Abstract
DNA mismatch repair (MMR) is an evolutionally conserved genome maintenance pathway and is well known for its role in maintaining replication fidelity by correcting biosynthetic errors generated during DNA replication. However, recent studies have shown that MMR preferentially protects actively transcribed genes from mutation during both DNA replication and transcription. This review describes the recent discoveries in this area. Potential mechanisms by which MMR safeguards actively transcribed genes are also discussed.
Keywords: H3K36me3, MSH6, replication timing, actively-transcribed genes, transcription-coupled repair, mutation frequency
1. Introduction
DNA mismatch repair (MMR) is commonly regarded as a replication-coupled system that ensures replication fidelity by correcting misincorporated nucleotides during DNA replication [1–4]. The importance of MMR in genome maintenance is underscored by the fact that defects in MMR cause cancers, including hereditary and sporadic colorectal cancers [2, 5, 6]. The typical MMR reaction in human cells involves three major steps: repair initiation, mismatch excision, and DNA resynthesis. The initiation reaction occurs when mismatch recognition protein MutSα (a MSH2-MSH6 heterodimer) or MutSβ (a MSH2-MSH3 heterodimer) binds to a mismatch, which triggers interactions and communications between MutSα (or MutSβ), PCNA (proliferating cell nuclear antigen), RFC (replication factor C), and MutLα (a MLH1PMS2 heterodimer). This leads to the recruitment of exonuclease 1 (Exo1) to a strand break located in the newly synthesized strand. In the excision step, Exo1 carries out DNA excision from the nick up to and beyond the mismatch in a manner dependent on MutSα (or MutSβ), MutLα, and replication protein A (RPA). Finally, DNA polymerase δ conducts the gap-filling reaction using the continuous (parental) DNA strand as a template, in concerted interactions with PCNA, RPA, and replication factor C (RFC), followed by DNA ligase I-catalyzed nick ligation.
The MutSα mismatch recognition protein is a key factor in MMR. Cells usually express more MSH6 than MSH3 and thus maintain a MutSα:MutSβ ratio of 10:1 [7, 8]. In addition to recognizing mismatches, MutSα also recognizes a variety of DNA lesions that are normally repaired by other DNA repair pathways [9], such as 8-oxo-guanine processed by the base excision repair (BER) pathway, UV-induced thymine-thymine dimers and polycyclic aromatic hydrocarbon-induced DNA adducts removed by the nucleotide excision repair (NER) pathway, and O6-methylguanine adducts repaired by the methylguanine methyltransferase suicide pathway [9–14]. Although this new MutSα activity is thought to play an important role in preventing severely damaged cells from proliferating via triggering apoptosis [9, 15], the underlying biology is unclear.
Our group has recently identified the histone mark H3K36me3 (trimethylated lysine 36 of histone protein H3) as an important MMR factor in human cells [16]. H3K36me3 physically interacts with the Pro-Trp-Trp-Pro (PWWP) domain located in the MSH6 subunit of MutSα and recruits MutSα to replicating chromatin. Depleting H3K36me3 or disrupting the H3K36me3-MutSα interaction leads to a mutator phenotype similar to cells with defective MMR genes [16]. It is also well known that H3K36me3 is highly enriched in gene bodies and actively transcribed regions [17, 18] and is associated with transcription elongation and splicing [19, 20]. Is H3K36me3-mediated MMR involved in transcription? Recent studies from several groups using various approaches, including chromatin-immunoprecipitation sequencing (ChIP-Seq) and whole genome sequencing of model cell lines, and bioinformatics analyses of cancer genomes, have revealed a common fact: H3K36me3-mediated MMR preferentially protects exons and actively transcribed genes from mutation [21–24], suggesting that H3K36me3-mediated MMR maintains genome stability not only in DNA replication, but also in transcription. The classical MMR function in replication has been extensively reviewed [4, 25–28]. Thus, this review will focus on the most recent developments demonstrating MMR’s preferential protection of actively transcribed genes, particularly in an H3K36me3-dependent manner.
2. Mismatch repair preferentially protects DNA in euchromatin
It was discovered long ago that spontaneous mutations are not evenly distributed within the human genome. Mutations occur much less frequently in euchromatin and protein-coding genes than in heterochromatin and other chromatin regions, respectively [29–31]. It was therefore postulated that the differences in mutation frequency between euchromatin and heterochromatin or between proteincoding regions and other chromatin regions are due to the timing of replication [30, 31] and the efficiency of DNA repair [32] in these chromatin regions. It was not realized until recently that both of these mechanisms are related to MMR.
