vs. low). There were no significant differences in rates of MACE, death, MI, or unplanned revascularization between operator cohorts from hospital discharge to 1-year follow-up (adjusted HR for MACE: 0.99, 95% CI 0.96–1.01 for intermediate vs. low; HR 1.01, 95% CI 0.99–1.04 for high versus low).
Conclusions:
Unadjusted 1-year outcomes following PCI were worse for older adults treated by operators with higher annual volume; however, patients treated by these operators had more cardiovascular comorbidities. After risk adjustment, higher operator volume was associated with lower in-hospital mortality and no difference in post-discharge MACE.
Keywords: percutaneous coronary intervention, outcomes research, morbidity/mortality, complex stenting, volume-outcome relationship
INTRODUCTION
Multiple studies have demonstrated an association between percutaneous coronary intervention (PCI) operator volume and short-term outcomes, including in-hospital mortality, major bleeding, acute kidney injury, and need for urgent coronary artery bypass grafting surgery, but absolute differences across operators are small.1–10 Other contemporary studies have failed to show any association between operator volume and outcomes and between hospital volume and outcomes in patients undergoing PCI for ST segment elevation myocardial infarction (STEMI).11–13 Moreover, nearly all studies evaluated only in-hospital outcomes. Patients undergoing PCI remain at high risk for adverse outcomes over longer-term follow-up, with a ~20% risk of death, myocardial infarction (MI), and repeat revascularization in the first year following PCI.14, 15 Suboptimal stent deployment (underexpansion, incomplete apposition, incomplete lesion coverage, and/or edge dissection) may occur with greater frequency among low volume operators and is associated with greater risk of adverse outcomes over long-term follow-up.16–20 Low-volume operators also use drug-eluting stents less often than high-volume operators and attempt fewer lesions per cardiac catheterization lab visit, both of which may ultimately result in higher rates of long-term adverse events due to re-stenosis and progression of untreated lesions.10 The relationship between operator volume and outcomes informs professional society recommendations,21 but a complete understanding of the volume-outcome relationship should take into account differences in long-term outcomes.
We analyzed data from the National Cardiovascular Data Registry’s (NCDR) CathPCI registry to Centers for Medicare and Medicaid (CMS) claims data to evaluate the association between operator PCI volume and one-year outcomes, including all-cause death, hospitalization for MI, or unplanned coronary revascularization.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study sample
The NCDR CathPCI registry, jointly administered by the American College of Cardiology (ACC) and the Society for Cardiovascular Angiography and Interventions (SCAI), has been previously described.22 It collects data from consecutive patients undergoing PCI at > 1500 hospitals in the United States (~90% of PCI centers), recording information on patient and hospital characteristics, including patient presentation, lesion and procedural details, peri-procedural and discharge medications, and in-hospital outcomes.23 Variables collected are determined and defined by physician work groups; data collection forms and dictionaries are available at the NCDR’s website. Data collected are subject to the NCDR’s comprehensive data quality program, which includes data quality report specifications for capture and transmission, as well as auditing.24 For patients ≥ 65 years old with fee-for-service Medicare, CathPCI data have been linked with CMS claims data using direct patient identifiers, allowing for ascertainment of longitudinal outcomes.
For this study, we included all PCI procedures in the linked dataset from July 1, 2009 through December 31, 2014. The CathPCI data collection form first collected National Provider Identification (NPI) number, which allows for unique identification of the operator for each PCI, in July 1, 2009, and CMS-linked data was available through December 31, 2014. Starting with patients ≥ 65 undergoing PCI during the study period (n = 1,297,833), we excluded patients unable to be linked to CMS claims data (n = 20,248), those ineligible for fee-for-service Medicare (n = 546,636), and any procedure missing operator’s NPI number (n = 7,305). Characteristics of included and excluded patients are described in Supplemental Table 1. For analyses of post-discharge events, we excluded patients that died during the index hospitalization (n = 15,280).
Definitions and outcomes
All study definitions were derived from the CathPCI data dictionary. The primary outcome for this analysis was 1-year major adverse coronary events (MACE), defined as the composite of all-cause death, hospitalization for MI, or unplanned coronary revascularization, occurring from the time of PCI to 1-year follow-up. Hospitalization for MI was defined as readmission with a primary diagnosis code of 410.x1. Unplanned coronary revascularization was defined as either PCI or coronary artery bypass grafting surgery (CABG) > 60 days following the index PCI, or, for procedures occurring ≤ 60 days following the index PCI, PCI or CABG associated with a primary discharge diagnosis of MI, unstable angina, heart failure, arrhythmia, or cardiac arrest.25–28 Secondary outcomes were the individual components of the MACE outcome as well as major bleeding, defined as a hemorrhagic stroke, hemoglobin drop ≥ 3 g/dl, red blood cell transfusion, or procedural intervention to stop bleeding during the index hospitalization, or readmission for bleeding (ICD-9 codes 430–432, 578.X, 719.1X, 423.0, 599.7, 626.2, 626.6, 626.8, 627.0, 627.1, 786.3, 784.7, 459.0).29 We also report appropriateness of PCI by operator volume stratum, which was based on the 2012 Appropriate Use Criteria for Coronary Revascularization, and determined using a validated algorithm.30–32 We performed landmark analyses of in-hospital deaths, death and major bleeding from the time of PCI to 30-day follow-up, and MACE from the time of hospital discharge to 1-year follow-up.
The total number of PCI procedures performed or attempted for each operator was counted using each operator’s unique NPI number, and each operator’s average annual volume was calculated by dividing the operator’s total number of PCI procedures by the number of days the operator was active during the study period (date of last PCI procedure – date of first PCI procedure) to derive PCIs per day and multiplying by 365. Operator volume was counted in the CathPCI database prior to CMS linkage or the exclusion of any patients aside from those that underwent procedures missing operator’s NPI number (< 1% of procedures in CathPCI),10 so each operator’s total number of PCI procedures included those performed in all patients, < 65 as well as ≥ 65 years old. Since the NPI number is a unique ID that carries across hospitals, operator volumes could be counted without regard to where procedures were performed, as long as procedures were performed in hospitals that participate in the CathPCI registry.
As the American Heart Association (AHA)/ACC/SCAI clinical competence statement recommends that operators perform an average of ≥ 50 PCIs/year to maintain competence, operators performing < 50 PCIs/year were defined as low-volume operators.21 Operators performing 50–100 and > 100 PCIs/year were defined as intermediate- and high-volume operators, respectively.
To estimate lifetime experience, we counted the number of years each operator performed at least one PCI between 2009 and 2013, and divided operators into groups by years of experience: 0–1, 2–4, > 4.
Statistical analysis
Patient, procedural, and hospital characteristics are presented for high-, intermediate-, and low-volume operators, with categorical variables presented as frequencies (percentages) and continuous variables presented as medians (25th, 75th percentiles). Pearson χ2 tests and Kruskal-Wallis tests were used for comparing categorical and continuous variables, respectively. A p-value threshold of < 0.05 was used to define statistical significance.
For 30-day mortality and major bleeding, we performed logistic regression with a robust sandwich covariance matrix to generate unadjusted and risk-adjusted odds ratios with 95% confidence intervals (CI) for intermediate- and high-volume operators with low-volume operators as a reference. Covariates for adjustment included all variables comprising variables included in the CathPCI in-hospital mortality risk score,33 along with year of PCI and other variables selected by expert opinion, including demographic variables, cardiac risk factors, details of the coronary anatomy and index PCI procedure, and discharge medications (Full list of covariates in Supplemental Methods). The use of a robust sandwich covariance matrix accounts for clustering of patient outcomes within operators. We repeated these analyses within the subgroups of patients with ST segment elevation MI (STEMI), unstable angina/non-ST segment elevation MI (UA/NSTEMI), and stable angina.
To examine outcomes from the time of PCI until 1-year follow-up, we plotted unadjusted cumulative incidence curves for MACE and its individual components for high-, intermediate-, and low-volume operators. Unadjusted MACE and mortality were compared for patients undergoing PCI by high-, intermediate-, and low-volume operators using the log-rank test; for unadjusted comparisons of the incidence of readmission for MI and unplanned revascularization by operator volume, Fine and Gray sub-distribution hazards were used to account for the competing risk of mortality.34 To quantify the effect of operator volume on MACE and all-cause mortality from time of PCI to 1-year post-PCI, we estimated a Cox proportional hazards model with a robust sandwich covariance matrix to account for clustering within operators. Risk-adjusted hazard ratios comparing high- and intermediate-volume operators with low-volume operators were derived. Due to violation of the proportional hazard assumption for operator volume, separate hazard ratios were estimated for in-hospital and post-discharge periods. We repeated this process for the outcomes of readmission for MI and unplanned revascularization at one year. Covariates for adjustment were the same as for in-hospital and 30-day outcomes. For all outcomes, we report unadjusted and adjusted hazard ratios (HRs) with 95% CIs for high- and intermediate-volume operators, with low-volume operators as the reference. We repeated these analyses treating operator volume as a continuous variable, and report unadjusted and risk-adjusted HRs with associated 95% confidence interval (CI) for a 50 unit increase in annual PCI volume.
