Abstract
Lysosomes, the cell’s degradation center, are filled with acidic hydrolases. Lysosomes generate nutrient-sensitive signals to regulate the import of H+, hydrolases, and endocytic and autophagic cargos, and the export of the degradation products (catabolites). In response to environmental and cellular signals, lysosomes change their positioning, number, morphology, size, composition, and activity within minutes to hours to meet the changing cellular needs. Ion channels in the lysosome are essential transducers that mediate signal-initiated Ca2+/Fe2+/Zn2+ release and H+/Na+/K+-dependent changes of membrane potential across the perimeter membrane. Dysregulation of lysosomal ion flux impairs lysosome movement, membrane trafficking, nutrient-sensing, membrane repair, organelle membrane contact, and lysosome biogenesis and adaptation. Hence, activation and inhibition of lysosomal channels by synthetic modulators may tune lysosome function to maintain cellular health and promote cellular clearance in lysosome storage disorders.
Keywords: TRPML1, TPC2, mTOR, TFEB, Lysosomal Exocytosis, Lysosomal Storage Disease
Lysosome function
Lysosomes are traditionally viewed as macromolecule degradation center of the cell expressing more than 60 types of acidic hydrolases (Kolter and Sandhoff, 2005). Recent studies, however, have clearly demonstrated that lysosomes are also a signaling hub that hosts the major nutrient sensors in the cell (Bar-Peled et al., 2012; Settembre et al., 2013; Zoncu et al., 2011b). The “degradation” and “signaling” arms of lysosome function regulate each other reciprocally, such that degradation products, i.e., catabolites, are often the nutrient-sensitive signals that in turn control the duration and rate of degradation (Perera and Zoncu, 2016). Instructed by these signals, lysosomes undergo constant movement, membrane fusion and fission, exocytosis, proliferation (biogenesis and reformation), and self-repair (Perera and Zoncu, 2016; Skowyra et al., 2018). At resting conditions, each mammalian cell contains hundreds of primary and secondary (i.e., endolysosomes, autolysosomes, and phagolysosomes) lysosomes that are heterogeneous in protein and lipid composition, size (diameter = 100-500 nm), and positioning (e.g., peripheral vs. perinuclear) (Huotari and Helenius, 2011; Perera and Zoncu, 2016; Xu and Ren, 2015). Upon nutrient starvation and refeeding, these lysosome parameters change rapidly and reversibly to meet the changing cellular needs. In this review, we discuss recent advances in our understandings of the signals and signal transducers that regulate lysosomal membrane trafficking, formation of lysosome-organelle membrane contact sites, nutrient-sensing, biogenesis, and adaptation (Fig. 1).
Fig 1. Function and regulation of lysosomes.
Receiving inputs from both endocytic and autophagic pathways, lysosomes are the degradation center in the cell under both normal and starvation conditions. The degradation products, i.e., catabolites, are released through exporters, vesicular trafficking, lysosomal exocytosis, or interorganelle material exchange at membrane contact sites. Lysosomes are formed de novo through endosome maturation, or reformed from autolysosomes or endolysosome hybrids. Lysosomes form membrane contact sites with ER and other organelles, at which export of lipids and ions takes place. Lysosomal membrane proteins bridge the information of cytoplasmic signaling and luminal degradation. Lysosomal ion channels, by mediating signal-dependent lysosomal ion flux, participate in various lysosomal functions, including lysosomal membrane trafficking, catabolite export, nutrient sensing, and mTOR signaling. Triggered by various cellular cues, lysosomal Ca2+ release regulates lysosomal trafficking, lysosomal exocytosis, autophagic clearance of damaged mitochondria, plasma membrane repair, lysosomal membrane repair, TFEB nuclear translocation, and lysosome biogenesis.
Degradation
Lysosomes are the primary sites of cellular recycling in which proteins, complex lipids, polysaccharides, and nucleotides are broken down by proteases, lipases, glycosidases, and nucleases into their respective monogenetic building-block molecules: amino acids (AAs), monosaccharides, free fatty acids, and nucleosides for reuse in biosynthetic pathways (Kolter and Sandhoff, 2005) (Fig. 1). Mutations in the hydrolases result in accumulation of the cargo substrates, which in turn causes lysosomal insufficiency at the cellular level, and more than 50 types of lysosome storage diseases (LSDs) at the organismal level (Kolter and Sandhoff, 2005).
Membrane trafficking: movement, fusion, and fission
Lysosomes are mobile vesicles that undergo frequent membrane trafficking, i.e., fusion and fission, with other intracellular compartments (Luzio et al., 2007). Lysosomes receive cargo materials delivered from endocytosis and autophagy (Fig. 1). Exogenous endocytic cargos are fed into lysosomes through the endosome maturation process (Fig. 1), which also includes lysosomal acidification and hydrolase delivery via mannose-6-phosphate receptors (Huotari and Helenius, 2011). Conversely, intracellular autophagic cargos, e.g., damaged mitochondria, are packed into autophagosomes and delivered to lysosomes through autophagosome-lysosome fusion (Gatica et al., 2018). During these processes, lysosomes, with the aid of motor proteins, move constantly in both anterograde (nucleus-to-plasma membrane) and retrograde directions (Du et al., 2016; Li et al., 2016b). Degradation products, i.e., catabolites, are exported into the cytoplasm or extracellular space through multiple efflux mechanisms: permease and transporter, vesicular trafficking, exchange at lysosome-organelle membrane contact sites, and lysosomal exocytosis (Xu and Ren, 2015) (see Fig. 1). Whereas insoluble lipids and empty mannose-6-phosphate receptors are retrogradely transported to the Trans-Golgi-network (TGN) for reuse (Huotari and Helenius, 2011; van der Kant and Neefjes, 2014; Xu and Ren, 2015), catabolite-containing lysosomes are anterogradely transported and release the contents into the extracellular space through lysosomal exocytosis (fusion with the plasma membrane) (Medina et al., 2011) (Fig. 1).
Lysosome-organelle membrane contact
Catabolite export may also employ the non-vesicular, inter-organelle material exchange pathways at lysosome-organelle membrane contact sites (Phillips and Voeltz, 2016; van der Kant and Neefjes, 2014) (see Fig. 1). Lysosomes form membrane contact sites with all types of organelles, including ER, mitochondria, and peroxisomes (Phillips and Voeltz, 2016; van der Kant and Neefjes, 2014). Free cholesterol can be exported from lysosomes to the ER and peroxisomes through lysosome-ER and lysosome-peroxisome membrane contact sites (Chu et al., 2015; van der Kant et al., 2013; Zhao andRidgway, 2017). At ER-lysosome membrane contact sites, ER may sequester lysosomal Ca2+ (Kilpatrick et al., 2013), and ER Ca2+ can refill lysosomal Ca2+ stores (Wang et al., 2017). Additionally, membrane contact sites may also regulate the fission and positioning of endosomes and lysosomes (Rowland et al., 2014).
Nutrient sensing
Lysosomes provide a mobile physical platform that monitors nutrient levels in the cell (Settembre et al., 2013). Whereas the yeast vacuole (a lysosome equivalent) is the major storage site for nutrients, in mammalian cells, the majority of the building-block molecules used in biosynthesis are supplied from lysosomes (Kolter and Sandhoff, 2005). The mammalian target of rapamycin complex 1 (mTORC1) kinase that integrates the information of cellular nutrients and growth signals, is primarily localized on the lysosomal membrane (Zoncu et al., 2011a). By forming a protein complex with lysosomal GTPases (e.g., Rags and Rheb), the V-ATPase, and amino acid exporters (e.g., SLC38A9), mTORC1 “senses” both the luminal and cytosolic pools of amino acids (Perera and Zoncu, 2016). Upon amino acid starvation, mTORC1 dissociates from the lysosomal membrane and its substrates (see Fig. 1), resulting in halted protein synthesis but elevated autophagy (Settembre et al., 2013). Lysosomes also host AMP-activated protein kinase (AMPK), the primary glucose sensor in the cell (Perera and Zoncu, 2016; Zhang et al., 2017). Glucose starvation activates AMPK by increasing the AMP/ATP ratio or production of fructose 1, 6-bisphosphatase, a glycolytic metabolite (Zhang et al., 2017). Moreover, SLC38A9, as well as the cholesterol transporter NPC1, are involved in the sensing of lysosomal sterol lipids (Castellano et al., 2017). Hence, lysosomes are focal sensing sites of cellular nutrients.
