Abstract
Background:
Multidrug resistance (MDR) transporter proteins such as P-glycoprotein (P-gp) efflux a variety of chemotherapeutic drugs from acute myeloid leukemia (AML) blasts leading to clinical drug resistance.
Methods:
This study examined heterogeneity of MDR functional efflux by AML blasts using two flow cytometry bioassays. Bone marrow specimens (N = 50) from elderly patients with newly diagnosed AML were analyzed for CD34+ blasts with MDR efflux function. Efflux was measured with a fluorescent dye (DiOC2) as a surrogate for oncology drugs that are substrates for MDR efflux. P-gp-mediated efflux was differentiated from non-P-gp MDR activities using zosuquidar, a highly selective P-gp modulator. The bioassays included a zosuquidar-dependent DiOC2 accumulation bioassay that measured only P-gp. The second method, termed the efflux bioassay, could detect P-gp and other non-P-gp efflux depending on bioassay culture conditions.
Results:
62% of the specimens were considered positive for blasts with P-gp function, and 26% of such P-gp-positive specimens also exhibited zosuquidar-resistant (i.e., non-P-gp) MDR efflux activity. 37% of P-gp-negative AML blast specimens displayed zosuquidar-resistant MDR function in the efflux bioassay.
Conclusions:
These results confirm the heterogeneous nature of MDR efflux pumps in AML blasts, and provide support for the hypothesis that non-P-gp MDR contributed to negative results with zosuquidar in AML trials like ECOG-ACRIN E3999.
Keywords: multidrug resistance, drug transporters, acute myeloid leukemia
Introduction
Multidrug resistance (MDR) efflux pumps capable of eliminating chemotherapeutic drugs from tumor cells contribute to clinical drug resistance in acute myeloid leukemia (AML) and other cancers (1–6). Expression of MDR in acute myeloid leukemia (AML) blasts is well known for redundancy with multiple molecular species capable of actively eliminating drugs from the neoplastic cells (4–6). Heterogeneity of MDR mechanisms has been hypothesized as a significant factor in the failed clinical studies for zosuquidar, a P-glycoprotein (P-gp) -specific MDR modulator (1, 2).
Much of MDR activity is mediated by transmembrane proteins belonging to the superfamily of ATP-binding cassette (ABC) transporters (1,6). Predominant among these transporters is P-gp, encoded by the ABCB1/MDR1 gene. Expression of P-gp by AML blasts is highly related to resistance to multiple chemotherapeutic agents (5, 7–11), including anthracyclines such as daunorubicin. In addition to P-gp, other MDR mediators include the multidrug resistance proteins 1 and 2 (MRP1/ABCC1; MRP-2/ABCC2), and breast cancer resistance protein (BCRP/ABCG2) (1, 2, 6).
A key strategy to overcome P-gp MDR in AML and other cancers has been the development of small molecule, P-gp inhibitory drugs (1–4, 6). Early generations of P-gp modulators acted on multiple efflux pumps, altered the pharmacokinetic (PK) profiles of concomitantly administered chemotherapy, and exacerbated chemotherapy toxicities (12–17). Dose reductions of the chemotherapy were therefore needed to minimize toxicities, which compromised efficacy. Nevertheless, there were some positive results with these early P-gp modulators in AML clinical studies, most notably with quinine and cyclosporine A (CsA), relatively broad-spectrum MDR modulators with activities vs. P-gp, MRP1, BCRP, and other transporters (1–3, 12–17).
Subsequent generations of P-gp modulators were developed to displayed greater specificity for P-gp, less reactivity vs. other efflux pumps, and to minimally alter PK of concomitant chemotherapy (1, 2). Zosuquidar, a third generation P-gp modulator, fits the desired characteristics of P-gp specificity and minimal PK alterations of concomitant chemotherapy (18–27). However, results from a pivotal zosuquidar clinical trial (E3999) in elderly AML conducted by the ECOG-ACRIN Cancer Research Group were negative (27). Elderly patients with AML were treated in study E3999 with daunorubicin (P-gp substrate) and cytarabine, with and without zosuquidar. P-gp was identified as an independent adverse prognostic factor for overall survival, but zosuquidar failed to provide improve survival vs. placebo in patients with P-gp-positive blasts. Non-P-gp drug resistance mechanisms were speculated as significant contributors for the lack of efficacy of zosuquidar in E3999 (1, 2, 27).
An important patient stratification variable in clinical trials of P-gp modulation has been the identification and relative quantification of blast expression and function of P-gp and other MDR mediators. Flow cytometry is an efficient technique for measuring MDR functional activities (21, 26, 27, 29–35), whereby leukemic blasts can be shown to efflux or eliminate fluorescent substrate drugs (e.g., daunorubicin) or dye surrogates like rhodamine-123 (Rh123) and 3,3’-diethyloxacarbocyanine iodide (DiOC2). There are two basic functional bioassay formats using these methods, the efflux and the accumulation bioassays.