Most active protein-coding genes that play essential roles in a cell reside in euchromatin and are replicated early [33–35]. Recent cancer genome studies [22, 36] have revealed that somatic mutations in MMR-proficient tumors occur less frequently in early replicating euchromatin and more frequently in late replicating heterochromatin. However, in tumors displaying microsatellite instability (MSI), which is often associated with a defective MMR system [4], mutations are no more frequent in late replicating heterochromatin than in early replicating euchromatin [22, 36]. This indicates that MMR accounts for the observed difference in mutation frequency between euchromatin and heterochromatin, suggesting that MMR preferentially prevents mutation accumulation in euchromatin. Consistent with human studies, MMR’s preferential protection of open chromatin has also been reported recently in yeast and plants [37, 38], implying that all organisms use the same mechanism to ensure the stability of important genetic materials.
3. H3K36me3-MSH6 co-enrichment determines local chromatin mutation frequency
MMR functions in the context of chromatin in vivo [39, 40]. We justified this concept when we found that the histone mark H3K36me3 recruits MutSα to replicating chromatin through its interaction with the PWWP domain in the MSH6 subunit of MutSα [16]. Therefore, it is reasonable to believe that the chromatin distribution and abundance of H3K36me3 could influence local MMR activity and mutation frequency. Our recent ChIP-Seq analysis revealed that this is indeed the case [21]. We found that H3K36me3 is more widely distributed than MutSα, but all MutSα-enriched genes are also abundant in H3K36me3, further indicating that MutSα is recruited to chromatin via H3K36me3. However, like spontaneous mutations [29–31], H3K36me3 and MutSα are not evenly distributed in the genome. Instead, they are more enriched in euchromatin, exons, and 3’ gene bodies than in heterochromatin, introns, and 5’ gene bodies, respectively. Correspondingly, the mutation frequencies in euchromatin, exons, and 3’ gene bodies are much lower than those in heterochromatin, introns, and 5’ gene bodies, respectively. These observations indicate that H3K36me3/MutSα abundance is inversely correlated to local mutation frequency [21].
Because actively transcribed protein-coding genes are replicated early [33–35] and MMR is known for its role in maintaining replication fidelity [4, 25–28], one would assume that replication-associated MMR ensures the stability of early replicating genes/chromatin. Indeed, studying the relationship between replication timing, H3K36me3/MutSα enrichment, and mutation frequency revealed that chromatin regions highly enriched for H3K36me3/MutSα tend to replicate earlier and have a lower relative mutation frequency than regions with fewer H3K36me3 signals [21], suggesting that H3K36me3-mediated MMR significantly contributes to the replication accuracy of early replicating genes/chromatin.
4. H3K36me3-mediated MMR preferentially protects actively transcribed genes
In addition to playing roles in DNA repair [16, 21], H3K36me3 was initially identified for its involvement in transcription [41–43]. However, its actual role(s) in transcription are still unclear. Since transcription requires chromatin in an open structure, the transcribed regions are vulnerable to attack by various DNA damage reagents during transcription [44, 45]. These DNA lesions must be removed before transcription to maintain both transcription accuracy and gene stability. Although transcription-coupled nucleotide excision repair (TC-NER) has been shown to safeguard the genome during transcription [46], the narrow DNA substrate specificity of NER may limit its role in transcription-coupled repair. In contrast, MutSα can recognize almost all non-WatsonCrick DNA structures (i.e., mismatches and damaged bases or nucleotides) and can be efficiently recruited to transcription sites by H3K36me3, making MMR an ideal system to deal with all kinds of DNA lesions produced during transcription.
