Abstract
Objective:
This study evaluates the effect of unilateral lingual sensory loss on the spatial and temporal dynamics of jaw movements during pig chewing.
Design:
X-ray Reconstruction of Moving Morphology (XROMM) was used to reconstruct the 3-dimensional jaw movements of 6 pigs during chewing before and after complete unilateral lingual nerve transection. The effect of the transection were evaluated at the temporal and spatial level using Multiple Analysis of Variance. Temporal variables include gape cycle and phase durations, and the corresponding relative phase durations. Spatial variables include the amplitude of jaw opening, jaw yaw, and of mandibular retraction-protraction.
Results:
The temporal and spatial dynamics of jaw movements did not differ when chewing ipsi-versus contralateral to the transection. When compared to pre-transection data, 4 of the 6 animals showed significant changes in temporal characteristics of the gape cycle following the transection, irrespective of chewing side, but the specific response to the lesion was highly dependent on the animal. On the other hand, in affected individuals the amplitude of jaw movements was altered similarly in all 3 dimensions: jaw opening and protraction-retraction increased whereas jaw yaw decreased.
Conclusion:
The variable impact of this injury in this animal model suggests that individuals use different compensatory strategies to adjust or maintain the temporal dynamics of the gape cycle. Because the amplitude of jaw movements are more adversely affected than their timing, results suggest that maintaining the tongue-jaw coordination is critical and this can come at the expense of bolus handling and masticatory performance.
Keywords: chewing, jaw movements, lingual nerve denervation, lingual nerve transection, gape cycle, XROMM
Introduction
The rich mechanoreceptor innervation of the tongue suggests that sensorimotor integration from the tongue is critical for feeding, both in terms of conveying information on the texture of foods in the oral cavity but also on the movements and state of structures of the oral cavity (Haggard & de Boer, 2014). The lingual nerve (LN), a branch of the mandibular division of the trigeminal nerve (CN V3) serves two populations of lingual mechanoreceptors: 1) densely-populated superficial receptors that provide the tongue with its extreme tactile sensitivity and 2) deep receptors conveying information about tongue position in the absence of tactile stimuli (Trulsson & Essick, 1997). Synaptic coupling between these receptors and ipsilateral jaw-muscle motoneurons ensures precision and safety of tongue movements in coordination with the jaw during many oral behaviors, including food ingestion and mastication (Minato et al., 2009; Turker, Johnsen, Sowman, & Trulsson, 2006). Moreover, although mastication is primarily driven by jaw movements, the tongue is critical for food processing because of its role in bolus formation, transport, and positioning between the teeth. Indeed, the CNS uses lingual sensory information to control tongue shape and pressure to produce appropriate bolus consistency and lingual propulsive forces to facilitate swallowing (Takahashi, Miyamoto, Terao, & Yokoyama, 2007). Thus it is not surprising that the anterior part of the tongue, which is in constant contact with food, is more highly modulated than the glossopharyngeal-innervated posterior part (Pouderoux & Kahrilas, 1995).
The goal of this study is to evaluate the effect of unilateral LN injury on the dynamics of mastication in an animal model (pig, Sus scrofa, Linnaeus 1758). The LN is susceptible to iatrogenic injuries during many dental and other oral procedures because 1) of its close association to the lingual cortical plate of the mandible and the lingual rim of the third molar alveolus, 2) it lies relatively unprotected under the mucosa of the oral floor, and 3) its position is altered by tongue movements (Iwanaga, 2017; Mendes, de Carvalho Leite Leal Nunes, & de Almeida Lopes, 2013; Pogrel, Renaut, Schmidt, & Ammar, 1995). For example, the vast majority of LN injuries occur during third molar extractions and periodontal and mandibular implant surgeries (Hillerup, 2007; Klazen et al., 2018). In fact, 53% of oral and maxillofacial surgeons report having patients with LN injuries, 46% have had patients with permanent LN injuries during their career, and 76% report patient-recovery complicated by LN anesthesia, dysesthesia or paresthesia, with approximately 19% of these having permanent sensory impairment (Graff-Radford & Evans, 2003; Robert, Bacchetti, & Pogrel, 2005). Intubation, laryngoscope placement and oral cancer treatments, including radiotherapy, removal of submandibular gland tumors or tongue resections, cause an additional unknown number of LN injuries annually (e.g., Bodin, Jaghagen, & Isberg, 2004; Jaghagen, Bodin, & Isberg, 2008; Magboul & Joel, 2011; Renes, Zwart, Scheffer, & Renes, 2011).
Patients with LN injuries report adverse effects on eating and other oral behaviors (Jaghagen et al., 2008; Pogrel, Jergensen, Burgon, & Hulme, 2011; Renton & Yilmaz, 2012). In fact, LN-injured patients report more of a negative impact on daily life than those with inferior alveolar nerve injuries (Jacks, Zuniga, Turvey, & Schalit, 1998). This may be because mastication and oral sensory exposure significantly affect feelings of satiation by altering circulating ghrelin levels (Hogenkamp & Schioth, 2013; Li et al., 2011; Zijlstra, Mars, Stafleu, & de Graaf, 2010). Moreover, altered lingual sensation affects cortical motor and sensory centers. In humans, short-term topical anesthesia to the tongue decreases primary sensory and motor cortex activation impacting the control of swallowing (Teismann et al., 2007). Following LN transection in rats, the face motor cortex exhibits changes in as little as 1 week but is more pronounced after 21 days. This may reflect motor compensation strategies resulting from adaptations in motor output in response to altered sensory input (Adachi, Lee, Hu, Yao, & Sessle, 2007).
Here, we investigate the immediate effects of LN transection on the spatial and temporal characteristics of jaw movements during mastication in pigs, a commonly used animal model for masticatory dynamics (Herring et al., 2011). Transection of the LN in the oral floor results in complete general and special (i.e., taste) sensory loss from the ipsilateral anterior 2/3 of the tongue. We hypothesize that unilateral elimination of lingual afferents will alter both the temporal dynamics and the amplitude of jaw movements during the gape cycle and that there will be a more pronounced effect when chewing ipsilateral to the lesion. In addition to understanding the effect of unilateral nerve lesions on ipsi-versus contralateral chewing dynamics, identifying the changes that occur in this animal model following LN lesion can inform our understanding of the physiological and biomechanical consequences of LN damage in humans.
Materials & Methods
Animals, surgeries, CT scans and study design
Marker-based X-ray Reconstruction Of Moving Morphology (XROMM) (Brainerd et al., 2010) was used to quantify masticatory kinematics in six 3-to-4-month-old female pigs chewing size-standardized (2 cm × 2 cm × 1 cm) pieces of apple. Radiopaque tantalum markers (1.0 mm in diameter) were surgically implanted into the bones and teeth of the skull and jaw of each animal while they were under isoflurane anesthesia. Prior to and following this surgery, pigs were trained to enter, feed and exit a custom-built restraint system. Once trained, control data were recorded daily over the course of 1–2 weeks. Animals were then again anesthetized and the left LN of each animal was transected. During this procedure, the tongue was reflected to the right to expose the floor of the oral cavity on the left. An incision was made in the oral floor mucosa, and the nerve was identified and isolated from the sublingual gland, submandibular duct, and vessels. Two loops of suture were placed around the nerve as far caudally as possible, and the nerve was transected between the loops. Each end of the nerve, with suture loops attached for post-mortem identification, were then pushed away from each other into the surrounding tissue to prevent the two ends from rejoining. The incision was sutured closed. Following a minimum 24-hour recovery, daily recording sessions resumed for one week. Once sufficient treatment data were recorded, animals were euthanized and dissections were performed to verify success of the transection.
During and following the data collection period, animals were CT-scanned to produce the 3D bone models in Avizo (FEI, Hillsboro, OR, USA) used to extract kinematic data (Brainerd et al., 2010). In vivo scanning was done under isoflurane anesthesia at The Ohio State University College of Veterinary Medicine (Columbus, OH, USA) on a GE Lightspeed Ultra CT scanner. Post-mortem scanning was done locally at Holzer Clinic (Athens, OH, USA) on a Phillips Brilliance 64 scanner. All aspects of this study complied with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines, and were conducted with approval of the Ohio University Institutional Animal Care and Use Committee (protocol #12-U-009).