The efflux bioassay involves loading of cells with a fluorescent MDR substrate drug or dye, and incubation of the cells at 37°C to allow for dye efflux (29, 30, 32). Controls include incubation of cells at 4°C to define total dye loading, and incubation at 37°C with and without an efflux inhibitor (e.g., zosuquidar, CsA). The efflux bioassay can detect multiple MDR pump activities depending on the incubation conditions and specificities of the modulator and substrate drug or dye. The efflux bioassay was used in the present study to measure P-gp and non-P-gp efflux activities in AML blasts.
The accumulation bioassay involves culturing cells at 37°C with fluorescent dye alone or with dye plus a MDR modulator like P-gp-specific zosuquidar (21, 26, 31). In this case, P-gp-positive cells accumulate little of the substrate dye when cultured without zosuquidar because of continual P-gp efflux activity. P-gp-positive cells as cultured with dye plus P-gp modulator accumulate substantially more dye because efflux activity would be inhibited. Cells lacking P-gp accumulate the same level of dye regardless if P-gp modulator is present or not. The accumulation bioassay is therefore specific to the modulator, since only cells expressing the specific efflux pump will demonstrate a positive response with enhanced dye or drug accumulation. The accumulation assay was used in the presence study to unequivocally differentiate P-gp-positive from P-gp-negative leukemic blast efflux activities.
Described herein is an analysis of leukemic blast MDR function with bone marrow aspirates from 50 patients with newly diagnosed AML. Comparative CD34+ blast responses were examined using the accumulation and efflux bioassays with zosuquidar to differentiate P-gp from non-P-gp efflux activities. This cellular analysis clearly identifies MDR heterogeneity in the leukemic cell populations, including blasts that are 1) MDR-negative by DiOC2 efflux or accumulation bioassays, 2) only P-gp-positive given the constraints of the bioassays, and 3) obviously positive for at least one other MDR efflux pump in blasts expressing or lacking appreciable P-gp.
Methods
AML Patient Specimens
Bone marrow aspirates were collected from 50 elderly AML patients prior to enrollment in zosuquidar Phase I/II and II clinical trials: (26, 27). The patient samples were from 2 independent cohorts in studies of newly diagnosed primary and secondary AML: Study E3999 and a separate Phase I/II trial sponsored by Kanisa Pharmaceuticals (KAN-979). All patients were enrolled with written informed consent and IRB approval in accordance with the Declaration of Helsinki. Eligible patients for cohort 1 (Phase I/II) have been previously described (26) and were 55 to 75 years of age. Eligible patients for cohort 2 (Phase II) have been previously described as well (27) and were >60 years of age. All other eligibility criteria were similar between cohorts, such as ECOG performance status, acceptable levels of serum metabolites (e.g., bilirubin, creatinine), and a resting left ventricular cardiac ejection fraction of greater than 45%.
Selection of the 50 patient specimens presented for this study differed by cohort. For cohort 1, both bioassays were conducted on all samples from the first 18 screened patients with sufficient cell numbers in the aspirate to do both bioassays. Seventy-two percent (13/18) of cohort 1 samples were considered P-gp-positive by the DiOC2 accumulation bioassay, P-gp ratio ≥1.30. The 32 specimens from cohort 2 were selected based on availability (i.e., cryopreserved vial counts >4) and previously determined P-gp functional activity using the Rh123 efflux assay (27). Cohort 2 specimens included 18 specimens considered P-gp-positive with the DiOC2 accumulation bioassay (18/32, 56%). Collectively, 31 of 50 (62%) specimens/patients were considered to have P-gp-positive blasts.
Miscellaneous reagents
DiOC2 was purchased from Invitrogen (Carlsbad, CA). Fluorochrome-labeled anti-CD antibodies were purchased from BD Biosciences (La Jolla, CA), including anti-CD45-PerCP and -APC-Cy7, anti-CD3-APC and -PerCE-Cy5.5, anti-CD34-APC, anti-CD11b-PE and -PE-Cy7, and anti-CD56-PE. Phosphate-buffered saline (PBS), and RPMI 1640 supplemented with HEPES were purchased from BioWhittaker Molecular Applications (Rockland, ME). Zosuquidar was prepared by Formatech (Andover, MA) under contract with Kanisa Pharmaceuticals (San Diego, CA).
Functional Bioassays with AML Patient Specimens
White cells were enriched from bone marrow aspirates by elimination of red blood cells (RBC) using either ammonium chloride RBC lysis (cohort 1) or Ficoll-Paque step-gradient purification (cohort 2) (26, 27, 31). Development experiments established that comparable P-gp responses are observed with peripheral blood natural killer (NK) cells enriched by RBC lysis or gradient purification, as discussed (31). The white cells from cohort 1 specimens were used fresh, while those from cohort 2 had been cryopreserved. The cells were washed with serum-free RPMI-1640 and resuspended to 107 cells /mL in RPMI-1640. An aliquot of the cells was treated with saturating concentrations of PerCP-labeled anti-CD45 and APC-conjugated anti-CD34 (20–30 minutes, room temp., in the dark). The cells were subsequently washed, resuspended to 107 cells /mL in RPMI-1640, and aliquoted into 2 15-mL conical centrifuge tubes for further processing for the accumulation (0.10 mL/tube, 1.0 × 106 cells) and efflux bioassays (0.15 mL/tube, 1.5 × 106 cells). Development work established that saturation of P-gp modulation with zosuquidar occurs at approximately 1–10 ng/mL in serum-free cultures of P-gp-positive normal NK cells and K562/R7 leukemia cell line (26,31), as documented by others (21). Both the accumulation and efflux bioassays used 100 ng/mL final zosuquidar concentration for P-gp-specific modulation as described below.
