Abstract
Herpes simplex virus type 1 (HSV) keratitis is a leading cause of infectious blindness. Clinical disease occurs variably throughout the cornea from epithelium to endothelium and recurrent HSV stromal keratitis is associated with corneal scarring and neovascularization. HSV keratitis can be associated with ocular pain and subsequent neutrophic keratopathy. Host cell interactions with HSV trigger an inflammatory cascade responsible not only for clearance of virus but also for progressive corneal opacification due to inflammatory cell infiltrate, angiogenesis, and corneal nerve loss. Current antiviral therapies target viral replication to decrease disease duration, severity and recurrence, but there are limitations to these agents. Therapies directed towards viral entry into cells, protein synthesis, inflammatory cytokines and vascular endothelial growth factor pathways in animal models represent promising new approaches to the treatment of recurrent HSV keratitis.
Keywords: herpes simplex virus, HSV keratitis, dendritic keratitis, stromal keratitis, host cell, antiviral, acyclovir, inflammation
I. Herpes keratitis:
Herpes simplex virus type 1 (HSV) is the leading cause of infectious blindness in the developed world. HSV infection can occur at any location in the eye; the most common presentation is epithelial or dendritic keratitis. Herpes stromal keratitis can result in progressive corneal opacification and vision loss with recurrent disease. The host cell’s response to HSV plays an important role in the pathogenesis of HSV infection in the eye. Novel therapeutics targeting these host cell interactions with HSV may provide additional antiviral and anti-inflammatory treatment options.
a. Virus life cycle
The herpes simplex virus type 1 (HSV) is a member of the alpha subfamily of herpesviruses. It is a DNA virus composed of a linear double stranded DNA genome packaged within an icosahedral capsid. An inner tegument composed of a layer of mRNAs and proteins, and an outer lipid bilayer envelope containing glycoproteins cover the capsid. For virus to gain entry into cells, glycoproteins on this outer envelope, including gB, gC, gD, gH, and gL, interact with each other and some cell surface receptors to facilitate penetration of the capsid through the plasma membrane of the host. Heparan sulfate via its interaction with gB and/or gC allows virus attachment to cells followed by the binding of viral gD with 3-OS heparan sulfate, herpes viral entry mediator (HVEM), or nectin-1, which is required for the capsid delivery into the cytoplasm.1 Depending on the cell type the virus can fuse with the host cell or undergo endocytosis for entry.2 The newly delivered capsid is then transported to the nucleus for viral gene replication using host cell DNA polymerase and production of new virions which are released from the cell to infect other nearby cells. The release is facilitated by removal of heparan sulfate moieties on infected cells by the host enzyme heparanase.3 HSV can continue replicating in cells or it can enter latency, by traveling to the trigeminal ganglion by way of corneal nerves. Because of this establishment of latency and reactivation from latency, HSV can exist for the lifetime of the infected host.
b. Epidemiology
By adulthood, the seroprevalence of HSV has been reported to be 50% in the United States and up to 90% in parts of Africa.4,5 The seroprevalence of HSV has been decreasing in the US and other countries; delay in HSV seropositivity may result in increasing incidence of ocular HSV. The incidence of new HSV keratitis (both epithelial and stromal disease) is 18–25 per 100,000 with recurrence rates estimated to be 50% at 5 years and over 60% at 20 years.6,7 Most epidemiologic studies have occurred in Western countries and it is possible that the global burden of visual impairment related to ocular HSV is higher than prior estimates.
There is no clear evidence of differential susceptibility to HSV infection based on gender; there have been variable reports of increased incidence of HSV keratitis in women and increased susceptibility to recurrences of HSV keratitis in men.8 Based on studies in humans and animals, females produce a more robust immune response to viruses compared to males with resultant resistance to viruses, including HSV, but greater immunopathology.9 In contrast, males have a more intense and severe response to viral infections and may be more susceptible to reactivation compared to females.10 These gender differences in susceptibility to infection may also affect response to potential therapies.
c. Clinical definitions and presentation
Primary HSV infection can occur in children or young adults by way of direct contact through mucous membranes, often presenting as conjunctivitis with eyelid vesicles. The majority of cases of ocular HSV result from reactivation of latent HSV infection from the trigeminal ganglion with viral shedding in the cornea. Reactivation of HSV has been triggered by various environmental factors, including stress, fever, and ultraviolet light exposure as well as iatrogenic factors such as laser treatment or topical corticosteroids.11 Ocular HSV is most often a unilateral disease, but bilateral disease has been reported in 2–19% of cases, particularly in immunosuppressed patients.12,13
Epithelial keratitis
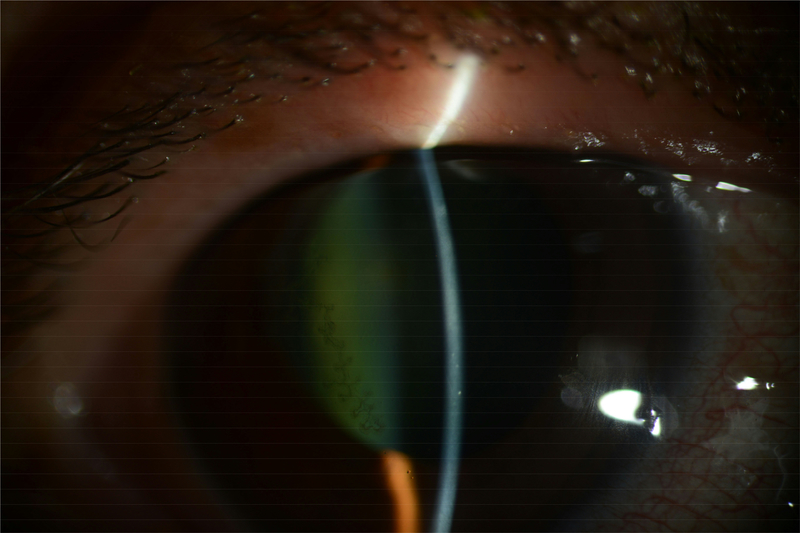
Over 60% of new cases of ocular HSV present with corneal epithelial disease, progressing from punctate keratitis to dendritic keratitis or a geographic ulcer.14 Patients initially present with symptoms of eye pain, tearing, redness, and foreign body sensation. Dendritic keratitis is characterized by branching lesions on the epithelium with terminal bulbs; these terminal bulbs contain live virus (Figure 1). These lesions can coalesce to form a geographic ulcer with discrete flat edges that can change conformation as infection spreads. Corneal sensation is diminished in the affected eye due to associated decline in subbasal corneal nerves.15 A sequela of this disruption in corneal sensation and diminished corneal nerves includes neurotrophic keratopathy, which can result in persistent corneal epithelial defects, corneal melt and perforation. Although epithelial lesions can resolve on their own, antiviral therapy is used to speed the resolution of these lesions and limit the extent of disease.
Figure 1.
Clinical slit lamp photograph of a patient with HSV epithelial keratitis demonstrating a dendrite with branching lesions and terminal bulbs seen with fluorescein staining. (Image courtesy of Dr. Joel Sugar and Dr. Julie Goldman)
Stromal keratitis
Herpes stromal keratitis (HSK) accounts for 20–48% of recurrent cases of ocular HSV infection and is the form of HSV keratitis that is associated with long-term vision loss due to corneal scarring and neovascularization.16 With each recurrence of HSK, there is additional stromal immune response in the cornea with progressive opacification and neovascularization.
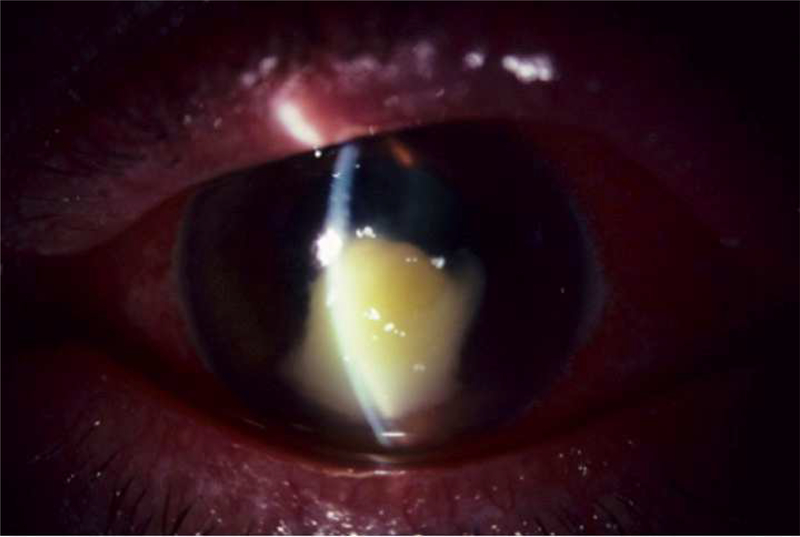
There are several forms of HSK which have been described including, 1) necrotizing stromal keratitis and 2) non-necrotizing immune stromal or disciform keratitis. Necrotizing stromal keratitis is characterized by fulminant stromal infiltration with or without epithelial ulceration likely from viral invasion of the corneal stroma (Figure 2); patients may be at high risk for corneal melt due to the robust inflammatory response within the cornea.17 Immune stromal keratitis is the more common manifestation of HSK and presents with stromal inflammation without necrosis and generally intact overlying epithelium. Immune stromal keratitis can also present as an immune ring which represents antigen-antibody immune complex deposition in the stroma. Disciform keratitis is used to describe focal stromal edema, often located in the inferior cornea with keratic precipitates. Chronic recurrent stromal keratitis can lead to the development of stromal scarring, corneal thinning, neovascularization and lipid deposition.