Lysosome adaptation and heterogeneity.
Nutrient-derived signals regulate lysosome function, trafficking, and adaptation (Perera and Zoncu, 2016; Settembre et al., 2013). Lysosomal degradation is likely regulated by multiple feedback mechanisms: an increase in cargo vesicles, e.g., autophagosomes, can stimulate degradation, and the end products of degradation can inhibit degradation (Perera and Zoncu, 2016; Settembre et al., 2013). Indeed, removing metabolites in cell culture medium (amino acid starvation) serves as the most commonly-used experimental paradigm to stimulate autophagic degradation (Gatica et al., 2018). In addition to promoting autophagosome formation, amino acid starvation also boosts lysosome function, as manifested by increases in the delivery of V-ATPase, membrane fusion and fission, and directional movement (Zhou et al., 2013). Lysosomal signals may recruit multiple adaptation mechanisms to sustain lysosome consumption (see Fig. 1). First, nutrient-dependent changes in the mTOR activity and phosphoinositide levels generate new lysosomes from tubulated autolysosomes (autophagic lysosome reformation) (Li et al., 2016b; Rong et al., 2012). Second, an ESCRT-dependent membrane self-repair process increases the pool of “healthy” lysosomes (Skowyra et al., 2018). Third, a transcriptional factor EB (TFEB)-dependent, lysosome-to-nucleus transcriptional program is triggered to facilitate the expression of genes for lysosome biogenesis (Settembre et al., 2011). Hence, catabolite-sensitive signals, through their effectors mTOR, AMPK, Rags, and various lipid kinases (McCartney et al., 2014; Perera and Zoncu, 2016), play essential roles in lysosome regulation. Non-nutritional signals, e.g., other lysosomal stressors such as oxidants and pathogen invasion, may also activate lysosome adaptation (Fig. 1), often through the shared signal transducers and effectors such as mTOR and TFEB (Perera and Zoncu, 2016; Settembre et al., 2013; Zhang et al., 2016).
While lysosomes can be synchronized in their fusion/reformation cycle upon stimulation, under resting and basal states, lysosomes are largely un-synchronized and heterogeneous in the individual cells: primary vs. secondary lysosomes, endolysosomes vs. autolysosomes, activated (acidified) vs. resting (neutral) lysosomes, and perinuclear vs. peripheral lysosomes (Bright et al., 2016; Johnson et al., 2016). Conceivably, the same set of nutrient-sensitive signals, transducers, and effectors, at their ambient levels, determine the basal rate of degradation. Because increasing cargo load and experimentally changing the catabolite levels have profound effects on lysosomal functions and signaling, we propose that the heterogeneity of individual lysosomes reflects the differences in their cargo and catabolite contents (Perera and Zoncu, 2016; Settembre et al., 2013), and subsequent differences in the recruitment of transducers and effectors.
Lysosome ion homeostasis and membrane potential
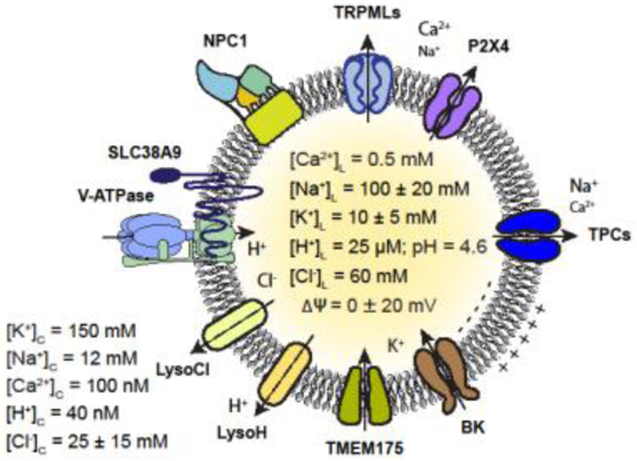
Lysosomal ion channels and transporters establish the concentration gradients for H+, Na+, K+, Ca2+, and Cl−, and lysosomal membrane potential (Δψ) across lysosomal membranes (Figs. 1, 2).
Fig 2. Lysosomal ion channels and transporters.
Compared with cytoplasm, the lysosome lumen contains high H+, Ca2+, and Na+, but low K+. At resting conditions, lysosomal Δψ is close to 0 mV. Lysosomal channels that have been characterized using lysosomal patch-clamp include Na+/Ca2+-permeable TRPMLs, P2X4, and TPCs, and K+-selective channels BK/LysoKvca and TMEM175. V-ATPase is the proton pump that acidifies lysosomes. The molecular identities of lysosomal Cl− and H+ conductances are not yet known. SLC38A9 is the lysosomal arginine sensor and transporter; NPC1 is the lysosomal cholesterol transporter.
H+:
A hallmark feature of lysosomes is an acidic luminal pH (Kolter and Sandhoff, 2005; Mindell, 2012). During endosome maturation, lysosomes are gradually acidified by the V-ATPase to reach a plateau pHLumen (pHL) of 4.6 (Johnson et al., 2016) (Fig. 2). pHL for individual lysosomes, however, is heterogeneous depending on location and nutrient status in the cell (Bright et al., 2016; Johnson et al., 2016), which regulates the activities of both V-ATPase and an unidentified H+ “leak” conductance (Cang et al., 2014).
Ca2+:
Lysosomes serve as small-volume, mobile intracellular Ca2+ stores, with [Ca2+]L ~0.5 mM, 5,000-fold higher than [Ca2+]cytosoi (Yang et al., 2018). A putative Ca2+/H+ exchanger was proposed to drive lysosomal Ca2+ uptake (Christensen et al., 2002; Morgan et al., 2011), but recent studies suggest that ER Ca2+ may refill lysosome stores independent of pH (Garrity et al., 2016). In the latter model, ER Ca2+ release channel IP3 receptors, together with a putative lysosomal Ca2+ uptake channel/transporter, mediate Ca2+ exchange at ER-lysosome membrane contact sites (Wang et al., 2017; Yang et al., 2018). Lysosomal Ca2+ release regulates most lysosomal functions (Li et al., 2013a; Li et al., 2016b; Phillips and Voeltz, 2016; Xu and Ren, 2015), including lysosome movement, membrane trafficking, and membrane contact sites formation.
Na+/K+:
The lysosome lumen was thought to be high in K+ and low in Na+, suggesting that like ER, there are no substantial concentration gradients of Na+ or K+ across lysosomal membranes (Morgan et al., 2011; Steinberg et al., 2010). This view has been challenged by several recent whole-endolysosome patch-clamp studies showing the presence of multiple Na+-selective and K+-selective channels in the lysosome (Cang et al., 2015; Cao et al., 2015b; Wang et al., 2017; Wang et al., 2012). Additionally, isolated lysosomes are reported to contain high [Na+]L and low [K+]L (Wang et al., 2012) (Fig. 2). The ion transporters that establish the lysosomal Na+ and K+ gradients are not yet known, but disruptions of the gradients using Na+/H+ and K+/H+ ionophores are known to impair various lysosomal functions, including lysosomal acidification and catabolite export (Wang et al., 2017; Wyant et al., 2017).
Cl−:
[Cl−]L is estimated to be 60-80 mM using the pH-insensitive probe Clensor (Chakraborty et al., 2017). Lysosome Cl−, maintained by CLC family Cl−/H+ exchangers, may provide counterions to support acidification (Graves et al., 2008; Jentsch and Pusch, 2018; Stauber and Jentsch, 2013).