The Efflux Bioassay.
The cells for the efflux assay were further diluted to 0.4 mL with RPMI-1640 before addition of 0.1 mL RPMI-1640 containing 500 ng/mL DiOC2 (100 ng/mL final DiOC2, and 3 × 106 cells/mL). The cells were incubated 45 minutes at 37°C to allow dye accumulation. The cells were subsequently washed with ice-cold RPMI-1640 and resuspended with the same medium, before being distributed into 6 chilled 12 × 75 mm polystyrene tubes, 300 μL/tube (~2.5 × 105 cells/tube). These tubes of cells were organized into 3 groups of duplicate tubes. The first 2 groups were inoculated with 200 μl/tube of RPMI-1640 and incubated 90 ± 10 minutes at (a) 4°C for baseline dye loading determinations (active transport inhibited), and (b) 37°C for maximal efflux activity. The third efflux group was inoculated with 200 μL/tube RPMI-1640 containing 250 ng/mL zosuquidar, yielding a saturating 100 ng/mL final zosuquidar concentration. These latter tubes were also incubated at 37°C for 90 minutes to determine the effects of selective P-gp modulation on dye efflux. Following incubation, the processed cells were stored on ice until flow cytometry analysis as previously described (27, 29, 32). A target of 25K events was collected for each of the duplicate tubes/group.
Accumulation Assay.
An aliquot of anti-CD-stained cells (100 μL of 107/mL) was diluted to 1.2 mL with RPMI-1640, of which 300 μL was distributed into each of 4 chilled tubes (2.5 × 105/tube). All tubes were inoculated with 100 μL ice-cold medium containing 10 ng/mL DiOC2. The tubes were distributed into 2 groups, the first group of duplicate tubes was inoculated with 100 μL medium alone and the second group with 100 μL medium containing 500 ng/mL zosuquidar (100 ng/mL final concentration). The bioassay cultures were incubated at 37°C (water bath) for 60 ± 5 minutes, before washing and resuspending with ice-cold RPMI-1640. The washed cells were stored on ice until flow cytometry analysis (21, 26, 31). A target of 25K events was collected for each of duplicate tubes/group.
Data analysis
Descriptive statistics, 2-tailed Fisher exact test and Spearman rank correlation analyses were conducted using Statistica software (StatSoft, Inc., Tulsa, OK). Ratios and percentage determinations were calculated as illustrated by the equations shown in Table I. Five key calculations using mean fluorescence intensity (MFI) values allowed for cross comparisons between blast populations in the two bioassays. The calculations include (1) P-gp accumulation ratio, (2) P-gp efflux ratio, (3) percent unimpeded efflux for total MDR activity, (4) percent efflux in the presence of zosuquidar, and (5) percent inhibition of efflux by zosuquidar. The P-gp accumulation and P-gp efflux ratios identify only presence or absence of P-gp activity and are particularly important because they have been described and used in association with multiple zosuquidar AML clinical trials (23, 24, 26, 27, 31), including as a companion diagnostic (26). The other measures are important because they reveal MDR heterogeneity as described subsequently.
Table I.
Summary of Calculations for P-gp-specific and MDR Non-specific Efflux Indices using Mean Fluorescence Intensity (MFI) Values from Flow Cytometry of DiOC2 and CD34+ Leukemic Blasts
| Calculations Derived from MFI Values (DiOC2) of CD34+ Blast Cultures |
|---|
| 1. P-gp Accumulation Ratio = Accumulation w/Zos, 37°C ÷ Accumulation w/o Zos, 37°C |
| 2. P-gp Efflux Ratio = Efflux w/Zos, 37°C ÷ Efflux, 37°C |
| 3. % Efflux (MDR Non-specific) = (Efflux, 4°C − Efflux, 37°C)*100 ÷ Efflux, 4°C |
| 4. % Efflux w/Zos (MDR Non-specific) = (Efflux w/Zos, 37°C − Efflux,37°C)*100 ÷ Efflux w/Zos |
| 5. % Inhibition of Efflux by Zos = (1-((Efflux, 4°C − Efflux, w/Zos, 37°C) ÷ (Efflux, 4°C − Efflux, 37°C)))*100 |
Results
Patient Demographics and Disease Characteristics.