Figure 2.
Clinical slit lamp photograph of a patient with HSV necrotizing stromal keratitis showing fulminant necrosis of the corneal stroma with epithelial ulceration. (Image courtesy of Dr. Joel Sugar)
Endotheliitis and Uveitis
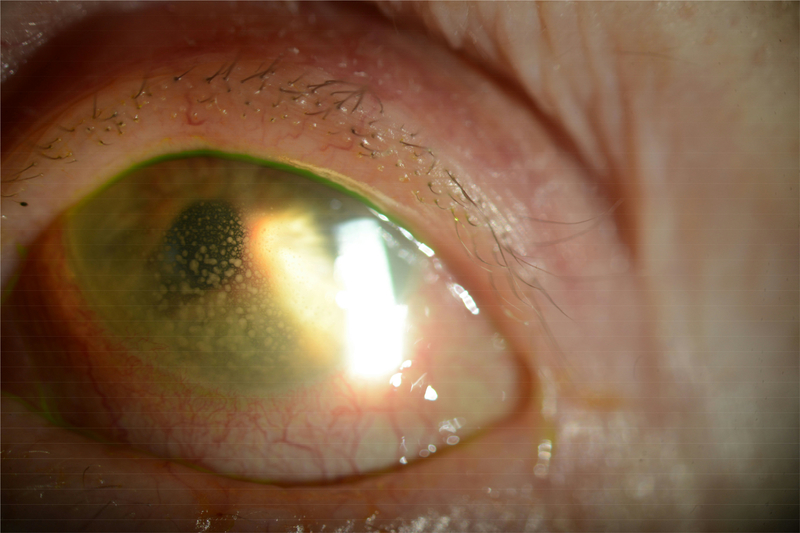
HSV endotheliitis can also be described as disciform keratitis with associated endothelial dysfunction, focal stromal edema and keratic precipitates (Figure 3). HSV has been identified by PCR from aqueous specimens of patients with endotheliitis.11 There can also be anterior chamber inflammation with the endotheliitis or in isolation as HSV uveitis. Patients may present with elevated intraocular pressure due to inflammation of the trabecular meshwork or trabeculitis which responds to topical corticosteroids. HSV uveitis can also be associated with iris atrophy seen as transillumination defects on slit lamp examination.
Figure 3.
Clinical slit lamp photograph of a patient with history of HSV stromal keratitis who presented several years after last flare of keratitis with active HSV uveitis with large granulomatous keratic precipitates on the endothelium. (Image courtesy of Dr. Joel Sugar)
II. Pathophysiology of HSV keratitis
a. Overview of pathophysiology
In order for HSV to cause clinical signs of infection in the cornea, it must evade the multiple barriers created by the host, including the innate immune response early in disease and the subsequent delayed adaptive immune response resulting from viral antigen presentation. HSV gains entry to corneal epithelial cells through interaction of specific viral and host receptors. HSV uses host cell DNA polymerase to replicate and create viral progeny which then exit the cell and move on to infect neighboring cells. Virus then travels from corneal nerves to the trigeminal ganglion where it can enter latency only to reactivate and cause recurrent infection.
i. Epithelial keratitis
HSV infects corneal epithelial cells, initially manifesting as punctate keratopathy and then coalescing to dendritic keratitis as virus spreads from cell to cell, possibly following the pattern of the subbasal plexus of corneal nerves to create branching lesions.16 Actively replicating infectious virus is generally found at the borders or terminal bulbs of the dendritic lesion or ulcer. This damage may further progress to form a geographic ulcer, characterized by an enlarged central region of dead cells. Within 12–24 hours of infection as viral replication occurs, epithelial cells swell causing disruption of the superficial layers with eventual cell lysis.18 In cell cultures of HSV, infected corneal epithelial cells begin to form syncytia resembling the clinical appearance of the dendritic lesion; this cell to cell spread enables HSV to evade the immune system.19
Type I interferons (IFN-a/b) produced by infected corneal epithelial cells limit the spread of virus by making neighboring cells less permissive to viral entry and production.19,20 HSV-specific IgG and sIgA present in the tear film and stroma may also help prevent spread of virus in the epithelium during this stage. These lesions frequently resolve spontaneously; however, in cases of immunocompromise or inappropriate corticosteroid use, uncontrolled viral spread may result in the penetration and rupture of the basement membrane and initiation of stromal disease.16
ii. Stromal keratitis
Herpes stromal keratitis (HSK) is primarily immune-mediated inflammation although viral particles can persist in latent or active states in the corneal stroma or can spread from epithelial infection.21 In mice infected with a strain of HSV that could not move from the sensory ganglion back to the cornea, HSK developed because virus was still present in the cornea.22 Studies have demonstrated the presence of HSV DNA and latency associated transcript in corneas of patients with history of HSV keratitis supporting the likelihood of corneal latency.23,24 Latent corneal HSV infection could be removed through penetrating keratoplasty, but in reality may provide a source for recurrent infection in the corneal graft.25
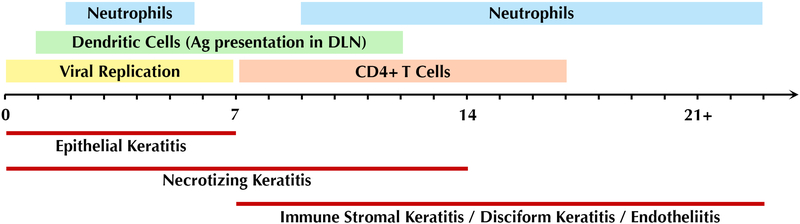
HSK is driven by interactions between HSV-infected resident corneal cells and infiltrating inflammatory cells. Much of our understanding of these processes has been generated through the use of mouse and rabbit models of infection. In humans, HSK is generally the result of recurrent infection, whereby virus latently harbored in the trigeminal ganglia reactivates and tracks along corneal nerves in a retrograde manner to infect the cornea. Virus replication in the cornea triggers innate immune signaling with production of cytokines and chemokines by epithelial and stromal corneal cells. Starting within 24 hours, there is an influx of inflammatory cells, predominantly neutrophils, but also natural killer (NK) cells, dendritic cells, and macrophages, which at this stage function to limit viral spread within the tissue. Dendritic cells are the major antigen presenting cells that phagocytose viral particles and infected cells and track to the draining lymph nodes to generate an adaptive immune response. Starting at 7 days post infection (dpi), and peaking at 1421 days, CD4+ T cells reach the cornea, coinciding with the clinical phase of the disease (Figure 4). CD4+ T cells produce various cytokines such as IFN-g and IL-17, which drive the infiltration and activation of a second wave of neutrophils thought to be more pathogenic in nature. Further production of cytokines and extracellular matrix-degrading proteases by these neutrophils drive the characteristic inflammation and tissue destruction seen in HSK. The recruitment of inflammatory cells causes corneal clouding that obscures the visual axis and can lead to blindness.
Figure 4.
Timeline of clinical course and pathogenesis of HSV keratitis.
iii. Endotheliitis/uveitis
HSV endotheliitis often occurs in conjunction with stromal keratitis or uveitis. HSV DNA has been detected in endothelial cells, in the aqueous fluid, and in the trabecular meshwork of patients with HSV endotheliitis or uveitis.26 HSV has been found to invade endothelial cells with reduction in cell density and pleomorphism. In vivo confocal microscopy has identified pseudoguttata, infiltration of inflammatory cells and enlargement of intercellular gaps and an overall decrease in endothelial cell density in eyes affected by HSV endotheliitis.27
b. Inflammatory Cascade and Sequelae
i. Immune cell subsets in pathogenesis
Most work to dissect the immunological aspects of herpetic keratitis has made use of mouse models of primary corneal infection, which are thought to mimic the processes of HSK in humans. Likewise, in this section we will focus on information garnered from murine infection models, while bearing in mind one important caveat. In humans, the vast majority of HSK develops as a result of recurrent infection, in which primary infection may have arisen from locations other than the cornea. Nevertheless, primary corneal infection of mice seems to provoke a similar immune response with similar kinetics to the situation of reactivation of latent virus traveling from the trigeminal ganglia to the cornea. In both cases, replicating virus is cleared from the cornea by 7 dpi. Upon infection, corneal epithelial cells initiate the innate immune response by secreting cytokines and chemokines that serve as the first homing signals for various cells of the immune system.
