Abstract
Background:
Adherence to endocrine therapy for breast cancer is often inadequate, in part due to medication out-of-pocket costs. Numerous states have enacted parity laws to limit patient cost-sharing for oral anticancer drugs. We sought to estimate the impact of these laws on patient copayments for and adherence to oral endocrine therapy for breast cancer.
Methods:
Utilizing administrative health insurance claims data from 2007–2014 in the Clinformatics™ Data Mart Database (OptumInsight), we identified female patients aged 18–64 years with invasive cancer or ductal carcinoma in situ of the breast who initiated endocrine therapy and were enrolled in fully insured health plans in states that either enacted parity legislation between 2008 and 2013 or had not yet enacted legislation by 2015. Differences-in-differences analysis was used to compare copayments for and adherence to endocrine therapy during the one-year period before and after each year of legislation enactment.
Results:
We identified 6,900 individuals with 7,778 unique drug therapy courses. Parity legislation was associated with significant decreases in the 25th percentile copayments for anastrozole of $4.39 (95% CI: −4.52 to −4.26; p<0.001) and for exemestane of $3.08 (95% CI: −4.80 to −1.35; p<0.001). Median copayment for exemestane decreased $10.25 (95% CI: −12.61 to −7.89; p<0.001). Higher median monthly copayment was significantly associated with higher risk of medication non-adherence (adjusted RR 1.006 per dollar increase; p<0.001).
Conclusions:
Parity laws had a modest effect on lowering the cost of anastrozole and exemestane, but more focused efforts to limit endocrine therapy out-of-pocket costs may have a greater impact on medication adherence.
Keywords: oral parity, breast cancer, endocrine therapy, out-of-pocket cost, adherence
Precis:
In this study of administrative claims from a large national insurer, enactment of parity legislation was associated with modestly lower monthly copayments for exemestane and anastrozole. Lower monthly copayment was associated with decreased risk of endocrine therapy non-adherence.
Introduction
Oral endocrine therapy is an integral component of hormone receptor-positive breast cancer treatment that has been shown to lower the risk of disease recurrence and death.1,2 As such, the National Comprehensive Cancer Network guidelines recommend adjuvant endocrine therapy with tamoxifen or an aromatase inhibitor, depending on menopausal status, for a minimum of 5 years after surgery for early-stage estrogen receptor-positive breast cancer.3 However, over a quarter of patients may be non-adherent to these medications, in part due to medication out-of-pocket costs.4–6 Efforts to lower the cost-sharing burden of these drugs may improve therapy adherence and ultimately disease outcomes.
To date, 43 states and the District of Columbia have enacted oral parity laws that limit patient out-of-pocket costs of oral anticancer medications, typically covered under a patient’s pharmacy benefit, to that of intravenous anticancer medications, typically covered under the medical benefit.7 These laws apply only to state-regulated insurance plans (i.e., fully insured plans) that do not fall under the Employee Retirement Income Security Act, and federal legislation is pending.8 While these laws have not been shown to consistently reduce out-of-pocket spending for oral anticancer medications exclusive of endocrine therapy, they were associated with decreased spending for less expensive drugs.9 We aimed to investigate whether parity laws impacted the cost of and adherence to endocrine therapies.
Methods
Cohort Selection
We performed a retrospective cohort study using national administrative health insurance claims data from 2007–2014 obtained from the Clinformatics™ Data Mart Database (OptumInsight, Eden Prairie, MN), which is a de-identified database from a large national insurance provider. This study was deemed exempt from review by the Institutional Review Board of Stanford University.
We selected female patients aged 18 to 64 years with a diagnosis of invasive cancer or ductal carcinoma in situ of the breast, who were enrolled in fully insured commercial health insurance plans that were subject to state laws (Appendix A). Oral endocrine therapy administration was identified from pharmacy claims using National Drug Codes for anastrozole, exemestane, letrozole, and tamoxifen (Appendix B).
Our treatment group consisted of patients who resided in states that had enacted oral parity legislation between 2008 and 2013 (Appendix C) and who had initiated oral endocrine therapy with an index claim either in the 12 months prior to January 1st of the year their respective state’s law was enacted or in the 12 months after December 31st of the year the law was enacted. Our control group consisted of patients who resided in states that had not yet enacted oral parity legislation as of January 1st, 2015 (Appendix C) and who had also initiated oral endocrine therapy between 2007 and 2014. Additionally, all patients were required to have continuous health plan enrollment over an 18-month period inclusive of the year of endocrine therapy initiation as well as at least one additional claim for the same drug within the same calendar year to allow accurate calculation of comorbidity index and adherence measures, respectively.