Bone marrow aspirates from 50 elderly patients with newly diagnosed AML were analyzed for this study, 32 from E3999 and 18 from KAN-979 (Table II). A modestly distinguishing feature between the 2 cohorts was the proportion of samples deemed positive for P-gp, 56% P-gp-positive for E3999 samples and 72% for the KAN-979 cohort. This was by design, since accumulation and efflux results had been obtained for the KAN-979 trial, and it was desired that more results of P-gp-negative also be obtained. Detailed descriptions of patients in studies E3999 and KAN-979 have been previously published (26,27).
Table II.
Demographics and disease characteristics of the AML patients
| Source of Patient Specimen | |||
|---|---|---|---|
| Parameter | Subset of E3999 | KAN-979 | Combined Subsets |
| Number of Patients, N | N = 32 | N = 18 | N = 50 |
| P-gp pos, N, (%) | 18 (56) | 13 (72) | 31 (62) |
| Age, Median | 70 | 67 | 68 |
| Age, <70 yrs., N, (%) | 16 (50) | 14 (78) | 30 (60) |
| Male | 18 (56) | 9 (50) | 27 (54) |
| Female | 14 (44) | 9 (50) | 23 (46) |
| De Novo AML | 19 (59) | 11 (61) | 30 (60) |
| Prior MDS | 13 (41) | 7 (39) | 20 (40) |
| ECOG PS | |||
| PS, 0 | 7 (22) | 7 (39) | 14 (28) |
| PS, 1 | 15 (47) | 10 (56) | 25 (50) |
| PS, 2 | 8 (25) | 1 (3) | 9 (18) |
| PS, 3 | 2 (6) | 0 (0) | 2 (4) |
| Cytogenetics | |||
| Favorable | 1 (3) | 1 (3) | 2 (4) |
| Intermediate | 11 (34) | 9 (50) | 20 (40) |
| Unfavorable | 6 (19) | 5 (28) | 11 (22) |
| Unknown | 14 (44) | 3 (17) | 17 (34) |
Identification of Four Leukemic Blast MDR Phenotypes with Accumulation and Efflux Bioassays.
Bone marrow specimens of the AML patients were simultaneously tested for leukemic blast activities in the accumulation and efflux bioassays by flow cytometry, using DiOC2 MFI values as indicators of MDR-substrate movement into and out of the blast populations. Zosuquidar was used as a P-gp-selective modulator, allowing for identification of district MDR phenotypes of leukemic blasts based on substrate (DiOC2) retention or efflux under different conditions.
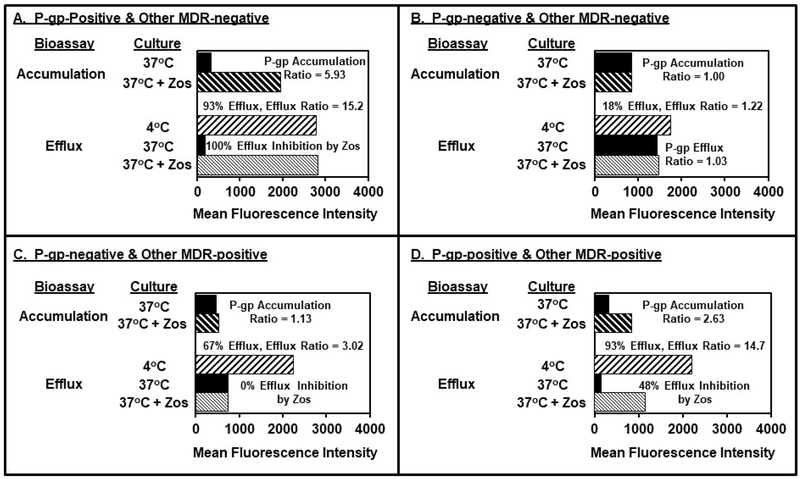
Four functional MDR phenotypes of AML blast specimens were identified using the accumulation and efflux bioassays as illustrated by 4 respective bone marrow samples in panels A-D of Figure 1. Panel A illustrates the responses of a representative AML specimen with only appreciable P-gp activity in the bioassays. In the accumulation bioassay, untreated blasts cultured in medium alone exhibited a MFI of 328, while zosuquidar-treated cells had a MFI of 1,945. This yielded a P-gp accumulation ratio of 5.93 (ratio = 1,945 ÷ 328 = 5.93).
Figure. 1.
Identification of Four Leukemic Blast MDR Phenotypes with Accumulation and Efflux Bioassays. Bioassay responses of 4 representative AML specimens are shown in Panels A-D. Panel A illustrates a “P-gp-positive, other MDR-negative” specimen with a positive P-gp accumulation ratio, and efflux that was totally inhibited by zosuquidar. Panel B shows a “P-gp-negative, other MDR-negative” specimen with minimal blast responses in the presence and absence of zosuquidar with either bioassays. Panel C shows a “P-gp-negative, other MDR-positive” specimen that displayed a negative P-gp-accumulation ratio and efflux that was insensitive to the modulatory effects of zosuquidar. Panel D shows a “P-gp-positive, other MDR-positive” specimen, where the distinguishing feature of the P-gp-positive blast population, is efflux that can be only partially inhibited by zosuquidar.