Our recent studies have provided evidence suggesting that H3K36me3-mediated MMR is involved in protecting actively transcribed genes from mutation during transcription [21]. We observed that a number of very actively transcribed genes (including some critical tumor suppressor genes), which are highly enriched for H3K36me3/MSH6, had higher mutation frequencies than genes with less transcriptional activity and less H3K36me3/MutSα abundance. This phenomenon does not accord with the inverse relationship between H3K36me3/MutSα enrichment and local mutation frequency, and it cannot be explained by replication-associated mutation, as actively transcribed genes would have been better protected by H3K36me3mediated MMR during replication. However, extensive exposure to DNA damage reagents and inefficient repair of DNA lesions in actively transcribed genes during transcription could solve the puzzle, because actively transcribed genes are persistently exposed in the open chromatin structure during transcription and thus suffer more DNA damage-induced mutations than less actively transcribed genes. This assumption is supported by the fact that actively transcribed genes displayed a higher H2O2-induced mutation frequency than less actively transcribed ones in MMR-deficient, but BER- and NER-proficient cells [21]. It is obvious that the higher mutation frequency is directly related to MMR activity and transcription. If these mutations were induced by H2O2-caused damage during DNA replication, where actively transcribed genes and less actively transcribed genes can be damaged with equal probabilities, it is difficult to imagine why DNA polymerases preferentially induce mutations only in actively transcribed genes. Taken together, the evidence shows that, in addition to its mismatch correction function coupled with DNA replication, MMR also maintains the stability of actively transcribed genes by directly or indirectly removing DNA lesions associated with transcription. This could adequately explain recent cancer genome data showing that mutations preferentially occur in active genes in tumors defective in MMR [23, 24].
5. Proposed mechanisms for MMR’s involvement in transcription-coupled repair
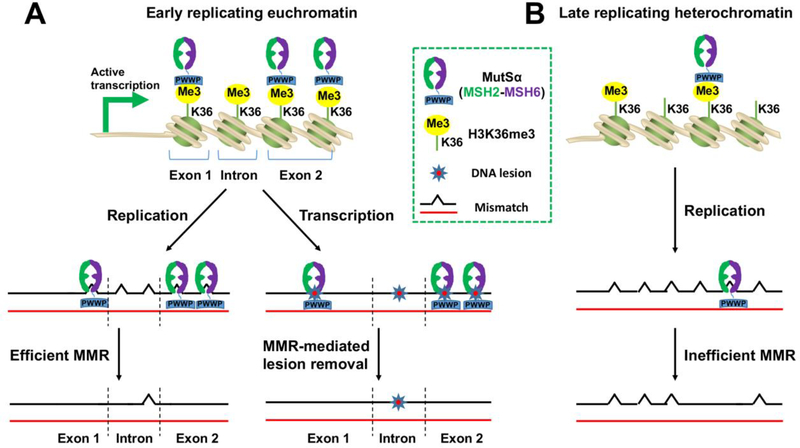
As described above, emerging evidence suggests that MMR preferentially protects actively transcribed genes during both DNA replication and transcription (see Figure 1). Like in replication, MutSα can be recruited to transcribed chromatin through its interaction with H3K36me3. There are at least four possible ways that MMR can participate in transcription-associated lesion removal. First, MutSα directly recognizes DNA lesions [9–14] and initiates the downstream MMR reaction. Second, MutSα identifies DNA damages first and then recruits TC-NER factors to the damage site in the transcribed strand to process the lesion via the NER pathway. Third, TC-NER proteins recognize DNA lesions, but MMR proteins (MutSα and MutLα) facilitate the recruitment of other essential repair factors, as proposed by Cantor and colleagues [47]. Finally, a damaged base/nucleotide can be converted to another base/nucleotide by enzymes (i.e., APOBEC3) to form a mismatch, which is then processed by MMR.
Figure 1. Mechanisms by which MMR selectively safeguards actively transcribed genes.
Actively transcribed genes reside in euchromatin, which is not only replicated early, but also highly enriched for H3K36me3 and MutSα. As such, mispairs generated during DNA replication in actively transcribed genes are efficiently repaired by the H3K36me3-mediated MMR system. Similarly, the MMR system acts to directly or indirectly remove DNA lesions created during active transcription (A). In contrast, heterochromatin is replicated late and less enriched for H3K36me3 and MutSα, thus not all mismatches can be corrected in heterochromatin (B).
MMR is a strand-specific reaction that targets the newly synthesized strand for error correction [1–4]. This strand-specific reaction is directed either by a pre-existing strand break (e.g., the ends of Okazaki fragments) or a nick generated by the MutLα endonuclease activity [3, 4, 48]. How the MMR system removes the strand-specific damage during transcription is unknown. However, previous studies have provided some hints. MMR has been shown to occur in non-dividing cells exposed to high levels of DNA damage, a reaction called non-canonical MMR [49–51]. Recent studies by Modrich and colleagues [48, 52, 53] have revealed that covalently closed circular DNA containing a lesion or a helix perturbation that MutSα recognizes stimulates PCNA loading and subsequently activates the MutLα endonuclease activity. Although the endonucleolytic cleavage by MutLα lacks strand bias in vitro, it has been proposed that in vivo interactions between PCNA and MutSα [54] and/or unidentified DNA signals confer strand-specificity on the reaction. Alternatively, strand-specific nicks can be generated by TC-NER factors XPG and XPF [46]. Therefore, once H3K36me3 recruits MutSα to actively transcribed chromatin containing a DNA lesion, strand-specific lesion removal targeting the damaged transcribed strand will occur.