Outcome Measures and Study Covariates
Our primary outcome on the claims level was monthly copayment amount, normalized to a 30-day supply of medication and exclusive of deductibles, given that an individual plan’s deductible would not be specific to oral anticancer drugs and may have been met with other services. Furthermore, only a small proportion of claims (4%) in our sample had non-zero deductible payments. Coinsurance amounts were not available in the database. All prices were converted to real 2014 dollars using the medical component of the U.S. Consumer Price Index.10
On the person-drug level, copayment amounts were summarized as the median of all claims for the same drug, normalized to a 30-day supply, for the same patient. Variable medication possession ratios (MPRs) were estimated on a per-patient, per-drug basis by calculating the ratio of sum of days supplied divided by number of days between the start of the first prescription and the scheduled end date of the last prescription (i.e., fill date plus number of days supplied).11,12 Patients with an MPR ≥ 0.8 for a particular drug were considered adherent to that drug.4,6 Given the potential of overestimating true adherence rates by using this methodology, we also performed a sensitivity analysis by calculating fixed MPRs using the ratio of sum of days supplied divided by number of days from the start of the first prescription to the end of the calendar year.
Statistical Analysis
Given that some states enacted parity laws at differing times during the study period, we performed a differences-in-differences analysis using data the year before and after parity legislation in states that did so, while using non-parity law states to provide controls for these before and after comparisons.9,13,14 A figure detailing this experimental design is presented in Appendix D. Differences in baseline characteristics between parity and non-parity states were described and assessed using Pearson’s chi-square test. Univariable quantile regression for each drug was used to compare median copayment amounts between pre-and post-law periods in states enacting the law after changes to generic formulation. Multivariable quantile regression for the 25th, 50th, 75th, 90th, and 95th percentiles of copayment amounts for oral endocrine therapy was used to estimate the impact of parity law enactment for each drug separately using a differences-in-differences approach and adjusting for year, plan type and geography. In addition, multivariable linear regressions with robust standard errors were performed to estimate the effect of parity law enactment on mean copayment amount for each drug.
Poisson regression models with log link and robust standard errors were used to estimate the association between median monthly copayment on the person-drug level and non-adherence. Multivariable models adjusted for drug type and demographic characteristics, including age, Charlson comorbidity score, and year of treatment. Sensitivity analyses of adherence were performed using copayment dichotomized at $20, a cutoff defined in previous research,5 and median monthly out-of-pocket spending, inclusive of copayment and deductible amounts. All tests were two-sided with an alpha level of 0.05. Statistical analyses were performed using SAS software (version 9.4, SAS Institute, Cary, North Carolina) and R Statistical Software (version 3.4, R Foundation for Statistical Computing, Vienna, Austria). Further details on our analysis can be found in Appendix E.
Results
Patient Characteristics
We identified 6,900 individuals with 41,063 pharmacy claims and 7,778 unique drug therapy courses, representing an average of 1.1 different drugs utilized per patient in the first year of endocrine therapy. The most prevalent therapy was tamoxifen (40% of all treatment courses). Approximately 21% of our cohort resided in states subject to parity legislation. Additional baseline characteristics are presented in Table 1.
Table 1.
Baseline demographic characteristics by residence in states subject to parity laws and those not subject to them.