XROMM data collection
During recording sessions, animals were imaged using biplanar fluoroscopy with two synchronized high-speed digital video cameras mounted on the output ports of the image intensifiers. Original fluoroscopy videos are stored on the XROMM portal. On average, radiation exposures were set at 80 kVp and 4.5 mA. Following the XROMM workflow (Brainerd et al., 2010), the standard two-step calibration routine was implemented at the beginning and end of each recording session, resulting in still fluoroscopy images used for distortion correction and 3D calibration of the space covered by both fluoroscopes. First, a perforated steel sheet with standardized hole-spacing and sizes (item #9255T641, McMaster-Carr, Robinson, NJ, USA) was imaged in each fluoroscopy view to correct for the distortion characteristic of X-ray images. Second, the field of view covered by both fluoroscopes was calibrated by exposing a custom-built cube of 4 plastic sheets containing 64 radiopaque tantalum beads placed in a 4 × 4 fashion 2.5 cm apart from one another. The 3D positioning of each of these calibration beads is used as the reference system when calculating direct linear transformations.
Fluoroscopy videos were processed using XMALab version 1.5.0 (Knorlein, Baier, Gatesy, Laurence-Chasen, & Brainerd, 2016). The 2D position of each tantalum marker was digitized frame-by-frame in each distortion-corrected fluoroscopy view. The 3D coordinates of each marker was then determined using the direct linear transformations calculated from the calibration cube. 3D coordinates were filtered using a low-pass Butterworth filter (25Hz cutoff frequency). The standard deviation of the 3D distance between markers implanted in the same bone was used to quantify measurement error (Brainerd et al., 2010) (Table 1). By registering the 3D coordinates of the implanted markers to their respective reference coordinates in the CT scans, singular value decomposition was used to calculate rigid body motions of the skull and jaw.
Table 1.
Summary of measurement errors and precision thresholds by animal.
|
Measurement error (of rigid body motion) |
Precision threshold (for movement detection) |
||||
|---|---|---|---|---|---|
| Animal ID | Skull beads | Jaw beads | Ry | Rz | Tx |
| 5 | 0.14 mm | 0.17 mm | 0.13° | 0.10° | 0.07 cm |
| 6 | 0.73 mm | 0.70 mm | 0.08° | 0.12° | 0.07 cm |
| 7 | 0.65 mm | 0.55 mm | 0.11° | 0.14° | 0.06 cm |
| 9 | 0.47 mm | 0.47 mm | 0.62° | 0.32° | 0.05 cm |
| 10 | 0.45 mm | 0.38 mm | 0.10° | 0.12° | 0.09 cm |
| 13 | 0.46 mm | 0.47 mm | 0.27° | 0.35° | 0.04 cm |
Measurement error is quantified by the average standard deviation of the 3D distance between markers implanted in the same rigid body (i.e., skull or jaw). Precision thresholds for movement detection for Ry, Rz and Tx (see Material and Methods for more details).
Rigid body motions were then assigned to the corresponding 3D models in Maya (Autodesk Inc., San Rafael, CA, USA) to animate the sequences frame-by-frame and quantify jaw movements. For each animal, a joint coordinate system (JCS) was created to quantify jaw movements with respect to the skull. The JCS consisted of 3 perpendicular axes oriented rostrocaudally (X), dorsoventrally (Y) and mediolaterally (Z) and positioned at the temporomandibular joint (TMJ) (Figure 1). The JCS was parented to the skull so that the movements of the lower jaw were measured independently of skull position and motion. In this JCS, there are 6 degrees of freedom: 3 rotations (Rx, Ry, and Rz) and 3 translations (Tx, Ty, Tz) (Brainerd et al., 2010; Menegaz, Baier, Metzger, Herring, & Brainerd, 2015; Montuelle, Olson, Curtis, Sidote, & Williams, 2018).
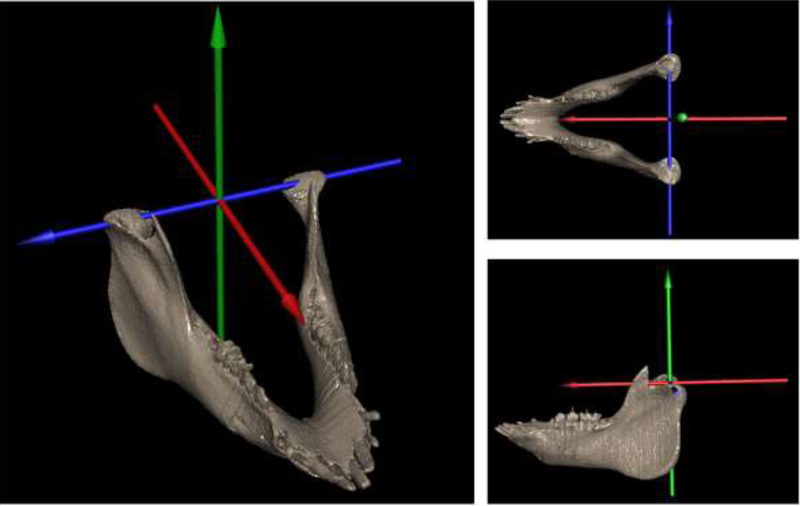
Figure 1.
A) Jaw 3D model with the joint coordinate system used to calculate rotations (Rx, Ry, and Rz) and translations (Tx, Ty and Tz) of the jaw relative to the skull during chewing. The skull has been removed to allow visibility of the JCS. Rotations of the jaw about Rz (blue arrow) were used to determine gape cycles whereas rotations about Ry (green arrow) was used to determine chewing side.
To determine the precision of motion reconstruction, the frozen head of each animal was imaged post mortem following the same biplanar fluoroscopy and XROMM protocol as for in vivo data. Because the lower jaw should not move with respect to the skull when frozen, there should be no rotation or translation of the jaw relative to the skull. The standard deviation from zero of measurements in each of the six degrees of freedom (Rx, Ry, Rz and Tx, Ty, Tz) indicates digitizing noise, and determines the precision threshold at which movements can be confidently interpreted as real biological motion (Table 1). Below this threshold, observations are discarded as noise (Brainerd et al., 2010; Menegaz et al., 2015).
In accordance with previous observations of pig chewing (Brainerd et al., 2010; Menegaz et al., 2015), jaw movements during feeding in pigs are characterized by three main degrees of freedom: rotation around the mediolateral axis (i.e., jaw pitch, Rz), rotation around the dorsoventral axis (i.e., jaw yaw, Ry), and translation along the anteroposterior axis (i.e., protraction-retraction, Tx). Variation in each of these three degrees of freedom were greater than their respective precision threshold and could thus be confidently interpreted as real biological movements (see Table 1). For the remaining degrees of freedom, Rx, Ty and Tz, none were consistently greater than their respective precision threshold and thus were removed from further analysis.
Variables and statistical analysis
Temporal variables.
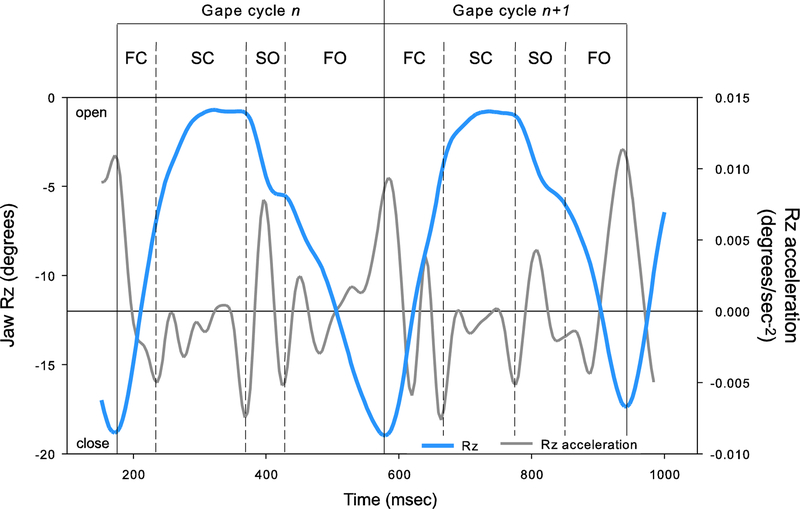
For each gape cycle, we measured the important temporal parameters of chewing to determine whether sensory disturbance of the tongue impact chewing dynamics. Total gape cycle duration was calculated as the difference in milliseconds between two successive maximum gapes (Figure 2). Within each cycle, the second derivation of Rz (i.e., Rz acceleration) was used to determine the start and end of four phases (see Montuelle et al. 2018): fast closing (FC), slow closing (SC), slow opening (SO), and fast opening (FO) (Figure 2). The duration of each phase was calculated in absolute (milliseconds) and relative (to gape cycle duration, %) units to allow for comparisons.
Figure 2.