6. Perspectives
In summary, recent studies have identified a new MMR function, i.e., as a genome-maintenance system that preferentially protects actively transcribed chromatin from mutation in an H3K36me3-dependent manner during both DNA replication and transcription. While MMR’s role in replication has been well studied, its participation in transcription-associated lesion removal was controversial [55–57] but is currently being re-discovered [21]. However, the mechanism of the latter remains to be investigated. In addition, the H3K36me3-mediated MMR seems to protect exons better than introns within actively transcribed genes. We observed that mutation frequency is higher in introns than in exons in MMR-proficient human cells, but the relationship is inverted in cells depleted of H3K36me3 or disrupted in the H3K36me3-MSH6 interaction [21]. Frigola et al. analyzed the whole genome expression and mutation data of several hundred MMR-proficient and MMR-deficient tumors deposited in the TCGA database and demonstrated that mismatches in exonic DNA are repaired more efficiently than their intronic counterparts in a H3K36me3-dependent manner [24]. Since introns are usually transcribed together with their corresponding exons, how does the MMR system selectively repair DNA lesions in exons? Frigola et al. [24] suggest that a crosstalk between the RNA splicing machinery and MMR could account for the differential mutation frequencies in exons and introns. Alternatively, since H3K36me3 is more enriched in exons than introns, Schwartz et al. suggest that H3K36me3 levels define exon-intron boundaries during mRNA synthesis and that H3K36me3’s preferential recruitment of MutSα to exon-containing nucleosomes allows MMR to specifically target exons [58]. In addition, RNA polymerase II is known to be an important sensor for TC-NER [46], but do MMR proteins and RNA polymerase II communicate with each other? Recent studies have identified somatic mutations of lysine 36 (e.g., mutated to methionine and isoleucine) and glycine 34 (e.g., to arginine and valine) of the transcription-associated histone H3.3 as cancer drivers for pediatric gliomas and other malignancies [59–64]. Do these mutations block MMR function in transcription? All these questions await future thorough investigations.
Acknowledgements
We thank Jonathan Feinberg for helpful comments, and funds from the National Institutes of Health (GM112702 and CA192003), the National Natural Science Foundation of China (31370766, 31570814, and 81630077), the NSFC-ISF joint Research Program (31461143005), the National Key R&D Program of China 2016YFC1303300, and the Tsinghua-Peking Joint Center for Life Sciences. G.-M.L. holds the Reece A. Overcash, Jr. Chair for Research on Colon Cancer.
The abbreviations used are:
- MMR
mismatch repair
- H3K36me3
histone H3 lysine 36 trimethylation
- PCNA
proliferation cellular nuclear antigen
- RPA
replication protein A
- RFC
replication factor C
- PWWP domain
Proline-Tryptophan-Tryptophan-Proline motif
- BER
base excision repair
- NER
nucleotide excision repair
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kolodner R, Biochemistry and genetics of eukaryotic mismatch repair, Genes & development, 10 (1996) 1433–1442. [DOI] [PubMed] [Google Scholar]
- [2].Modrich P, Lahue R, Mismatch repair in replication fidelity, genetic recombination, and cancer biology, Annu Rev Biochem, 65 (1996) 101–133. [DOI] [PubMed] [Google Scholar]
- [3].Kunkel TA, Erie DA, Eukaryotic Mismatch Repair in Relation to DNA Replication, Annual review of genetics, 49 (2015) 291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li GM, Mechanisms and functions of DNA mismatch repair, Cell research, 18 (2008) 85–98. [DOI] [PubMed] [Google Scholar]
- [5].Kinzler KW, Vogelstein B, Lessons from hereditary colorectal cancer, Cell, 87 (1996) 159–170. [DOI] [PubMed] [Google Scholar]
- [6].Kolodner RD, Marsischky GT, Eukaryotic DNA mismatch repair, Curr Opin Genet Dev, 9 (1999) 89–96. [DOI] [PubMed] [Google Scholar]