| Total |
Non-Parity States |
Parity States |
||||
|---|---|---|---|---|---|---|
| Patient Characteristics | No.a | No.a | (%) | No.a | (%) | Pb |
| Total | 7778 | 6172 | (100.0) | 1606 | (100.0) | |
| Oral endocrine therapy | 0.096 | |||||
| Anastrozole | 2392 | 1930 | (31.3) | 462 | (28.8) | |
| Exemestane | 805 | 651 | (10.5) | 154 | (9.6) | |
| Letrozole | 1454 | 1142 | (18.5) | 312 | (19.4) | |
| Tamoxifen | 3127 | 2449 | (39.7) | 678 | (42.2) | |
| Year of index claim | <0.001 | |||||
| 2007–2008 | 1782 | 1748 | (28.3) | 34 | (2.1) | |
| 2009–2010 | 1836 | 1415 | (22.9) | 421 | (26.2) | |
| 2011–2012 | 2312 | 1558 | (25.2) | 754 | (46.9) | |
| 2013–2015 | 1848 | 1451 | (23.5) | 397 | (24.7) | |
| Age, years | 0.335 | |||||
| 18–44 | 1017 | 806 | (13.1) | 211 | (13.1) | |
| 45–54 | 3266 | 2562 | (41.5) | 704 | (43.8) | |
| 55–59 | 1842 | 1474 | (23.9) | 368 | (22.9) | |
| 60–64 | 1653 | 1330 | (21.5) | 323 | (20.1) | |
| Charlson-Deyo comorbidity score |
0.366 | |||||
| 0 | 7096 | 5641 | (91.4) | 1455 | (90.6) | |
| 1 | 573 | 450 | (7.3) | 123 | (7.7) | |
| 2 or higher | 109 | 81 | (1.3) | 28 | (1.7) | |
| Diagnosis | 0.853 | |||||
| Invasive cancer | 6950 | 5517 | (89.4) | 1433 | (89.2) | |
| Carcinoma in-situ | 828 | 655 | (10.6) | 173 | (10.8) | |
| Geographic region | <0.001 | |||||
| Northeast | 349 | 179 | (2.9) | 170 | (10.6) | |
| Midwest | 2614 | 2158 | (35.0) | 456 | (28.4) | |
| South | 2215 | 1464 | (23.7) | 751 | (46.8) | |
| West | 2600 | 2371 | (38.4) | 229 | (14.3) | |
| Insurance plan type | <0.001 | |||||
| HMO | 2269 | 1864 | (30.2) | 405 | (25.2) | |
| POS | 5121 | 4060 | (65.8) | 1061 | (66.1) | |
| Otherc | 388 | 248 | (4.0) | 140 | (8.7) | |
Number of unique drug therapy courses, defined as single drug type per patient. Patients may have had multiple (up to 4) drug therapy courses.
Pearson’s chi-squared p value
Includes preferred provider organization, exclusive provider organization, and indemnity plans
Abbreviations: HMO (health maintenance organization); POS (point of service)
Copayment Costs
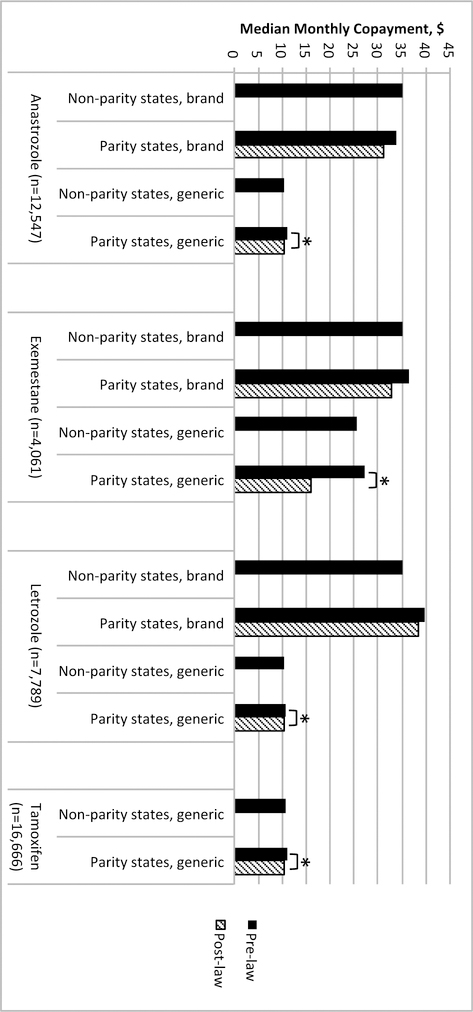
Median monthly copayment amounts for anastrozole, exemestane, letrozole, and tamoxifen were $10.95, $31.62, $21.90, and $10.51, respectively. During the study period, generic formulations for anastrozole, exemestane, and letrozole were introduced, while all pharmacy claims for tamoxifen in our study were for its generic formulation. Figure 1 characterizes the median monthly copayments for both brand and generic formulations of each drug in non-parity states and the change in copayments before and after law enactment in parity states. Median monthly copayments decreased significantly for generic formulations of each drug after parity legislation in states subject to the laws, with the greatest decrease for exemestane of $11.43 (p=0.001). Absolute differences in median monthly copayments for generic anastrozole, letrozole, and tamoxifen were small (difference <$1.00, p<0.001 for each).