Δψ:
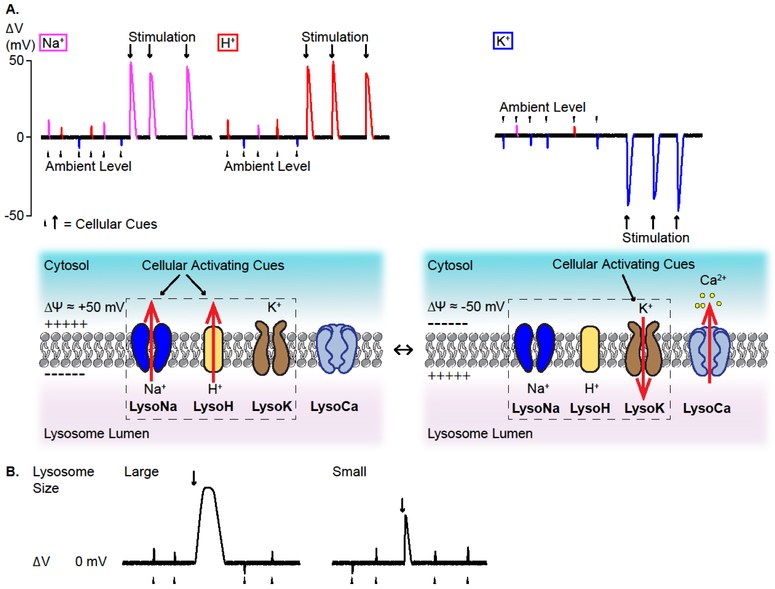
Given the steep concentration gradients of Na+, K+, and H+, lysosomal Δψ (=ψcytosoi-ψlumen) is determined by the relative permeabilities of these ions (Wang et al., 2017). For a lysosome with a diameter of 0.3 μm, there are only 9 ×105 (in molecule) Na+, 9 ×104 K+, and 200 H+ in the lumen (assuming [Na+]L= 100 mM, [K+]L = 10 mM, and [H+]L = 25 μM; Fig. 2). Theoretically, if this lysosome has 10 channels with a single-channel conductance of 10 pS and 0.1 open probability, at a driving force of 10 mV, 600 ions would pass across the lysosomal membrane per millisecond. However, upon channel openings, luminal ion composition would be altered quickly (in milliseconds) to reduce the electrochemical gradients (Fig. 3). Hence, luminal ion depletion and large or lasting changes in Δψ would not occur in situ (Fig. 3). The reported lysosomal Δψ values are scattered but clustered at small negative values (Morgan et al., 2011), e.g. −19 mV in macrophage phagolysosomes (Koivusalo et al., 2011). In the current-clamped vacuolin-enlarged lysosomes at assumed high [Na+]L, Δψ is found to be slightly positive (e.g. +20 mV) (Cang et al., 2013; Wang et al., 2017). We propose that, under ambient levels of cellular signals, “resting” lysosomal Δψ is near 0 mV (± 20 mV; Figs. 2,3). Upon stimulation, lysosome-associated cellular cues activate various resident Na+, H+, and K+ channels to rapidly but transiently change Δψ of individual lysosomes to regulate various Δψ-dependent lysosomal functions such as catabolite export (Fig. 3).
Fig 3. Regulation of lysosomal membrane potential.
A. Lysosomal membrane potential (Δψ) is determined by the relative permeability of the lysosomal membranes to H+, Na+, and K+, through an unidentified H+-leak conductance (LysoH), LysoNa channels (e.g., TPCs), and LysoK channels (e.g., BK/LsyoKvca and TMEM175). TPCs are Na+ channels activated by PI(3,5)P2; TMEM175 is a K+ leak channel; BK channels are activated by both voltage and Ca2+. Lysosomal Δψ is depolarized when LysoH and LysoNa channels are open (left panels), and hyperpolarized when LysoK channels are open (right panels). In the resting conditions, lysosome Δψ fluctuates around 0 mV (±20 mV), due to minimal activation of lysosomal ion channels by ambient levels of cellular signals. Large Δψ changes (± 50 mV) occur when the levels of lysosomal ion channel-activating signals are high. Activation of LysoK reduces or reverses lysosomal Δψ, providing a larger driving force for LysoCa-mediated lysosomal Ca2+ release. B. Lysosome size may influence the duration and amplitude of lysosome Δψ changes. Luminal ion composition changes more dramatically in small-sized lysosomes. Hence, larger lysosomes exhibit bigger and longer lasting Δψ changes upon identical (in strength and duration) stimulation.
Lysosomal Ca2+ and Na+ channels
There exist at least three types of lysosomal Ca2+ (LysoCa) channels in the mammalian cells: TRPML1-3, TPC1-2, and P2X4 (Fig. 2), all of which have been directly measured and verified by direct recordings on native lysosomes. As many lysosomal functions are Ca2+ dependent, cellular cues may regulate them by activating LysoCa channels (Yang et al., 2018). Unlike TRPMLs and P2X4, TPCs, although Ca2+-permeable, are Na+-selective (Cang et al., 2013; Guo et al., 2017; Wang et al., 2012), and hence are more likely to be involved in direct regulation of lysosomal Δψ- and Na+-dependent functions. The focus of the current review is to discuss how cellular cues regulate lysosomal functions through modulation of channels in the lysosomal membranes. Hence, additional candidate LysoCa and LysoNa channels that are only inferred by pharmacology or antibodies are not discussed here.
Mucolipin TRP channels (TRPMLs).
The mucolipin subfamily of transient receptor potential (TRP) channels, TRPML1-3, are tetrameric 6 transmembrane (TM) channels that are localized exclusively on endosomes and lysosomes (Cheng et al., 2010) (see Table 1). Whereas TRPML1 is ubiquitously expressed, TRPML2 and TRPML3 are more restrictively expressed (Cheng et al., 2010; Xu and Ren, 2015). Although TRPML1 has been most extensively studied, genetic and cell biological studies of mammalian TRPML2 and TRPML3, as well as non-mammalian, e.g., Drosophila and C. elegans TRPMLs, suggest conserved roles of mucolipin channels in regulating lysosomal functions (Grimm et al., 2017). Loss-of-function mutations of human TRPML1 cause type IV mucolipidosis (ML-IV), a LSD with the symptoms of neurodegeneration and muscular dystrophy (Boudewyn and Walkley, 2018). Di-leucine motifs at the intracellular N- and C-termini are responsible for the lysosomal localization of TRPML1 (Xu and Ren, 2015). Whole-endolysosome patch-clamp studies reveal that mammalian TRPML1-3 channels are permeable to both Ca2+ and Na+, as well as K+ and heavy metal ions such as Fe2+ and Zn2+ (Xiong and Zhu, 2016). Recent high-resolution structural studies have confirmed that the cationic selectivity is determined by negatively charged amino acid residues in the pore loop, and the activation gate is made of the S6 helices (Chen et al., 2017; Schmiege et al., 2017).
Table 1.
Lysosome ion channels and their functions.