Regression models were created for the overall dataset, and separately for the STEMI subgroup, the unstable angina/non-STEMI (UA/NSTEMI) subgroup, the stable angina subgroup, and subgroups of patients undergoing left main PCI, PCI of bifurcation lesions, and PCI of chronic total occlusions (CTO). To estimate the interaction between operator volume and estimated lifetime experience, we determined the association between operator volume and outcomes in the cohort of patients that underwent PCI in 2014, and separately among patients that underwent PCI by operators with 0–1, 2–4, and > 4 years of experience between 2009 and 2013.
For all analyses, a HR or OR < 1 indicates that higher PCI operator volume is associated with lower odds of the outcome compared with lower operator volume, and a HR or OR > 1 indicates that higher PCI operator volume is associated with higher odds of the outcome compared with lower operator volume.
All statistical analyses were performed by the Duke Clinical Research Institute using SAS version 9.3. The Duke University Medical Center Institutional Review Board granted a waiver of informed consent and authorization for this study, as data are collected for CathPCI without individual patient identifiers.
RESULTS
From July 1, 2009 to December 31, 2014, 8,936 operators performed 723,644 PCIs at 1,460 sites (Figure 1). We classified 3,505 (39.2%) operators that performed < 50 PCIs/year as low-volume operators, 2,878 (32.2%) that performed 50–100 PCIs/year as intermediate-volume operators, and 2,553 (28.6%) that performed > 100 PCIs/year as high-volume operators.
Figure 1:
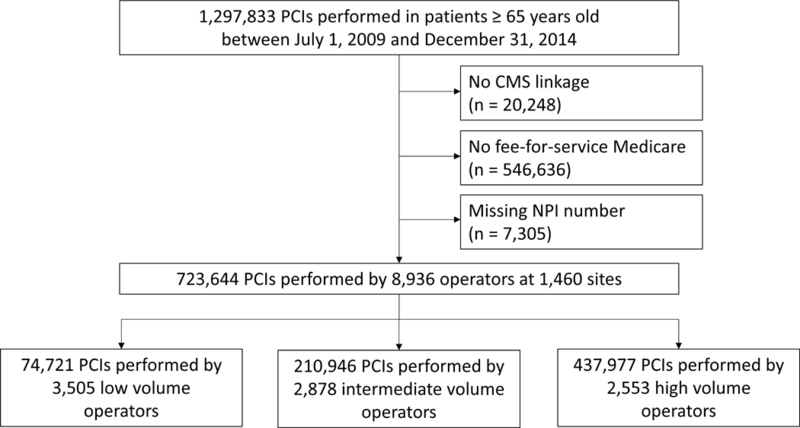
Study flow PCI, percutaneous coronary intervention; CMS, Centers for Medicare and Medicaid Services; NPI, National Provider Identification. Low-volume operators performed < 50 PCIs/year; intermediate-volume operators performed 50–100 PCIs/year; high-volume operators performed > 100 PCIs/year
Compared with high- and intermediate-volume operators, low-volume operators were more likely to practice in the western region of the U.S. (Table 1). They less frequently practiced at teaching hospitals and their hospitals had lower median annual PCI volumes (484 vs. 541 vs. 770 PCIs/year for low- vs. intermediate- vs. high-volume operators; p < 0.0001).
Table 1:
Hospital and patient characteristics by operator volume
| Overall (n = 723,644 PCIs; 8,936 operators) |
High (> 100 PCIs/year) (n = 437,977 PCIs; 2,553 operators) | Intermediate (50–100 PCIs/year) (n = 210,946 PCIs; 2,878 operators) | Low (< 50 PCIs/year) (n = 74,721 PCIs; 3,505 operators) | P value | |
|---|---|---|---|---|---|
| Hospital characteristics | |||||
| Hospital region | < 0.0001 | ||||
| Northeast | 104,044 (14.4%) | 68,760 (15.7%) | 27,577 (13.1%) | 7,707 (10.3%) | |
| Midwest | 204,514 (28.3%) | 133,946 (30.6%) | 56,238 (26.7%) | 14,330 (19.2%) | |
| South | 316,948 (43.8%) | 192,835 (44.0%) | 89,851 (42.6%) | 34,262 (45.9%) | |
| West | 98,114 (13.6%) | 42,436 (9.7%) | 37,280 (17.7%) | 18,398 (24.6%) | |
| Urban location | 395,016 (54.6%) | 249,338 (56.9%) | 106,636 (50.6%) | 39,042 (52.3%) | < 0.0001 |
| No. of beds | 393 (261, 576) | 404 (270, 587) | 364 (250, 540) | 366 (250, 569) | < 0.0001 |
| Annual PCI volume | 663 (406, 1083) | 770 (490, 1231) | 541 (340, 859) | 484 (268, 847) | < 0.0001 |
| Teaching hospital* | 333,131 (46.0%) | 220,510 (50.4%) | 85,423 (40.5%) | 27,207 (36.4%) | < 0.0001 |
| Private/community hospital | 641,075 (88.6%) | 386,876 (88.3%) | 187,723 (89.0%) | 66,476 (89.0%) | < 0.0001 |
| Patient characteristics | |||||
| Age | 74 (69, 80) | 74 (69, 80) | 74 (69, 80) | 74 (69, 79) | 0.0002 |
| Male | 448,322 (62.0%) | 270,732 (61.8%) | 130,939 (62.1%) | 46,651 (62.4%) | 0.002 |
| White | 662,486 (91.6%) | 404,367 (92.3%) | 192,200 (91.1%) | 65,919 (88.2%) | < 0.0001 |
| Admit source | < 0.0001 | ||||
| Emergency dept | 251,165 (34.7%) | 142,341 (32.5%) | 80,119 (38.0%) | 28,705 (38.4%) | |
| Transfer in | 129,335 (17.9%) | 85,911 (19.6%) | 33,633 (15,9%) | 9,791 (13.1%) | |
| Other | 342,418 (47.3%) | 209,294 (478%) | 96,978 (46.0%) | 36,146 (48.4%) | |
| BMI | 28 (25, 32) | 28 (25, 32) | 28 (25, 32) | 28 (25, 32) | < 0.0001 |
| GFR | 67 (52, 85) | 67 (52, 85) | 68 (52, 85) | 68 (53, 86) | < 0.0001 |
| Prior MI | 196,693 (27.2%) | 121,856 (27.8%) | 55,706 (26.4%) | 19,131 (25.6%) | < 0.0001 |
| Prior CHF | 111,603 (15.4%) | 70,498 (16.1%) | 30,937 (14.7%) | 10,168 (13.6%) | < 0.0001 |
| HF within 2 weeks | 97,340 (13.5%) | 60,451 (13.8%) | 27,913 (13.2%) | 8,976 (12.0%) | < 0.0001 |
| LV systolic dysfunction | 90,579 (12.5%) | 56,565 (12.9%) | 25,884 (12.3%) | 8,130 (10.9%) | < 0.0001 |
| Cardiogenic shock within 24 hours | 21,785 (3.0%) | 12,321 (2.8%) | 6,976 (3.3%) | 2,488 (3.3%) | < 0.0001 |
| Cardiac arrest within 24 hours | 12,573 (1.7%) | 7,093 (1.6%) | 4,020 (1.9%) | 1,460 (2.0%) | < 0.0001 |
| Diabetes | 269,926 (37.3%) | 165,231 (37.7%) | 77,520 (36.8%) | 27,175 (36.4%) | < 0.0001 |
| Cerebrovascular disease | 122,238 (16.9%) | 76,600 (17.5%) | 34,323 (16.3%) | 11,315 (15.1%) | < 0.0001 |
| Peripheral vascular disease | 115,744 (16.0%) | 72,681 (16.6%) | 32,577 (15.4%) | 10,486 (14.0%) | < 0.0001 |
| Hypertension | 626,220 (86.5%) | 381,203 (87.0%) | 171,054 (85.8%) | 63,963 (85.6%) | < 0.0001 |
| Chronic lung disease | 133,221 (18.4%) | 83,611 (19.1%) | 37,596 (17.8%) | 12,014 (16.1%) | < 0.0001 |
| Current/recent smoker | 100,217 (13.9%) | 61,172 (14.0%) | 29,156 (13.8%) | 9,889 (13.2%) | 0.05 |
| Dyslipidemia | 583,235 (80.6%) | 356,374 (81.4%) | 168,008 (79.7%) | 58,853 (78.8%) | < 0.0001 |
| Prior PCI | 246,607 (34.1%) | 152,378 (34.8%) | 69,455 (32.9%) | 24,774 (33.2%) | < 0.0001 |
| Prior CABG | 163,621 (22.6%) | 102,379 (23.4%) | 45,590 (21.6%) | 15,652 (21.0%) | < 0.0001 |
| Admission symptoms | < 0.0001 | ||||
| No symptoms | 55,855 (7.7%) | 32,540 (7.4%) | 16,674 (7.9%) | 6,641 (8.9%) | |
| Atypical chest pain | 23,541 (3.3%) | 14,395 (3.3%) | 6,747 (3.2%) | 2,399 (3.2%) | |
| Stable angina | 133,145 (16.9%) | 73,951 (16.9%) | 34,787 (16.5%) | 13,407 (17.9%) | |
| Unstable angina | 280,997 (38.8%) | 175,768 (40.1%) | 78,456 (37.2%) | 26,773 (35.8%) | |
| NSTEMI | 146,333 (20.2%) | 89,260 (20.4%) | 43,130 (20.5%) | 13,943 (18.7%) | |
| STEMI | 94,616 (13.1%) | 51,988 (11.9%) | 31,099 (14.7%) | 11,529 (15.4%) | |
| Appropriate PCI | 576,831 (79.7%) | 350,648 (80.1%) | 158,186 (79.7%) | 57,997 (77.6%) | < 0.0001 |
| PCI status | < 0.0001 | ||||
| Elective | 321,495 (44.4%) | 195,377 (44.6%) | 91,413 (43.3%) | 34,705 (46.5%) | |
| Urgent | 294,611 (40.7%) | 183,490 (41.9%) | 84,228 (39.9%) | 26,893 (36.0%) | |