P-gp accumulation ratio values from this study of 50 AML specimens ranged from 0.94 to10.99, with increasing ratios indicative of increasing P-gp function. The distinction between P-gp-positive and P-gp-negative specimens as defined by this bioassay will be addressed subsequently. Basically, accumulation ratios ≥ 1.30 identify specimens with blasts exhibiting appreciable P-gp function as described previously (26, 31).
Efflux bioassay results with this P-gp-positive specimen are also shown in Panel A. The blasts were loaded with DiOC2 as evidenced by a MFI of 2,796 in the 4°C control group. Blast MFI was reduced to 185 following 37°C incubation for 90 min., reflecting 93% efflux. Zosuquidar totally inhibited efflux as indicated by the MFI of 2,825, which was not appreciably different from that seen for the 4°C control cultures (MFI 2,796). Associated calculations yielded 0% efflux in the presence of zosuquidar or 100% inhibition of efflux by zosuquidar, and a P-gp efflux ratio of 15.3 vs. the 37°C unimpeded efflux group (MFI 2,825 ÷ 185 = 15.3, not shown). These results indicate that essentially all efflux activity observed with this Panel A bone marrow specimen could be attributed to P-gp, i.e., “other MDR-negative” for the purposes of this study. This was the predominant phenotype observed, 46% (23 of 50) of all samples.
It should be noted that almost all samples in this study exhibited some degree of efflux at 37°C incubation. This dye movement across the plasma membrane is due to an ill-defined mix of active pumping mechanisms and passive flux. This was expected from other studies where it has been concluded that efflux < 40% is considered nonspecific because it is low, variable, and resistant to inhibition by efflux modulators like CsA (27, 29, 32). For the purposes of this study, samples that exhibited ≥ 40% dye loss are considered efflux-positive by definition.
Panel B of Figure 1 illustrates the minimal responses of an AML blast specimen lacking appreciable P-gp and other MDR activities in the bioassays (double negative). The blasts exhibited nearly identical DiOC2 MFI in the absence and presence of zosuquidar using the accumulation bioassay, yielding an accumulation P-gp ratio of 1.00. This representative blast specimen exhibited little efflux at 37°C as demonstrated by 18% total dye efflux, which was not appreciably modulated by culture with zosuquidar (17% total dye efflux and P-gp efflux ratio of 1.03). Double negative specimens accounted for 24% (12 of 50) of all specimens.
Panel C of Figure 1 shows representative efflux responses of a P-gp-negative blast population, with substantial zosuquidar-resistant MDR functionality. Results with the P-gp accumulation bioassay were negative bases on the accumulation ratio of 1.13. For the efflux bioassay, appreciable dye loading was evident (MFI 2,246), as was unimpeded 37°C efflux (MFI 743, 67% efflux vs. baseline). Significantly, parallel cultures of these blasts demonstrated substantial efflux in the presence of zosuquidar, and comparable dye loss (67%) was observed vs. the unimpeded 37°C group (P-gp efflux ratio = 1.00). This zosuquidar-resistant efflux activity is presumably not mediated by P-gp and, since it was inhibited in the 4°C cultures, is likely energy-dependent activity of other transporters. This phenotype of P-gp-negative, other MDR-positive accounted for 14% (7 of 50) of all specimens.
Panel D of Figure 1 shows an example blast specimen with both P-gp and non-P-gp MDR activities. The specimen was clearly P-gp-positive by the accumulation bioassay (P-gp accumulation ratio = 2.64). Ninety-three percent efflux was observed, which was only partially modulated (48%) by zosuquidar. Thus, the sample is P-gp-positive, and has zosuquidar-resistant efflux activity too. This phenotype accounted for 16% (8 of 50) of all specimens, although 26% (8 of 31) of P-gp-positive specimens.