| Lysosomal Ion Channel |
Permeability | Subcellular Localization |
Lysosome Targeting Motif1 |
Acidic pHL
dependence |
Endogenous Agonists |
Endogenous Inhibitors |
Synthetic Agonists |
Synthetic Inhibitors |
Lysosome Function | Human Disease & Mouse KO phenotype |
|---|---|---|---|---|---|---|---|---|---|---|
| TRPML1 | Cation, non-selective Ca2+ > K+ = Na+ > Cs+ | LEL | L577L578 L15L16 | ↑ | PI(3,5)P2, ROS |
PI(4,5)P2,
sphingomyelin, adenosine |
ML-SAs, SF-51, MK6-83 |
ML-SIs | Lysosomal exocytosis; retrograde transport; plasma membrane repair TFEB activation; ROS sensing |
ML-IV; NPC; AD |
| TRPML2 | Cation, none-selective Na+
~ K+ ~ Cs+, Ca2+ ~ Fe2+ |
RE, LEL, PM | PI(3,5)P2 | ML-SAs | ML-SIs | lysosome trafficking regulation | Impaired immune response | |||
| TRPML3 | Cation, none-selective Ca2+ > Na+ >K+ » Cs+ | EE, LEL, PM | ↓ | PI(3,5)P2 | PI(4,5)P2 | ML-SAs, SFs |
ML-SIs | Exosome release | ||
| TPC1 | Na+/Ca2+ | EE, LEL | L83I84 | ↑ | PI(3,5)P2, voltage sphingosine (?), NAADP (?), |
Cytoplasmic ATP, mTOR |
Tetrandrine (?), Ned 19 (?) |
Amino acid export; endolysosomal excitability; pHLhomeostasis; cellular ATP sensor; ER-endosome membrane contact site formation |
Reduced virus entry; impaired exercise endurance after fasting |
|
| TPC2 | Na+/Ca2+
PNa/Pca >
10,
PNa/Pk > 60 |
LEL | L11L12 | PI(3,5)P2, NAADP (?) |
Cytoplasmic ATP, mTOR, Mg2+ |
Tetrandrine (?), Ned19 (?) |
Amino acid export;
pHLhomeostasis autophagy regulation; cellular ATP sensor; lysosome trafficking regulation |
Hypercholesterolemia; impaired exercise endurance after fasting; PD; NAFLD; reduced virus entry |
||
| P2X4 | Ca2+, Na+ | LEL, PM | L22I23
Y372xxV Y378xxGL |
↓ | Luminal ATP | Lysosome fusion | ||||
| TMEM175 | K+,
PK/PNa 30 |
EE, LEL | L499L500(?) | ↓ | 4-AP, Zn2+ | pHL homeostasis;
autophagosome-lysosome fusion; lysosome resting Δψ maintenance |
PD | |||
| SLO1 (BK) | K+ | LEL, MT, PM | L488L489,
L734I735 (?) |
↓ | Ca2+ | NS1619 | Paxillin, IBTX |
Lysosomal Ca2+ release and
refilling; lysosome Δψ control |
Various neurological symptoms;
impaired glucose homeostasis |
Based on the human protein
Abbreviations:
4-AP: 4-aminopyridine; ATP: adenosine triphosphate; EE: early endosome; ER: endoplasmic reticulum; IBTX: iberiotoxin; LEL: late endosome and lysosome; ML-SAs: TRPML1 synthetic agonists; ML-SIs: TRPML1 synthetic inhibitors; MT: mitochondria; mTOR: mammalian target of rapamycin; NAADP: nicotinic acid adenine dinucleotide phosphate; pHL: lysosome luminal pH; PM: plasma membrane; RE: recycling endosome; ROS; reactive oxygen species; TFEB: transcription factor EB
AD: Alzheimer’s disease; NAFLD: non-alcoholic fatty liver disease; NPC: Niemann-Pick type C disease; ML-IV: type IV mucolipidosis; PD: Parkinson’s disease
Endogenous agonists: PI(3,5)P2 and ROS.
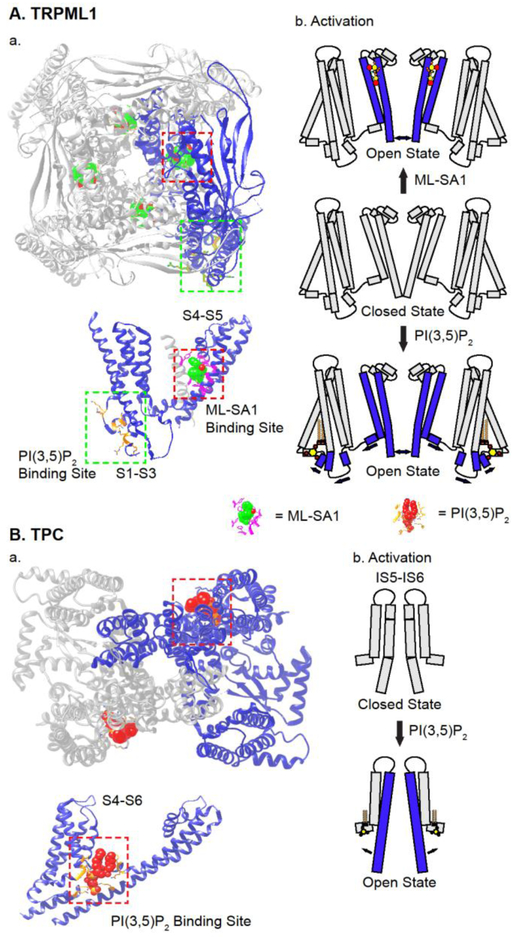
PI(3,5)P2 is a late endosome and lysosome (LEL)-specific phosphoinositide that regulates several trafficking steps of lysosomes (McCartney et al., 2014). Inhibiting PI(3,5)P2 synthesis genetically or pharmacologically causes lysosomal trafficking defects mimicking ML-IV (TRPML1 null mutant) cells (McCartney et al., 2014). PI(3,5)P2 potently activates whole-lysosomal TRPML1-3 and Drosophila TRPML channels by binding to positively-charged amino acid residues in the cytosolic N-terminal domain, as modelled in the high-resolution structures (Chen et al., 2017; Dong et al., 2010; Hirschi et al., 2017)Fig. 4). PI(3,5)P2 binding may lead to conformational changes in the S2-S3 linker to open the S6 gate (Fig. 4) (Chen et al., 2017; Hirschi et al., 2017). Whereas cellular PI(3,5)P2 levels are shown to change prior to lysosomal trafficking events, in PI(3,5)P2-deficient cells, many lysosomal functions are defective, including retrograde movement, exocytosis, and reformation (McCartney et al., 2014; Xu and Ren, 2015; Zolov et al., 2012). Hence, TRPMLs may serve as an essential signal transducer for lysosomal PI(3,5)P2. Consistently, mutations in the PI(3,5)P2 binding sites of TRPML1 (see Fig. 4) affect PI(3,5)P2-dependent lysosomal functions (Li et al., 2016b). However, other PI(3,5)P2 effectors may also contribute to the regulation of these lysosomal functions (McCartney et al., 2014).
Fig 4. Structural mechanisms of ligand-dependent activation of lysosomal TRPML and TPC channels.
A. High-resolution structures of TRPML1. a) The upper panel shows the top view of TRPML1 in the tetrameric assembly. Red and green boxes indicate the ML-SA1 and PI(3,5)P2 binding sites, respectively. The lower panels illustrate the ML-SA1 and PI(3,5)P2 binding sites in one single TRPML1 subunit, b) Carton illustrations of ligand-induced channel opening. B. High-resolution structures of TPC2. a) The upper panel shows the top view of TPC2 in the dimeric assembly. The PI(3,5)P2 binding sites are enclosed in the red box. The lower panel illustrates the PI(3,5)P2 binding sites in one single TPC2 subunit, b) Carton illustrations of PI(3,5)P2-induced channel opening.
Reactive oxygen species (ROS) are environmental stress signals that regulate a number of cellular functions, including autophagosome and lysosome biogenesis (Ravi et al., 2016; Zhang et al., 2016). ROS levels are elevated upon mitochondrial damage, and this triggers mitophagy to remove damaged mitochondria and excessive ROS, working as a negative feedback mechanism to maintain cellular health (Zhang et al., 2016) (Fig. 1). ROS induce nuclear translocation of TFEB, a transcriptional regulator of autophagosome and lysosome biogenesis (Settembre et al., 2011), in a TRPML1- and lysosomal Ca2+-dependent manner (Zhang et al., 2016). ROS directly and robustly activate lysosomal TRPML1 channels, suggesting that TRPML1 may function as a signal transducer for ROS to regulate lysosome function (Ravi et al., 2016; Zhang et al., 2016). Consistent with this hypothesis, ROS sensitivity of TRPML1 is shown to be required for ROS-induced TFEB activation and mitophagy (Zhang et al., 2016). Generally speaking, because TRPML1 is activated by more than one cellular cue, to test which activation mechanism is key to a specific function, it is necessary to introduce knock-in mutations at agonist-specific binding sites.
Endogenous inhibitors: mTOR, PI(4,5)P2, sphingomyelin, adenosine.
mTOR was recently shown to phosphorylate and inhibit TRPML1 (Li et al., 2016a; Onyenwoke et al., 2015; Sun et al., 2018). As TRPML1 is activated during amino acid starvation (Medina et al., 2015), it is possible that starvation-induced mTOR inhibition serves as a nutrient-derived signal to modulate TRPML1-mediated Ca2+ release. Two cell surface lipids reportedly inhibit TRPML1: PI(4,5)P2 and sphingomyelin, and this inhibition is proposed to prevent TRPML1 from being active in non-lysosomal compartments (Shen et al., 2012; Zhang et al., 2012). However, PI(4,5)P2 was recently reported to be generated on the lysosomal membrane to regulate mTOR-dependent lysosome reformation (Rong et al., 2012). As lysosome reformation is a Ca2+-dependent process (Li et al., 2016b), it is possible that PI(4,5)P2 inhibition of TRPML1 plays an important role in lysosome reformation. Lysosomal PI(4,5)P2 and sphingomyelin levels are aberrantly elevated in some LSD cells (De Leo et al., 2016; Shen et al., 2012). Likewise, in adenosine deaminase-deficient cells, luminal adenosine accumulation may inhibit TRPML1 to cause lysosomal dysfunction (Zhong et al., 2017). Hence, pathogenic TRPML1 inhibition may underlie the trafficking defects in many LSDs.