| Emergency | 104,729 (14.5%) | 57,576 (13.2%) | 34,369 (16.3%) | 12,784 (17.1%) | |
| Salvage | 2,517 (0.4%) | 1,360 (0.3%) | 853 (0.4%) | 304 (0.4%) | |
| Discharge Medications | |||||
| Aspirin | 671,417 (96.1%) | 408,429 (96.5%) | 194,610 (95.8%) | 68,378 (95.1%) | < 0.0001 |
| P2Y12 inhibitor | 674,862 (95.7%) | 410,615 (96.0%) | 195,493 (95.3%) | 68,754 (94.8%) | < 0.0001 |
| Statin | 609,710 (88.4%) | 369,974 (88.6%) | 177,470 (88.4%) | 62,266 (87.3%) | < 0.0001 |
| Beta blocker | 568,723 (83.0%) | 347,100 (83.7%) | 164,628 (82.5%) | 56,995 (80.7%) | < 0.0001 |
, residency/fellowship program; BMI, body mass index; GFR, glomerular filtration rate, calculated by the Modification of Diet in Renal Disease equation; MI, myocardial infarction; CHF, congestive heart failure; HF, heart failure; LV, left ventricular; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
There were also significant differences in patient characteristics for PCIs performed by low-, intermediate-, and high-volume operators. Low-volume operators more frequently performed PCI on patients admitted through the emergency department than intermediate- or high-volume operators, and more frequently performed PCI for STEMI (15.4 vs. 14.7 vs. 11.9% for low- vs. intermediate- vs. high-volume operators, p < 0.0001) and PCI with an emergency indication (17.1 vs. 16.3 vs. 13.2% for low- vs. intermediate- vs. high-volume operators, p < 0.0001). Patients undergoing PCI by low-volume operators had a lower prevalence of known chronic vascular disease and risk factors than those undergoing PCI by high- and intermediate-volume operators. Discharge prescription rates of aspirin, P2Y12 inhibitors, statins, and beta blockers were similar among operator types.
Low-volume operators, as compared with intermediate- and high-volume operators, less frequently attempted 2 or more lesions in a single visit to the cardiac catheterization lab (22.8 vs. 25.4 vs. 28.8% for low- vs. intermediate- vs. high-volume operators) (Table 2). High-volume operators used drug eluting stents slightly more often than low and intermediate volume operators (69.3% vs. 68.4% vs. 71.6% for low- vs. intermediate- vs. high-volume operators) Low-volume operators less frequently attempted left main, bifurcation, or chronic total occlusion lesions, but differences in the proportion of PCIs these types of procedures comprised between high- and low-volume operators were small (< 2%).
Table 2:
PCI characteristics by operator volume
| Overall (n = 723,644 PCIs; 8,936 operators) |
High (> 100 PCIs/year) (n = 437,977 PCIs; 2,553 operators) | Intermediate (50–100 PCIs/year) (n = 210,946 PCIs; 2,878 operators) | Low (< 50 PCIs/year) (n = 74,721 PCIs; 3,505 operators) | P value | |
|---|---|---|---|---|---|
| Lesion segment | < 0.0001 | ||||
| Left main | 17,508 (2.4%) | 12,006 (2.7%) | 4,263 (2.0%) | 1,239 (1.7%) | |
| Proximal LAD | 107,708 (14.9%) | 64,314 (14.7%) | 74,108 (35.1%) | 26,577 (35.6%) | |
| pRCA/mLAD/pLCx | 253,480 (35.0%) | 152,795 (34.9%) | 74,108 (35.1%) | 26,577 (35.6%) | |
| Other | 342,147 (47.3%) | 207,207 (47.3%) | 99,952 (47.4%) | 34,988 (46.8%) | |
| Previously treated lesion | 58,792 (8.1%) | 35,826 (8.2%) | 16,842 (8.0%) | 6,124 (8.2%) | 0.03 |
| Vein graft lesion | 55,062 (7.6%) | 33,465 (7.6%) | 16,058 (7.6%) | 5,539 (7.4%) | 0.19 |
| Chronic total occlusion | 17,545 (17.5%) | 10,695 (18.7%) | 4,997 (15.8%) | 1,853 (16.3%) | < 0.0001 |
| Bifurcation lesion | 80,112 (11.1%) | 49,305 (11.3%) | 23,363 (11.1%) | 7,444 (10.0%) | < 0.0001 |
| Lesion length | 16 (12, 23) | 16 (12, 24) | 15 (12, 23) | 15 (12, 20) | < 0.0001 |
| No. lesions attempted in lab visit | < 0.0001 | ||||
| 1> | 527,321 (72.9%) | 312,128 (71.3%) | 157,489 (74.7%) | 57,704 (77.2%) | |
| 2 | 155,520 (21.5%) | 98,319 (22.5%) | 43,211 (20.5%) | 13,990 (18.7%) | |
| ≥ 3 | 40,803 (5.6%) | 27,530 (6.3%) | 10,246 (4.9%) | 3,027 (4.1%) | |
| Radial access | 87,714 (12.1%) | 59,479 (13.6%) | 23,483 (11.1%) | 4,752 (6.4%) | < 0.0001 |
| Unfractionated heparin | 367,617 (50.9%) | 226,456 (51.8%) | 106,509 (50.6%) | 34,652 (46.5%) | < 0.0001 |
| Bivalirudin | 434,174 (60.0%) | 259,810 (59.4%) | 127,792 (60.6%) | 56,582 (62.4%) | < 0.0001 |
| Glycoprotein IIb/IIIa inhibitor | 159,510 (22.1%) | 88,154 (20.2%) | 53,116 (25.2%) | 18,240 (24.5%) | < 0.0001 |
| Drug eluting stent | 509,776 (70.5%) | 313,739 (71.6%) | 144,233 (68.4%) | 51,804 (69.3%) | < 0.0001 |
| Fluoroscopy time (minutes) | 12 (8, 19) | 12 (7, 19) | 13 (8, 20) | 13 (8, 21) | < 0.0001 |
| Contrast volume (mL) | 180 (130, 235) | 175 (126, 230) | 180 (140, 240) | 185 (140, 250) | < 0.0001 |
| IVUS performed | 13,358 (20.8%) | 8,862 (22.1%) | 3,406 (18.6%) | 1,090 (18.7%) | < 0.0001 |
| Successful PCI | 673,076 (93.5%) | 409,851 (94.0%) | 194,666 (92.8% | 68,559 (92.3%) | < 0.0001 |
LAD, left anterior descending coronary artery; pLCx, proximal left circumflex coronary artery; mLAD, mid-LAD coronary artery; pRCA, proximal right coronary artery; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention. Successful PCI was defined as successful dilation of all lesions attempted.
Association between operator volumes and short-term outcomes
Overall, 15,319 patients (2.2%) died while hospitalized. The incidence of in-hospital death was 2.4% for low-volume operators, 2.3% for intermediate-volume operators, and 2.0% for high-volume operators (Table 3) (OR 0.95, 95% CI 0.89–1.01 for intermediate vs. low; OR 0.82, 95% CI 0.77–0.87 for high vs. low). When patients were stratified by type of presentation, patients undergoing PCI for STEMI by high- and intermediate-volume operators had a significantly lower incidence of death than those undergoing PCI by low volume operators, but operator volume category was not associated with mortality for patients presenting with UA/NSTEMI or stable angina. When patients were stratified by lesion type, patients undergoing PCI for bifurcation, left main, and CTO lesions had lower in-hospital mortality when procedures were performed by high-volume operators compared with low-volume operators; only patients undergoing PCI for CTO lesions had lower mortality when procedures were performed by intermediate-volume operators compared with low-volume operators. After adjustment for baseline patient and hospital characteristics, both high- and intermediate-volume operators had lower in-hospital mortality than low-volume operators (OR 0.92, 95% CI 0.86–0.96 for intermediate vs. low; OR 0.79, 95% CI 0.75–0.83 for high vs. low). Risk-adjusted outcomes in subgroups largely paralleled unadjusted outcomes and are presented in Table 3.