Representative waves of 2 gape cycles from a pig chewing on apple showing ventral rotation (Rz; solid blue line) of the lower jaw and the corresponding acceleration of Rz (solid grey line). Gape cycles were defined between two consecutive maximum gapes and were split into four phases based on the acceleration of Rz: fast closing (FC), slow closing (SC), slow opening (SO), and fast opening FO.
Kinematic variables.
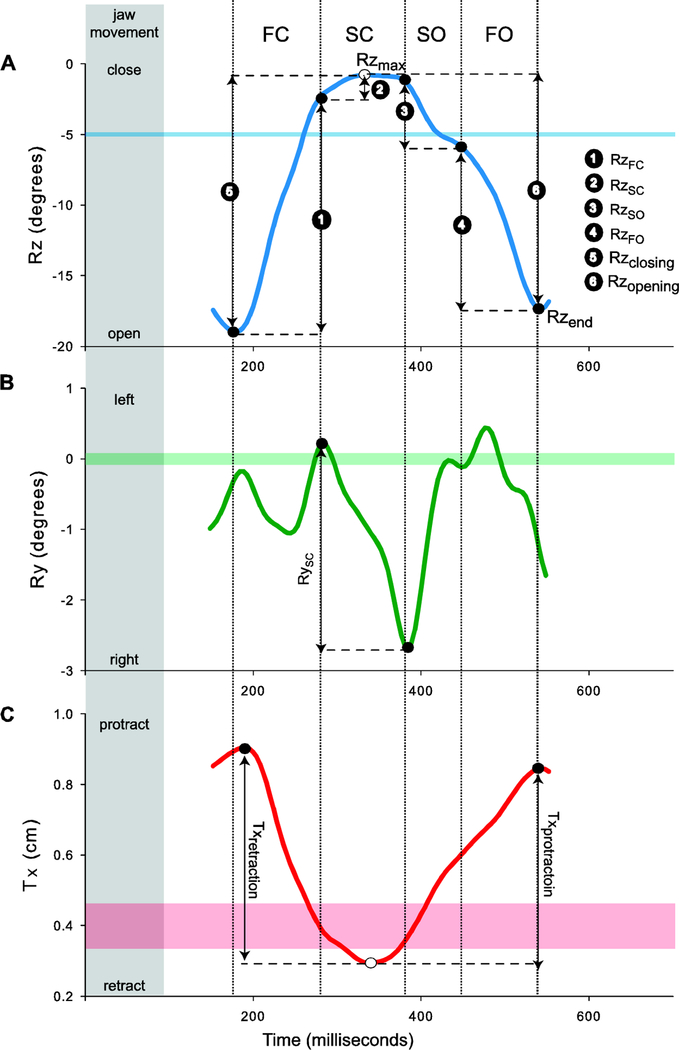
In accordance with previous observations of chewing in pigs (Brainerd et al., 2010; Menegaz et al., 2015), the primary degree of freedom in jaw movements during chewing is rotation around a mediolateral axis running through the condyles (Rz). Rhythmic opening-closing cycles of the jaw (i.e., gape cycles) during chewing are determined as the change in Rz over time, with the start and end of each cycle determined at the time of maximum jaw depression (i.e., maximum gape and minimum jaw Rz) (Figures 2 and 3A). The total amplitude of ventral jaw rotation during jaw opening (Rzopening), reflecting the primary degree of freedom, was calculated as the difference between maximum jaw elevation (Rzmax; i.e., minimum gape during SC) and maximum jaw depression at the end of the gape cycle (Rzend; i.e., maximum gape at the end of the cycle) (Figure 3A). The total amplitude of ventral rotation of the jaw may relate to performance issues during intra-oral bolus manipulation during jaw opening. Indeed, gape opening can be interpreted as the risk of dropping and losing food during chewing (i.e., the wider the gape opens, the more likely food may exit the oral cavity). Note that we did not directly investigate the amplitude of dorsal rotation during jaw closing simply because this was virtually identical to the ventral rotation of jaw opening given that the teeth typically come into occlusion at the end of jaw closing, providing a natural limitation to this movement. The relative amounts of jaw elevation achieved during each FC and SC, and similarly for jaw depression during SO and FO, were investigated by scaling the absolute phase amplitude to the associated total amplitude of dorsal (closing) or ventral (opening) rotation (Figure 3A). This allowed us to understand the contribution of each phase to the overall movement of the jaw during the opening or closing portion of the gape cycle.
Figure 3.
Representative kinematic waves illustrating Rx, Ry and Tz for a single gape cycle. Their respective precision thresholds (horizontal shaded bars). The variables extracted from each wave are also shown. A) The amplitude of Rx during each closing (1, 2) or opening (3, 4) phase of the gape cycle was scaled to the total amplitude of jaw closing (5) or opening (6), respectively. The amplitude of ventral jaw rotation during jaw opening (Rzopening) was calculated as the difference between maximum jaw elevation (Rzmax) and maximum jaw depression at the end of the gape cycle (Rzend). B) The amplitude of Ry during SC (RySC)was calculated as the difference between the medial-most rotational position of the lower jaw at the end of SC and the lateral-most position recorded at the beginning of SC. Absolute values of Ry were used to account for the sign change between left and right chews. The chew shown is a left chew indicated by the negative Ry. C) The amplitude of jaw retraction (Txretraction) was calculated as the difference between minimum and initial Tx position whereas the amplitude of jaw protraction (Txprotraction) was calculated as the difference between final and minimum Tx position.
The second largest degree of freedom detected in our dataset was jaw yaw (Ry), representing rotation about a dorsoventral axis. Since the JCS was defined using the right hand rule, an increase in Ry (i.e., positive Ry) during SC corresponds to jaw rotation towards the left side of the animal, indicating a right chew (Figure 3B). Conversely, a decrease in Ry (i.e., negative Ry) during SC corresponds to jaw rotation towards the animal’s right side, indicating a left chew. During SC, Ry reflects the buccal to lingual occlusal movement of the working-side (i.e., chewing side) teeth resulting in food breakdown. Jaw yaw may be impacted if placement and maintenance of food between the teeth is negatively affected by LN injury. To determine whether LN transection affected food breakdown, we calculated the absolute amplitude of Ry during SC as the difference between the medial-most rotational position of the lower jaw at the end of SC and the lateral-most position recorded at the beginning of SC (Figure 3B). We used the absolute amplitude because animals alternate chewing sides during chewing, resulting in a decreasing Ry during left side chews whereas right side chews are characterized by an increasing Ry during SC.
The final degree of freedom in jaw movement that was detected in our study was the anteroposterior translation of the mandibular condyles with respect to the skull (i.e., Tx). This represents the protraction-retraction of the jaw at the condyles, with decrease in Tx indicating mandibular retraction whereas increase in Tx indicates mandibular protraction (Figure 3C). Maximum condylar retraction is close to the instant of minimum gape during SC whereas maximum protraction is close to the instant of maximum gape (Figure 3C). In other words, the condyles retract during jaw-closing and then at the end of jaw-closing, they reverse and begin to protract until the start of the next gape cycle, although it may not strictly coincide with the start of FC (see Figure 3). For each gape cycle, initial, minimum and final Tx position of the condyles were used to calculate the amplitude of jaw retraction (i.e., difference between minimum and initial Tx position; Txretraction) and protraction (i.e., the difference between final and minimum Tx position; Txprotraction).
The extent to which jaw movements are affected by unilateral lingual sensory disturbances was evaluated by testing the significance of differences in jaw kinematics before and after unilateral left LN transection. All temporal and kinematic variables were analyzed using a Multiple Analysis of Variance (MANOVA) coupled with univariate F-ratio’s. The MANOVA was designed with animal as a random factor, treatment as a fixed factor (control versus left LN transection) and chewing side (contralateral versus ipsilateral to transection) nested into treatment. Chewing side was nested into the treatment factor because control chewing side (left versus right) are not equivalent to treatment chewing side (ipsilateral versus contralateral). The associated interactions were tested as well, but removed from the final design when they were non-significant. Overall, the treatment × side × animal and treatment × side interactions were non-significant for all but one variable (the amplitude of jaw retraction). However, the treatment × animal interaction term was significant for all kinematic variables indicating that left LN lesion affected jaw movements differently in each animal. Consequently, each animal was analyzed separately, and the effects of the treatment were tested at the individual level. Sample by animal are presented in Table 2.
Table 2.