Other modulators: luminal acidic pH and cAMP/PKA.
Luminal pH modulates the channel activities of TRPML1 and TRPML3 (Miao et al., 2015) (see Table 1). Hence, cellular cues affecting lysosome acidification may regulate lysosome functions via TRPML-dependent mechanisms. In the uro-epithelial cells, pathogen invasion induces lysosome alkalization to trigger TRPML3- and Ca2+-dependent exosome release (Miao et al., 2015). cAMP signaling is known to regulate lysosome acidification and function, but the underlying mechanisms are not clear (Rahman et al., 2016). In parietal cells, PKA increases the activity of TRPML1 that is localized in the tubulovesicles (Sahoo et al., 2017; Vergarajauregui et al., 2008). Future studies may reveal whether cAMP/PKA signaling regulates lysosome function through TRPML1.
TRPML-specific synthetic agonists and inhibitors.
Small-molecule synthetic modulators have been used to probe TRPML-dependent lysosomal functions (Li et al., 2013b; Shen et al., 2012). ML-SA (synthetic agonist) compounds, by binding to a hydrophobic pocket above the S5-S6 gate (Fig. 4) (Schmiege et al., 2017), specifically activate whole-lysosomal TRPMLs, but not other lysosomal ion channels. Binding mutations were reported to selectively abolish ML-SA1 activation without interfering with PI(3,5)P2 activation (Schmiege et al., 2017). Hence, synthetic agonists may provide a powerful tool to acutely activate and inhibit TRPML1, linking channel activity with specific lysosomal functions. For examples, ML-SA activation of TRPML1 is sufficient to trigger lysosomal exocytosis, TFEB activation, and retrograde transport, and the effects were abolished in TRPML1 knockout (KO) cells (Li et al., 2016b; Xu and Ren, 2015; Zhang et al., 2016). Moreover, many lysosomal functions mediated by endogenous activation of TRPML1 are blocked by ML-SIs (synthetic inhibitors) (Li et al., 2016b; Zhang et al., 2016).
Two-pore TPC channels.
TPC1-2 are dimeric 12TM cation channels that are localized exclusively on endosomes and lysosomes, via di-leucine motifs (Calcraft et al., 2009; Xu and Ren, 2015). TPCs, ubiquitously expressed in mammalian tissues (Grimm et al., 2017; Guo et al., 2016; Xu and Ren, 2015) have been shown to be K+-impermeable Na+-selective cation channels in lysosomal electrophysiological analyses (Grimm et al., 2017; Wang et al., 2012). In cell studies, TPCs are believed to be lysosomal Ca2+-release channels (Grimm et al., 2017; Wang et al., 2012).
NAADP, PI(3,5)P2, and sphingosine.
TPCs were first suggested to mediate NAADP-dependent Ca2+ release from endolysosomes (Brailoiu et al., 2009; Calcraft et al., 2009). Small NAADP-activated Ca2+ currents were reported in TPC2-overexpressing cells in some, but not other studies (Grimm et al., 2017). In contrast, PI(3,5)P2 robustly activates TPC currents in all lysosomal electrophysiological studies (Cang et al., 2013; She et al., 2018; Wang et al., 2012). Hence, TPCs are unlikely to be the direct target of NAADP, as suggested by photo-labelling studies using TPC knockouts (Lin-Moshier et al., 2012). NAADP-mediated Ca2+ release and TPCs reportedly facilitate formation of endosome-ER membrane contact sites (Kilpatrick et al., 2017) and Ebola virus entry (Grimm et al., 2017; Kilpatrick et al., 2017; Sakurai et al., 2015). However, the highly potent inhibitors of the NAADP receptor, e.g., Ned-19, which are widely used in NAADP/TPC cell studies to draw firm conclusions, barely block whole-lysosomal TPC currents (Wang et al., 2012) (Zhang and Xu, manuscript in preparation). Sphingosine is also shown to trigger TPC1-dependent Ca2+ release and subsequent TFEB activation (Hoglinger et al., 2015). However, sphingosine failed to directly activate TPCs in the lysosomal recordings (Zhang and Xu, unpublished observation).
Several recent studies demonstrated that mammalian TPCs are PI(3,5)P2-activated Na+-selective channels (PNa/Pca > 10 and PNa/Pk > 60) (Guo et al., 2017; Wang et al., 2012). In contrast, plant TPC1 was found to be Ca2+-selective. Structure-guided mutational analyses have identified the determinants in the pore loop responsible for Ca2+ vs. Na+ selectivity (Guo et al., 2017). High-resolution structural studies have demonstrated that PI(3,5)P2 opens the channel gate by binding to positively-charged amino acid residues in the S4-S5 link and S6 (She et al., 2018) (Fig. 4). In the absence of PI(3,5)P2, the background Na+-permeability is minimal and lysosomal Δψ is independent of TPCs (Zhang and Xu, unpublished observation); in the presence of PI(3,5)P2, lysosomal Δψ is depolarized via TPCs (Wang et al., 2017) (Fig. 3). Hence, TPCs are essential signal transducers for PI(3,5)P2 to regulate lysosomal Δψ (Cang et al., 2013).
ATP, mTOR,pH, and voltage.
TPCs are also modulated by several cytosolic and luminal factors. TPC2 is modulated by intracellular ATP through mTORC1 kinase (Cang et al., 2013). In pulmonary arterial smooth muscle cells, pharmacological inhibition of mTOR leads to TPC-dependent Ca2+ release (Ogunbayo et al., 2018). Hence, TPC2 may couple the metabolic status of the cell with lysosome function. TPC KO cells exhibit defects in autophagy regulation, lipid metabolism, Ebola virus infection, lysosome pH stability, and amino acid export (Grimm et al., 2017; Patel and Kilpatrick, 2018). It is not clear whether these TPC functions are mediated by mTOR inhibition or PI(3,5)P2 activation. It was presumed that mTOR inhibition plays an important role during amino acid starvation (Cang et al., 2013), however, PI(3,5)P2 levels are known to decrease dramatically upon amino acid starvation (McCartney et al., 2014; Zolov et al., 2012). Therefore, separating these effects may require knock-in mutations that selectively abolish PI(3,5)P2 activation or mTOR inhibition.
Lysosome pH and voltage may specifically regulate TPC1 but not TPC2 (Cang et al., 2013). The voltage-dependence of TPC1 is mediated by arginine residues in the S4 of the second repeat (Patel et al., 2017; She et al., 2018). The role of such regulation is unclear as TPC1 and TPC2 are believed to play similar or redundant roles in most TPC-dependent lysosomal functions (Grimm et al., 2017). It was recently reported that NAADP activation of TPC1 required arginine residues present only in TPC1 but not TPC2 (Patel et al., 2017). However, NAADP is believed by many investigators to activate both TPC1 and TPC2 (Grimm et al., 2017).
P2X4.
P2X4 is a trimeric 2TM channel that is permeable to both Na+ and Ca2+ when activated by ATP (Huang et al., 2014; Kawate et al., 2009). P2X4 is dually localized on both lysosomal and plasma membranes, and the lysosomal targeting requires both di-leucine and tyrosine motifs (Huang et al., 2014; Qureshi et al., 2007). Lysosomal P2X4 is activated by luminal ATP, and inhibited by acidic lysosomal pH (Clarke et al., 2000; Huang et al., 2014). Activation of P2X4-dependent Ca2+ release may drive homotypic lysosome fusion in a calmodulin-dependent manner (Cao et al., 2015a).
Lysosome K+ channels
Consistent with the presence of large K+ concentration gradient across the lysosomal membranes, there exist multiple K+-selective channels in the lysosome. Also consistently, K+ ionophores and change of cytosolic K+ are known to affect lysosomal Δψ (Cang et al., 2013; Wang et al., 2017).