- [7].Drummond JT, Genschel J, Wolf E, Modrich P, DHFR/MSH3 amplification in methotrexate-resistant cells alters the hMutSalpha/hMutSbeta ratio and reduces the efficiency of base-base mismatch repair, Proceedings of the National Academy of Sciences of the United States of America, 94 (1997) 10144–10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marra G, Iaccarino I, Lettieri T, Roscilli G, Delmastro P, Jiricny J, Mismatch repair deficiency associated with overexpression of the MSH3 gene, Proceedings of the National Academy of Sciences of the United States of America, 95 (1998) 8568–8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li GM, The role of mismatch repair in DNA damage-induced apoptosis, Oncol Res, 11 (1999) 393–400. [PubMed] [Google Scholar]
- [10].Mu D, Tursun M, Duckett DR, Drummond JT, Modrich P, Sancar A, Recognition and repair of compound DNA lesions (base damage and mismatch) by human mismatch repair and excision repair systems, Mol Cell Biol, 17 (1997) 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang H, Lawrence CW, Li GM, Hays JB, Specific binding of human MSH2.MSH6 mismatch-repair protein heterodimers to DNA incorporating thymine- or uracil-containing UV light photoproducts opposite mismatched bases, The Journal of biological chemistry, 274 (1999) 16894–16900. [DOI] [PubMed] [Google Scholar]
- [12].Ni TT, Marsischky GT, Kolodner RD, MSH2 and MSH6 are required for removal of adenine misincorporated opposite 8-oxo-guanine in S. cerevisiae, Mol Cell, 4 (1999) 439–444. [DOI] [PubMed] [Google Scholar]
- [13].Mazurek A, Berardini M, Fishel R, Activation of human MutS homologs by 8oxo-guanine DNA damage, The Journal of biological chemistry, 277 (2002) 8260–8266. [DOI] [PubMed] [Google Scholar]
- [14].Gu L, Wu J, Qiu L, Jennings CD, Li GM, Involvement of DNA mismatch repair in folate deficiency-induced apoptosis small star, filled, J Nutr Biochem, 13 (2002) 355363. [DOI] [PubMed] [Google Scholar]
- [15].Wu J, Gu L, Wang H, Geacintov NE, Li G-M, Mismatch repair processing of carcinogen-DNA adducts triggers apoptosis, Mol. Cell. Biol, 19 (1999) 8292–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li F, Mao G, Tong D, Huang J, Gu L, Yang W, Li GM, The Histone Mark H3K36me3 Regulates Human DNA Mismatch Repair through Its Interaction with MutSalpha, Cell, 153 (2013) 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K, High-resolution profiling of histone methylations in the human genome, Cell, 129 (2007) 823–837. [DOI] [PubMed] [Google Scholar]
- [18].Wagner EJ, Carpenter PB, Understanding the language of Lys36 methylation at histone H3, Nat Rev Mol Cell Biol, 13 (2012) 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo R, Zheng L, Park JW, Lv R, Chen H, Jiao F, Xu W, Mu S, Wen H, Qiu J, Wang Z, Yang P, Wu F, Hui J, Fu X, Shi X, Shi YG, Xing Y, Lan F, Shi Y, BS69/ZMYND11 reads and connects histone H3.3 lysine 36 trimethylation-decorated chromatin to regulated pre-mRNA processing, Mol Cell, 56 (2014) 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wen H, Li Y, Xi Y, Jiang S, Stratton S, Peng D, Tanaka K, Ren Y, Xia Z, Wu J, Li B, Barton MC, Li W, Li H, Shi X, ZMYND11 links histone H3.3K36me3 to transcription elongation and tumour suppression, Nature, 508 (2014) 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang Y, Gu L, Li G-M, H3K36me3-mediated mismatch repair preferentially protects actively transcribed genes from mutation, The Journal of biological chemistry, 293 (2018) 7811–7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Supek F, Lehner B, Differential DNA mismatch repair underlies mutation rate variation across the human genome, Nature, 521 (2015) 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Supek F, Lehner B, Clustered Mutation Signatures Reveal that Error-Prone DNA Repair Targets Mutations to Active Genes, Cell, 170 (2017) 534–547. [DOI] [PubMed] [Google Scholar]