Sample sizes for each animal by treatment and chewing side relative to the transection.
| Animal ID |
Ipsilateral (left) chews |
Contralateral (right) Chews |
Total chews | |||
|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | Control | Treatment | |
| 5 | 17 | 30 | 11 | 27 | 28 | 57 |
| 6 | 18 | 18 | 15 | 18 | 33 | 36 |
| 7 | 21 | 8 | 4 | 9 | 25 | 17 |
| 9 | 42 | 17 | 33 | 13 | 75 | 30 |
| 10 | 25 | 22 | 12 | 32 | 37 | 54 |
| 13 | 37 | 48 | 32 | 39 | 69 | 87 |
Results
Temporal variables.
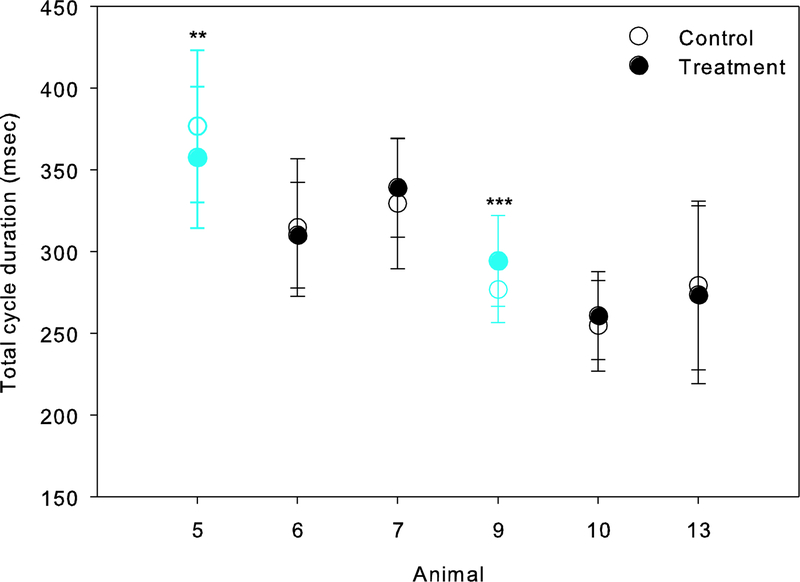
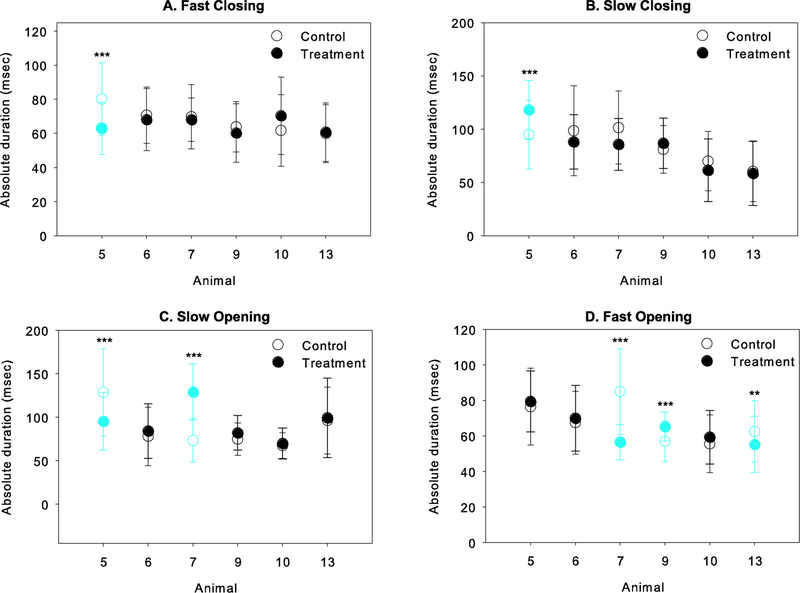
A summary of the statistical results comparing temporal characteristics of jaw movements between the control and treatment datasets are provided in Table 3. For total gape cycle duration, differences pre- and post-treatment were significant in two animals, animals 5 and 9 (Figure 4; Table 3). However, the effect of left LN transection differed between these animals: gape cycles were shorter after the transection in animal 5, but longer in animal 9. Pre- and post-treatment differences in absolute phase durations are shown in Figure 5. Overall, the effects of the transection on the timing of jaw movements were limited to 4 of the 6 animals studied, but again, each showed a different outcome. After LN transection in animal 5, the duration of FC and SO decreased but SC increased. In animal 7, SO was longer but FO was shorter post-treatment. FO was also significantly shorter in animal 13, but longer in animal 9. Finally, LN transection had no effect on any absolute phase durations in animals 6 and 10 (see Table 3).
Table 3.
Summary of statistical results comparing jaw temporal dynamics pre- and post-lingual nerve transection.
| Animal | ||||||
|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 9 | 10 | 13 | |
| Cycle duration F5,535 = 2.370, p = 0.038 |
F1,82=4.614 p=0.035 |
ns | ns | F1,102=13.020 p<0.001 |
ns | ns |
| Absolute phase durations | ||||||
| FCabs F5,535 = 4.539, p < 0.001 |
F1,82=17.654 p<0.001 |
ns | ns | ns | ns | ns |
| SCabs F5,535 = 4.272, p = 0.001 |
F1,82=11.529 p=0.001 |
ns | ns | ns | ns | ns |
| SOabs F5,535 = 9.817, p < 0.001 |
F1,82=13.179 p<0.001 |
ns | F1,39=27.877 p<0.001 |
ns | ns | ns |
| FOabs F5,535 = 9.054, p < 0.001 |
ns | ns | F1,39=16.481 p<0.001 |
F1,102=12.630 p=0.001 |
ns | F1,153=7.376 p=0.007 |
| Relative phase duration (% of total gape cycle duration) | ||||||
| FCrel F5,535 = 3.073, p = 0.010 |
F1,82=9.273 p=0.003 |
ns | ns | F1,102=4.784 p=0.031 |
ns | ns |
| SCrel F5,536 = 4.724, p < 0.001 |
F1,82=15.058 p<0.001 |
ns | F1,39=4.845 p=0.034 |
ns | ns | ns |
| SOrel F5,536 = 9.293, p < 0.001 |
F1,82=12.341 p=0.001 |
ns | F1,39=35.315 p<0.001 |
ns | ns | ns |
| FOrel F5,536 = 7.639, p < 0.001 |
ns | ns | F1,39=17.974 p<0.001 |
F1,102=4.244 p=0.042 |
ns | ns |
Table entries are the F-ratio’s corresponding to the treatment effect tested within each individual separately. The treatment effect had to be tested at the individual level, because the treatment × animal interaction term was significant (Wilk’s lambda λ = 0.775, F80,2308 = 3.467, p < 0.001; associated univariate F-ratio’s are provided under each variable name). The treatment × chew side interaction and the treatment × chew side × animal interaction were NS (Wilk’s lambda λ = 0.898, F80,3287 = 0.704, p = 0.978, and Wilk’s lambda λ = 0.973, F16,1054 = 0.704, p = 0.540, respectively). NS indicates that control vs treatment differences were not significant.
Figure 4.
Total gape cycle duration before (control, unfilled cirlces) and after (treatment, filled circles) unilateral LN transection. The treatment × animal interaction was significant with the treatment having the opposite effect on gape cycle duration in animals 5 and 9 (in blue; **, p<0.01; ***, p≤0.001, respectively), and no effect on the other animals.
Figure 5.
Effect of unilateral LN transection on absolute intra-cycle phase durations (control, unfilled circles; treatment, filled circles. Only those animals for which the absolute duration of one or more phases of the gape cycle differed significantly between control and treatment are shown. Blue symbols indicate a significant difference pre- and post-transection for that animal, with the significance level indicated as follows: *, p<0.05; **, p<0.01; ***, p≤0.001.
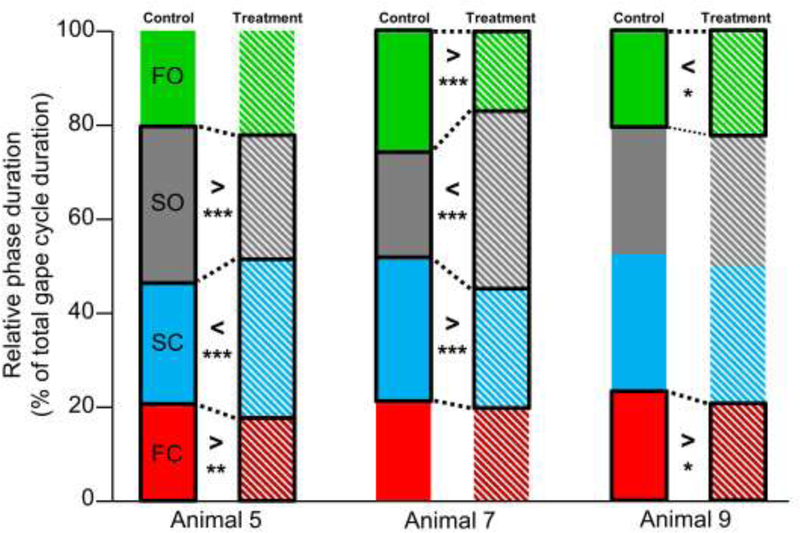
Pre- and post-treatment differences for relative phase durations were also limited to a subsample of animals (Figure 6; see Table 3). In accordance with what is observed in absolute duration, in animal 5, FC and SO were relatively shorter whereas the SC was relatively longer after the treatment. In contrast, animal 7 was characterized by a relatively longer SO but shorter SC and FO following the lesion. Finally, in animal 9, FC was shorter but FO was longer post-treatment. The lesion had no effect on relative phase durations in animals 6, 10 and 13 (see Table 3).