TMEM175.
Human TMEM175 are dimeric 12 TM K+- and Cs+-selective channels that are expressed in both early endosomes and LELs (Cang et al., 2015) (Table 1). Unlike canonic K+ channels that use the GYG motif as the selectivity filter, TMEM175 harbors a FSD motif on both TM1 and TM7 to achieve a high K+ over Na+ selectivity (Lee et al., 2017). Whereas overexpression of TMEM175 decreased lysosomal Δψ, knockout of TMEM175 in macrophages slightly increased lysosomal Δψ (Cang et al., 2015). These results are consistent with the early observations showing that lysosomes isolated from the liver cells are permeable to both Cs+ and K+ (Casey et al., 1978). Hence, TMEM175 may mediate a constitutively-active background K+ conductance in the lysosome (Cang et al., 2015) (Fig. 3). However, TMEM175 currents are rather small and indeed non-detectable in most isolated lysosomes (Wang et al., 2017), suggesting that TMEM175 might be further potentiated by certain cellular cues to reach more negative Δψ under physiological conditions. One potential candidate is luminal pH, and pH regulation of TMEM175 is required for maintaining lysosomal pH homeostasis during starvation (Cang et al., 2015) (Table 1). TMEM175 may also function as a negative regulator of autophagosome-lysosome fusion during starvation (Cang et al., 2015; Jinn et al., 2017). Hence, TMEM175 may be required for nutrient- and pH-dependent regulation of lysosome Δψ.
BK/LysoKVCa.
BK channels are tetrameric big-conductance Ca2+-activated Kv channels expressed at the plasma membrane of excitable cells as a negative regulator of membrane excitability. Two recent studies suggest that BK-like currents are also present in the lysosomes of both excitable and non-excitable cells (lysosome voltage-dependent Ca2+-activated K+ channels; LysoKVCa) (Cao et al., 2015b; Wang et al., 2017). Upon peri-lysosomal Ca2+ increase, lysosomal Δψ changes rapidly in a LysoKVCa dependent-manner (Cao et al., 2015b; Wang et al., 2017). Given the large conductance, opening of just one single LysoKVCa channel is sufficient to produce significant changes of lysosomal Δψ (Wang et al., 2017). A negative Δψ may increase the driving force for lysosomal Ca2+ release (Cao et al., 2015b; Wang et al., 2017) (Fig. 3), allowing LysoKVCa to regulate Ca2+-dependent lysosomal functions. In addition, LysoKVCa and its Ca2+ sensitivity are required for refilling of lysosomal Ca2+ stores (Wang et al., 2017; Yang et al., 2018). Hence, LysoKVCa is a positive regulator of lysosomal Ca2+ signaling. As ER-lysosome membrane contact sites may be required for Ca2+ refilling, it is possible that BK/LysoKVCa regulate ER-lysosome membrane contact site formation through a Δψ-dependent mechanism (Yang et al., 2018).
Lysosomal ion channels in lysosomal diseases
Lysosome Storage Disorders.
Mutations of TRPML1 cause ML-IV, and ML-IV patients cannot walk or talk (Boudewyn and Walkley, 2018). Due to the lysosomal trafficking defects, undigested biomolecules and lipofuscin accumulate in ML-IV lysosomes (Boudewyn and Walkley, 2018). LSDs may also be caused by human mutations that affect the production of cellular cues that activate or inhibit lysosomal channels (Dong et al., 2010). Mutations in NPC1 cause Niemann-Pick type C disease (NPC), a neurodegenerative LSD with cholesterol/sphingolipid accumulation (Castellano et al., 2017). Excessive sphingomyelin accumulation in the lysosome inhibits TRPML1 (Shen et al., 2012). Likewise, mutations in PIKfyve, the PI(3,5)P2-synthesizing enzyme, and OCRL, a PI(4,5)P2 phosphatase, also cause LSD-like symptoms (De Leo et al., 2016; Dong et al., 2010). In all these LSD cells, reduced lysosomal TRPML1 activity may underlie or contribute to trafficking defects and lysosome dysfunction.
Common neurodegenerative diseases.
Alzheimer’s (AD) and Parkinson’s (PD) are common neurodegenerative diseases, in which lysosome insufficiency is believed to be pathogenic (Lie and Nixon, 2018). Whereas impaired TRPML1 signaling may contribute to AD (Bae et al., 2014; Lee et al., 2015), genome-wide association studies (GWAS) have identified a link between TMEM175 and PD (Chang et al., 2017). TMEM175-deficient neurons are susceptible to α-synuclein aggregation (Jinn et al., 2017). A major cause to inherited and sporadic PD is the gain-of-function mutations in LRRK2 kinase (Hockey et al., 2015; Rivero-Rios et al., 2016), and LRRK2-dependent lysosomal defects are ameliorated by TPC2 inhibition (Hockey et al., 2015). Hence lysosomal channels may have a general role in lysosome-related diseases.
Small molecule lysosomal channel modulators in potential treatment of lysosomal diseases.
Synthetic modulators may mimic the endogenous agonists to regulate lysosomal functions. Small molecule TRPML1 agonists were sufficient to restore lysosome function in ML-IV patient fibroblasts carrying partial loss-of-function mutations (Grimm et al., 2017). In most LSDs, hydrolase deficiency causes primary lysosomal storage, which in turn affects lysosome function to cause secondary storage, resulting in a vicious cycle (Xu and Ren, 2015). As TRPML1 is a major regulator of lysosomal trafficking (Shen et al., 2012), activation of TRPML1 may facilitate lysosomal trafficking to promote cellular clearance and reduce lysosomal storage in many LSDs. Indeed, TRPML1 overexpression and ML-SAs can promote cholesterol clearance in NPC cells (Shen et al., 2012; Zhong et al., 2016; Zou et al., 2015). Likewise, BK agonists were reported to decrease cellular storage in a number of LSD fibroblasts (Zhong et al., 2016). Given the similarity between LSDs and common neurodegenerative diseases (Lie and Nixon, 2018), lysosomal channel modulators may promote cellular clearance in lysosome-related diseases in general.
Future directions
We had witnessed the discovery of many lysosomal ion channels, along with the cellular cues that regulate them. However, the molecular determinants for several lysosomal conductances have yet to be established. For instance, an unidentified H+ “leak” channel seems to be regulated by nutrient-sensitive cellular cues to affect lysosomal pH (Cang et al., 2014; Cang et al., 2013). Likewise, there exist several unidentified Cl− conductances in the lysosome (unpublished observations in the Xu lab), which are not mediated by the known lysosomal CLC transporters (Jentsch and Pusch, 2018). While lysosomal degradation is known to generate large amount of osmolytes, whether there exist water channels and osmo-sensitive channels is unclear. In order to study how cellular cues regulate lysosomal ion flux and Δψ changes in intact cells, it is necessary to develop lysosome-targeted voltage sensors and pH-insensitive luminal Ca2+/Na+/K+ sensors. Finally, super-resolution live imaging of lysosomes may help reveal how lysosomal Ca2+ release and Δψ changes are correlated with lysosomal membrane dynamics and membrane contact site formation.
Highlights:
Lysosomes generate nutrient-sensitive signals to regulate both the import of H+, hydrolases, and endocytic and autophagic cargos and the export of the degradation products.
In response to environmental and cellular signals, lysosomes change their positioning, number, morphology, size, composition, and activity within minutes to hours to meet the changing cellular needs.
There exist Na+, K+, and Ca2+-selective channels in the lysosome, to maintain the concentration gradients of H+, Na+, K+, and Ca2+ across lysosomal membrane.
Ion channels in the lysosome are essential transducers of cellular signals and are required for cellular homeostasis.
Signal-initiated lysosomal Ca2+ release and membrane potential changes regulate lysosome movement, membrane trafficking, nutrient-sensing, membrane repair, organelle membrane contact, and lysosome biogenesis.
Activation and inhibition of lysosomal channels may tune lysosome function to promote cellular clearance in lysosome storage disorders.
Outstanding Questions Box:
What are the molecular determinants for several lysosomal conductances? For instance, an unidentified H+ “leak” channel is regulated by nutrient-sensitive cellular cues to affect lysosomal pH. Likewise, there exist several unidentified Cl− conductances in the lysosome, which are not mediated by the known lysosomal CLC transporters.