Figure 1.
Median monthly copayment for brand and generic formulations of each drug by residence in states subject to parity laws and those not subject to them. *Difference is statistically significant with p<0.05 on univariable quantile regression.
On multivariable differences-in-differences quantile regression analysis, parity legislation was associated with significant decreases in the 25th percentile copayments for anastrozole of $4.39 (95% confidence interval [CI]: −4.52 to −4.26; p<0.001) and for exemestane of $3.08 (95% CI: −4.80 to −1.35; p<0.001). Copayment at the 25th percentile for letrozole increased $7.21 (95% CI: 7.17 to 7.26; p<0.001), but did not change significantly for tamoxifen (Table 2). At the 50th percentile, copayment for exemestane decreased $10.25 (95% CI: −12.61 to −7.89; p<0.001) after parity legislation. For the other drugs, estimates were effectively zero due to overlap of the cumulative distribution functions of copayment at the 50th percentile before and after parity legislation (see Appendix F). Additional analyses performed at the 75th, 90th, and 95th percentiles were also effectively zero due to the same reason. The use of dithering (i.e., adding small random perturbations to the copayment amounts) made no substantive difference to these estimates or conclusions. There was also no significant difference in mean copayment amount associated with parity legislation for any of the four drugs (Appendix G).
Table 2.
Multivariable differences-in-differences quantile regression analyzing change in copayment amounts associated with parity law enactment.
| Change Associated with Parity Laws | ||||
|---|---|---|---|---|
| Drug | Baseline Monthly Copayment, $ |
Estimate, $ | (95% CI) | P |
| Anastrozole | ||||
| 25th percentile | 10.00 | −4.39 | (−4.52 to −4.26) | <0.001 |
| 50th percentile | 10.95 | 0.00 | (--to --) | -- |
| 75th percentile | 25.30 | 0.00 | (--to --) | -- |
| Exemestane | ||||
| 25th percentile | 10.63 | −3.08 | (−4.80 to −1.35) | <0.001 |
| 50th percentile | 31.62 | −10.25 | (−12.61 to −7.89) | <0.001 |
| 75th percentile | 37.94 | 0.00 | (--to --) | -- |
| Letrozole | ||||
| 25th percentile | 10.25 | 7.21 | (7.17 to 7.26) | <0.001 |
| 50th percentile | 21.90 | 0.00 | (--to --) | -- |
| 75th percentile | 37.94 | 0.00 | (--to --) | -- |
| Tamoxifen | ||||
| 25th percentile | 8.18 | −0.21 | (−0.73 to 0.31) | 0.428 |
| 50th percentile | 10.51 | 0.00 | (--to --) | -- |
| 75th percentile | 11.68 | 0.00 | (--to --) | -- |
We further investigated the potential differential impact of parity legislation on generic versus brand-name drugs. On subset differences-in-differences analyses evaluating only generic drugs, copayment was significantly lower after legislation for anastrozole at the 25th percentile and for exemestane at the 50th percentile (Appendix H). On analyses evaluating only brand-name drugs, copayment was significantly lower after legislation for exemestane at the 25th and 50th percentiles, as well as for letrozole at the 25th percentile (Appendix I). Our results suggest that legislation was associated with lower copayment amounts for both generic and brand-name formulations but had a greater absolute impact on costs for brand-name drugs.
To test the assumptions of our differences-in-differences analysis, we additionally evaluated the change in copayment amounts for each drug across states in only the pre-law period and in only the post-law period. There did not appear to be significant differences in the copayment quarterly trends for each drug across states in the pre-law-only period, suggesting that the differences observed after legislation enactment may be related to the policy itself (Appendix J). In addition, we performed a placebo test by analyzing only claims in the post-law period for states that enacted the laws and compared them to claims from our control states. There were no statistically significant differences in copayment amounts across states for any of the drugs between the first 6 months and the latter 6 months of the post-law period (Appendix K).