a. Neutrophils
Neutrophils are the earliest responders to infection, and arrive to the cornea in large numbers in two distinct phases over the course the disease. Given the overlap between the first neutrophil influx at 2 to 5 dpi and the waning of active viral replication at 5 to 7 dpi, it was assumed that neutrophils have a major role in viral clearance from the cornea.28,29 Depletion studies using an antibody against the granulocyte marker Gr-1 showed delayed clearance of HSV-1 from the cornea;30 however, the interpretation of these results was later challenged when it was discovered that Gr-1 is also present on other cell types besides neutrophils.31 A later study using the neutrophil-specific Ly6G antibody showed no difference in virus clearance, although this was conducted in a mouse lung infection model. Based on review of the existing literature, we believe neutrophils play a beneficial role in the initial clearance of virus from the cornea, but given the uncertainty in the field, this is an important area for future research. On the other hand, it is well understood that the second wave of neutrophil infiltration coincides with the appearance of clinical symptoms, and is more destructive to the cornea.32 Starting around 8–9 dpi and intensifying until 21 dpi, neutrophils presumed to be attracted to the cornea and activated by CD4+ T cells degranulate and secrete inflammatory mediators, proteases, and free radicals, and are responsible for much of the tissue damage observed in HSK. Making up 70–80% of leukocytic infiltrate at this stage, neutrophils are also a major contributor to corneal opacity.28,29
b. Dendritic cells, macrophages, and natural killer cells
Although it was once thought that the homeostatic cornea is devoid of any immune cell component, it is now clear that the normal cornea contains multiple bone marrow-derived leukocytes that function in antigen uptake and presentation.33–35 Dendritic cells (DCs) are large phagocytic cells that sample their environment for non-self antigens, traffic to the draining lymph nodes, and present antigens to T cells via MHC-II to initiate the adaptive immune response. As part of the innate immune system, macrophages and natural killer (NK) cells survey tissue environments, with macrophages serving as phagocytic consumers of pathogens and NK cells poised to eliminate unhealthy cells based on decreased class I MHC expression.36 Within 24 hours after infection, DCs and macrophages increase in numbers and in activation status (MHC-II+) in the central cornea.37 Hu et al. went on to show that specific depletion of DCs in CD11c-DTR mice increased severity of keratitis, whereas specific depletion of macrophages with injection of clodronate liposomes produced no significant difference in disease. In contrast, through the use of ATG5-deficient CD11c-Cre mice it was recently shown that dendritic cell autophagy, which allows functional DC antigen processing and presentation, contributes to disease. Disruption of autophagy in dendritic cells alone produced a decrease in disease severity.38
c. CD4+ T cells
T lymphocytes (primarily CD4+ T cells) are thought to be the major drivers of lesion development in stromal keratitis.39–41 Early work showed that athymic (nude) mice lacking T and B cells succumb to HSV-1 corneal infection, but did not develop lesions of stromal keratitis.42 In the immunocompetent scenario, T cells appear in the cornea around 7 dpi, and continue to increase in numbers there through 21 dpi. A generally accepted view is that CD4+ T cells under a Th1 program producing IL-2 and IFN-γ in the cornea are responsible for the second wave neutrophil recruitment.25 Indeed, blocking these two cytokines with neutralizing antibodies was shown to slow the progression of HSK.43,44 With more recent advances in our knowledge of subtypes of CD4+ T cells came the understanding of the pro-inflammatory roles of interleukin-17 and Th17 cells in the cornea.45,46 Another CD4+ T cell subtype involved in the progression of keratitis but poorly understood is that of regulatory T cells (Treg), which are immunomodulatory and have the capacity to dampen the immune response through expression.47–49
While it is clear that CD4+ T cells are important players in the immunopathogenesis of HSV keratitis, their antigen specificity remains unresolved.25,41 Some studies have put forth the notion that CD4+ T cells react to autoantigens that have been unmasked during the course of infection.50,51 However, this idea has been refuted as cross reactivity between suspected autoantigens was not detected and no effect on HSK severity was observed, thus casting doubt on the molecular mimicry hypothesis.52,53 Instead, the model of bystander activation was put forth when it was found that nude mice reconstituted with T cells lacking specificity to viral antigens still developed stromal lesions, in addition to being incapable of clearing infection and succumbing to encephalitis.54,55
d. CD8+ T cells
While roles of CD4+ T cells have been studied extensively in HSK, the importance of other T cell types, such as CD8+ T cells, regulatory T cells (Tregs), and Th17 cells has historically been more elusive. Multiple lines of evidence have emerged in recent years, which contribute substantially to our understanding of these cell types in the pathogenesis and protection of HSK.
CD8+ (or cytotoxic) T cells generally function in the control of viral infections by sensing and targeting infected cells for destruction through recognition of viral peptides through T cell receptor (TCR) complexes and class I MHC binding.56 In keratitis, some investigators believe that CD8+ T cells play an important role in controlling recurrent infections. The investigators have shown that following primary infection, activated HSV-1 specific CD8 T cells migrate into the trigeminal ganglia where they associate with neuronal cell bodies and are retained as an aspect of immunological memory.57–60 More recently a separate group has refuted the findings and demonstrated a bystander role for CD8+ T cells during HSV-1 reactivation from latently infected mice.61 A substantial amount of work has been dedicated to understanding the repertoire of CD8 T cell receptors in herpes keratitis. Original observations suggested that CD8 T cell responses were directed almost entirely against a single epitope of glycoprotein B.62 It was later demonstrated that this immunodominant gB epitope accounts for closer to 50% of CD8+ T cells in the TG, and the authors provided a detailed account of the 18 subdominant epitopes that collectively define the TCR repertoire.63 An understanding of the epitope specificities of T cells can be useful in designing a vaccine against HSV-1.64 Others have focused on understanding specific subpopulations of CD8+ T cells and how they function in disease. Khan et al. found that symptomatic HSV-1 infected patients had less differentiated, monofunctional CD8+ T cells compared to asymptomatic patients seropositive for HSV-1, who had higher proportions of differentiated and multifunctional HSV-1 gB-specific CD8 T cells.65 These authors later found that asymptomatic seropositive individuals possessed CD8+ T effector memory cells that were primarily directed against three particular HSV-1 epitopes. Combination treatment of mice with a vaccine including these three epitopes and an adenoviral expression vector of the T cell attractant CXCL10 protected against virus shedding and reduced recurrent herpetic disease.66
ii. Host factors in control of inflammation
It remains unknown why certain individuals infected with HSV-1 develop symptoms of recurrent infection while others merely shed virus asymptomatically.67,68 These observations point to the importance of host factors in generating susceptibility to infection, and the interplay between pathogen and host response is still an area of active research.
a. Toll-like receptors
TLRs are a widely expressed component of the innate immune system with the ability to sense specific conserved motifs called pathogen associated molecular patterns (PAMPs). To date, 13 TLRs have been identified, 9 common to humans and mice, with each recognizing a specific PAMP or ligand.69 With respect to HSV-1 infection, it has been shown that TLRs 2, 3, and 9 have some role in sensing of viral products; TLR2 binds glycoproteins at the cell surface, while TLR3 and TLR9 in endosomes recognize double-stranded RNA and unmethylated CpG motifs, respectively.70,71 Binding of TLRs by these molecular patterns triggers a cascade of cellular signaling events, ultimately driving cytokine production, inflammation, and generation of an innate immune response.25 While activation of TLRs is an important early event in viral clearance and generation of a cellular response to infection, overactive inflammation can result in excessive infiltration into the cornea causing opacity and loss of vision.
b. Cytokines and chemokines
Cytokines and chemokines are small proteins (~5–20 kilodaltons) secreted in response to infection and other cellular stresses. They are produced by a broad range of cell types of the cornea and immune system, and by binding to their specific receptors, can trigger cellular activation, differentiation, and recruitment.20 Numerous cytokines are released at various stages in the development of keratitis, and here we will briefly focus on a few that have been studied most extensively.
Among the first cytokines secreted upon HSV-1 infection of the cornea are type I interferons (IFN-a and IFN-b), which can be produced by most cell types including corneal epithelial cells.72 IFN-a/b are important early controllers of viral replication; binding of IFNs to their cell surface receptors results in multiple signaling cascades and finally production of a variety of interferon-stimulated genes (ISGs) that have antiviral properties.20 Infectious spread and systemic dissemination is limited as neighboring cells become more resistant to infection.73 Several studies have shown that type I interferons are required for proper control of HSV-1 replication in the cornea.72,74 It was also shown that a functional type I interferon system is required for proper recruitment of immune cells to the site of infection.75
Interferon-γ (IFN-γ) or type II interferon is produced only by cells of the immune system, particularly Th1 cells, and binds to a distinct receptor from type I interferon. IFN-γ activates cells of the innate immune system to increase cytokine secretion, phagocytosis, and expression of class II MHC molecules.76 In the HSV-1 infected cornea, IFN-γ, produced by Th1 cells in combination with IL-2, is pro-inflammatory in action, driving infiltration of neutrophils and activation/maturation of antigen presenting cells.43,44,77
Additional pro-inflammatory cytokines IL-1, IL-6, and TNF-a have been shown to be major drivers of corneal inflammation, and various strategies to block these cytokines have proven effective in decreasing pathogenesis of HSK in mice.78–81 IL-6 in particular has also been implicated as a driver of corneal neovascularization, through stimulation of VEGF production.82,83 Similarly, IL-17 is a pro-inflammatory mediator secreted by Th17 cells, which also regulates VEGF production to promote corneal angiogenesis after HSV-1 infection.45,46,84
CXCL1 and CXCL2 specifically target the receptor CXCR2 on neutrophils and attract them to infiltrate the cornea.85,86 CXCR2-deficient mice infected with HSV-1 show minimal infiltration of PMNs into the cornea.87 CXCL10 is the only cytokine that is expressed in the cornea at detectable levels at baseline,88 and this chemokine acts solely through the receptor CXCR3 (expressed on macrophages, DCs and activated T cells).89 Multiple studies have shown that defects in the CXCL10-CXCR3 system result in uncontrolled viral replication and increased severity of HSK.90–92 CXCL10 has also been implicated in recruitment of CD8+ T cells to the infected tissues and contributes to protection from recurrent infection in murine models of UV-induced reactivation.93
While many of the cytokines produced in HSV keratitis are pro-inflammatory and promote leukocyte infiltration and tissue damage, IL-10 has been shown to have a more suppressive role by dampening immune responses. IL-10 produced by resident corneal cells and Tregs suppresses pro-inflammatory mediators and is protective against HSK.94 Administration of IL-10 after HSV-1 corneal infection resulted in decreased production of the pro-inflammatory cytokines IL-2 and IL-6, and decreased neutrophil infiltration and corneal opacity.85,95
c. Heparanase
The host cell enzyme heparanase (HPSE) has been shown to promote HSV infection and pathogenesis through multiple mechanisms. HPSE is involved in the maintenance of extracellular matrix homeostasis through the normal turnover of cell-associated heparan sulfate.96 HPSE is upregulated at the transcriptional level late in a productive infection with some dependence on the viral protein ICP34.5.3,97 Once expressed at a higher level, HPSE contributes to viral spread by removing heparan sulfate on the cell surface and allowing newly produced virions to be released to the surroundings.3 As heparan sulfate is the major viral attachment receptor, removal of this interaction between host cell surface and virus is important for viral progeny to spread to other cells. While these findings were originally described in the case of HSV-1 infection, other groups have shown that HPSE similarly contributes to release and pathogenesis of other viruses, including dengue virus, human papilloma virus, respiratory syncytial virus, and porcine respiratory and reproductive syncytial virus.98–100 Inhibition of HPSE with the small molecule OGT 2115 or shRNA knockdown in vivo resulted in significant decreases in virus release and spread in the cornea.3,97 HPSE also contributes to tissue damage and corneal inflammation by breaking down extracellular matrix and releasing growth factors such as VEGF, which are normally sequestered by chains of heparan sulfate. This notion is also supported by numerous reports in the cancer field, where HPSE has been shown to be a driver of angiogenesis, inflammation, and tumor metastasis.96,101 Finally, HPSE appears to have a regulatory role in transcription of proinflammatory cytokines like IL-1β, IL-6 and TNF-α, and when activated drives increased production of these transcripts.97 Viral infection also promotes translocation of HPSE to the nucleus, where it presumably could participate in regulation of selective transcription of key target genes.97 Although the details of its actions remain to be elucidated, HPSE may prove a valuable therapeutic target to control multiple pathological aspects of HSV infection and corneal inflammation.