Figure 6.
Effect of unilateral LN transection on relative (to gape cycle duration) intra-cycle phase durations: FC in red, SC in blue, SO in grey and FO in green. Solid boxes represent control data whereas hatched boxes represent treatment data. Only those animals for which the relative duration of one or more phases of the gape cycle differed significantly between control and treatment are shown. Black boxes and dotted lines indicate significant differences between control and treatment relative phase durations. The nature of the difference is indicated by > or < and the significance level (*, p<0.05; **, p<0.01; ***, p≤0.001).
Kinematic variables.
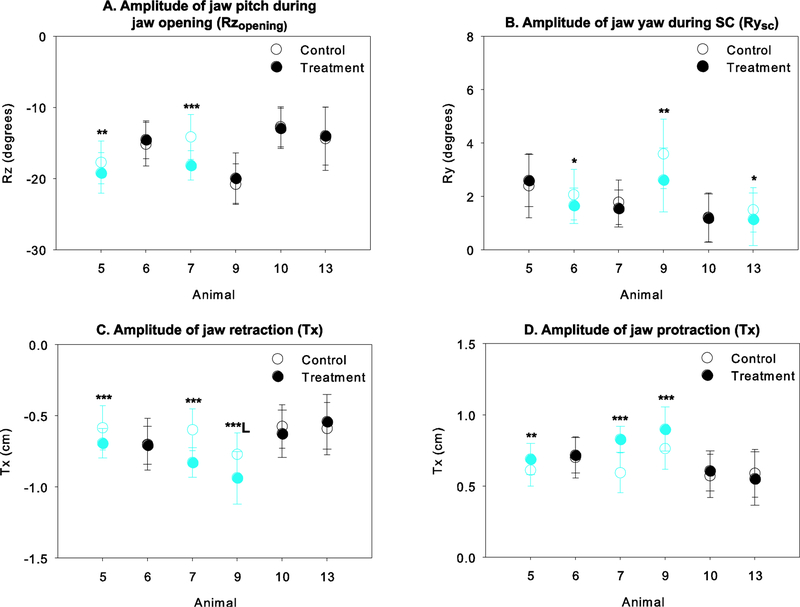
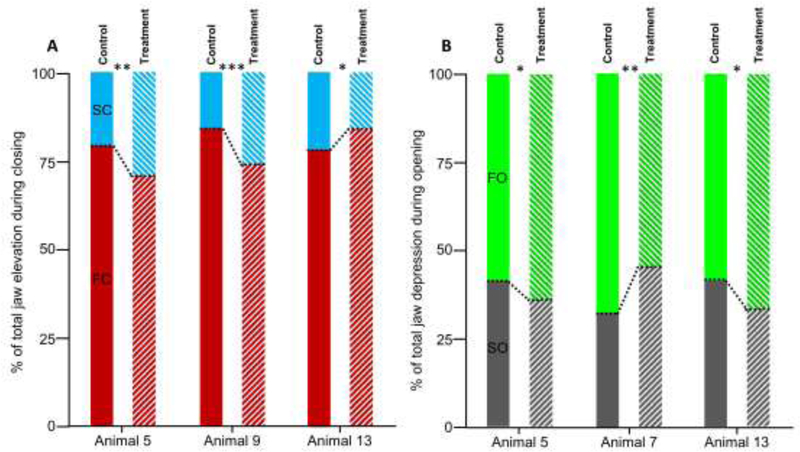
A summary of the statistical results comparing jaw kinematics between the control and treatment datasets are provided in Table 4. The absolute amplitude of jaw depression (Rzopening) was significantly greater after the LN transection in only two of the animals, animals 5 and 7 (Figure 7A). The relative amplitude of jaw closing during FC and SC was significantly altered after the transection in 3 of the 6 animals, albeit differently (Figure 8A). In animals 5 and 9, jaw rotation during FC was reduced and accordingly a relatively greater amount of Rz rotation occurs during SC. In contrast, in animal 13, more rotation occured during FC after the transection, and accordingly, less was achieved during SC. The relative amplitude of Rz rotation during jaw depression achieved during SO decreased significantly after the treatment in animal 5 and 13, but increased in animal 7 (Figure 8B). Accordingly, during FO, the relative amplitude of jaw depression increased in animal 5 and 13, but decreased in animal 7.
Table 4.
Summary of statistical results comparing jaw kinematics pre- and post-lingual nerve transection.
| Animal | ||||||
|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 9 | 10 | 13 | |
| Dorsoventral rotation of the lower jaw (i.e., jaw pitch, Rz) | ||||||
| RZopening F5,535 = 3.974, p = 0.002 |
F1,82= 5.333 p=0.023 |
ns | F1,39= 23.026 p<0.001 |
ns | ns | ns |
| RZFCrel and RZSCrel1 F5,535 = 5.052, p < 0.001 |
F1,82=7.567 p=0.007 |
ns | ns | F1,102=11.357 p=0.001 |
ns | F1,153=5.611 p=0.019 |
| RZSOrel and RZFOrel1 F5,535 = 5.851, p < 0.001 |
F1,82=5.499 p=0.021 |
ns | F1,39=9.975 p=0.003 |
ns | ns | F1,153=11.238 p=0.001 |
| Mediolateral rotation of the lower jaw (i.e., jaw yaw, Ry) | ||||||
| RYSC F5,535 = 2.907, p = 0.005 |
ns | F1,66=4.379 p=0.040 |
ns | F1,102=9.392 p=0.003 |
ns | F1,153=5.854 p=0.017 |
| Anteroposterior translation of the mandibular condyle (i.e., Tx) | ||||||
| TXretraction F5,535 = 8.130, p < 0.001 |
F1,82=13.932 p<0.001 |
ns | F1,39=34.310 p<0.001 |
F1,57=22.584 p=0.001 {L} |
ns | ns |
| TXprotraction F5,535 = 7.488, p < 0.001 |
F1,82=10.298 p=0.002 |
ns | F1,39=30.931 p<0.001 |
F1,102=16.814 p<0.001 |
ns | ns |
Table entries are the F-ratio’s corresponding to the treatment effect tested within each individual separately. The treatment effect had to be tested at the individual level, because the treatment × animal interaction term was significant (Wilk’s lambda λ = 0.653, F45,2360 = 5.240, p < 0.001; associated univariate F-ratio’s are provided under each variable name). The treatment × side × animal and treatment × side interactions were non-significant (Wilk’s lambda λ = 0.854, F80,3503 = 0.919, p = 0.692, and Wilk’s lambda λ = 0.970, F18,1050 = 0.886, p = 0.596, respectively) for all but the absolute amplitude of jaw retraction, TXretraction. {L} indicates the chewing side for which significant differences were observed pre- and post-treatment. NS indicates that control vs treatment differences were not significant.
Because relative Rz amplitude during FC and SC are expressed as a percentage of the total amplitude of jaw closing, they both change in concert and the statistical results are identical for each phase. Similarly for the relative Rz amplitude during SO and FO during jaw opening.
Figure 7.
Effect of unilateral LN transection on the A) amplitude of jaw pitch during jaw opening (Rzopening), B) amplitude of jaw yaw during SC (Rysc), C) amplitude of jaw retraction (Tx) and D) amplitude of jaw protraction (Tx). Control data are shown with unfilled symbols whereas treatment data are shown with solid symbols. In C and D, retraction is indicated by negative numbers whereas protraction is indicated by positive numbers. Blue symbols indicate a significant difference pre- and post-transection for that animal, with the significance level indicated as follows: *, p<0.05; **, p<0.01; ***, p≤0.001. In D, animal 9 exhibit a significant decrease in condylar retraction for left chews only, as indicated by L.
Figure 8.