How are lysosomal Na+, and K+, and Ca2+ channels activated by endogenous cellular cues and nutrient signals?
While lysosomal degradation is known to generate large amount of osmolytes, whether there exist water channels and osmo-sensitive channels is unclear.
In order to study how cellular cues regulate lysosomal ion flux and membrane potential changes in intact cells, it is necessary to develop lysosome-targeted voltage sensors and pH-insensitive luminal Ca2+/Na+/K+ sensors.
Super-resolution live imaging of lysosomes may help reveal how lysosomal Ca2+ release and membrane potential changes are correlated with lysosomal membrane dynamics and lysosome-organelle membrane contact site formation.
Acknowledgements
We apologize to colleagues whose works are not cited due to space limitations. The works in the authors’ laboratories are supported by NIH grants (NS062792, AR060837, and DK115474 to H.X). We appreciate the encouragement and helpful comments of other members of the Xu laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bae M, Patel N, Xu H, Lee M, Tominaga-Yamanaka K, Nath A, Geiger J, Gorospe M, Mattson MP, and Haughey NJ (2014). Activation of TRPML1 clears intraneuronal Abeta in preclinical models of HIV infection. J Neurosci 34, 11485–11503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Schweitzer LD, Zoncu R, and Sabatini DM (2012). Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewyn LC, and Walkley SU (2018). Current concepts in the neuropathogenesis of mucolipidosis type IV. J Neurochem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, et al. (2009). Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol 186, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright NA, Davis LJ, and Luzio JP (2016). Endolysosomes Are the Principal Intracellular Sites of Acid Hydrolase Activity. Curr Biol 26, 2233–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. (2009). NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Aranda K, Seo YJ, Gasnier B, and Ren D. (2015). TMEM175 Is an Organelle K(+) Channel Regulating Lysosomal Function. Cell 162, 1101–1112. [DOI] [PubMed] [Google Scholar]
- Cang C, Bekele B, and Ren D. (2014). The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat Chem Biol 10, 463–469. [DOI] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, and Ren D. (2013). mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 152, 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhong XZ, Zou Y, Murrell-Lagnado R, Zhu MX, and Dong XP (2015a). Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J Cell Biol 209, 879–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhong XZ, Zou Y, Zhang Z, Toro L, and Dong XP (2015b). BK Channels Alleviate Lysosomal Storage Diseases by Providing Positive Feedback Regulation of Lysosomal Ca2+ Release. Dev Cell 33, 427–441. [DOI] [PubMed] [Google Scholar]
- Casey RP, Hollemans M, and Tager JM (1978). The permeability of the lysosomal membrane to small ions. Biochim Biophys Acta 508, 15–26. [DOI] [PubMed] [Google Scholar]
- Castellano BM, Thelen AM, Moldavski O, Feltes M, van der Welle RE, Mydock-McGrane L, Jiang X, van Eijkeren RJ, Davis OB, Louie SM, et al. (2017). Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 355, 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Leung K, and Krishnan Y. (2017). High lumenal chloride in the lysosome is critical for lysosome function. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, International Parkinson's Disease Genomics C, and Me Research T, Kerchner GA, Ayalon G, et al. (2017). A metaanalysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet 49, 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, She J, Zeng W, Guo J, Xu H, Bai XC, and Jiang Y. (2017). Structure of mammalian endolysosomal TRPML1 channel in nanodiscs. Nature 550, 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Shen D, Samie M, and Xu H. (2010). Mucolipins: Intracellular TRPML1-3 channels. FEBS Lett 584, 2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, and Swanson JA (2002). pH-dependent regulation of lysosomal calcium in macrophages. J Cell Sci 115, 599–607. [DOI] [PubMed] [Google Scholar]
- Chu BB, Liao YC, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL, and Song BL (2015). Cholesterol transport through lysosome-peroxisome membrane contacts. Cell 161, 291–306. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Benham CD, Bridges A, George AR, and Meadows HJ (2000). Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J Physiol 523 Pt 3, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo MG, Staiano L, Vicinanza M, Luciani A, Carissimo A, Mutarelli M, Di Campli A, Polishchuk E, Di Tullio G, Morra V, et al. (2016). Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nat Cell Biol 18, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, and Xu H. (2008). The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 455, 992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, et al. (2010). PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat Commun 1, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du W, Su QP, Chen Y, Zhu Y, Jiang D, Rong Y, Zhang S, Zhang Y, Ren H, Zhang C, et al. (2016). Kinesin 1 Drives Autolysosome Tubulation. Dev Cell 37, 326–336. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, and Xu H. (2016). The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatica D, Lahiri V, and Klionsky DJ (2018). Cargo recognition and degradation by selective autophagy. Nat Cell Biol 20, 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves AR, Curran PK, Smith CL, and Mindell JA (2008). The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453, 788–792. [DOI] [PubMed] [Google Scholar]
- Grimm C, Butz E, Chen CC, Wahl-Schott C, and Biel M. (2017). From mucolipidosis type IV to Ebola: TRPML and two-pore channels at the crossroads of endo-lysosomal trafficking and disease. Cell Calcium 67, 148–155. [DOI] [PubMed] [Google Scholar]
- Guo J, Zeng W, Chen Q, Lee C, Chen L, Yang Y, Cang C, Ren D, and Jiang Y. (2016). Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature 531, 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zeng W, and Jiang Y. (2017). Tuning the ion selectivity of two-pore channels. Proc Natl Acad Sci U S A 114, 1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi M, Herzik MA Jr., Wie J, Suo Y, Borschel WF, Ren D, Lander GC, and Lee SY (2017). Cryo-electron microscopy structure of the lysosomal calcium-permeable channel TRPML3. Nature 550, 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, and Patel S. (2015). Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci 128, 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger D, Haberkant P, Aguilera-Romero A, Riezman H, Porter FD, Platt FM, Galione A, and Schultz C. (2015). Intracellular sphingosine releases calcium from lysosomes. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, Murrell-Lagnado R, and Dong XP (2014). P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J Biol Chem 289, 17658–17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, and Helenius A. (2011). Endosome maturation. Embo J 30, 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, and Pusch M. (2018). CLC Chloride Channels and Transporters: Structure, Function, Physiology, and Disease. Physiol Rev 98, 1493–1590. [DOI] [PubMed] [Google Scholar]
- Jinn S, Drolet RE, Cramer PE, Wong AH, Toolan DM, Gretzula CA, Voleti B, Vassileva G, Disa J, Tadin-Strapps M, et al. (2017). TMEM175 deficiency impairs lysosomal and mitochondrial function and increases alpha-synuclein aggregation. Proc Natl Acad Sci U S A 114, 2389–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Ostrowski P, Jaumouille V, and Grinstein S. (2016). The position of lysosomes within the cell determines their luminal pH. J Cell Biol 212, 677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, and Gouaux E. (2009). Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460, 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Hockey LN, Yates E, Futter CE, and Patel S. (2017). An Endosomal NAADP-Sensitive Two-Pore Ca(2+) Channel Regulates ER-Endosome Membrane Contact Sites to Control Growth Factor Signaling. Cell Rep 18, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Schapira AH, Futter CE, and Patel S. (2013). Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J Cell Sci 126, 60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M, Steinberg BE, Mason D, and Grinstein S. (2011). In situ measurement of the electrical potential across the lysosomal membrane using FRET. Traffic 12, 972–982. [DOI] [PubMed] [Google Scholar]
- Kolter T, and Sandhoff K. (2005). Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annual review of cell and developmental biology 21, 81–103. [DOI] [PubMed] [Google Scholar]
- Lee C, Guo J, Zeng W, Kim S, She J, Cang C, Ren D, and Jiang Y. (2017). The lysosomal potassium channel TMEM175 adopts a novel tetrameric architecture. Nature 547, 472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, et al. (2015). Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep 12, 1430–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RJ, Xu J, Fu C, Zhang J, Zheng YG, Jia H, and Liu JO (2016a). Regulation of mTORC1 by lysosomal calcium and calmodulin. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Garrity AG, and Xu H. (2013a). Regulation of membrane trafficking by signalling on endosomal and lysosomal membranes. J Physiol 591, 4389–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, and Xu H. (2016b). A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol 18, 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang X, Zhang X, Zhao M, Tsang WL, Zhang Y, Yau RG, Weisman LS, and Xu H. (2013b). Genetically encoded fluorescent probe to visualize intracellular phosphatidylinositol 3,5-bisphosphate localization and dynamics. Proc Natl Acad Sci U S A 110, 21165–21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, and Nixon RA (2018). Lysosome trafficking and signaling in health and neurodegenerative diseases. Neurobiol Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, and Marchant JS (2012). Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J Biol Chem 287, 2296–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, and Bright NA (2007). Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8, 622–632. [DOI] [PubMed] [Google Scholar]