- [24].Frigola J, Sabarinathan R, Mularoni L, Muiños F, Gonzalez-Perez A, LópezBigas N, Reduced mutation rate in exons due to differential mismatch repair, Nature genetics, 49 (2017) 1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kunkel TA, Erie DA, Eukaryotic Mismatch Repair in Relation to DNA Replication, Annual review of genetics, 49 (2015) 291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li GM, New insights and challenges in mismatch repair: getting over the chromatin hurdle, DNA repair, 19 (2014) 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Modrich P, Mechanisms in eukaryotic mismatch repair, J Biol Chem, 281 (2006) 30305–30309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kolodner RD, A personal historical view of DNA mismatch repair with an emphasis on eukaryotic DNA mismatch repair, DNA repair, 38 (2016) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schuster-Böckler B LB, Chromatin organization is a major influence on regional mutation rates in human cancer cells, Nature, 488 (2012) 504–507. [DOI] [PubMed] [Google Scholar]
- [30].Wolfe KH, Sharp PM, Li WH, Mutation rates differ among regions of the mammalian genome, Nature, 337 (1989) 283–285. [DOI] [PubMed] [Google Scholar]
- [31].Ellegren H, Smith NGC, Webster MT, Mutation rate variation in the mammalian genome, Curr Opin Genet Dev, 13 (2003) 562–568. [DOI] [PubMed] [Google Scholar]
- [32].Bohr VA, Phillips DH, Hanawalt PC, Heterogeneous DNA damage and repair in the mammalian genome [published erratum appears in Cancer Res 1988. March 1;48(5):1377], Cancer Res, (1987). [PubMed] [Google Scholar]
- [33].Hiratani I, Ryba T, Itoh M, Rathjen J, Kulik M, Papp B, Fussner E, Bazett-Jones DP, Plath K, Dalton S, Rathjen PD, Gilbert DM, Genome-wide dynamics of replication timing revealed by in vitro models of mouse embryogenesis, Genome research, 20 (2010) 155–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hiratani I, Ryba T, Itoh M, Yokochi T, Schwaiger M, Chang CW, Lyou Y, Townes TM, Schubeler D, Gilbert DM, Global reorganization of replication domains during embryonic stem cell differentiation, PLoS Biol, 6 (2008) e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lubelsky Y, Prinz JA, DeNapoli L, Li Y, Belsky JA, MacAlpine DM, DNA replication and transcription programs respond to the same chromatin cues, Genome research, 24 (2014) 1102–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zheng CL, Wang NJ, Chung J, Moslehi H, Sanborn JZ, Hur JS, e.a EA. Collisson, Transcription restores DNA repair to heterochromatin, determining regional mutation rates in cancer genomes, Cell Reports, 9 (2014) 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Belfield EJ, Ding ZJ, Jamieson FJC, Visscher AM, Zheng SJ, Mithani A, Harberd NP, DNA mismatch repair preferentially protects genes from mutation, Genome research, 28 (2018) 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sun L, Zhang Y, Zhang Z, Zheng Y, Du L, Zhu B, Preferential Protection of Genetic Fidelity within Open Chromatin by the Mismatch Repair Machinery, The Journal of biological chemistry, 291 (2016) 17692–17705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ataian Y, Krebs JE, Five repair pathways in one context: chromatin modification during DNA repair, Biochem Cell Biol, 84 (2006) 490–504. [DOI] [PubMed] [Google Scholar]
- [40].Schöpf B, Bregenhorn S, Quivy JP, Kadyrov FA, Almouzni G, Jiricny J, Interplay between mismatch repair and chromatin assembly, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) 1895–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bell O, Wirbelauer C, Hild M, Scharf AN, Schwaiger M, MacAlpine DM, Zilbermann F, van Leeuwen F, Bell SP, Imhof A, Garza D, Peters AH, Schubeler D, Localized H3K36 methylation states define histone H4K16 acetylation during transcriptional elongation in Drosophila, The EMBO journal, 26 (2007) 4974–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA, A chromatin landmark and transcription initiation at most promoters in human cells, Cell, 130 (2007) 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kim A, Kiefer CM, Dean A, Distinctive signatures of histone methylation in transcribed coding and noncoding human beta-globin sequences, Mol Cell Biol, 27 (2007) 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jinks-Robertson S, Bhagwat AS, Transcription-associated mutagenesis, Annu Rev Genet, 48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khobta A, Epe B, Interactions between DNA damage, repair, and transcription, Mutat Res, 736 (2012) 5–14. [DOI] [PubMed] [Google Scholar]
- [46].Hanawalt PC, Spivak G, Transcription-coupled DNA repair: two decades of progress and surprises, Nat Rev Mol Cell Biol, 9 (2008) 958–970. [DOI] [PubMed] [Google Scholar]