Table 3:
In-hospital mortality by operator volume
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
|---|---|---|
| Overall | ||
| High vs. low volume | 0.81 (0.77–0.87)* | 0.79 (0.75–0.83)* |
| Intermediate vs. low volume | 0.95 (0.89–1.01) | 0.91 (0.86–0.96)* |
| STEMI only | ||
| High vs. low volume | 0.90 (0.85–0.96)* | 0.87 (0.82–0.92)* |
| Intermediate vs. low volume | 0.92 (0.86–0.99)* | 0.90 (0.84–0.95)* |
| UA/NSTEMI only | ||
| High vs. low volume | 0.95 (0.87–1.05) | 0.87 (0.80–0.95) * |
| Intermediate vs. low volume | 1.02 (0.92–1.13) | 0.98 (0.89–1.08) |
| Stable angina only | ||
| High vs. low volume | 0.82 (0.55–1.22) | 0.76 (0.51–1.12) |
| Intermediate vs. low volume | 1.09 (0.72–1.65) | 1.05 (0.69–1.58) |
| Chronic total occlusion | ||
| High vs. low volume | 0.55 (0.45–0.67)* | 0.71 (0.60–0.86)* |
| Intermediate vs. low volume | 0.80 (0.66–0.98)* | 0.86 (0.72–1.04) |
| Bifurcation lesion | ||
| High vs. low volume | 0.78 (0.68–0.90)* | 0.77 (0.67–0.88)* |
| Intermediate vs. low volume | 0.91 (0.79–1.06) | 0.89 (0.77–1.02) |
| Left main lesion | ||
| High vs. low volume | 0.72 (0.57–0.91)* | 0.73 (0.60–0.89)* |
| Intermediate vs. low volume | 1.15 (0.91–1.46) | 1.07 (0.87–1.31) |
p < 0.05. MI, myocardial infarction; OR, odds ratio; CI, confidence interval; STEMI, ST segment elevation MI; UA, unstable angina; NSTEMI, non-ST segment elevation MI; CTO, chronic total occlusion. Low-volume operators performed < 50 PCIs/year; intermediate-volume operators performed 50–100 PCIs/year; high-volume operators performed > 100 PCIs/year
In the first 30 days, 24,097 patients died; the cumulative incidence of mortality was 3.5% for low-volume operators, 3.5% for intermediate volume operators, and 3.2% for high volume operators (OR 1.00, 95% CI 0.95–1.06 for intermediate vs. low; OR 0.92, 95% CI 0.88–0.97 for high vs. low) (Supplement Table 2). After risk adjustment, 30-day mortality remained lower for patients treated by high-volume operators compared with low-volume operators, but not for intermediate-volume operators compared with low-volume operators. By contrast, operator volume group had no association with the incidence of 30-day bleeding, on unadjusted or risk-adjusted analyses (risk-adjusted OR 1.00, 95% CI 0.94–1.06 for intermediate vs. low; risk-adjusted OR 0.96, 95% CI 0.91–1.01 for high vs. low) (Supplement Table 2). In the subgroup of patients with UA/NSTEMI, patients treated by intermediate- and high-volume operators had a lower incidence of 30-day bleeding than those treated by low-volume operators on risk-adjusted analyses.
Association between operator volumes and 1-year outcomes
Overall 109,735 patients (15.2%) had a MACE event over the course of 1-year follow-up. The unadjusted 1-year cumulative incidence of MACE was 15.9% for low-volume operators, 16.2% for intermediate-volume operators, and 16.5% for high-volume operators (Figure 2 and Supplemental Table 3) (p = 0.004 by log-rank test). When patients were stratified by presentation subgroup, those undergoing PCI for UA/NSTEMI and stable angina had cumulative incidences of MACE that were significantly different by operator volume stratum. When stratified by lesion subtype, patients undergoing PCI for CTO and bifurcation lesions had cumulative incidences of MACE that were significantly different by operator volume stratum.
Figure 2:
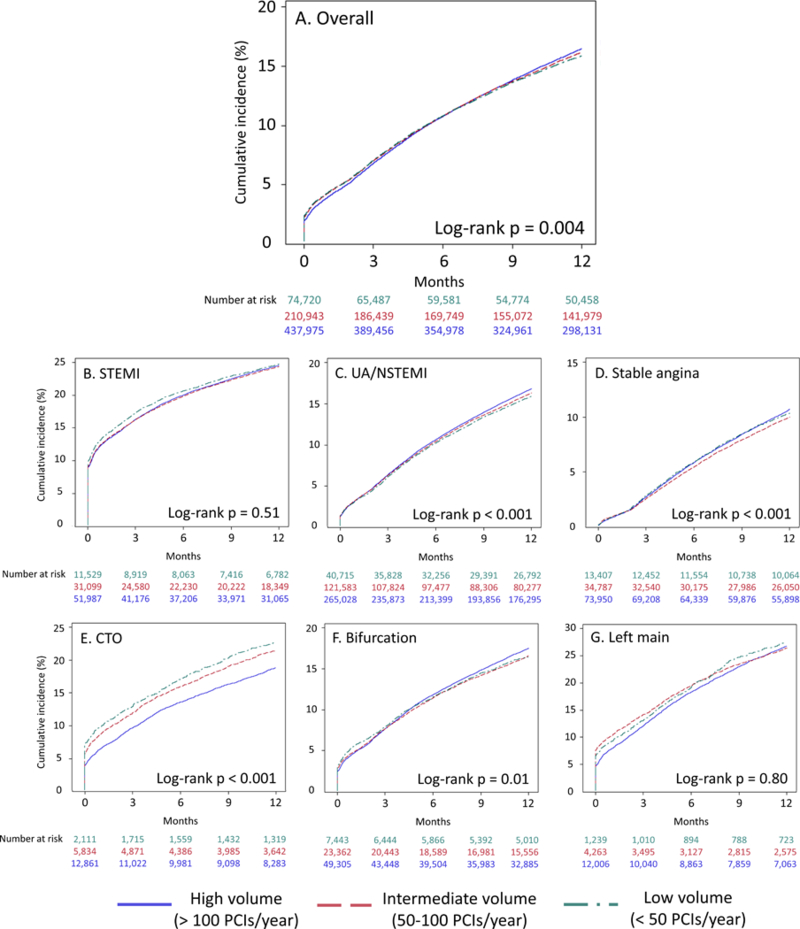
1-year cumulative incidence of MACE by operator volume overall (A), in patients presenting with STEMI (B), UA/NSTEMI (C), and stable angina (D), and those undergoing PCI of chronic total occlusion (E), bifurcation (F), and left main (G) lesions MACE, major adverse cardiovascular events (all-cause death, readmission for myocardial infarction, unplanned revascularization); STEMI, ST segment elevation myocardial infarction; UA, unstable angina; NSTEMI, non-ST segment elevation myocardial infarction; CTO, chronic total occlusion. Operator volume defined as in Figure 1.
The proportional hazards assumption was not satisfied for the outcome of MACE or mortality from the time of PCI to 1-year follow-up, so Cox modeling could not be performed. The proportional hazards assumption was satisfied for the time from hospital discharge to 1-year follow-up for both outcomes.
On unadjusted analyses, both high- and intermediate-volume operators had a higher incidence of MACE from hospital discharge to 1-year follow-up, but these differences were attenuated and non-significant after adjustment for baseline risk factors (adjusted HR 0.99, 95% CI 0.96–1.01 for intermediate vs. low; adjusted HR 1.01, 95% CI 0.99–1.04 for high vs. low) (Table 4). There were no significant differences in 1-year post-discharge MACE in any presentation or lesion subgroup on unadjusted analyses; patients undergoing PCI for stable angina by intermediate-volume operators had a nominally significant lower risk of MACE than low-volume operators on risk-adjusted analysis.