Adherence Outcomes
Among our cohort, the proportions of patients adherent during the first year of therapy to anastrozole, exemestane, letrozole, and tamoxifen were 92.7%, 92.1%, 91.3%, and 90.9%, respectively, as measured using the variable medication possession ratio (MPR) methodology. Higher median monthly copayment was significantly associated with higher risk of non-adherence (adjusted risk ratio [aRR] 1.006 per dollar increase; p<0.001; Table 3). Neither drug type nor the interaction between median monthly copayment and drug type were significantly associated with adherence. Grouping the three aromatase inhibitors into a single classification also did not change the results. Our analyses were supported by the outcomes of our sensitivity analyses (Appendix L-M). Monthly copayment ≥$20 was associated with higher risk of medication non-adherence but did not reach statistical significance (aRR 1.241; p=0.058), while median total out-of-pocket spending, inclusive of deductible amounts, was significantly associated with higher risk of non-adherence (aRR 1.003; p<0.001). Using the fixed MPR methodology, adherence rates for anastrozole, exemestane, letrozole, and tamoxifen were lower at 84.9%, 78.3%, 83.7%, and 84.7%, respectively. Higher median monthly copayment remained significantly associated with higher risk of non-adherence even after utilizing this alternative definition of adherence.
Table 3.
Association of non-adherence to endocrine therapy with copayment amount.
| Univariable Analysis |
Multivariable Analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | RR | (95% CI) | P | aRR | (95% CI) | P |
| Median monthly copaymenta, $ | 1.004 | (1.002, 1.006) | 0.001 | 1.006 | (1.003, 1.008) | <0.001 |
| Age, years | ||||||
| 18–44 | 1.625 | (1.243, 2.125) | <0.001 | 1.561 | (1.187, 2.053) | 0.002 |
| 45–54 | 1.508 | (1.211, 1.879) | <0.001 | 1.504 | (1.208, 1.873) | <0.001 |
| 55–59 | 1.297 | (1.013, 1.661) | 0.039 | 1.279 | (1.002, 1.633) | 0.048 |
| 60–64 | Reference | -- | -- | Reference | -- | -- |
| Charlson-Deyo comorbidity score | ||||||
| 0 | Reference | -- | -- | Reference | -- | -- |
| 1 | 1.350 | (1.053, 1.731) | 0.018 | 1.427 | (1.115, 1.825) | 0.005 |
| 2 or higher | 0.901 | (0.457, 1.777) | 0.764 | 1.002 | (0.524, 1.915) | 0.995 |
| Year of index claim | ||||||
| 2007–2008 | 0.962 | (0.816, 1.133) | 0.640 | 0.869 | (0.735, 1.028) | 0.102 |
| 2009–2010 | 0.212 | (0.160, 0.282) | <0.001 | 0.195 | (0.146, 0.259) | <0.001 |
| 2011–2012 | 0.279 | (0.221, 0.351) | <0.001 | 0.274 | (0.218, 0.346) | <0.001 |
| 2013–2015 | Reference | -- | -- | Reference | -- | -- |
| Oral endocrine therapy | ||||||
| Anastrozole | 0.806 | (0.673, 0.964) | 0.019 | 0.865 | (0.722, 1.037) | 0.116 |
| Exemestane | 0.875 | (0.676, 1.134) | 0.314 | 0.793 | (0.601, 1.048) | 0.103 |
| Letrozole | 0.954 | (0.781, 1.165) | 0.645 | 0.999 | (0.814, 1.226) | 0.992 |
| Tamoxifen | Reference | -- | -- | Reference | -- | -- |
Calculated on a per patient, per drug level and normalized to a 30-day prescription.
Abbreviations: RR (risk ratio of non-adherence); aRR (adjusted risk ratio of non-adherence)
Discussion
Parity laws regulating fully insured health plans have been enacted by a majority of states since 2008 to limit patient cost sharing for oral anticancer medications to an amount no greater than that for infused anticancer medications. However, given differences in coverage for both infused and oral medications across various health plans, the effect that state laws have had on patient out-of-pocket spending for cancer therapy remains unclear. A federal bill, the Cancer Drug Coverage Parity Act,8 was recently introduced in Congress to regulate self-funded plans that fall under the Employee Retirement Income Security Act, and an understanding of the impact of state laws will be important to the ongoing federal legislative debate. Using an administrative health insurance claims database from a large national insurer, we performed a differences-in-differences analysis to explore the effects of parity legislation enactment on copayments for endocrine therapy in fully insured health plans. We found that parity legislation significantly reduced monthly copayments for exemestane and anastrozole, but increased copayments for letrozole, in states subject to the laws compared to states that had not yet enacted parity legislation. Furthermore, a reduction in monthly copayment amount was significantly associated with higher likelihood of adherence.