- McCartney AJ, Zhang Y, and Weisman LS (2014). Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays 36, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, et al. (2015). Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 17, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, et al. (2011). Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev Cell 21, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Li G, Zhang X, Xu H, and Abraham SN (2015). A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell 161, 1306–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA (2012). Lysosomal acidification mechanisms. Annu Rev Physiol 74, 69–86. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Platt FM, Lloyd-Evans E, and Galione A. (2011). Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem J 439, 349–374. [DOI] [PubMed] [Google Scholar]
- Ogunbayo OA, Duan J, Xiong J, Wang Q, Feng X, Ma J, Zhu MX, and Evans AM (2018). mTORC1 controls lysosomal Ca(2+) release through the two-pore channel TPC2. Sci Signal 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyenwoke RU, Sexton JZ, Yan F, Diaz MC, Forsberg LJ, Major MB, and Brenman JE (2015). The mucolipidosis IV Ca2+ channel TRPML1 (MCOLN1) is regulated by the TOR kinase. Biochem J 470, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Churamani D, and Brailoiu E. (2017). NAADP-evoked Ca(2+) signals through two-pore channel-1 require arginine residues in the first S4-S5 linker. Cell Calcium 68, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, and Kilpatrick BS (2018). Two-pore channels and disease. Biochim Biophys Acta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RM, and Zoncu R. (2016). The Lysosome as a Regulatory Hub. Annual review of cell and developmental biology 32, 223–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MJ, and Voeltz GK (2016). Structure and function of ER membrane contact sites with other organelles. Nat Rev Mol Cell Biol 17, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Paramasivam A, Yu JC, and Murrell-Lagnado RD (2007). Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci 120, 3838–3849. [DOI] [PubMed] [Google Scholar]
- Rahman N, Ramos-Espiritu L, Milner TA, Buck J, and Levin LR (2016). Soluble adenylyl cyclase is essential for proper lysosomal acidification. J Gen Physiol 148, 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi S, Pena KA, Chu CT, and Kiselyov K. (2016). Biphasic regulation of lysosomal exocytosis by oxidative stress. Cell Calcium 60, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Rios P, Fernandez B, Madero-Perez J, Lozano MR, and Hilfiker S. (2016). Two-Pore Channels and Parkinson's Disease: Where's the Link? Messenger (Los Angel) 5, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Liu M, Ma L, Du W, Zhang H, Tian Y, Cao Z, Li Y, Ren H, Zhang C, et al. (2012). Clathrin and phosphatidylinositol-4,5-bisphosphate regulate autophagic lysosome reformation. Nat Cell Biol 14, 924–934. [DOI] [PubMed] [Google Scholar]
- Rowland AA, Chitwood PJ, Phillips MJ, and Voeltz GK (2014). ER contact sites define the position and timing of endosome fission. Cell 159, 1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo N, Gu M, Zhang X, Raval N, Yang J, Bekier M, Calvo R, Patnaik S, Wang W, King G, et al. (2017). Gastric Acid Secretion from Parietal Cells Is Mediated by a Ca(2+) Efflux Channel in the Tubulovesicle. Dev Cell 41, 262–273 e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Kolokoltsov AA, Chen CC, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, and Davey RA (2015). Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science 347, 995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiege P, Fine M, Blobel G, and Li X. (2017). Human TRPML1 channel structures in open and closed conformations. Nature 550, 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. (2011). TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, and Ballabio A. (2013). Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol 14, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J, Guo J, Chen Q, Zeng W, Jiang Y, and Bai XC (2018). Structural insights into the voltage and phospholipid activation of the mammalian TPC1 channel. Nature 556, 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, et al. (2012). Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun 3, 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra ML, Schlesinger PH, Naismith TV, and Hanson PI (2018). Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber T, and Jentsch TJ (2013). Chloride in vesicular trafficking and function. Annu Rev Physiol 75, 453–477. [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Huynh KK, Brodovitch A, Jabs S, Stauber T, Jentsch TJ, and Grinstein S. (2010). A cation counterflux supports lysosomal acidification. J Cell Biol 189, 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Yang Y, Zhong XZ, Cao Q, Zhu XH, Zhu X, and Dong XP (2018). A negative feedback regulation of MTORC1 activity by the lysosomal Ca(2+) channel MCOLN1 (mucolipin 1) using a CALM (calmodulin)-dependent mechanism. Autophagy 14, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kant R, and Neefjes J. (2014). Small regulators, major consequences - Ca(2)(+) and cholesterol at the endosome-ER interface. J Cell Sci 127, 929–938. [DOI] [PubMed] [Google Scholar]
- van der Kant R, Zondervan I, Janssen L, and Neefjes J. (2013). Cholesterol-binding molecules MLN64 and ORP1L mark distinct late endosomes with transporters ABCA3 and NPC1. J Lipid Res 54, 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergarajauregui S, Oberdick R, Kiselyov K, and Puertollano R. (2008). Mucolipin 1 channel activity is regulated by protein kinase A-mediated phosphorylation. Biochem J 410, 417–425. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang X, Gao Q, Lawas M, Yu L, Cheng X, Gu M, Sahoo N, Li X, Li P, et al. (2017). A voltage-dependent K(+) channel in the lysosome is required for refilling lysosomal Ca(2+) stores. J Cell Biol 216, 1715–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al. (2012). TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 151, 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyant GA, Abu-Remaileh M, Wolfson RL, Chen WW, Freinkman E, Danai LV, Vander Heiden MG, and Sabatini DM (2017). mTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 171, 642–654 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, and Zhu MX (2016). Regulation of lysosomal ion homeostasis by channels and transporters. Sci China Life Sci 59, 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, and Ren D. (2015). Lysosomal physiology. Annu Rev Physiol 77, 57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhao Z, Gu M, Feng X, and Xu H. (2018). Release and uptake mechanisms of vesicular Ca(2+) stores. Protein Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CS, Hawley SA, Zong Y, Li M, Wang Z, Gray A, Ma T, Cui J, Feng JW, Zhu M, et al. (2017). Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548, 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, et al. (2016). MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun 7, 12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li X, and Xu H. (2012). Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc Natl Acad Sci U S A 109, 11384–11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, and Ridgway ND (2017). Oxysterol-Binding Protein-Related Protein 1L Regulates Cholesterol Egress from the Endo-Lysosomal System. Cell Rep 19, 1807–1818. [DOI] [PubMed] [Google Scholar]
- Zhong XZ, Sun X, Cao Q, Dong G, Schiffmann R, and Dong XP (2016). BK channel agonist represents a potential therapeutic approach for lysosomal storage diseases. Scientific reports 6, 33684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Zou Y, Sun X, Dong G, Cao Q, Pandey A, Rainey JK, Zhu X, and Dong XP (2017). Inhibition of Transient Receptor Potential Channel Mucolipin-1 (TRPML1) by Lysosomal Adenosine Involved in Severe Combined Immunodeficiency Diseases. J Biol Chem 292, 3445–3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, and Shen HM (2013). Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res 23, 508–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolov SN, Bridges D, Zhang Y, Lee WW, Riehle E, Verma R, Lenk GM, Converso-Baran K, Weide T, Albin RL, et al. (2012). In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci U S A 109, 17472–17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, and Sabatini DM (2011a). mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334, 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, and Sabatini DM (2011b). mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Hu B, Arpag S, Yan Q, Hamilton A, Zeng YS, Vanoye CG, and Li J. (2015). Reactivation of Lysosomal Ca2+ Efflux Rescues Abnormal Lysosomal Storage in FIG4-Deficient Cells. J Neurosci 35, 6801–6812. [DOI] [PMC free article] [PubMed] [Google Scholar]