Table 4:
1-year post-discharge MACE and all-cause mortality by operator volume
| MACE | Mortality | |||
|---|---|---|---|---|
| Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|
| Overall | ||||
| High vs. low volume | 1.07 (1.04–1.11)* | 1.01 (0.99–1.04) | 1.11 (1.07–1.15)* | 1.04 (1.00–1.08) |
| Intermediate vs. low volume | 1.03 (1.00–1.06)* | 0.99 (0.96–1.01) | 1.07 (1.03–1.11)* | 1.01 (0.97–1.05) |
| STEMI only | ||||
| High vs. low volume | 1.04 (0.98–1.10) | 0.98 (0.93–1.04) | 1.07 (1.00–1.15) | 1.01 (0.94–1.09) |
| Intermediate vs. low volume | 1.01 (0.95–1.07) | 0.97 (0.91–1.03) | 1.06 (0.98–1.14) | 1.00 (0.93–1.08) |
| UA/NSTEMI only | ||||
| High vs. low volume | 1.07 (1.04–1.11)* | 1.00 (0.97–1.04) | 1.13 (1.08–1.19)* | 1.04 (1.00–1.09) |
| Intermediate vs. low volume | 1.03 (0.99–1.06) | 1.00 (0.97–1.04) | 1.08 (1.03–1.13)* | 1.04 (0.99–1.09) |
| Stable angina only | ||||
| High vs. low volume | 1.04 (0.97–1.11) | 0.98 (0.92–1.04) | 1.01 (0.92–1.11) | 0.93 (0.84–1.02) |
| Intermediate vs. low volume | 0.96 (0.89–1.03) | 0.93 (0.87–0.99)* | 0.94 (0.85–1.04) | 0.89 (0.84–1.02) |
| Chronic total occlusion | ||||
| High vs. low volume | 0.92 (0.82–1.04) | 1.06 (0.93–1.21) | 0.92 (0.78–1.08) | 1.10 (0.93–1.31) |
| Intermediate vs. low volume | 1.00 (0.88–1.14) | 1.01 (0.87–1.16) | 1.02 (0.86–1.21) | 1.03 (0.85–1.23) |
| Bifurcation lesion | ||||
| High vs. low volume | 1.13 (1.05–1.22)* | 1.07 (0.99–1.16) | 1.11 (1.01–1.23)* | 1.05 (0.95–1.16) |
| Intermediate vs. low volume | 1.03 (0.95–1.12) | 1.00 (0.92–1.09) | 0.99 (0.89–1.10) | 0.95 (0.85–1.06) |
| Left main lesion | ||||
| High vs. low volume | 1.05 (0.91–1.20) | 0.97 (0.84–1.12) | 1.13 (0.94–1.35) | 1.01 (0.84–1.22) |
| Intermediate vs. low volume | 0.91 (0.79–1.06) | 0.97 (0.84–1.12) | 1.00 (0.83–1.22) | 0.89 (0.73–1.10) |
p < 0.05. MACE, major adverse cardiovascular events (all-cause death, readmission for myocardial infarction, unplanned revascularization); HR, hazard ratio; all other abbreviations per Table 3. Low-volume operators performed < 50 PCIs/year; intermediate-volume operators performed 50–100 PCIs/year; high-volume operators performed > 100 PCIs/year
The unadjusted cumulative incidence of 1-year mortality was 9.5% for low-volume operators, 9.9% for intermediate-volume operators, and 9.8% for high-volume operators (Figure 3 and Supplemental Table 3) (p = 0.03 by log-rank test). When patients were stratified by presentation subgroup, only those presenting with UA/NSTEMI had a cumulative incidence of 1-year mortality that was significantly different by operator volume stratum. When stratified by lesion subtype, patients undergoing PCI for CTO had a cumulative incidence of mortality that was significantly different by operator volume stratum.
Figure 3:
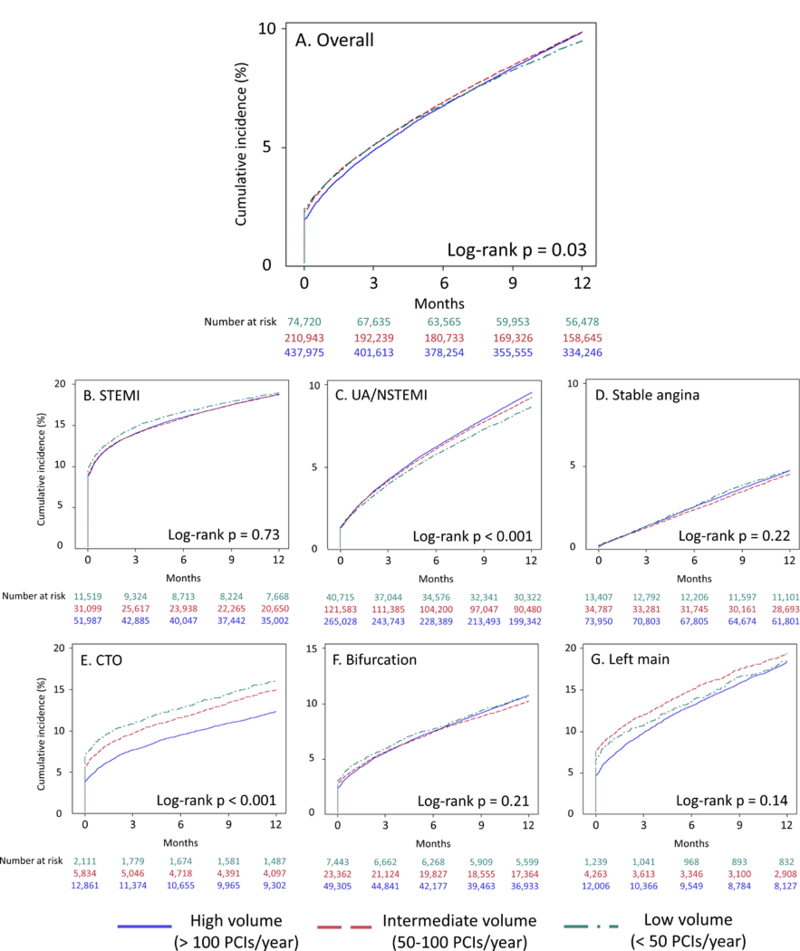
1-year cumulative incidence of all-cause mortality by operator volume overall (A), in patients presenting with STEMI (B), UA/NSTEMI (C), and stable angina (D), and those undergoing PCI of chronic total occlusion (E), bifurcation (F), and left main (G) lesions All abbreviations as in Figure 2; operator volumes defined as in Figures 1 and 2.
On unadjusted analyses, both high- and intermediate-volume operators had a higher incidence of mortality from hospital discharge to 1-year follow-up than low-volume operators, but there was no difference in mortality on risk-adjusted analyses (adjusted HR 1.01, 95% CI 0.97–1.05 for intermediate vs. low; adjusted HR 1.04, 95% CI 1.00–1.08 for high vs. low) (Table 4). There were no differences in mortality by operator volume stratum among any presentation or lesion subgroup on risk-adjusted analyses.
The unadjusted rate of readmission for MI and unplanned repeat revascularization was higher for high-volume operators than low-volume operators. After risk adjustment, these differences were attenuated and non-significant, and rates of readmission for recurrent MI and unplanned revascularization did not differ significantly by operator cohort in the overall population (Supplemental Table 4). Risk-adjusted rates of readmission for recurrent MI and unplanned revascularization were not different by operator cohort in any presentation or lesion subgroup except for patients undergoing left main PCI, in which patients undergoing PCI by intermediate-volume operators had a nominally significant lower risk of unplanned revascularization than low-volume operators.
Operator volume, when analyzed as a continuous variable, was linearly associated with mortality from the time of PCI to 1-year follow-up, up to a threshold of 200 PCIs annually: For every 50 PCI increase in operator volume up to 200, there was a corresponding 1% decrease in the risk-adjusted hazard of 1-year death (HR 0.99, 95% CI 0.98–1.00, p = 0.02) (Supplemental Table 5). This finding was driven by an inverse association between operator volume and in-hospital mortality (OR 0.95, 95% CI 0.94–0.97); there was no association between operator volume and risk-adjusted post-discharge mortality (HR 1.00, 95% CI 0.99–1.01). No significant association was observed between operator volume and 1-year mortality in any presentation subgroup. There was no significant association between operator volume, expressed continuously, and the risk-adjusted incidence of 1-year MACE (HR 1.00, 95% CI 0.99–1.00 per 50 PCI increase in annual operator volume) nor the risk-adjusted incidence of MACE from discharge to 1-year follow-up (HR 1.01, 95% CI 1.00–1.01 per 50 PCI increase in annual operator volume). There was a direct association between operator volume and the incidence of 1-year unplanned revascularization, which persisted after adjustment for baseline risk factors (risk-adjusted HR 1.02, 95% CI 1.01–1.03 per 50 PCI increase in annual operator volume up to 200).
Interaction between operator experience and outcomes
In 2014, 6,524 operators performed 115,137 PCIs; 652 of these operators (10.0%) had been active 0–1 years between 2009 and 2013, 1,469 (22.5%) 2–4 years, and 4,403 (67.5%) > 4 years. Operators with 0–1 year of experience were more likely to be low volume operators than those with more experience (58.5% of operators with 0–1 year experience, 32.6% of those with 2–4 years experience, 30.5% of those with > 4 years experience, p < 0.001) (Supplemental Table 6).
For PCIs performed in 2014, the association between operator volume and in-hospital mortality among all operators was similar to that during the entire study period; high and intermediate volume operators had lower unadjusted and risk-adjusted in-hospital mortality than low volume operators. For operators with 0–1 year experience, there was no significant association between operator volume and in-hospital mortality and the point estimate for the effect approached 1.0 (Table 5). By contrast, for operators with 2–4 and > 4 years experience, high volume operators had significantly lower unadjusted and risk-adjusted in-hospital mortality than low volume operators. Similar to the overall cohort, there was no association between operator volume and 1-year post-discharge mortality or MACE in any operator experience subset.