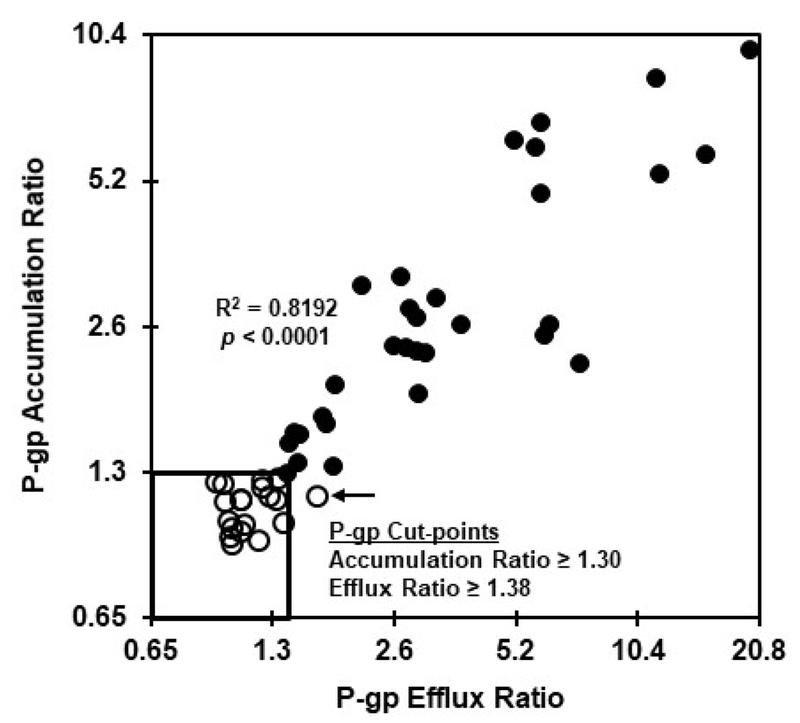
The P-gp accumulation and efflux ratios are highly correlated and comparably identify patient samples as being positive or negative for blasts with P-gp function.
Figure 2 presents the correlation between P-gp ratios obtained with the accumulation and efflux bioassays for the entire patient sample population (N = 50). These measures were highly related as indicated by an R2 of 0.8182 (p < 0.0001) for the overall data set. It has been previously reported that samples with zosuquidar-dependent accumulation ratios < 1.30 can be considered negative for P-gp function (26, 31). This ratio cut-point value was used to differentiate P-gp-positive from P-gp-negative samples for this study.
Figure. 2.
Comparison of P-gp ratios generated with the accumulation and efflux bioassays. Fifty AML specimens were tested in the presence and absence of zosuquidar in the DiOC2 accumulation and efflux bioassays as described in the legend to figure 1. P-gp accumulation and efflux ratios were calculated as described for Table 1. Samples that tested P-gp-negative using the accumulation bioassay are indicated by open symbols, while P-gp-positive samples are denoted as solid symbols. The accumulation and efflux bioassays were comparably effective at differentiating P-gp-positive vs. –negative AML specimens.
Nineteen of the 50 tested samples (38%) in this study exhibited an accumulation ratio < 1.30 (i.e., P-gp-negative) and are depicted as open symbols in Figures 2–4 to allow for monitoring of this population across bioassays and calculations. Results with the corresponding P-gp efflux bioassay ratios for these 19 samples were complicated by the outlier (Grubb’s test) efflux result identified by the arrow in Figure 2. Excluding that outlier sample yielded a P-gp efflux ratio mean (± standard dev.) of 1.127 ± 0.136 and an upper 95% confidence interval of 1.38 as a cut-point for differentiation between P-gp-negative vs. P-gp-positive samples with the efflux bioassay.
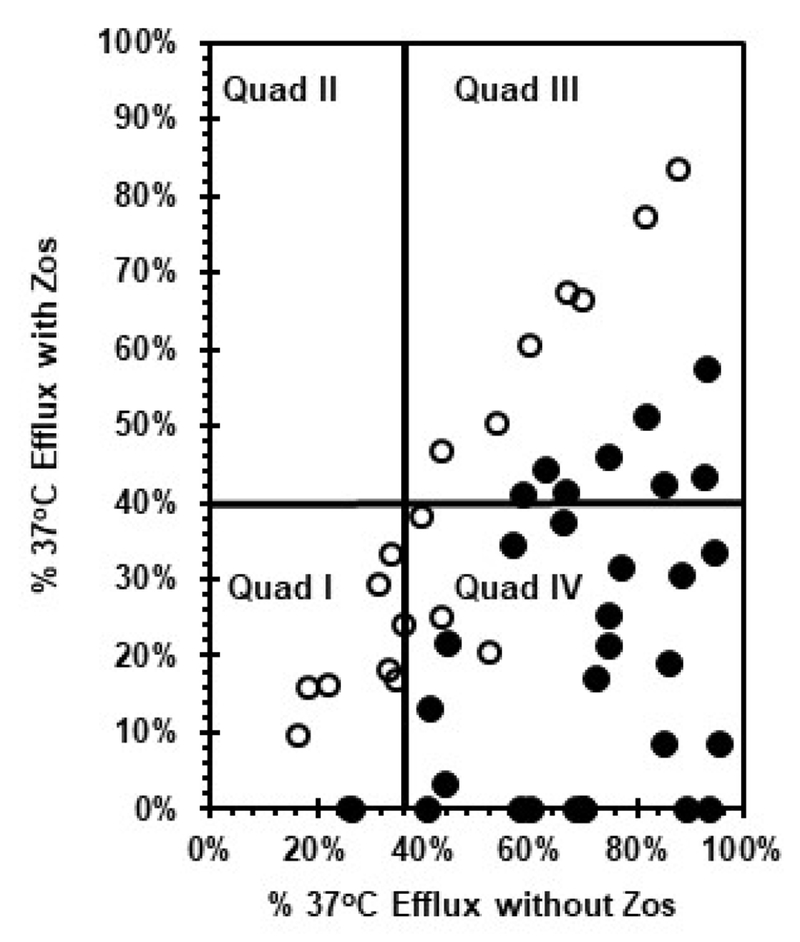
Figure 4.