Prior research by Dusetzina et al. has suggested that the impact of state oral parity laws on the overall cost of drugs has been variable, with an estimated increase in out-of-pocket costs of approximately $150 per month for the top 5% most expensive drugs but a decrease of $20 per month for the lowest 25% of drugs after enactment of parity legislation.9 In addition, the proportion of drugs with $0 out-of-pocket cost in fully insured health plans increased from 15% to 53%, compared to 12% to 18% in self-funded plans.9 These data suggest that parity laws may interestingly have a greater effect on reducing the cost of relatively less expensive drugs.
Oral endocrine therapies were not included in the aforementioned analysis, but the results of our current study are generally consistent with the finding by Dusetzina et al. that parity laws decreased spending for drugs with baseline out-of-pocket costs less than $50.9 In only states subject to parity laws, median monthly copayment decreased significantly for generic formulations of all four drugs after parity legislation, with the greatest decrease for exemestane of over $11. On multivariable differences-in-differences analysis controlling for residence in a state subject to parity laws, we found that parity legislation significantly decreased monthly copayment for exemestane, the endocrine therapy with the highest baseline cost, by $3.08 and $10.25 at the 25th and 50th percentiles, respectively. When considering brand-name formulations of exemestane only, the magnitude of these declines increased to $23.04 and $16.42, respectively.
Legislation enactment was also associated with decreased copayment for anastrozole by $4.39 and increased copayment for letrozole of $7.21 at the 25th percentiles, but without any measurable difference at the 50th percentile for either drug. This implies that the distribution of lesser copayments for anastrozole and letrozole changed as a result of the laws, but did not change for the majority of fills. It is unclear why copayments increased for letrozole without further details on the structure of each health plan’s medical and pharmacy benefits. However, one possible explanation is that for plans with initially favorable coverage of letrozole, perhaps via tiered pharmacy benefits, copayments were increased without exceeding the cost-sharing amount on medical services and thus satisfying parity with infused therapies, a concern that has been introduced previously.7
Given the concept of oral parity, these laws theoretically reduce patient spending only when the baseline out-of-pocket cost for an oral drug exceeds the out-of-pocket cost for medical services, which would typically be paid by the patient for administration of an infused drug. With a median copayment of over $30 per month, exemestane possibly exceeded this threshold for most health plans included in our sample. Consequently, we observed the greatest decrease in patient spending for exemestane after enactment of parity legislation. However, the impact of parity laws on patient out-of-pocket costs is as dependent on a health plan’s benefits structure as on a specific drug’s market price or cost to the insurer. This may help to explain why we observed changes at the 25th and 50th percentiles, but not at the upper end of each distribution. One hypothesis is that the lower end of the endocrine therapy copay distribution in our sample comprised primarily of health plans that had very favorable coverage of medical services, including infused chemotherapy, while the opposite was true at the upper end of the distribution. If so, the greatest effect after parity legislation would be seen on drugs in health plans with lower initial copayment amounts.
One analysis of health insurance claims data from privately insured nonelderly patients found that annual out-of-pocket spending for targeted oral anticancer drugs between 2001 and 2011 was less than half of that for intravenous anticancer drugs, despite rising costs of oral drugs to the insurer.15 The authors hypothesized that the lack of significant coinsurance requirements for pharmacy benefits in the studied health plans contributed to these results. A report by the actuarial firm Milliman also concluded that health plans with pharmacy benefits that lack significant patient coinsurance would likely experience little effect from parity legislation given a low baseline cost-sharing burden on patients for oral drugs.16 Similarly, the lack of coinsurance data in our database possibly underestimates the change in patient out-of-pocket costs, though the effect would be less for lower-cost drugs.