Table 5:
Outcomes by operator volume and overall experience
| In-hospital mortality | 1-year post-discharge mortality | 1-year post-discharge MACE | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Overall† | ||||||
| High vs. low volume | 0.77 (0.68–0.87)* |
0.76 (0.68–0.85)* |
1.10 (1.00–1.21)* |
1.04 (0.94–1.15) |
1.12 (1.04–1.21)* |
1.05 (0.97–1.14) |
| Intermediate vs. low volume | 0.92 (0.80–1.05) |
0.88 (0.78–0.99)* |
1.07 (0.96–1.18) |
1.01 (0.91–1.13) |
1.08 (0.99–1.17) |
1.02 (0.94–1.11) |
| 0–1 years experience‡ | ||||||
| High vs. low volume | 0.99 (0.69–1.43) |
1.02 (0.72–1.45) |
0.97 (0.75–1.20) |
0.94 (0.72–1.24) |
0.99 (0.79–1.23) |
0.98 (0.77–1.25) |
| Intermediate vs. low volume | 0.92 (0.63–1.34) |
0.95 (0.67–1.35) |
0.89 (0.66–1.20) |
0.83 (0.61–1.13) |
0.90 (0.70–1.16) |
0.87 (0.66–1.13) |
| 2–4 years experience‡ | ||||||
| High vs. low volume | 0.77 (0.59–1.00)* |
0.75 (0.60–0.95)* |
1.21 (0.99–1.49) |
1.12 (0.90–1.38) |
1.10 (0.94–1.29) |
1.02 (0.87–1.20) |
| Intermediate vs. low volume | 0.90 (0.68–1.19) |
0.83 (0.65–1.06) |
1.16 (0.93–1.45) |
1.15 (0.92–1.45) |
1.02 (0.86–1.21) |
1.00 (0.84–1.20) |
| > 4 years experience‡ | ||||||
| High vs. low volume | 0.77 (0.66–0.90)* |
0.74 (0.64–0.85)* |
1.14 (1.02–1.28)* |
1.08 (0.95–1.22) |
1.19 (1.08–1.30)* |
1.10 (1.00–1.22) |
| Intermediate vs. low volume | 0.93 (0.79–1.10) |
0.88 (0.76–1.02) |
1.10 (0.97–1.25) |
1.03 (0.90–1.18) |
1.15 (1.04–1.27)* |
1.07 (0.97–1.19) |
; p < 0.05;
, PCIs performed in 2014 only;
, number of years active (performed at least 1 PCI) between 2009 and 2013
HR, hazard ratio; CI, confidence interval. Low-volume operators performed < 50 PCIs/year; intermediate-volume operators performed 50–100 PCIs/year; high-volume operators performed > 100 PCIs/year
DISCUSSION
In this nationally representative study, we found that operators performing fewer than 50 PCIs annually, the ACC/AHA/SCAI recommended minimum number of procedures to maintain competency, had a higher risk-adjusted rate of in-hospital mortality, but did not have a higher risk-adjusted hazard of death or MACE over 1-year post-discharge follow-up than higher volume operators. On unadjusted analyses, high-volume operators had a higher incidence of long-term MACE and mortality than low volume operators, but high-volume operators performed more complex PCI on patients at higher risk of long-term cardiovascular events. When operator volume was analyzed continuously, higher operator volume was associated with lower risk-adjusted mortality over 1-year follow-up, but this finding is driven entirely by in-hospital death, and there was no association between operator volume and post-discharge mortality or MACE. Overall, given that 44% of operators nationwide perform fewer than 50 PCIs annually,10 these findings are reassuring that the small inverse association between operator volume and short-term outcomes is not compounded by a similar association between operator volume and long-term outcomes given current case selection patterns.
Over two dozen studies have now considered the association between PCI operator volume and outcomes.10–12, 35 The most largest contemporary study, an analysis of the nationally-representative CathPCI database, found a modest, though significant, inverse relationship between operator volume and in-hospital mortality, broadly consistent with the totality of evidence from the stent era.10 However, only two studies have described the association between operator volume and > 30 day outcomes. Mueller et al. found no association between operator experience and the 24-month incidence of death, myocardial infarction or clinically-driven revascularization in a 300-patient, 6-operator, single-center study.36 A substudy of the Enhanced Suppression of the Platelet glycoprotein IIb/IIIa Receptor with Integrilin Therapy (ESPRIT) trial involving 1338 patients divided operators into those performing < 100 PCIs (n = 91) and ≥ 100 PCIs annually (n = 1136), and found that there was no association between operator volume cohort and 1-year death, MI, or target vessel revascularization.37 Though our study includes only patients ≥ 65 years old, it represents the largest, most contemporary, and most nationally representative examination of the association between operator volume and long-term outcomes.
We found, most importantly, that the association between operator volume and outcomes, including MACE and mortality, is not static from the time of PCI to 1-year follow-up. On unadjusted and risk-adjusted analyses, high- and intermediate- volume operators have a lower incidence of in-hospital mortality than low-volume operators, defined as per the ACC/AHA/SCAI’s clinical competency document, which is consistent across presentation and lesion type subgroups. However, from the time of hospital discharge to 1-year follow-up, low-volume operators had a lower unadjusted rate of 1-year MACE, mortality, and unplanned revascularization than intermediate- or high-volume operators. However, case mix differed for low-, intermediate-, and high-volume operators, with low-volume operators more frequently performing PCI for STEMI and less frequently performing PCI on patients with established cardiovascular disease and cardiovascular risk factors. Though differences in case mix were modest, after adjustment for baseline patient and lesion characteristics, differences in MACE and mortality between operator cohorts were attenuated and non-significant from the time of hospital discharge to 1-year follow-up. When we analyzed operator volume as a continuous variable, an approach that affords maximal power to detect a significant association between operator volume and outcomes, we found a small inverse association between operator volume and 1-year mortality following PCI. This association is driven by in-hospital events, as there was no significant association between operator volume and 1-year post-discharge MACE or mortality, though there was a direct association between operator volume, analyzed continuously, and risk-adjusted unplanned revascularization. However, this association may be related to practice patterns of high-volume operators: Higher volume operators more often perform PCIs in patients with clinical and angiographic risk factors for restenosis (multi-vessel PCI, chronic total occlusion, diabetes mellitus),38, 39 and may be more likely than lower volume operators to perform PCI for worsening angina.
Our findings may seem at odds with volume-outcome analyses in other specialties. For example, in patients undergoing surgical procedures for colon cancer, breast cancer, head and neck cancer, higher surgeon volume is associated with a reduced incidence of long-term mortality.40 While there are various explanations for the operator volume-outcome relationship, the most commonly acknowledged one is the “practice makes perfect” hypothesis, which holds that higher volumes lead to better operative decision-making and technique.41 This holds true for in-hospital outcomes, with a modest, but statistically significant, relationship between greater operator PCI volume and lower risk of mortality.10 When examining longer-term outcomes we hypothesized that the same relationship would hold based on an assumption that lower volume operators would be technically less proficient than higher volume operators. High-volume operators use techniques that are associated with reduced adverse outcomes following PCI like radial access and greater drug-eluting stent use.10 It is possible that they may also use techniques to reduce suboptimal stent deployment, which is associated with higher rates of stent thrombosis and re-stenosis, ultimately leading to unplanned revascularization, recurrent MI, or death.16–20 The CathPCI dataset does not allow for granularity regarding stent implantation technique; however, the difference in short-term in-hospital outcomes and lack of difference in long-term outcomes between high-, intermediate-, and low-volume operators indicates that if operator volume is associated with technical proficiency, the effect is manifest prior to hospital discharge. Long-term outcomes following PCI may be more likely to be a consequence of underlying disease than anything that happened during the procedure itself.
Our results have implications for professional society guidelines. Though operators performing fewer than the ACC/AHA/SCAI’s minimum recommendation of 50 PCIs annually have a higher rate of in-hospital mortality following PCI, the relationship between operator volume and short-term mortality is modest and of uncertain clinical significance, especially considering that these low-volume operators may play a role in maintaining critical access to primary PCI for STEMI.10 Intriguingly, this association does not appear to hold for operators in their first years of independent practice, suggesting that high-intensity fellowship training may have residual benefits as an operator transitions to independent practice, or that hospitals may exercise greater oversight over these new operators. When composing operator volume recommendations, professional society guideline committees need to balance maximizing patient safety with maintaining access to critical emergency procedures, and an approach that focuses on identifying and disseminating best practices among all operators, regardless of volume, may be preferable to strict volume recommendations.10, 42 Since operator volume is not associated with long-term outcomes among patients surviving the index hospitalization, the guidelines should consider volume standards in the context of overall care quality, including process performance measures relevant to the post-PCI population, such as prescription of secondary prevention strategies aimed at improving in-hospital and long-term outcomes.