c. Viral evasion mechanisms
Multiple mechanisms exist by which HSV-1 can subvert host immune responses and cellular machinery to promote virus propagation and persistence.102 It is worth briefly taking note of the cellular pathways that the virus has evolved to bypass; understanding the importance of these host factors and seeking to bolster these cellular responses may yield valuable therapeutic options.
Given that toll-like receptor (TLR), RIG-I-like receptor (RLR), and cytosolic DNA sensing pathways are major initial indicators of viruses in cells, HSV-1 has developed a number of ways to avoid early detection.102 Viral downregulation of these sensors or the signaling pathways controlling them is one strategy HSV-1 employs to pass undetected.103,104 Another key viral protein ICP0 accomplishes this through direct ubiquitination of target host proteins, such as interferon gamma inducible protein 16 (IFI16).105 By tagging this key player in the cytosolic DNA sensing pathway with ubiquitin residues, the virus is able to avoid one arm of the innate immune response. In other cases, viral kinases can alter the phosphorylation status of transcription factors to interfere with nuclear translocation and expression of target genes, as in the case of interferon regulatory factor 3 (IRF3) and nuclear factor (NF)-kB by viral kinase US3.106,107 Other major cellular defense mechanisms disrupted by the virus include MHC class I antigen presentation,108 DNA damage response pathway,109 autophagy,110 endoplasmic reticulum stress,111 and necroptosis.112
iii. Control of corneal neovascularization
Inappropriate blood vessel formation in the normally transparent and avascular cornea is a major cause of the vision loss and blindness due to herpetic keratitis. Evidence suggests that increased corneal angiogenesis is the result of increased production or unopposed action of various growth factors, with vascular endothelial growth factor (VEGF) as the main driver.113 Blocking VEGF by multiple means decreased blood vessel formation in the cornea and decreased severity of keratitis.113–115 It appears that neutrophils infiltrating into the cornea produce matrix metalloproteases (MMPs), MMP9 in particular, which break down the extracellular matrix and provide the substrates for vessel formation.116 A pathological cycle is set in motion whereby increased neovascularization allows increased access of neutrophils and other leukocytes into the cornea, which then release more MMPs and drive additional neovascularization.116 Another critical contributor to corneal neovascularization was found to be depletion of soluble VEGF receptor (sVEGFR) that occurs upon infection.46,117 In the healthy cornea, sVEGFR is constitutively present and maintains a majority of VEGF in a sequestered and inactive state. Upon corneal infection, an imbalance is generated via a loss of sVEGFR, which allows free VEGF to bind cell surface receptor VEGFR2 and promote angiogenesis.46 It was subsequently found that IL-17 also participates in the angiogenic process in the cornea by regulating VEGF and sVEGFR expression.84 As inhibition of the host factors VEGF and MMP9 has proven effective in mitigating neovascularization and severity of keratitis in mouse models, these strategies show promise as therapeutics in human disease.
iv. Effects on the ocular surface and corneal tissues
One of the downstream consequences of HSV corneal infection is the effect on corneal nerves with loss of the sub-basal nerve plexus with resultant diminished corneal sensation. This loss of corneal sensation and normal blink reflex can eventually lead to neurotrophic keratopathy, a degenerative condition that can lead to the development of epithelial breakdown, superinfection, and even corneal melt. In vivo confocal microscopy studies have demonstrated a significant decrease in the sub-basal nerve plexus in eyes of patients with HSV keratitis compared to controls which corresponded to corneal sensation testing; the unaffected eyes in HSV keratitis patients also had diminished corneal nerve density compared to controls.15 Corneal nerve density loss occurs early in the course of disease during acute infection with progressive loss corresponding to the total duration of disease. In a mouse model, mice lost corneal sensation 8 days after HSV 1 infection; mice were also found to have upregulation of the nerve guidance molecule, semaphorin 7A, with reinnervation by 30 days post infection without functional recovery of corneal sensation.118
The theory behind why corneal nerves are damaged during HSV infection has been debated. In a study by Hu using a leukocyte marker, P-selectin glycoprotein ligand-1, knockout mouse, there was more significant corneal nerve loss but decreased corneal leukocytes in the KO compared to wild type mouse. There were also higher viral titers in these KO mice indicating that virus but not leukocyte infiltration played a role in corneal nerve loss.37 However, a study by Yun, et al, demonstrated that in a mouse model deficient of CD4 T cells, corneal nerves were able to regenerate following HSV infection in association with recovery of blink reflex and decreased inflammation.119 Hyperinnervation of the cornea by sympathetic nerves after loss of sensory nerves has been identified in mouse models of HSV; blocking this innervation of sympathetic fibers by excising the superior cervical ganglion resulted in decreased severity of HSK.120
Recurrent unilateral HSV keratitis has also been associated with bilateral tear dysfunction, even during disease quiescence. One study demonstrated hyperosmolarity and reduction in tearing and tear stability in unaffected eyes of patients with history of HSV keratitis, likely due to loss of corneal sensation affecting input to the afferent pathways of tear secretion.121 Patients with unilateral HSV keratitis may have ocular pain related to the development of bilateral dry eye disease. In another study, patients with unilateral HSV keratitis reported higher discomfort levels and visual symptoms associated with dry eye in unaffected eyes compared to normal controls.122 Dry eye and/or neuropathic pain are unfortunate repercussions of the disruption in corneal nerves by HSV; ocular pain can occur even well after the active viral infection has resolved.123 In one study, patients with quiescent HSV keratitis reported significant impairment in quality of life due to ocular pain compared to normal controls and other bilateral ocular surface diseases.124 Patients with more frequent relapses of disease had lower quality of life related to pain, possibly due to memory of severe pain associated with acute infection or to chronic bilateral ocular surface disease from recurrent keratitis.
III. Treatment approaches
a. Antiviral
Current antiviral therapy for HSV is targeted to inhibition of viral replication. Three systemic (acyclovir, valacyclovir and famciclovir) and two topical (trifluridine and ganciclovir gel) therapies are currently available in the United States for HSV infection, although only the topical therapies are approved by the FDA specifically for the treatment of HSV keratitis.125 Acyclovir is available in topical form in Europe and other countries. Acyclovir is a nucleoside analog that inhibits DNA polymerase, thereby inhibiting HSV viral replication. Acyclovir is phosphorylated by HSV thymidine kinase and mutations in thymidine kinase can lead to acyclovir resistance. Acyclovir has a higher affinity for HSV infected cells compared to topical anti-viral therapies such as trifluridine, resulting in less toxicity to non-infected cells. Valacyclovir is the prodrug of acyclovir and has 3–5 times the bioavailability compared to acyclovir, with serum levels similar to intravenous administration of acyclovir.126 Famciclovir is a guanosine analog used to treat various herpesviruses and is used less commonly than acyclovir and valacyclovir.