While endocrine therapies for breast cancer are typically less expensive than newer targeted anticancer therapies, their out-of-pocket costs may still create significant financial barriers to initial access and to therapy adherence.4–6,17–19 Rates of adherence to adjuvant endocrine therapy for breast cancer have been reported between 75% and 88% in the commercially insured population,5,17,20 but as low as 63% in the Medicare population.4,21 In this commercially insured, non-elderly population, we observed adherence rates between 90% and 92% in the first year of endocrine therapy. While our rates are higher than other studies, much of this discrepancy is likely related to differences in the definition of adherence. Unlike other studies that calculated medication possession ratios (MPRs) over a fixed time interval,4,5 we utilized variable MPRs12 to observe the effects of copayment on adherence in the one year before and one year after parity enactment without biasing against those who initiated therapy later in the year. However, this methodology, along with inclusion of only patients with at least two pharmacy fills within the first year of therapy, likely overestimates true adherence rates among our study population. We performed additional analyses by recalculating adherence rates using the number of days from the first prescription to the end of the calendar year, with results that are more consistent with prior research. However, this methodology may underestimate adherence by penalizing those who discontinued or switched therapy before the end of the year.
Nevertheless, consistent with previous research,4–6,19,22,23 we found that higher copayments and total out-of-pocket costs, along with younger age and higher comorbidity score, were associated with increased risk of non-adherence to endocrine therapy, using both methodologies for the calculation adherence. Multiple studies have found out-of-pocket costs as low as $20-$30 per month to be associated with significantly lower adherence to these medications.5,6,17 We estimate that a decrease in monthly copayment of $10 may decrease the risk of non-adherence to endocrine therapy by 6%, while reducing copayment below $20 may decrease the risk of non-adherence by over 20%. Consequently, parity laws may have had a modest but beneficial effect on overall endocrine therapy adherence. Improved understanding and mitigation of modifiable factors that influence endocrine therapy adherence may improve breast cancer outcomes, including recurrence risk, disease-free survival, and quality of life.24–27
Given that some states enacted parity laws at differing times during the study period, while other states had not enacted a law during that time, our study design leveraged the benefits of a natural experiment to control for unmeasured confounders. However, several important limitations to our study exist. While our primary analyses focused on copayments, this outcome may not represent total patient out-of-pocket costs net of supplemental insurance and discounts. In addition, as discussed earlier, coinsurance details were not available in the database, and more detailed demographic and clinical information, such as disease stage or prior treatments, was not available to include in our adjusted models. Finally, our analyses focused on secondary non-adherence only among patients with at least two prescription fills. Due to the nature of claims data containing information on only filled prescriptions, we were unable to comment on the effect of copayment amounts on primary non-adherence, i.e., not filling the initial prescription, which likely contributes to lower overall medication adherence. Further research is required to investigate these effects.
Despite these limitations, our results can inform future discussions of state and federal oral parity legislation, as well as other efforts to lower out-of-pocket cost of anticancer drugs and of endocrine therapies in particular. Parity laws appeared to have a modest effect on lowering the cost of exemestane and anastrozole. While legislation appeared to lower copayments for both generic and brand-name formulations of exemestane, the absolute impact was greatest on copayments for brand-name drugs given their high baseline cost. Lower copayments were associated with improved adherence across all drugs, and more focused efforts to limit out-of-pocket costs for endocrine therapy may have a greater impact on medication adherence.
Supplementary Material
Acknowledgements:
Data for this project were accessed using the Stanford Center for Population Health Sciences (PHS) Data Core. The PHS Data Core is supported by a National Institutes of Health (NIH) National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and from internal Stanford funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
No conflicts of interest to disclose.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet Lond Engl 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 2010;11(12):1135–1141. doi: 10.1016/S1470-2045(10)70257-6 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast cancer Version 1.2018. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Published 2018.
- 4.Farias AJ, Du XL. Association Between Out-Of-Pocket Costs, Race/Ethnicity, and Adjuvant Endocrine Therapy Adherence Among Medicare Patients With Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol 2017;35(1):86–95. doi: 10.1200/JCO.2016.68.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farias AJ, Hansen RN, Zeliadt SB, Ornelas IJ, Li CI, Thompson B. Factors Associated with Adherence to Adjuvant Endocrine Therapy Among Privately Insured and Newly Diagnosed Breast Cancer Patients: A Quantile Regression Analysis. J Manag Care Spec Pharm 2016;22(8):969–978. doi: 10.18553/jmcp.2016.22.8.969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 2011;29(18):2534–2542. doi: 10.1200/JCO.2010.33.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kircher SM, Meeker CR, Nimeiri H, et al. The Parity Paradigm: Can Legislation Help Reduce the Cost Burden of Oral Anticancer Medications? Value Health 2016;19(1):88–98. doi: 10.1016/j.jval.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 8.Lance LHR 1409 −115th Congress (2017–2018): Cancer Drug Parity Act of 2017 https://www.congress.gov/bill/115th-congress/house-bill/1409. Published March 17, 2017. Accessed April 18, 2018.