Stratification of AML patients based on CD34+ blast efflux activities in the absence and presence of zosuquidar. Efflux bioassay results from 50 AML specimens are shown as percent 37°C efflux in the presence of zosuquidar (x-axis) vs. percent 37°C efflux in the absence of zosuquidar (y-axis). Solid symbols indicate specimens identified as P-gp-positive, while open symbols denote specimens lacking P-gp function.
The P-gp accumulation and efflux cut-points are identified by the inset box shown in Figure 2. These results confirm that the P-gp ratios for the accumulation and efflux assays identify the same set of samples as P-gp-positive vs. P-gp-negative as defined herein. Consequently, all conclusions using samples designated as P-gp-negative or P-gp-positive by the accumulation assay are considered applicable to conclusions with the efflux method.
Identification of independent and concomitant P-gp and Non-P-gp MDR functions by AML blasts with the accumulation and efflux bioassays.
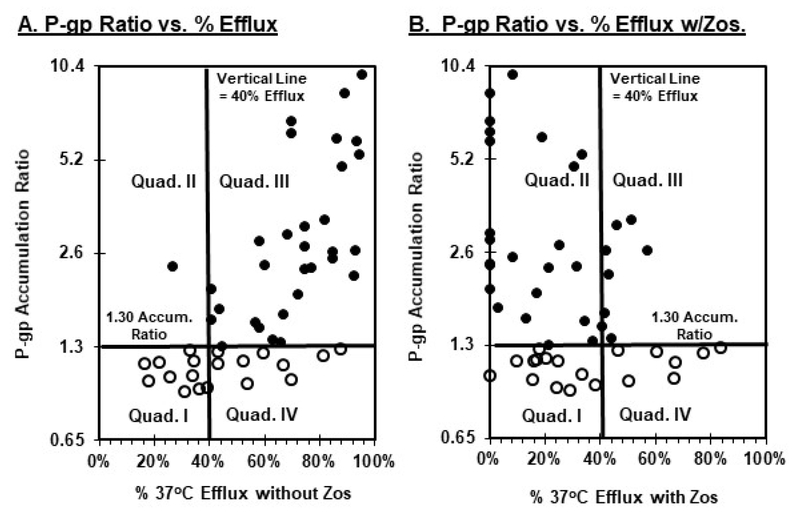
Panel A of Figure 3 shows the correlation between the accumulation P-gp ratio and percent unimpeded 37°C efflux (i.e., without zosuquidar). Almost all samples (97%) deemed P-gp-positive by the accumulation assay (closed symbols) exhibited substantial (≥ 40%) unimpeded 37°C efflux. The samples start to register as P-gp-positive by the P-gp accumulation ratio (i.e., ratio ≥ 1.30) at approximately 40% efflux (Fig. 3, vertical lines panels A and B). These results corroborate the contention that ≥ 40% efflux is a reasonable demarcation between efflux-positive vs. non-specific dye movement as described previously (27, 29, 32). It is readily apparent as illustrated by the open symbols in quadrant IV of Panel A that a substantial percentage of P-gp-negative samples (9 of 19, 47%) also exhibited appreciable unimpeded (≥ 40%) efflux.
Figure 3.
Identification of independent and concomitant P-gp and non-P-gp MDR function by AML blasts with the accumulation and efflux bioassays. Bioassay results were obtained as described in the legend to figure 1. Solid symbols identify specimens identified as P-gp-positive, while open symbols denote specimens lacking P-gp function. Panel A illustrates P-gp accumulation ratios (x-axis) vs. percent 37°C efflux in the absence of zosuquidar (y-axis). Panel B shows P-gp accumulation ratios (x-axis) vs. percent 37°C efflux in the presence of zosuquidar (y-axis).
Panel B of Figure 3 shows the relationships between the P-gp accumulation ratio and percent efflux in the presence of zosuquidar for P-gp-positive and P-gp-negative populations. Two important observations were revealed. First, as shown by quadrant III, a substantial percentage of samples with P-gp-positive blasts (8 of 31, 26%) exhibited positive (>40%) efflux in the presence of zosuquidar (i.e., zosuquidar-resistant MDR efflux). It remains to be determined whether such expression of multiple MDR species reflects concomitant function by individual blasts, or if subsets of the total blast population preferentially express one or the other individual MDR pumps.
Nevertheless, the second key finding in Panel B of Figure 3 is that non-P-gp MDR activity was also observed in an appreciable subpopulation of P-gp-negative samples (quadrant IV, 7 of 19, 37%), with somewhat more substantial non-P-gp efflux (upwards of 80%+) than observed with the closed symbol P-gp-positive fraction in quadrant III (40–60%+). These findings of zosuquidar-resistant MDR activities in P-gp-positive and P-gp–negative blast samples have potentially important implications for design of companion diagnostics with P-gp modulators like zosuquidar as addressed in the Discussion.