Topical trifluridine is a pyrimidine nucleoside analog that is still a commonly used therapy for epithelial keratitis; however, it is administered up to 9 times per day and can be associated with epithelial toxicity. Topical ganciclovir gel is a purine nucleoside that inhibits viral DNA replication; it is administered 5 times/day and has less epithelial toxicity than trifluridine. While there are no studies directly comparing the efficacy of topical trifluridine and ganciclovir in the treatment of HSV epithelial keratitis, extrapolation from studies comparing each drug to topical acyclovir suggests similar efficacy.127
The Herpetic Eye Disease studies (HEDS) found that oral acyclovir prophylaxis reduced the risk for recurrence of stromal keratitis by 50% among patients who previously had stromal keratitis.128 There was no benefit from addition of oral acyclovir to HSV epithelial keratitis treated with topical trifluridine or to HSK treated with topical corticosteroids and trifluridine.129
Interferon interferes with viral replication and has been shown to have antiviral activity against HSV; topical formulations of interferon α2B have been used as eye drops in HSV epithelial keratitis, however they are not approved by the FDA and are not readily available for use.125 Epithelial debridement has also been used as a primary treatment or in combination with antiviral therapy for HSV epithelial keratitis; however, there have been reports of inconsistent efficacy and recurrent infection following debridement.127
b. Anti-inflammatory
The primary cause of vision loss related to ocular HSV infection is related to the damaging effects of inflammation in the cornea. Currently, the primary method of treatment for inflammation related to HSV keratitis is corticosteroid therapy. The HEDS studies confirmed that topical corticosteroid reduced the progression or persistence of inflammation related to HSK compared to placebo by 68%.130 There was no significant difference in visual outcomes between the placebo and corticosteroid group at 6 months. Topical corticosteroid therapy is also the primary treatment for HSV endotheliitis and uveitis along with a systemic antiviral agent.
Topical cyclosporine has also been used as an alternative anti-inflammatory therapy in nonnecrotizing HSK, including patients who were not responsive to topical corticosteroids.131 While not widely used, this therapy may be useful in patients who have a steroid response since cyclosporine does not increase intraocular pressure.
Amniotic membrane transplants have also been proposed as a potential treatment for HSV keratitis and have been used in patients with recurrent epithelial keratitis and necrotizing stromal keratitis along with antiviral therapy. Amniotic membrane has anti-inflammatory effects on activated neutrophils, lymphocytes and macrophages, reduces levels of inflammatory cytokines, IL-2 and IFN-γ, and may release nerve growth factor, allowing for improved wound healing.132
c. Limitations of current treatment approaches
Current antiviral therapies are limited to inhibiting viral replication. These treatments do not cure latent infection nor do they address the damaging inflammatory response within the cornea that can develop with HSV infection. Antiviral resistance can occur; the prevalence of acyclovir resistance is 0.5% in immunocompetent patients and 15% in immunocompromised patients.133 In a study of patients who had recurrence of HSK in spite of antiviral prophylaxis, ocular fluid samples confirmed HSV with genotypic resistance to acyclovir.134 Rates of acyclovir resistance have been rising in immunocompromised patients, particularly individuals who have received hematopoietic stem cell transplants. Mutations in the UL23 gene encoding thymidine kinase account for the overwhelming majority of acyclovir resistant cases, but mutations on the UL30 gene for DNA polymerase can also cause drug resistance.133 While other antiviral therapies such as foscarnet and cidofovir can be used in cases of acyclovir resistance, these drugs have significant systemic toxicities. Topical ganciclovir can be used for acyclovir-resistant HSV epithelial keratitis, but there is limited data on its use in HSK and HSV endotheliitis.
There are limitations to the use of topical corticosteroids for the treatment of the inflammatory sequelae of HSV keratitis, including corneal scarring, neovascularization and neurotrophic keratopathy. Topical corticosteroids can be associated with steroid response glaucoma and cataract formation which can affect visual acuity. Topical corticosteroids can also cause recurrence of epithelial keratitis.135
Finally, neither antiviral nor anti-inflammatory therapies are effective in the treatment of diminished corneal sensation and eventual neurotrophic keratopathy that can develop in many ocular HSV patients.
d. Novel therapies
There are a number of different promising targets for novel antiviral therapies, including viral and host cell targets.
Viral entry targets
Molecules targeting host cell receptors, including 3-O-sulfated heparan sulfate, and HSV envelope glycoproteins such as gD can inhibit HSV entry and spread. These molecules competitively bind to either host or viral glycoprotein receptors thereby inhibiting viral attachment and entry into cells.
Cationic membrane penetrating peptides, G1 and G2, bind to 3-OS-HS and block the binding of HSV glycoprotein, gD, preventing viral entry into cells in in vitro and ex vivo models.136 One study found that the use of G2 with acyclovir had the greatest antiviral effect on HSV infected cells compared to G2 or acyclovir alone.136 Another study examining the slow release of G2 peptide through a contact lens demonstrated significant suppression of HSV in both viral entry and spread in in vivo and ex vivo models of pig and human corneas.137 A DNA or RNA aptamer that binds to gD is another potential antiviral therapy which blocks HSV entry and overall infectivity by reducing viral spread when used prophylactically prior to infection and therapeutically after infection. 138
The enzyme heparanase is another potential therapeutic target in preventing viral spread and damaging inflammatory effects associated with HSV. Heparanase is upregulated by HSV and results in viral egress, breakdown of extracellular matrix and release of proinflammatory and proangiogenic factors. The use of a heparanase inhibitor such as OGT 2115 on human corneal epithelial cells infected with HSV resulted in a decrease in the spread of HSV to neighboring cells and a decrease in pro-inflammatory cytokines, IL-1β, IL-6, and TNF-α.97 Heparanase may represent a unique therapeutic target because of its combined antiviral and anti-inflammatory effects.
Viral replication targets
Two systemic medications, amenavir and pritelivir, which inhibit the helicase-primase enzyme complex in HSV during viral replication have been studied in separate phase II clinical trials in patients with genital HSV. Initial studies demonstrated decreased viral shedding and time to lesion healing with these drugs compared to valacyclovir.139 Pritelivir is also being studied in a phase II trial in acyclovir-resistant mucocutaneous herpes in immunocompromised patients. Another target of viral replication, SC93305, developed from tyrosine-phosphorylation-regulated kinase inhibitors, may be a target for acyclovir resistant strains of HSV and exhibits dosedependent suppression of HSV viral replication.140 Finally, a small molecule inhibitor of TANKbinding kinase 1 (TBK1), BX795, suppresses HSV viral protein synthesis with antiviral activity on par with trifluridine in animal models of ocular HSV infection.141
Another promising antiviral strategy that has emerged recently is CRISPR/Cas9 targeting of viral genomes present in latently infected neurons. The clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 nuclease system was first described as a mechanism used by bacteria to defend against foreign nucleic acids.142 Researchers have taken advantage of this system to specifically remove or knock out gene segments of interest and study their functions. The specificity of the technique is dictated by the sequence of a 17–20 nucleotide guide RNA (gRNA), which is designed by the researcher to direct the Cas9 nuclease to a particular gene target. An exciting advance in the herpesvirus field came when van Diemen et al. demonstrated that CRISPR/Cas9 targeting of viral genes effectively blocks viral replication and establishment of latency.143 Efficacy was demonstrated for Epstein-Barr virus, human cytomegalovirus, and HSV-1. Furthermore, these authors showed that cells harboring latent viral genomes could also be depleted of their invading viral nucleic acids in this way.143 This approach offers great potential towards a cure for HSV as all currently available therapies have no effect on viral latency. It will be interesting to see whether this mechanism can be used in vivo to delete viral genomes from latently infected neurons.
Anti-inflammatory and Anti-angiogenic mediators
In addition to pursuing host and viral receptors involved in HSV infection, several molecules involved in the inflammation and angiogenesis associated with recurrent HSK have been targeted as potential therapies.
Retinoic acid has been shown to decrease the progression of HSK by stabilizing regulatory T cells (Treg) and reducing the expression of proinflammatory cytokines in mouse models.144 Azacytidine, a DNA methyltransferase inhibitor, is another target of Treg cell activity to diminish corneal lesions in HSK. This drug is FDA approved for the treatment of myelodysplastic syndrome and helps to balance regulatory T cell function over other proinflammatory CD4 T cell subsets by demethylating Treg.49 Immune modifying nanoparticle therapy was found to decrease the infiltration of inflammatory cells, including HVEM positive PMNs, in a mouse model of corneal HSV-1 infection, thereby decreasing corneal infiltrates and preserving corneal sensation.145 Another study examined the use of pigment epithelial-derived factor (PEDF) and docosahexaenoic acid (DHA) in a rabbit model of HSK; rabbits treated with PEDF+DHA had a reduction in corneal epithelial lesions and neovascularization, more rapid improvement in inflammatory cell infiltration by day 14 post infection, and regeneration of corneal nerves with improvement in corneal sensation.146 Other lipid mediators such as aspirin-triggered resolvin D1 have also been found to have potential therapeutic effects on severity of stromal keratitis lesions and corneal neovascularization.147
MicroRNA are non-coding RNA involved in regulation of gene expression. Several microRNAs have been found to play a role in the pathogenesis of HSV keratitis. Mir155 is upregulated in ocular HSV infection with resultant influx of inflammatory T cells, Th1 and Th17, into the cornea; when nanoparticles were used to silence mir155, mice had decreased corneal stromal lesions and neovascularization.47
Corneal angiogenesis develops in HSK with subsequent opacification and scarring. Nanoparticles used to silence Mir132 resulted in diminished corneal neovascularization and corneal stromal infiltrates in mice infected with HSV.148 Other targets to corneal angiogenesis include inhibition of downstream molecules involved in the vascular endothelial growth factor (VEGF) pathway. A Src kinase inhibitor molecule administered topically or systemically reduced the severity of HSK lesions and corneal angiogenesis in mouse models.149 Finally, use of subconjunctival soluble Robo4, which activates an antiangiogenic pathway that effects VEGF signaling, reduced corneal angiogenesis in HSV infected mice compared to wild type controls.150
Conclusions
The host cell responds to HSV infection with a complex series of events of immunopathogenesis, manifesting as either epithelial, stromal, or endothelial keratitis. Innate and adaptive immune responses are responsible for clearing active HSV infection, but also cause damaging inflammation within the cornea which can lead to neovascularization and scarring. While antiviral and antiinflammatory therapies help to reduce the duration, severity and risk for recurrent HSV keratitis, there are numerous limitations of currently used treatments. Host cell receptors and the subsequent immune response triggered by infection represent novel therapeutic targets.