Limitations
This study is a retrospective analysis, and is subject to unmeasured confounding. Though our risk-adjusted analyses account for measured severity of patient presentation, it is possible, for example, that higher volume operators are more likely to attempt PCI in patients with unmeasured variables indicating poor long-term prognosis, which would mask true differences in long-term outcomes. With regard to short-term outcomes, it is possible that operators with poorer outcomes and/or less skill receive fewer referrals and have low volumes for that reason. PCIs analyzed in this report were performed between 2009 and 2014, and practice patterns may have changed in the intervening years; however, PCI has generally grown safer over the years, attenuating the operator-volume association.1, 10 As we did not find an association between operator volume and long-term outcomes during our study period, it is not expected that one would exist at present. Furthermore, this analysis only includes patients ≥ 65 years old, but older adults comprise a growing percentage of patients undergoing PCI,43, 44 and there is no reason to suspect that the relationship between operator volume and outcomes should be affected by patient age. Our analysis does exclude a number of low-volume operators who performed PCI on younger patients, but did not perform a single PCI on a patient in the linked database, so our descriptive analysis of the proportion of high-, intermediate-, and low-volume operators underestimates the proportion of low-volume operators; an analysis of PCIs performed on all patients in CathPCI, not just those > 65 years old, found that 44% of operators performed < 50 PCIs annually, compared with 39% in this analysis.10 Moreover, as we were unable to capture unplanned index vessel or index lesion revascularization, we examined all unplanned revascularizations; differences in operator technique would not affect the hazard of future unplanned revascularization in non-index vessels, and including non-index vessel revascularization may add statistical noise that obscures true operator-related differences in rates of index vessel or index lesion revascularization. In addition, CathPCI does not include variables that could be used to approximate overall experience, such as number of years in practice, total lifetime volume, or board certification. We were able to estimate number of years in practice, but this estimation is imprecise, and operators who performed PCIs in non-CathPCI hospitals may have been counted among the operators with 0–1 years of experience. Though the CathPCI registry captures PCI procedures performed in > 90% of U.S. cardiac catheterization labs, it does not capture procedures performed in Veterans Administration or Department of Defense hospitals, and operators performing PCIs in these hospitals and hospitals that participate in the CathPCI registry will have their procedure volumes undercounted. Despite the above limitations, CathPCI is a nationally-representative, quality-controlled database, and our analysis of > 700,000 PCIs performed by nearly 9,000 operators represents the most comprehensive examination of the association between operator volume and long-term outcomes to date.
CONCLUSIONS
Low annual operator PCI volume, defined as fewer than 50 PCIs annually, is associated with worse in-hospital outcomes compared with higher operator volume, but is not associated with higher rates of 1-year MACE or mortality among patients older than 65 years.
Supplementary Material
CLINICAL PERSPECTIVE
What is new?
• Though high and intermediate volume percutaneous coronary intervention (PCI) operators have a lower incidence of in-hospital death than low-volume operators after adjustment for patient characteristics, there is no association between operator volume and 1-year post-discharge outcomes
• The associations between operator volumes and outcomes are consistent regardless of indication for PCI and in patients with complex lesion subtypes; however, there is no association between operator volume and in-hospital mortality among operators with 0–1 years experience
What are the clinical implications?
• Since the association between operator volumes and in-hospital mortality is small and operator volume is not associated with long-term outcomes among patients surviving the index hospitalization, clinical practice guidelines should consider volume standards in the context of overall care quality and should consider de-emphasizing operator volume as a quality measure
Acknowledgments
FUNDING SOURCES
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry. Dr. Fanaroff was funded by career development grants from the National Institutes of Health (5T32HL069749–13) and American Heart Association (17FTF33661087) during the conduct of the study.
Footnotes
DISCLOSURES
AC Fanaroff: Dr. Fanaroff reports research funding from Gilead Sciences outside the submitted work
P Zakroysky: Ms. Zakroysky reports no relevant disclosures
LA Kaltenback: Ms. Kaltenbach reports no relevant disclosures
D Wojdyla: Mr. Wojdyla reports no relevant disclosures
MW Sherwood: Dr. Sherwood reports honoraria/consulting fees from Janssen
MT Roe: Dr. Roe reports no relevant disclosures
TY Wang: Dr. Wang reports no relevant disclosures
ED Peterson: Dr. Peterson reports no relevant disclosures
HS Gurm: Dr. Gurm reports research funding from the National Institutes of Health and Blue Cross Blue Shield of Michigan, and consulting fees from Osprey Medical
MG Cohen: Dr. Cohen reports no relevant disclosures
JC Messenger: Dr. Messenger reports no relevant disclosures
SV Rao: Dr. Rao reports no relevant disclosures
REFERENCES
- 1.Hannan EL, Racz M, Ryan TJ, McCallister BD, Johnson LW, Arani DT, Guerci AD, Sosa J and Topol EJ. Coronary angioplasty volume-outcome relationships for hospitals and cardiologists. J Am Med Assoc. 1997;277:892–898. [PubMed] [Google Scholar]
- 2.McGrath PD, Wennberg DE, Malenka DJ, Kellett MA, Ryan TJ, O’Meara JR, Bradley WA, Hearne MJ, Hettleman B and Robb JF. Operator volume and outcomes in 12,988 percutaneous coronary interventions. J Am Coll Cardiol. 1998;31:570–576. [DOI] [PubMed] [Google Scholar]
- 3.Moscucci M, Share D, Smith D, O’Donnell MJ, Riba A, McNamara R, Lalonde T, Defranco AC, Patel K and Rogers EK. Relationship between operator volume and adverse outcome in contemporary percutaneous coronary intervention practice: an analysis of a quality-controlled multicenter percutaneous coronary intervention clinical database. J Am Coll Cardiol. 2005;46:625–632. [DOI] [PubMed] [Google Scholar]
- 4.Minges KE, Wang Y, Dodson JA, Normand S-LT, Rathore SS, Ting HH, Nallamothu BK, Messenger J, Krumholz HM and Curtis JP. Physician Annual Volume and In-Hospital Mortality Following Percutaneous Coronary Intervention. Circulation. 2011;124:A16550. [Google Scholar]
- 5.Badheka AO, Patel NJ, Grover P, Singh V, Patel N, Arora S, Chothani A, Mehta K, Deshmukh A and Savani GT. Impact of annual operator and institutional volume on percutaneous coronary intervention outcomes: a 5-year United States experience (2005–2009). Circulation. 2014;130:1392–1406. [DOI] [PubMed] [Google Scholar]
- 6.Malenka DJ, McGrath PD, Wennberg DE, Ryan TJ, Kellett MA, Shubrooks SJ, Bradley WA, Hettlemen BD, Robb JF and Hearne MJ. The relationship between operator volume and outcomes after percutaneous coronary interventions in high volume hospitals in 1994–1996: the northern New England experience. J Am Coll Cardiol. 1999;34:1471–1480. [DOI] [PubMed] [Google Scholar]
- 7.Harjai KJ, Berman AD, Grines CL, Kahn J, Marsalese D, Mehta RH, Schreiber T, Boura JA and O’Neill WW. Impact of interventionalist volume, experience, and board certification on coronary angioplasty outcomes in the era of stenting. Am J Cardiol. 2004;94:421–426. [DOI] [PubMed] [Google Scholar]
- 8.Mustafa M, Cohen M, Zapotulko K, Feinberg M, Miller M, Aueron F, Wasty N, Tanwir A and Rogal G. The lack of a simple relation between physician’s percutaneous coronary intervention volume and outcomes in the era of coronary stenting: a two‐centre experience. Int J Clin Pract. 2005;59:1401–1407. [DOI] [PubMed] [Google Scholar]
- 9.Cantor WJ, Hall R and Tu JV. Do operator volumes relate to clinical outcomes after percutaneous coronary intervention in the Canadian health care system? Am Heart J. 2006;151:902–908. [DOI] [PubMed] [Google Scholar]
- 10.Fanaroff AC, Zakroysky P, Dai D, Wojdyla D, Sherwood MW, Roe MT, Wang TY, Peterson ED, Gurm HS, Cohen MG, Messenger JC and Rao SV. Outcomes of Percutaneous Coronary Intervention in Relation to Procedural Characteristics and Operator Volumes in the United States. J Am Coll Cardiol. 2017;69:2913–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulme W, Sperrin M, Curzen N, Kinnaird T, De Belder MA, Ludman P, Kwok CS, Gale CP, Cockburn J, Kontopantelis E and Mamas MA. Operator volume is not associated with mortality following percutaneous coronary intervention: insights from the British Cardiovascular Intervention Society registry. Eur Heart J. 2018;39:1623–1634. [DOI] [PubMed] [Google Scholar]