Effect of unilateral LN transection on the contribution of Rz during constituent phases to A) jaw closing and B) jaw opening. Solid boxes represent control data whereas hatched boxes represent treatment data. The amount of Rz occurring during FC and SC (in red and blue, respectively) are shown in A as a percentage of total Rz during jaw closing. The relative amount of Rz occurring during FO and SO (in grey and green, respectively) are shown in B as a percentage of total Rz during jaw opening. Significance is indicated as follows: *, p<0.05; **, p<0.01; ***, p≤0.001.
Both the amplitude of jaw yaw (Ry) during SC and that of condylar retraction-protraction showed a consistent direction of change in half of the animals studied in which a post-treatment difference was observed. On one hand, Ry was significantly reduced after the transection in animals 6, 9 and 13 (Figure 7B). On the other hand, unilateral LN transection resulted in an increase in the amount of jaw retraction during jaw closing and protraction during jaw opening in animals 5, 7 and 9 (Figures 7C and 7D). Note however that, jaw retraction in animal 9, this was only the case for the left chews (Figure 7C) whereas the amplitude of jaw retraction did not differ for right chews.
Discussion
We initially hypothesized that unilateral LN transection would differentially affect ipsilateral and contralateral chewing cycle temporal and kinematic parameters because chewing is unilateral and the tongue is essential in placing and maintaining the bolus between the teeth during chewing. In our study, side-dependent effects were limited to a decrease in condylar retraction in only one of the six animals tested. For all other animals, treatment × side interaction terms were not significant, demonstrating that ipsi- and contralateral chewing cycles were affected similarly by loss of sensation in the anterior tongue, rejecting our initial hypothesis. This is likely because sensory information from other sources, e.g., the teeth and jaw muscles, play an integral role in the modulation of chewing. Because pigs routinely alternate chewing sides from one chew to the next (Herring & Scapino, 1973), a behavior we also observed, information from the contralateral side of the tongue could be used to inform food management ipsilateral to the lesion. This would also be consistent with previous research on other animal models suggesting that feed-forward and anticipatory mechanisms are used to alter the motor output for modulating gape cycle temporal parameters by incorporating information from prior chews (Ross et al., 2010; Ross et al., 2007).
In the context of our study, unilateral LN transection altered some aspects of jaw dynamics in every individual, either in their spatial and temporal components of jaw movements. Unilateral LN injury had only a moderate impact on the temporal parameters of jaw movements, and there was little consistency between the animals who did show pre- and post-transection differences. Looking more closely at the temporal parameters, it is interesting that jaw-closing phases were largely unaffected (Figures 5A-B) compared to the opening phases (Figures 5C-D). Whereas only 1 animal showed changes to the duration of the closing phases (animal 5), 4 of the 6 pigs showed post-lesion changes to SO, FO or both. During the slow phases of the gape cycle (i.e., SC and SO), the tongue is primarily engaged with the teeth to support the bolus and/or is beginning to manipulate the bolus for transport (Thexton & McGarrick, 1989). Maintaining temporal consistency in the forceful parts of the chewing cycle could be a mechanism to protect the tongue from injury because it preserves the timing of jaw-tongue coordination. We investigated relative phase durations to determine whether there were predictable tradeoffs between cycle phases. However, such trade-off in duration only occurred in two animals, albeit differently (Figure 6). Following the lesion, one animal (7) maintained gape cycle duration while significantly decreasing SC and increasing SO. In another animal (5), total gape cycle duration decreased, largely due to decreases in FC and SO, which more than offset the increase in SC. Thus there is no evidence that unilaterally eliminating lingual sensation results in reciprocal changes between phases.
Interestingly, unilateral disruption of lingual sensation from the anterior tongue affected the amplitude of jaw movements in all 3 dimensions (i.e., all 3 degrees of freedom characterizing jaw movements in pig chewing are affected), and more consistently between the animals tested. In summary, unilateral lingual nerve injury in pigs caused: (i) increase in jaw opening (Figure 7A), (ii) reduction of mediolateral jaw rotation during tooth-food-tooth contact (Figure 7B), and (iii) increase in condylar protraction-retraction (Figures 7C-D). Given the vast feedback from other oral tissues in producing and modulating jaw movements during chewing (Lund & Kolta, 2005; Sessle, 2011), the fact that a response was observed in some animals suggests that the effects of the loss of sensation itself can induce critical changes in masticatory gape cycle dynamics. In the animals exhibiting a response to the injury, there were far more consistent changes in the kinematic parameters as compared to the temporal parameters. Indeed, for each animal whose chewing movements were affected by the lesion, the amplitude of jaw movements changed in the same way but changes in timing were more variable. Thus while the temporal response may be highly individualized, the kinematic response may be more consistent across individuals.
The most interesting finding is that the amplitude of jaw yaw (Ry), or rotation of the jaw about a vertical axis, during SC was reduced after nerve transection in half of the animals tested (Figure 7B). A decrease in yaw during the primary phase in which food is broken down suggests a significant decrease in masticatory movements. Indeed, the mediolateral trajectory of the teeth that are in occlusion is directly dependent on the amount of mediolateral rotation that the jaw undergoes at the condyle. Therefore, based on our observations, the amount of medial displacement of the lower teeth relative to the upper teeth, and thus the efficiency of occlusion and masticatory movements in general may be impaired after LN transection. Reducing occlusion efficiency may have consequences at the level of the whole masticatory performance in that more or longer chewing cycles may be needed to reach swallowability if the grinding motion allowing tooth-food-tooth contact and food breakdown is not optimal. Additional data extracted from the same data set, including the amount of transverse occlusal movement that occurs, will allow testing this hypothesis more explicitly. Further investigation of the changes in occlusal contact dynamics would also be helpful in identifying the potential source of reduced masticatory performance. For example, it may be that tactile sensitivity facilitated by lingual mechanoreceptors is required for ipsilateral food placement and maintenance of food on the occlusal surfaces. Alternatively, there may be some fundamental change in occlusal contacts and the way that the upper and lower teeth interact due to negatively impacted central processing of lingual afferents that affects motor output. While it may be difficult to address the tongue-food interactions directly, it is possible to determine whether altered occlusion occurs after unilateral LN injury. Moreover, because of the tight coordination between jaw movements and tongue movements and deformation during chewing (Liu, Kayalioglu, Shcherbatyy, & Seifi, 2007; Liu, Shcherbatyy, Kayalioglu, & Seifi, 2009; Liu, Yamamura, Shcherbatyy, & Green, 2008), the potential linkages between altered occlusion and altered tongue movements and deformation as an indirect measure of tongue food-interactions can be assessed.
Contrary to jaw yaw that is reduced after unilateral LN transection, our results show that, in half of the animals studied, protraction-retraction of the mandibular condyles is amplified after unilateral LN transection (Figures 7C-D). Because both protraction and retraction are amplified similar amounts within each animal, it is unclear whether the impact of unilateral loss of lingual sensory receptors is affecting both equally or if one is just a response to the other to reset the jaw for the next gape cycle. If the latter, condylar protraction during opening is likely amplified to allow the tongue to clear the oral cavity and engage with the food and bring it back into the mouth at the initial stages of jaw closing and that a similar amount of retraction is required to ensure that the teeth occlude and the jaw fully closes. While this has clear functional ramifications for tongue-jaw coordination, this may also increase stress in the anterior and posterior surfaces of the temporomandibular joint which could potentially alter joint structure and mechanics over time.
Finally, the amplitude of jaw depression increased in two of the animals studied (Figure 7A). Greater jaw opening may have consequences in terms of managing the risk of dropping or losing food. Because of the precise sensorimotor control of tongue movements and deformation, sensory loss in the anterior tongue may negatively impact food and bolus manipulation, therefore increasing the risk of mishandling the food bolus. Obviously, the risk of losing or dropping food is at its greatest when the jaws are open. Therefore, an increase in jaw depression after lingual nerve injury may indicate that individuals become more cautious and amplify jaw movements to avoid biting their own tongue, particularly if the injury manifests as numbness or paresthesia. Sensation of the affected half of the tongue by surrounding tissues, such as the ipsilateral teeth and labial and buccal mucosa, may be involved in adjusting the movements of the jaw. Finally, closer inspection of the dynamics of jaw depression-elevation reveals that in two of the animals (5 and 9), relatively more jaw elevation occurs during the latter part of closing rather than during FC (Figure 8). A greater amount of jaw elevation during SC, as opposed to during FC, could provide additional support of the food on the occlusal surfaces, but may also reflect that altered lingual sensation may force individuals to be more cautious by emphasizing the slow phases of the jaw opening-closing movements.