Acknowledgments
Supported by grants: P30 EY001792 (AML, AMA, DS), NIH/NEI K12 EY021475 (AML), NIH/NEI R01 EY024710-A1 and R01 EY029426 (DS), NIH/NIAID R21AI128171–01A1 (DS), unrestricted departmental funding from Research to Prevent Blindness (AML, AMA, DS).
Footnotes
The authors report no conflicts of interest or disclosures for this manuscript submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. The Journal of clinical investigation. 2001;108(4):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agelidis AM, Shukla D. Cell entry mechanisms of HSV: what we have learned in recent years. Future virology. 2015;10(10):1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadigal SR, Agelidis AM, Karasneh GA, et al. Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nature communications. 2015;6:6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999–2010. The Journal of infectious diseases. 2014;209(3):325–333. [DOI] [PubMed] [Google Scholar]
- 5.Kasubi MJ, Nilsen A, Marsden HS, Bergstrom T, Langeland N, Haarr L. Prevalence of antibodies against herpes simplex virus types 1 and 2 in children and young people in an urban region in Tanzania. J Clin Microbiol. 2006;44(8):2801–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Survey of ophthalmology. 2012;57(5):448–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young RC, Hodge DO, Liesegang TJ, Baratz KH. Incidence, recurrence, and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: the effect of oral antiviral prophylaxis. Arch Ophthalmol. 2010;128(9):1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20(1):1–13. [DOI] [PubMed] [Google Scholar]
- 9.Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. Bioessays. 2012;34(12):1050–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X, Lundberg P, Tanamachi B, Openshaw H, Longmate J, Cantin E. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J Virol. 2001;75(6):3048–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavan-Langston D Herpes simplex of the ocular anterior segment. Curr Clin Top Infect Dis. 2000;20:298–324. [PubMed] [Google Scholar]
- 12.Derham AM, Chen E, Bunya VY, O’Malley RE. Bilateral Herpetic Keratitis After Bilateral Intravitreal Bevacizumab for Exudative Macular Degeneration. Cornea. 2017;36(7):878879. [DOI] [PubMed] [Google Scholar]
- 13.Darougar S, Wishart MS, Viswalingam ND. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol. 1985;69(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liesegang TJ, Melton LJ 3rd, Daly PJ, Ilstrup DM. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107(8):11551159. [DOI] [PubMed] [Google Scholar]
- 15.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117(10):1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Remeijer L, Osterhaus A, Verjans G. Human herpes simplex virus keratitis: the pathogenesis revisited. Ocular immunology and inflammation. 2004;12(4):255–285. [DOI] [PubMed] [Google Scholar]
- 17.Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18(2):127–143. [DOI] [PubMed] [Google Scholar]
- 18.Tabery HM. Early epithelial changes in recurrent herpes simplex virus keratitis: a noncontact photomicrographic study in vivo in the human cornea. Acta Ophthalmol Scand. 1998;76(3):349–352. [DOI] [PubMed] [Google Scholar]
- 19.Thakkar N, Jaishankar D, Agelidis A, et al. Cultured corneas show dendritic spread and restrict herpes simplex virus infection that is not observed with cultured corneal cells. Scientific reports. 2017;7:42559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macrophages Ellermann-Eriksen S. and cytokines in the early defence against herpes simplex virus. Virol J. 2005;2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol. 2009;54(2):226–234. [DOI] [PubMed] [Google Scholar]
- 22.Polcicova K, Biswas PS, Banerjee K, Wisner TW, Rouse BT, Johnson DC. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc Natl Acad Sci U S A. 2005;102(32):11462–11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brik D, Dunkel E, Pavan-Langston D. Herpetic keratitis: persistence of viral particles despite topical and systemic antiviral therapy. Report of two cases and review of the literature. Arch Ophthalmol. 1993;111(4):522–527. [DOI] [PubMed] [Google Scholar]
- 24.Higaki S, Fukuda M, Shimomura Y. Virological and molecular biological evidence supporting herpes simplex virus type 1 corneal latency. Jpn J Ophthalmol. 2015;59(2):131–134. [DOI] [PubMed] [Google Scholar]
- 25.Rowe AM, St Leger AJ, Jeon S, Dhaliwal DK, Knickelbein JE, Hendricks RL. Herpes keratitis. Progress in retinal and eye research. 2013;32:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue Y Review of clinical and basic approaches to corneal endotheliitis. Cornea. 2014;33 Suppl 11:S3–8. [DOI] [PubMed] [Google Scholar]
- 27.Hillenaar T, Weenen C, Wubbels RJ, Remeijer L. Endothelial involvement in herpes simplex virus keratitis: an in vivo confocal microscopy study. Ophthalmology. 2009;116(11):20772086 e2071–2072. [DOI] [PubMed] [Google Scholar]
- 28.Daheshia M, Kanangat S, Rouse BT. Production of key molecules by ocular neutrophils early after herpetic infection of the cornea. Exp Eye Res. 1998;67(6):619–624. [DOI] [PubMed] [Google Scholar]
- 29.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158(3):13831391. [PubMed] [Google Scholar]
- 30.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. Journal of Virology. 1996;70(2):898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geissmann F, Jung S, Littman DR. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity. 2003;19(1):71–82. [DOI] [PubMed] [Google Scholar]
- 32.Osorio Y, Wechsler SL, Nesburn AB, Ghiasi H. Reduced severity of HSV-1-induced corneal scarring in IL-12-deficient mice. Virus Res. 2002;90(1–2):317–326. [DOI] [PubMed] [Google Scholar]
- 33.Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43(3):639–646. [PubMed] [Google Scholar]
- 34.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. Journal of Leukocyte Biology. 2003;74(2):172–178. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura T, Ishikawa F, Sonoda KH, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46(2):497–503. [DOI] [PubMed] [Google Scholar]
- 36.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8(4):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu K, Harris DL, Yamaguchi T, von Andrian UH, Hamrah P. A Dual Role for Corneal Dendritic Cells in Herpes Simplex Keratitis: Local Suppression of Corneal Damage and Promotion of Systemic Viral Dissemination. PloS one. 2015;10(9):e0137123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Yin X, Stuart PM, Leib DA. Dendritic Cell Autophagy Contributes to Herpes Simplex Virus-Driven Stromal Keratitis and Immunopathology. mBio. 2015;6(6):e01426–01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendricks RL. Immunopathogenesis of viral ocular infections. Chem Immunol. 1999;73:120–136. [DOI] [PubMed] [Google Scholar]
- 40.Deshpande S, Banerjee K, Biswas PS, Rouse BT. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev Mol Med. 2004;6(8):1–14. [DOI] [PubMed] [Google Scholar]
- 41.Gimenez F, Suryawanshi A, Rouse BT. Pathogenesis of herpes stromal keratitis--a focus on corneal neovascularization. Prog Retin Eye Res. 2013;33:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metcalf JF, Hamilton DS, Reichert RW. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979;26(3):1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendricks RL, Tumpey TM, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992;149(9):3023–3028. [PubMed] [Google Scholar]
- 44.Tang Q, Chen W, Hendricks RL. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J Immunol. 1997;158(3):1275–1283. [PubMed] [Google Scholar]
- 45.Molesworth-Kenyon SJ, Yin R, Oakes JE, Lausch RN. IL-17 receptor signaling influences virus-induced corneal inflammation. J Leukoc Biol. 2008;83(2):401–408. [DOI] [PubMed] [Google Scholar]
- 46.Suryawanshi A, Veiga-Parga T, Rajasagi NK, et al. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187(4):1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhela S, Mulik S, Gimenez F, et al. Role of miR-155 in the pathogenesis of herpetic stromal keratitis. Am J Pathol. 2015;185(4):1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. In vitro-generated antigenspecific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol. 2008;82(14):6838–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varanasi SK, Reddy PB, Bhela S, Jaggi U, Gimenez F, Rouse BT. Azacytidine Treatment Inhibits the Progression of Herpes Stromal Keratitis by Enhancing Regulatory T Cell Function. J Virol. 2017;91(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panoutsakopoulou V, Sanchirico ME, Huster KM, et al. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15(1):137–147. [DOI] [PubMed] [Google Scholar]
- 51.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279(5355):13441347. [DOI] [PubMed] [Google Scholar]
- 52.Deshpande SP, Lee S, Zheng M, et al. Herpes simplex virus-induced keratitis: evaluation of the role of molecular mimicry in lesion pathogenesis. J Virol. 2001;75(7):3077–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verjans GM, Remeijer L, Mooy CM, Osterhaus AD. Herpes simplex virus-specific T cells infiltrate the cornea of patients with herpetic stromal keratitis: no evidence for autoreactive T cells. Invest Ophthalmol Vis Sci. 2000;41(9):2607–2612. [PubMed] [Google Scholar]