- [47].Guillemette S, Branagan A, Peng M, Dhruva A, Schärer OD, Cantor SB, FANCJ localization by mismatch repair is vital to maintain genomic integrity after UV irradiation, Cancer Res, 74 (2014) 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kadyrov FA, Dzantiev L, Constantin N, Modrich P, Endonucleolytic function of MutLalpha in human mismatch repair, Cell, 126 (2006) 297–308. [DOI] [PubMed] [Google Scholar]
- [49].Rodriguez GP, Romanova NV, Bao G, Rouf NC, Kow YW, Crouse GF, Mismatch repair-dependent mutagenesis in nondividing cells, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) 6153–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Crouse GF, Non-canonical actions of mismatch repair, DNA repair, 38 (2016) 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Peña-Diaz J, Bregenhorn S, Ghodgaonkar M, Follonier C, Artola-Borán M, Castor D, Lopes M, Sartori AA, Jiricny J, Noncanonical mismatch repair as a source of genomic instability in human cells, Mol Cell, 47 (2012) 669–680. [DOI] [PubMed] [Google Scholar]
- [52].Pluciennik A, Burdett V, Baitinger C, Iyer RR, Shi K, Modrich P, Extrahelical (CAG)/(CTG) triplet repeat elements support proliferating cell nuclear antigen loading and MutLα endonuclease activation, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) 12277–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pluciennik A, Dzantiev L, Iyer RR, Constantin N, Kadyrov FA, Modrich P, PCNA function in the activation and strand direction of MutLα endonuclease in mismatch repair, Proceedings of the National Academy of Sciences of the United States of America, 107 (2010) 16066–16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kawasoe Y, Tsurimoto T, Nakagawa T, Masukata H, Takahashi T, MutSα maintains the mismatch repair capability by inhibiting PCNA unloading, Elife, 5 (2016) e15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rochette PJ, Bastien N, McKay BC, Therrien JP, Drobetsky EA, Drouin R, Human cells bearing homozygous mutations in the DNA mismatch repair genes hMLH1 or hMSH2 are fully proficient in transcription-coupled nucleotide excision repair, Oncogene, 21 (2002) 5743–5752. [DOI] [PubMed] [Google Scholar]
- [56].Sonneveld E, Vrieling H, Mullenders LH, van Hoffen A, Mouse mismatch repair gene Msh2 is not essential for transcription-coupled repair of UV-induced cyclobutane pyrimidine dimers, Oncogene, 20 (2001) 538–541. [DOI] [PubMed] [Google Scholar]
- [57].Mellon I, Rajpal DK, Koi M, Boland CR, Champe GN, Transcription-coupled repair deficiency and mutations in human mismatch repair genes, Science, 272 (1996) 557–560. [DOI] [PubMed] [Google Scholar]
- [58].Schwartz S, Meshorer E, Ast G, Chromatin organization marks exon-intron structure, Nat Struct Mol Biol, 16 (2009) 990–995. [DOI] [PubMed] [Google Scholar]
- [59].Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N, Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma, Nature, 482 (2012) 226–231. [DOI] [PubMed] [Google Scholar]
- [60].Behjati S, Tarpey PS, et al. , Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone, Nature genetics, 45 (2013) 1479–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fang D, Gan H, Lee JH, Han J, Wang Z, Riester SM, L.e.a. Jin, The histone H3.3K36M mutation reprograms the epigenome of chondroblastomas, Science, 352 (2016) 1344–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Zhang J, Baker SJ, St P. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome, Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas, Nature genetics, 44 (2012) 251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bjerke L, Mackay A, Nandhabalan M, Burford A, Jury A, Popov S, Bax DA, Carvalho D, Taylor KR, Vinci M, Bajrami I, McGonnell IM, Lord CJ, Reis RM, Hargrave D, Ashworth A, Workman P, Jones C, Histone H3.3 Mutations Drive Pediatric Glioblastoma through Upregulation of MYCN, Cancer discovery, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Lu C, Jain SU, Hoelper D, Bechet D, Molden RC, Ran L, Murphy D.e.a., Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape, Science, 352 (2016) 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]