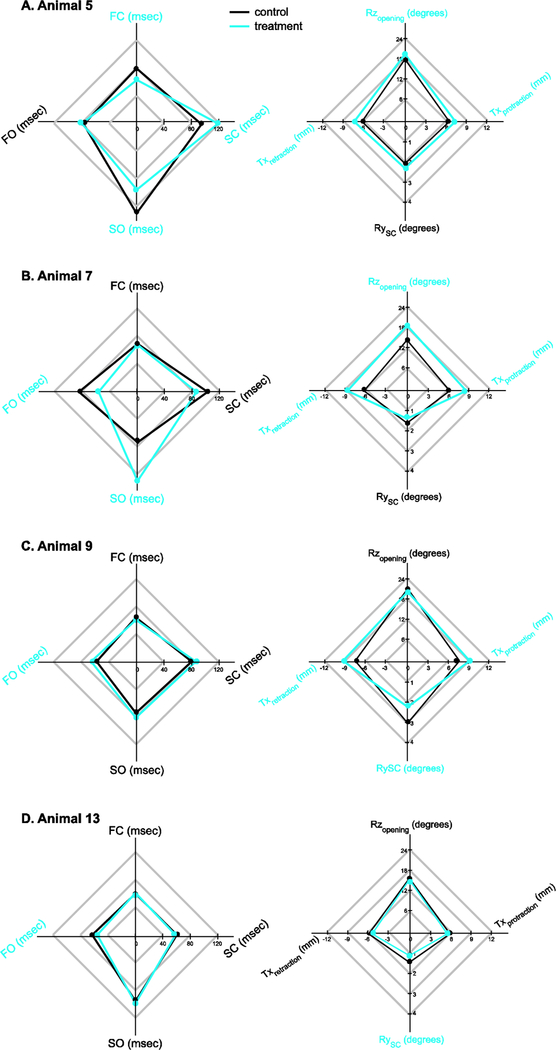
At the individual level, coupling the spatial and temporal results demonstrate parallel tradeoffs between jaw spatial dynamics and phase durations during the gape cycle (Figure 9). For example, in animal 5, we observe both a decrease in the duration and amplitude of jaw rotation during FC and SO, and increase in the duration and amplitude of jaw closing during SC (Figures 8 and 9A). In animal 7, increased Rz amplitude after the transection resulting in greater jaw opening was achieved by a longer and more pronounced SO, at the expense of a reduction in FO (Figure 9B). In animal 9, increased anterior translation of the jaw was associated with a longer FO phase, but there was no change in the amplitude of Rz during opening (Figure 9C). Likewise, the movements during jaw closing that were altered in these animals (yaw and jaw protraction) were not associated with changes in the duration of SC and/or FO. Finally in animal 13, there are no functional associations between the temporal and spatial changes we observed. The only temporal change occurred during opening (increased FO) whereas the only spatial change occurred with closing (jaw yaw during SC).
Figure 9.
Radar plots summarizing absolute cycles duration (left) and the amplitude of jaw movements for animals which showed significant differences before (control) and after (treatment, blue) unilateral lingual nerve transection. Variable names in blue are significantly different between the control and treatment for that animal.
The lack of consistent findings across all animals is surprising given the previous work in rats demonstrating ipsilateral loss of the jaw-opening reflex immediately following unilateral LN injury (Radwan & Thexton, 1993). The reflex is thought to be critical for regulating jaw movements during the gape cycle (Haraguchi et al., 1994; Lund, Kolta, Westberg, & Scott, 1998), and appears to mature during when animals begin to ingest solid foods (Thexton & McGarrick, 1984). The pigs used here were weaned at 3 weeks, and while not fully craniodentally mature, have reached the stage identified by (Huang, Zhang, & Herring, 1994) in which adult masticatory behavior and physiology have been acquired. On the other hand, perhaps major changes would have been observed over time, consistent with the more pronounced effects that have been shown to occur after 21 days with this injury in rats (Adachi et al., 2007). Moreover, the individual difference in responses observed following the transection may reflect the complexity of tongue and jaw movement during mastication and the potential for myriad compensatory changes to altered sensation. Understanding more clearly whether these changes in jaw movements are associated with changes in how the tongue moves and deforms to manipulate the bolus and whether masticatory performance is impacted, are both imperative for developing a more robust understanding of the effects of lingual nerve injury in humans. Nevertheless, our results demonstrating a more consistent effect of the transection on movement amplitude, as opposed to movement timing, identify a potentially important target for therapeutic intervention in patients with long-term lingual nerve injuries.
Conclusions
This is the first study to document the impact of unilateral LN injury on the dynamics of mastication in an animal model. On the one hand, unilateral sensory loss of the anterior tongue affects the spatial 3D dynamics (i.e., the amplitude of all 3 degrees of freedom) of jaw movements in many individuals. These are suggested to affect masticatory performance and to reduce the risk of losing or dropping food, and long-term can potentially affect tooth wear and joint mechanics. On the other hand, the fact that we did not find a consistent post-injury effect on the temporal dynamics of jaw movements (i.e., in gape cycle phase duration) in all animals indicates that individuals may differentially incorporate contralateral lingual or other populations of oral afferents to regulate chewing kinematics. Moreover, while some compensatory strategies may require coordinated changes in the temporal and spatial dynamics of jaw movements, this is not always the case. Finally, the fact that the magnitude of the jaw movements are more adversely affected as opposed to the timing of jaw movements, further suggests that maintaining the tongue-jaw coordination is critical and this can come at the expense of bolus handling and masticatory performance.
Highlights.
Lingual nerve injuries, which occur during routine dental and other oral procedures, affect feeding.
In the pig, unilateral denervation of the lingual nerve is associated with changes in the temporal and spatial dynamics of jaw movements during chewing.
Whereas the timing of jaw movements show a highly individualized response following the injury, changes in spatial dynamics are more consistent across individuals.
Maintaining tongue-jaw coordination is critical and this can come at the expense of bolus handling and masticatory performance.
Acknowledgments
The authors would like to thank the Ohio University Laboratory Animal Resources staff for their help with animal husbandry. We also would like to thank Dr. Andrew Niehaus at The Ohio State University College of Veterinary Medicine and Brooke Keener at the Holzer Clinic for their help with CT-scanning subjects and specimens. This work was supported by the National Institute of Dental and Craniofacial Research (1R15DE023668-01A1) and the National Science Foundation (MRI DBI-0922988 and IOS-1456810). The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
Abbreviations
- XROMM
X-ray Reconstruction Of Moving Morphology
- msec
millisecond
- FC
fast closing
- SC
slow closing
- SO
slow opening
- FO
fast opening
- LN
lingual nerve
- Rz
rotation around the mediolateral axis (i.e., pitch)
- Ry
rotation around the dorsoventral axis (i.e., yaw)
- Tx
translation along the rostrocaudal axis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi K, Lee JC, Hu JW, Yao D, & Sessle BJ (2007). Motor cortex neuroplasticity associated with lingual nerve injury in rats. Somatosensory & Motor Research, 24(3), 97–109. [DOI] [PubMed] [Google Scholar]
- Bodin I, Jaghagen EL, & Isberg A (2004). Intraoral sensation before and after radiotherapy and surgery for oral and pharyngeal cancer. Head & Neck, 26(11), 923–929. [DOI] [PubMed] [Google Scholar]
- Brainerd EL, Baier DB, Gatesy SM, Hedrick TL, Metzger KA, Gilbert SL, & Crisco JJ (2010). X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J Exp Zool A Ecol Genet Physiol, 313A(5), 262–279. [DOI] [PubMed] [Google Scholar]
- Graff-Radford SB, & Evans RW (2003). Lingual nerve injury. Headache, 43(9), 975–983. [DOI] [PubMed] [Google Scholar]
- Haggard P, & de Boer L (2014). Oral somatosensory awareness. Neuroscience & Biobehavioral Reviews, 47, 469–484. [DOI] [PubMed] [Google Scholar]
- Haraguchi N, Yamada Y, Furusawa H, Matsuo K, Oi K, Sato T, & Mizuno A (1994). Variation of the jaw-opening reflex during spontaneous mastication in rabbits. Brain Research Bulletin, 35(1), 93–95. [DOI] [PubMed] [Google Scholar]
- Herring S, Li Y, Liu Y, Liu Z, Popowics T, Rafferty K, & Wang K (2011). Oral Biology and Dental Models. In A. D. D. McAnulty Peter A Niels-Christian Ganderup, and Hastings Kenneth L. (Ed.), The Minipig in Biomedical Research (pp. 491–516): CRC Press [Google Scholar]
- Herring S, & Scapino R (1973). Physiology of feeding in miniature pigs. Journal of Morphology, 141(4), 427–460. [DOI] [PubMed] [Google Scholar]