- 54.Deshpande S, Zheng M, Lee S, et al. Bystander Activation Involving T Lymphocytes in Herpetic Stromal Keratitis. The Journal of Immunology. 2001;167(5):2902–2910. [DOI] [PubMed] [Google Scholar]
- 55.Gangappa S, Babu JS, Thomas J, Daheshia M, Rouse BT. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J Immunol. 1998;161(8):4289–4300. [PubMed] [Google Scholar]
- 56.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2(5):309–322. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee K, Biswas PS, Kumaraguru U, Schoenberger SP, Rouse BT. Protective and Pathological Roles of Virus-Specific and Bystander CD8+ T Cells in Herpetic Stromal Keratitis. The Journal of Immunology. 2004;173(12):7575–7583. [DOI] [PubMed] [Google Scholar]
- 58.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18(5):593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lang A, Nikolich-Zugich J. Development and Migration of Protective CD8+ T Cells into the Nervous System following Ocular Herpes Simplex Virus-1 Infection. The Journal of Immunology. 2005;174(5):2919–2925. [DOI] [PubMed] [Google Scholar]
- 60.Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virusspecific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104(9):3496–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mott KR, Gate D, Matundan HH, Ghiasi YN, Town T, Ghiasi H. CD8+ T Cells Play a Bystander Role in Mice Latently Infected with Herpes Simplex Virus 1. J Virol. 2016;90(10):50595067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. Journal of Virology. 1999;73(9):7619–7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virusspecific CD8+ T cell repertoire in C57BL/6 mice. J Immunol. 2011;186(7):3927–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jing L, Haas J, Chong TM, et al. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J Clin Invest. 2012;122(2):654–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khan AA, Srivastava R, Spencer D, et al. Phenotypic and functional characterization of herpes simplex virus glycoprotein B epitope-specific effector and memory CD8+ T cells from symptomatic and asymptomatic individuals with ocular herpes. J Virol. 2015;89(7):3776–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khan AA, Srivastava R, Chentoufi AA, et al. Bolstering the Number and Function of HSV-1Specific CD8(+) Effector Memory T Cells and Tissue-Resident Memory T Cells in Latently Infected Trigeminal Ganglia Reduces Recurrent Ocular Herpes Infection and Disease. J Immunol. 2017;199(1):186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shimomura Y, Higaki S. The kinetics of herpes virus on the ocular surface and suppression of its reactivation. Cornea. 2011;30 Suppl 1:S3–7. [DOI] [PubMed] [Google Scholar]
- 68.Toma HS, Murina AT, Areaux RG Jr, et al. Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol. 2008;23(4):249–273. [DOI] [PubMed] [Google Scholar]
- 69.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin X, Qin Q, Chen W, Qu J. Expression of toll-like receptors in the healthy and herpes simplex virus-infected cornea. Cornea. 2007;26(7):847–852. [DOI] [PubMed] [Google Scholar]
- 71.Ma Y, He B. Recognition of herpes simplex viruses: toll-like receptors and beyond. J Mol Biol. 2014;426(6):1133–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hendricks RL, Weber PC, Taylor JL, Koumbis A, Tumpey TM, Glorioso JC. Endogenously produced interferon alpha protects mice from herpes simplex virus type 1 corneal disease. J Gen Virol. 1991;72 ( Pt 7):1601–1610. [DOI] [PubMed] [Google Scholar]
- 73.Luker GD, Prior JL, Song J, Pica CM, Leib DA. Bioluminescence Imaging Reveals Systemic Dissemination of Herpes Simplex Virus Type 1 in the Absence of Interferon Receptors. Journal of Virology. 2003;77(20):11082–11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons Regulate the Phenotype of Wild-type and Mutant Herpes Simplex Viruses In Vivo. The Journal of Experimental Medicine. 1999;189(4):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conrady CD, Jones H, Zheng M, Carr DJ. A Functional Type I Interferon Pathway Drives Resistance to Cornea Herpes Simplex Virus Type 1 Infection by Recruitment of Leukocytes. J Biomed Res. 2011;25(2):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schoenborn JR, Dorschner MO, Sekimata M, et al. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat Immunol. 2007;8(7):732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang Q Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus- infected mouse corneas. Journal of Experimental Medicine 1996;184(4):1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biswas PS, Banerjee K, Kim B, Rouse BT. Mice Transgenic for IL-1 Receptor Antagonist Protein Are Resistant to Herpetic Stromal Keratitis: Possible Role for IL-1 in Herpetic Stromal Keratitis Pathogenesis. The Journal of Immunology. 2004;172(6):3736–3744. [DOI] [PubMed] [Google Scholar]
- 79.Fenton RR, Molesworth-Kenyon S, Oakes JE, Lausch RN. Linkage of IL-6 with neutrophil chemoattractant expression in virus-induced ocular inflammation. Investigative Ophthalmology & Visual Science. 2002;43(3):737–743. [PubMed] [Google Scholar]
- 80.Keadle TL, Usui N, Laycock KA, Miller JK, Pepose JS, Stuart PM. IL-1 and TNF-alpha are important factors in the pathogenesis of murine recurrent herpetic stromal keratitis. Invest Ophthalmol Vis Sci. 2000;41(1):96–102. [PubMed] [Google Scholar]
- 81.Veiga-Parga T, Gimenez F, Mulik S, Chiang EY, Grogan JL, Rouse BT. Controlling herpetic stromal keratitis by modulating lymphotoxin-alpha-mediated inflammatory pathways. Microbes Infect. 2013;15(10–11):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Experimental Eye Research. 2006;82(1):4654. [DOI] [PubMed] [Google Scholar]
- 83.Bryant-Hudson KM, Gurung HR, Zheng M, Carr DJ. Tumor necrosis factor alpha and interleukin-6 facilitate corneal lymphangiogenesis in response to herpes simplex virus 1 infection. Journal of virology. 2014;88(24):14451–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suryawanshi A, Veiga-Parga T, Reddy PB, Rajasagi NK, Rouse BT. IL-17A differentially regulates corneal vascular endothelial growth factor (VEGF)-A and soluble VEGF receptor 1 expression and promotes corneal angiogenesis after herpes simplex virus infection. J Immunol. 2012;188(7):3434–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tumpey TM, Cheng H, Yan XT, Oakes JE, Lausch RN. Chemokine synthesis in the HSV-1infected cornea and its suppression by interleukin-10. J Leukoc Biol. 1998;63(4):486–492. [DOI] [PubMed] [Google Scholar]
- 86.Yan XT, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39(10):1854–1862. [PubMed] [Google Scholar]
- 87.Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. CXCR2−/− Mice Show Enhanced Susceptibility to Herpetic Stromal Keratitis: A Role for IL-6-Induced Neovascularization. The Journal of Immunology. 2004;172(2):1237–1245. [DOI] [PubMed] [Google Scholar]
- 88.Carr DJJ, Chodosh J, Ash J, Lane TE. Effect of Anti-CXCL10 Monoclonal Antibody on Herpes Simplex Virus Type 1 Keratitis and Retinal Infection. Journal of Virology. 2003;77(18):10037–10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chensue SW. Molecular machinations: chemokine signals in host-pathogen interactions. Clin Microbiol Rev. 2001;14(4):821–835, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ariotti S, Beltman JB, Borsje R, et al. Subtle CXCR3-Dependent Chemotaxis of CTLs within Infected Tissue Allows Efficient Target Localization. J Immunol. 2015;195(11):5285–5295. [DOI] [PubMed] [Google Scholar]
- 91.Shen FH, Wang SW, Yeh TM, Tung YY, Hsu SM, Chen SH. Absence of CXCL10 aggravates herpes stromal keratitis with reduced primary neutrophil influx in mice. Journal of virology. 2013;87(15):8502–8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wuest TR, Carr DJJ. Dysregulation of CXCR3 Signaling due to CXCL10 Deficiency Impairs the Antiviral Response to Herpes Simplex Virus 1 Infection. The Journal of Immunology. 2008;181(11):7985–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Srivastava R, Khan AA, Chilukuri S, et al. CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8(+) TEM and CD8(+) TRM Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease. J Virol. 2017;91(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan XT, Zhuang M, Oakes JE, Lausch RN. Autocrine action of IL-10 suppresses proinflammatory mediators and inflammation in the HSV-1-infected cornea. J Leukoc Biol. 2001;69(1):149–157. [PubMed] [Google Scholar]
- 95.Tumpey TM, Elner VM, Chen SH, Oakes JE, Lausch RN. Interleukin-10 Treatment Can Suppress Stromal Keratitis Induced by Herpes-Simplex Virus Type-1. Journal of Immunology. 1994;153(5):2258–2265. [PubMed] [Google Scholar]
- 96.Goldberg R, Meirovitz A, Hirshoren N, et al. Versatile role of heparanase in inflammation. Matrix Biol. 2013;32(5):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Agelidis AM, Hadigal SR, Jaishankar D, Shukla D. Viral Activation of Heparanase Drives Pathogenesis of Herpes Simplex Virus-1. Cell Rep. 2017;20(2):439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo C, Zhu Z, Guo Y, et al. Heparanase Upregulation Contributes to Porcine Reproductive and Respiratory Syndrome Virus Release. J Virol. 2017;91(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Puerta-Guardo H, Glasner DR, Harris E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016;12(7):e1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thakkar N, Yadavalli T, Jaishankar D, Shukla D. Emerging Roles of Heparanase in Viral Pathogenesis. Pathogens. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Elkin M, Ilan N, Ishai-Michaeli R, et al. Heparanase as mediator of angiogenesis: mode of action. Faseb Journal. 2001;15(7):1661-+.. [DOI] [PubMed] [Google Scholar]