- 9.Dusetzina SB, Huskamp HA, Winn AN, Basch E, Keating NL. Out-of-Pocket and Health Care Spending Changes for Patients Using Orally Administered Anticancer Therapy After Adoption of State Parity Laws. JAMA Oncol November 2017. doi: 10.1001/jamaoncol.2017.3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers: Medical Care FRED, Federal Reserve Bank of St. Louis; https://fred.stlouisfed.org/series/CPIMEDSL. Published March 13, 2018. [Google Scholar]
- 11.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother 2006;40(7–8):1280–1288. doi: 10.1345/aph.1H018 [DOI] [PubMed] [Google Scholar]
- 12.Kozma CM, Dickson M, Phillips AL, Meletiche DM. Medication possession ratio: implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer Adherence 2013;7:509–516. doi: 10.2147/PPA.S40736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson JC, Whaley CM, Brown TT. Association of Reference Pricing with Drug Selection and Spending. N Engl J Med 2017;377(7):658–665. doi: 10.1056/NEJMsa1700087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA 2014;312(22):2401–2402. doi: 10.1001/jama.2014.16153 [DOI] [PubMed] [Google Scholar]
- 15.Shih Y-CT, Smieliauskas F, Geynisman DM, Kelly RJ, Smith TJ. Trends in the Cost and Use of Targeted Cancer Therapies for the Privately Insured Nonelderly: 2001 to 2011. J Clin Oncol 2015;33(19):2190–2196. doi: 10.1200/JCO.2014.58.2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitch K, Iwasaki K, Pyenson B. Parity for Oral and Intravenous/Injected Cancer Drugs Milliman; 2010. http://www.milliman.com/insight/research/health/Parity-for-oral-and-intravenous/injected-cancer-drugs/. Accessed May 18, 2018. [Google Scholar]
- 17.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst 2014;106(11). doi: 10.1093/jnci/dju319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat 2011;125(1):191–200. doi: 10.1007/s10549-010-0952-6 [DOI] [PubMed] [Google Scholar]
- 19.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol Off J Am Soc Clin Oncol 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 2008;26(4):556–562. doi: 10.1200/JCO.2007.11.5451 [DOI] [PubMed] [Google Scholar]
- 21.Neuner JM, Kamaraju S, Charlson JA, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Cancer Inst 2015;107(8). doi: 10.1093/jnci/djv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He W, Fang F, Varnum C, Eriksson M, Hall P, Czene K. Predictors of Discontinuation of Adjuvant Hormone Therapy in Patients With Breast Cancer. J Clin Oncol Off J Am Soc Clin Oncol 2015;33(20):2262–2269. doi: 10.1200/JCO.2014.59.3673 [DOI] [PubMed] [Google Scholar]
- 23.Wigertz A, Ahlgren J, Holmqvist M, et al. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res Treat 2012;133(1):367–373. doi: 10.1007/s10549-012-1961-4 [DOI] [PubMed] [Google Scholar]
- 24.Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment Adherence and Its Impact on Disease-Free Survival in the Breast International Group 1–98 Trial of Tamoxifen and Letrozole, Alone and in Sequence. J Clin Oncol Off J Am Soc Clin Oncol 2016;34(21):2452–2459. doi: 10.1200/JCO.2015.63.8619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Ray I, Dell’Aniello S, Bonnetain F, Azoulay L, Suissa S. Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat 2012;135(2):603–609. doi: 10.1007/s10549-012-2198-y [DOI] [PubMed] [Google Scholar]
- 26.Makubate B, Donnan PT, Dewar JA, Thompson AM, McCowan C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer 2013;108(7):1515–1524. doi: 10.1038/bjc.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCowan C, Wang S, Thompson AM, Makubate B, Petrie DJ. The value of high adherence to tamoxifen in women with breast cancer: a community-based cohort study. Br J Cancer 2013;109(5):1172–1180. doi: 10.1038/bjc.2013.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.