- Hillerup S (2007). Iatrogenic injury to oral branches of the trigeminal nerve: records of 449 cases. Clin Oral Investig, 11(2), 133–142. [DOI] [PubMed] [Google Scholar]
- Hogenkamp PS, & Schioth HB (2013). Effect of oral processing behaviour on food intake and satiety. Trends in Food Science & Technology, 34(1), 67–75. [Google Scholar]
- Huang X, Zhang G, & Herring SW (1994). Age changes in mastication in the pig. Comparative Biochemistry and Physiology. Comparative Physiology, 107(4), 647–654. [DOI] [PubMed] [Google Scholar]
- Iwanaga J (2017). The clinical view for dissection of the lingual nerve with application to minimizing iatrogenic injury. Clin Anat, 30(4), 467–469. [DOI] [PubMed] [Google Scholar]
- Jacks SC, Zuniga JR, Turvey TA, & Schalit C (1998). A retrospective analysis of lingual nerve sensory changes after mandibular bilateral sagittal split osteotomy. J Oral Maxillofac Surg, 56(6), 700–704; discussion 705. [DOI] [PubMed] [Google Scholar]
- Jaghagen EL, Bodin I, & Isberg A (2008). Pharyngeal swallowing dysfunction following treatment for oral and pharyngeal cancer--association with diminished intraoral sensation and discrimination ability. Head & Neck, 30(10), 1344–1351. [DOI] [PubMed] [Google Scholar]
- Klazen Y, Van der Cruyssen F, Vranckx M, Van Vlierberghe M, Politis C, Renton T, & Jacobs R (2018). Iatrogenic trigeminal post-traumatic neuropathy: a retrospective two-year cohort study. Int J Oral Maxillofac Surg [DOI] [PubMed]
- Knorlein BJ, Baier DB, Gatesy SM, Laurence-Chasen JD, & Brainerd EL (2016). Validation of XMALab software for marker-based XROMM. Journal of Experimental Biology, 219(Pt 23), 3701–3711. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang N, Hu L, Li Z, Li R, Li C, & Wang S (2011). Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young Chinese men. American Journal of Clinical Nutrition, 94(3), 709–716. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Kayalioglu M, Shcherbatyy V, & Seifi A (2007). Tongue deformation, jaw movement and muscle activity during mastication in pigs. Arch Oral Biol, 52(4), 309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Shcherbatyy V, Kayalioglu M, & Seifi A (2009). Internal kinematics of the tongue in relation to muscle activity and jaw movement in the pig. J Oral Rehabil, 36(9), 660–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZJ, Yamamura B, Shcherbatyy V, & Green JR (2008). Regional volumetric change of the tongue during mastication in pigs. J Oral Rehabil, 35(8), 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Kolta A, Westberg KG, & Scott G (1998). Brainstem mechanisms underlying feeding behaviors. Current Opinion in Neurobiology, 8(6), 718–724. [DOI] [PubMed] [Google Scholar]
- Lund JP, & Kolta A (2005). Adaption of the central masticatory pattern to the biomechanical properties of food. International Congress Series, 1284, 11–20. [Google Scholar]
- Magboul MM, & Joel S (2011). The video laryngoscopes blind spots and possible lingual nerve injury by the Gliderite rigid stylet--case presentation and review of literature. Middle East Journal of Anesthesiology, 20(6), 857–860. [PubMed] [Google Scholar]
- Mendes MB, de Carvalho Leite Leal Nunes CM, & de Almeida Lopes MC (2013). Anatomical relationship of lingual nerve to the region of mandibular third molar. J Oral Maxillofac Res, 4(4), e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegaz RA, Baier DB, Metzger KA, Herring SW, & Brainerd EL (2015). XROMM analysis of tooth occlusion and temporomandibular joint kinematics during feeding in juvenile miniature pigs. Journal of Experimental Biology, 218(Pt 16), 2573–2584. [DOI] [PubMed] [Google Scholar]
- Minato A, Ono T, Miyamoto JJ, Honda E, Kurabayashi T, & Moriyama K (2009). Preferred chewing side-dependent two-point discrimination and cortical activation pattern of tactile tongue sensation. Behavioural Brain Research, 203(1), 118–126. [DOI] [PubMed] [Google Scholar]
- Montuelle SJ, Olson R, Curtis H, Sidote J, & Williams SH (2018). Flexibility of feeding movements in pigs: effects of changes in food toughness and stiffness on the timing of jaw movements. Journal of Experimental Biology, 221(Pt 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogrel MA, Jergensen R, Burgon E, & Hulme D (2011). Long-term outcome of trigeminal nerve injuries related to dental treatment. J Oral Maxillofac Surg, 69(9), 2284–2288. [DOI] [PubMed] [Google Scholar]
- Pogrel MA, Renaut A, Schmidt B, & Ammar A (1995). The relationship of the lingual nerve to the mandibular third molar region: an anatomic study. J Oral Maxillofac Surg, 53(10), 1178–1181. [DOI] [PubMed] [Google Scholar]
- Pouderoux P, & Kahrilas PJ (1995). Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology, 108(5), 1418–1426. [DOI] [PubMed] [Google Scholar]
- Radwan Y, & Thexton AJ (1993). Recovery of the jaw-opening reflex after lesions of the lingual nerve in the rat. J Dent Res, 72(8), 1198–1205. [DOI] [PubMed] [Google Scholar]
- Renes SH, Zwart R, Scheffer GJ, & Renes S (2011). Lingual nerve injury following the use of an i-gel laryngeal mask. Anaesthesia, 66(3), 226–227. [DOI] [PubMed] [Google Scholar]
- Renton T, & Yilmaz Z (2012). Managing iatrogenic trigeminal nerve injury: a case series and review of the literature. Int J Oral Maxillofac Surg, 41(5), 629–637. [DOI] [PubMed] [Google Scholar]
- Robert RC, Bacchetti P, & Pogrel MA (2005). Frequency of trigeminal nerve injuries following third molar removal. J Oral Maxillofac Surg, 63(6), 732–735; discussion 736. [DOI] [PubMed] [Google Scholar]
- Ross CF, Baden AL, Georgi J, Herrel A, Metzger KA, Reed DA, … Wolff MS (2010). Chewing variation in lepidosaurs and primates. Journal of Experimental Biology, 213(4), 572–584. [DOI] [PubMed] [Google Scholar]
- Ross CF, Eckhardt A, Herrel A, Hylander WL, Metzger KA, Schaerlaeken V, … Williams SH (2007). Modulation of intra-oral processing in mammals and lepidosaurs. Integr Comp Biol, 47(1), 118–136. [DOI] [PubMed] [Google Scholar]
- Sessle BJ (2011). Face sensorimotor cortex: its role and neuroplasticity in the control of Orofacial movements. In Gossard J, Dubuc R & Kolta A (Eds.), Progress in Brain Research: Breath, walk and chew: the neural challenge (Vol. 188, pp. 71–82). [DOI] [PubMed] [Google Scholar]
- Takahashi T, Miyamoto T, Terao A, & Yokoyama A (2007). Cerebral activation related to the control of mastication during changes in food hardness. Neuroscience, 145(3), 791–794. [DOI] [PubMed] [Google Scholar]
- Teismann IK, Steinstraeter O, Stoeckigt K, Suntrup S, Wollbrink A, Pantev C, & Dziewas R (2007). Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neuroscience, 8, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thexton AJ, & McGarrick JD (1984). Maturation of brainstem reflex mechanisms in relation to the transition from liquid to solid food ingestion. Brain Behavior and Evolution, 25(2–3), 138–145. [DOI] [PubMed] [Google Scholar]
- Thexton AJ, & McGarrick JD (1989). Tongue movement in the cat during the intake of solid food. Arch Oral Biol, 34(4), 239–248. [DOI] [PubMed] [Google Scholar]
- Trulsson M, & Essick GK (1997). Low-threshold mechanoreceptive afferents in the human lingual nerve. J Neurophysiol, 77(2), 737–748. [DOI] [PubMed] [Google Scholar]
- Turker KS, Johnsen SE, Sowman PF, & Trulsson M (2006). A study on synaptic coupling between single orofacial mechanoreceptors and human masseter muscle. Experimental Brain Research, 170(4), 488–500. [DOI] [PubMed] [Google Scholar]
- Zijlstra N, Mars M, Stafleu A, & de Graaf C (2010). The effect of texture differences on satiation in 3 pairs of solid foods. Appetite, 55(3), 490–497. [DOI] [PubMed] [Google Scholar]