- 102.Su C, Zhan G, Zheng C. Evasion of host antiviral innate immunity by HSV-1, an update. Virol J 2016;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peri P, Mattila RK, Kantola H, et al. Herpes simplex virus type 1 Us3 gene deletion influences toll-like receptor responses in cultured monocytic cells. Virol J. 2008;5:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sen J, Liu X, Roller R, Knipe DM. Herpes simplex virus US3 tegument protein inhibits Tolllike receptor 2 signaling at or before TRAF6 ubiquitination. Virology. 2013;439(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Johnson KE, Chikoti L, Chandran B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol. 2013;87(9):50055018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang K, Ni L, Wang S, Zheng C. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-kappaB activation. J Virol. 2014;88(14):7941–7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang S, Wang K, Lin R, Zheng C. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J Virol. 2013;87(23):12814–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahn K, Meyer TH, Uebel S, et al. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus protein ICP47. Embo Journal. 1996;15(13):3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 109.Luftig MA. Viruses and the DNA Damage Response: Activation and Antagonism. Annu Rev Virol. 2014;1(1):605–625. [DOI] [PubMed] [Google Scholar]
- 110.Orvedahl A, Alexander D, Tallóczy Z, et al. HSV-1 ICP34.5 Confers Neurovirulence by Targeting the Beclin 1 Autophagy Protein. Cell Host & Microbe. 2007;1(1):23–35. [DOI] [PubMed] [Google Scholar]
- 111.Li S, Kong L, Yu X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit Rev Microbiol. 2015;41(2):150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang Z, Wu SQ, Liang Y, et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17(2):229–242. [DOI] [PubMed] [Google Scholar]
- 113.Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75(20):9828–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim B, Tang Q, Biswas PS, et al. Inhibition of Ocular Angiogenesis by siRNA Targeting Vascular Endothelial Growth Factor Pathway Genes. The American Journal of Pathology. 2004;165(6):2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng M, Schwarz MA, Lee S, Kumaraguru U, Rouse BT. Control of Stromal Keratitis by Inhibition of Neovascularization. The American Journal of Pathology. 2001;159(3):10211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. Journal of Clinical Investigation. 2002;110(8):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ambati BK, Nozaki M, Singh N, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443(7114):993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chucair-Elliott AJ, Zheng M, Carr DJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Investigative ophthalmology & visual science. 2015;56(2):1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yun H, Rowe AM, Lathrop KL, Harvey SA, Hendricks RL. Reversible nerve damage and corneal pathology in murine herpes simplex stromal keratitis. J Virol. 2014;88(14):7870–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yun H, Lathrop KL, Hendricks RL. A Central Role for Sympathetic Nerves in Herpes Stromal Keratitis in Mice. Invest Ophthalmol Vis Sci. 2016;57(4):1749–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.M’Garrech M, Rousseau A, Kaswin G, et al. Impairment of lacrimal secretion in the unaffected fellow eye of patients with recurrent unilateral herpetic keratitis. Ophthalmology. 2013;120(10):1959–1967. [DOI] [PubMed] [Google Scholar]
- 122.Jabbarvand M, Hashemian H, Khodaparast M, Rafatnejad A, Beheshtnejad A, Salami A. Do Unilateral Herpetic Stromal Keratitis and Neurotrophic Ulcers Cause Bilateral Dry Eye? Cornea. 2015;34(7):768–772. [DOI] [PubMed] [Google Scholar]
- 123.Galor A, Moein HR, Lee C, et al. Neuropathic pain and dry eye. Ocul Surf. 2018;16(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reynaud C, Rousseau A, Kaswin G, M’Garrech M, Barreau E, Labetoulle M. Persistent Impairment of Quality of Life in Patients with Herpes Simplex Keratitis. Ophthalmology. 2017;124(2):160–169. [DOI] [PubMed] [Google Scholar]
- 125.White M, Chodosh J Herpes simplex virus keratitis: a treatment guideline. 2014.
- 126.Tsatsos M, MacGregor C, Athanasiadis I, Moschos MM, Hossain P, Anderson D. Herpes simplex virus keratitis: an update of the pathogenesis and current treatment with oral and topical antiviral agents. Clin Exp Ophthalmol. 2016;44(9):824–837. [DOI] [PubMed] [Google Scholar]
- 127.Wilhelmus KR. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst Rev. 2015;1:CD002898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339(5):300–306. [DOI] [PubMed] [Google Scholar]
- 129.Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101(12):1871–1882. [DOI] [PubMed] [Google Scholar]
- 130.Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101(12):1883–1895; discussion 1895–1886. [DOI] [PubMed] [Google Scholar]
- 131.Rao SN. Treatment of herpes simplex virus stromal keratitis unresponsive to topical prednisolone 1% with topical cyclosporine 0.05%. Am J Ophthalmol. 2006;141(4):771–772. [DOI] [PubMed] [Google Scholar]
- 132.Cheng AMS, Tseng SCG. Self-Retained Amniotic Membrane Combined With Antiviral Therapy for Herpetic Epithelial Keratitis. Cornea. 2017;36(11):1383–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frobert E, Burrel S, Ducastelle-Lepretre S, et al. Resistance of herpes simplex viruses to acyclovir: an update from a ten-year survey in France. Antiviral Res. 2014;111:36–41. [DOI] [PubMed] [Google Scholar]
- 134.Rousseau A, Boutolleau D, Titier K, et al. Recurrent herpetic keratitis despite antiviral prophylaxis: A virological and pharmacological study. Antiviral Res. 2017;146:205–212. [DOI] [PubMed] [Google Scholar]
- 135.Wilhelmus KR, Dawson CR, Barron BA, et al. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Herpetic Eye Disease Study Group. Br J Ophthalmol. 1996;80(11):969–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park PJ, Antoine TE, Farooq AV, Valyi-Nagy T, Shukla D. An investigative peptide-acyclovir combination to control herpes simplex virus type 1 ocular infection. Investigative ophthalmology & visual science. 2013;54(9):6373–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jaishankar D, Buhrman JS, Valyi-Nagy T, Gemeinhart RA, Shukla D. Extended Release of an Anti-Heparan Sulfate Peptide From a Contact Lens Suppresses Corneal Herpes Simplex Virus-1 Infection. Investigative ophthalmology & visual science. 2016;57(1):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yadavalli T, Agelidis A, Jaishankar D, et al. Targeting Herpes Simplex Virus-1 gD by a DNA Aptamer Can Be an Effective New Strategy to Curb Viral Infection. Mol Ther Nucleic Acids. 2017;9:365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Birkmann A, Zimmermann H. HSV antivirals - current and future treatment options. Curr Opin Virol. 2016;18:9–13. [DOI] [PubMed] [Google Scholar]
- 140.Zinser E, Krawczyk A, Muhl-Zurbes P, et al. A new promising candidate to overcome drug resistant herpes simplex virus infections. Antiviral Res. 2018;149:202–210. [DOI] [PubMed] [Google Scholar]
- 141.Jaishankar D, Yakoub AM, Yadavalli T, et al. An off-target effect of BX795 blocks herpes simplex virus type 1 infection of the eye. Sci Transl Med. 2018;10(428). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dualRNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Diemen FR, Kruse EM, Hooykaas MJ, et al. CRISPR/Cas9-Mediated Genome Editing of Herpesviruses Limits Productive and Latent Infections. PLoS Pathog. 2016;12(6):e1005701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jaggi U, Varanasi SK, Bhela S, Rouse BT. On the role of retinoic acid in virus induced inflammatory response in cornea. Microbes Infect. 2018;20(6):337–345. [DOI] [PubMed] [Google Scholar]
- 145.Edwards RG, Kopp SJ, Ifergan I, et al. Murine Corneal Inflammation and Nerve Damage After Infection With HSV-1 Are Promoted by HVEM and Ameliorated by Immune-Modifying Nanoparticle Therapy. Invest Ophthalmol Vis Sci. 2017;58(1):282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.He J, Neumann D, Kakazu A, et al. PEDF plus DHA modulate inflammation and stimulate nerve regeneration after HSV-1 infection. Exp Eye Res. 2017;161:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rajasagi NK, Bhela S, Varanasi SK, Rouse BT. Frontline Science: Aspirin-triggered resolvin D1 controls herpes simplex virus-induced corneal immunopathology. J Leukoc Biol. 2017;102(5):1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mulik S, Xu J, Reddy PB, et al. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am J Pathol. 2012;181(2):525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharma S, Mulik S, Kumar N, Suryawanshi A, Rouse BT. An anti-inflammatory role of VEGFR2/Src kinase inhibitor in herpes simplex virus 1-induced immunopathology. J Virol. 2011;85(12):5995–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gimenez F, Mulik S, Veiga-Parga T, Bhela S, Rouse BT. Robo 4 Counteracts Angiogenesis in Herpetic Stromal Keratitis. PLoS One. 2015;10(12):e0141925. [DOI] [PMC free article] [PubMed] [Google Scholar]