- 12.Inohara T, Kohsaka S, Yamaji K, Amano T, Fujii K, Oda H, Uemura S, Kadota K, Miyata H and Nakamura M. Impact of Institutional and Operator Volume on Short-Term Outcomes of Percutaneous Coronary Intervention: A Report From the Japanese Nationwide Registry. JACC Cardiovasc Interv. 2017;10:918–927. [DOI] [PubMed] [Google Scholar]
- 13.Kumbhani DJ, Cannon CP, Fonarow GC, Liang L, Askari AT, Peacock WF, Peterson ED and Bhatt DL. Association of hospital primary angioplasty volume in ST-segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302:2207–13. [DOI] [PubMed] [Google Scholar]
- 14.Stolker JM, Cohen DJ, Kennedy KF, Pencina MJ, Lindsey JB, Mauri L, Cutlip DE and Kleiman NS. Repeat revascularization after contemporary percutaneous coronary intervention. Circ Cardiovasc Interv. 2012;5:772–782. [DOI] [PubMed] [Google Scholar]
- 15.Latif F, Kleiman NS, Cohen DJ, Pencina MJ, Yen C-H, Cutlip DE, Moliterno DJ, Nassif D, Lopez JJ and Saucedo JF. In-Hospital and 1-Year Outcomes Among Percutaneous Coronary Intervention Patients With Chronic Kidney Disease in the Era of Drug-Eluting Stents: A Report From the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) Registry. JACC Cardiovasc Interv. 2009;2:37–45. [DOI] [PubMed] [Google Scholar]
- 16.Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, Hess O, Meier B and Windecker S. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115:2426–2434. [DOI] [PubMed] [Google Scholar]
- 17.Uren N, Schwarzacher S, Metz J, Lee D, Honda Y, Yeung A, Fitzgerald P and Yock P. Predictors and outcomes of stent thrombosis. An intravascular ultrasound registry. Eur Heart J. 2002;23:124–132. [DOI] [PubMed] [Google Scholar]
- 18.Doi H, Maehara A, Mintz GS, Yu A, Wang H, Mandinov L, Popma JJ, Ellis SG, Grube E and Dawkins KD. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv. 2009;2:1269–1275. [DOI] [PubMed] [Google Scholar]
- 19.Fujii K, Carlier SG, Mintz GS, Yang Y-m, Moussa I, Weisz G, Dangas G, Mehran R, Lansky AJ and Kreps EM Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol. 2005;45:995–998. [DOI] [PubMed] [Google Scholar]
- 20.Brodie BR, Cooper C, Jones M, Fitzgerald P and Cummins F. Is adjunctive balloon postdilatation necessary after coronary stent deployment? Final results from the POSTIT trial. Catheter Cardiovasc Interv. 2003;59:184–192. [DOI] [PubMed] [Google Scholar]
- 21.Harold JG, Bass TA, Bashore TM, Brindis RG, Brush JE Jr., Burke JA, Dehmer GJ, Deychak YA, Jneid H, Jollis JG, Landzberg JS, Levine GN, McClurken JB, Messenger JC, Moussa ID, Muhlestein JB, Pomerantz RM, Sanborn TA, Sivaram CA, White CJ and Williams ES ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures: a report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training (writing committee to revise the 2007 clinical competence statement on cardiac interventional procedures). Circulation. 2013;128:436–72. [DOI] [PubMed] [Google Scholar]
- 22.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS and Williams JF. The American College of Cardiology-National Cardiovascular Data Registry™(ACC-NCDR™): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–2245. [DOI] [PubMed] [Google Scholar]
- 23.Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS and Masoudi FA. The NCDR CathPCI Registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013;99:297–303. [DOI] [PubMed] [Google Scholar]
- 24.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ and Reynolds MR. The National Cardiovascular Data Registry (NCDR) data quality brief: the NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. [DOI] [PubMed] [Google Scholar]
- 25.Curtis JP, Schreiner G, Wang Y, Chen J, Spertus JA, Rumsfeld JS, Brindis RG and Krumholz HM. All-cause readmission and repeat revascularization after percutaneous coronary intervention in a cohort of Medicare patients. J Am Coll Cardiol. 2009;54:903–907. [DOI] [PubMed] [Google Scholar]
- 26.Krumholz HM, Lin Z, Drye EE, Desai MM, Han LF, Rapp MT, Mattera JA and Normand S-LT. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swaminathan RV, Rao SV, McCoy LA, Kim LK, Minutello RM, Wong SC, Yang DC, Saha-Chaudhuri P, Singh HS, Bergman G and Feldman DN. Hospital Length of Stay and Clinical Outcomes in Older STEMI Patients After Primary PCI: A Report From the National Cardiovascular Data Registry. J Am Coll Cardiol. 2015;65:1161–1171. [DOI] [PubMed] [Google Scholar]
- 28.Inohara T, Kohsaka S, Miyata H, Sawano M, Ueda I, Maekawa Y, Fukuda K, Jones PG, Cohen DJ, Zhao Z, Spertus JA and Smolderen KG. Prognostic Impact of Subsequent Acute Coronary Syndrome and Unplanned Revascularization on Long-Term Mortality After an Index Percutaneous Coronary Intervention: A Report From a Japanese Multicenter Registry. J Am Heart Assoc. 2017;6:e006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao SV, Dai D, Subherwal S, Weintraub WS, Brindis RS, Messenger JC, Lopes RD and Peterson ED. Association between periprocedural bleeding and long-term outcomes following percutaneous coronary intervention in older patients. JACC Cardiovasc Interv. 2012;5:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai NR, Bradley SM, Parzynski CS, Nallamothu BK, Chan PS, Spertus JA, Patel MR, Ader J, Soufer A and Krumholz HM. Appropriate use criteria for coronary revascularization and trends in utilization, patient selection, and appropriateness of percutaneous coronary intervention. J Am Med Assoc. 2015;314:2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan PS, Patel MR, Klein LW, Krone RJ, Dehmer GJ, Kennedy K, Nallamothu BK, Weaver WD, Masoudi FA and Rumsfeld JS. Appropriateness of percutaneous coronary intervention. J Am Med Assoc. 2011;306:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK and Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update. J Am Coll Cardiol. 2012;59:857–881. [DOI] [PubMed] [Google Scholar]
- 33.Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK and Klein LW. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine JP and Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 35.Strom JB, Wimmer NJ, Wasfy JH, Kennedy K and Yeh RW. Association Between Operator Procedure Volume and Patient Outcomes in Percutaneous Coronary Intervention. Circ Cardiovasc Qual Outcomes. 2014;7:560–566. [DOI] [PubMed] [Google Scholar]
- 36.Mueller C, Hodgson JM, Brutsche M, Bestehorn H-P, Marsch S, Perruchoud AP, Roskamm H and Buettner HJ. Operator experience and long term outcome after percutaneous coronary intervention. Can J Cardiol. 2003;19:1047–1051. [PubMed] [Google Scholar]
- 37.Madan M, Nikhil J, Hellkamp AS, Pieper KS, Labinaz M, Cohen E, Buller CE, Cantor WJ, Seidelin P and Ducas J. Effect of operator and institutional volume on clinical outcomes after percutaneous coronary interventions performed in Canada and the United States: a brief report from the Enhanced Suppression of the Platelet glycoprotein IIb/IIIa Receptor with Integrilin Therapy (ESPRIT) study. Can J Cardiol. 2009;25:e269–e272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato M, Kimura T, Morimoto T, Nishikawa H, Uchida F, Suzuki H, Hayashi Y, Kadota K, Mitsudo K and Investigators J-CR. Comparison of five-year outcome of sirolimus-eluting stent implantation for chronic total occlusions versus for non-chronic total occlusion (from the j-Cypher registry). Am J Cardiol. 2012;110:1282–1289. [DOI] [PubMed] [Google Scholar]
- 39.Al Muradi H, Mehra A, Okolo J, Vlachos H, Selzer F, Marroquin OC, Skelding K, Holper EM, Williams DO and Abbott JD. Clinical presentation and predictors of target vessel revascularization after drug-eluting stent implantation. Cardiovasc Revasc Med. 2012;13:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morche J, Mathes T and Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finlayson SR. The volume-outcome debate revisited. Am Surg. 2006;72:1038–1042. [PubMed] [Google Scholar]
- 42.Kumbhani DJ and Nallamothu BK. PCI Volume Benchmarks: Still Adequate for Quality Assessment in 2017? J Am Coll Cardiol. 2017;69:2925–2928. [DOI] [PubMed] [Google Scholar]
- 43.Masoudi FA, Ponirakis A, Yeh RW, Maddox TM, Beachy J, Casale PN, Curtis JP, De Lemos J, Fonarow G and Heidenreich P. Cardiovascular care facts: a report from the national cardiovascular data registry: 2011. J Am Coll Cardiol. 2013;62:1931–1947. [DOI] [PubMed] [Google Scholar]
- 44.Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, Moore JW, Moussa I, Oetgen WJ and Varosy PD. Trends in US Cardiovascular Care: 2016 Report from 4 ACC National Cardiovascular Data Registries. J Am Coll Cardiol. 2016:23279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