Stratification of AML patients based on efflux activities for CD34+ blasts in the absence and presence of zosuquidar.
Figure 4 shows the relationships between unimpeded dye efflux at 37°C vs. efflux in the presence of zosuquidar as exhibited by this set of samples. Quadrant I identifies blasts with low DiOC2 efflux activity (<40%) in the presence and absence of zosuquidar. Quadrant II would represent cells capable of efflux only if zosuquidar was present, but no such cells were detected. Quadrant III illustrates blasts capable of substantial efflux in the presence and absence of zosuquidar. Patients with blasts registering in quadrants I, II, and III would be predicted to not benefit greatly from treatment with zosuquidar, either because the specimens did not exhibit efflux or because the efflux observed was resistant to zosuquidar. Quadrant IV illustrates specimens from the optimal target patient population for zosuquidar, patients with blasts capable of ≥ 40% DiOC2 efflux when zosuquidar is absent (37°C efflux), but which display < 40% efflux in the presence of drug.
Model companion diagnostic scenarios with single and two-step diagnostic processes based on MDR bioassays.
Table III presents 2 scenarios involving AML patient selection for a hypothetical MDR modulator clinical trial with a single vs. two-step companion diagnostic algorithm using sample data from these 50 AML specimens. This analysis is a comparison of blast sample characteristics for patients that would be selected based on a P-gp-restricted companion diagnostic for zosuquidar, versus specimen/patient selection following a secondary determination based on zosuquidar-resistant (non-P-gp) efflux activities.
Table III.
Model Companion Diagnostic Scenarios with Single and Two-step Selection Processes Based on MDR Bioassays.
| Primary Selection (N = 50) | Secondary Selection | Total Selection | ||||
|---|---|---|---|---|---|---|
| Group | Accumulation Assay Selectiona | Number Selected (%) | Efflux + | Efflux + with Zosb | Total Selected | Comments |
| 1 | P-gp-negative | 19 (38%) | Not Done | Not Done | 19 | Includes 37% Other MDR+ |
| 2 | P-gp-positive | 31 (62%) | Not Done | Not Done | 31 | Includes 26% Other MDR+ |
| 3 | P-gp-negative | 19 (38%) | 9/19 (47%) | 7/19 (37%) | 12/19 | Optimal Control for Gp. 4 |
| 4 | P-gp-positive | 31 (62%) | 30/31 (97%) | 8/31 (26%) | 23/31 | Eliminates Zosuquidar Unresponsive Patients |
Conclusions from the accumulation bioassay, percent of specimens exhibiting a P-gp accumulation ratio ≥1.30, i.e., P-gp-positive.
Results from the efflux bioassay, percent of P-gp-negative and –positive samples exhibiting ≥40% efflux in the presence of zosuquidar.
Groups 1 and 2 of Table III show results from a single-step P-gp selection process using either the zosuquidar P-gp accumulation or efflux bioassay. Group 1, shown as open symbols in Figure 2–4 and considered P-gp-negative, totaled 19 patients (38% of initial screened patients). P-gp-positive Group 2 totaled 31 patients, 62% of the total screened population. As demonstrated in Figures 3 and 4, both of these groups included patients with blasts having appreciable zosuquidar-resistant MDR function which could compromise interpretations of zosuquidar AML experiments or clinical trials.
Groups 3 and 4 of Table III show the respective patient/sample phenotypes following a secondary screening for zosuquidar-resistant MDR function. Thus, 9 of 19 (47%) of P-gp-negative specimens exhibited unimpeded 37°C efflux, 78% (7 of 9) of which were resistant to the inhibitory effects of zosuquidar. Exclusion of these 7 P-gp-negative, but MDR-positive samples/patients (7 of 19, 37%), would yield 12 selected patients from an original cohort of 19 P-gp-negative samples following the primary screen. Twenty-six percent (8/31, 26%) of P-gp-positive specimens also exhibited zosuquidar-resistant efflux activity. Exclusion of these zosuquidar-resistant samples/patients would yield 23 selected patients from an original cohort of 31 patient with P-gp-positive blasts identified with the initial P-gp screen. Collectively, this secondary determination would have identified 15 of 50 total patient specimens (30% of total) as having zosuquidar-resistant MDR activities which should be accounted for in companion diagnostic or pharmacodynamics biomarker evaluations of this drug.
Discussion
This analysis of 50 AML specimens for MDR function using zosuquidar, a P-gp-selective modulator, and two ex vivo leukemic blast bioassays confirms the heterogeneous nature of MDR efflux pump function by AML blasts. Moreover, it supports the supposition that function of multiple transporters could have circumvented or obscured efficacy of P-gp modulators in AML clinical trials like E3999 with zosuquidar, and offers clues to the design of companion diagnostic strategies that take into account redundancy or compensatory drug resistance mechanisms that can allow tumor cell escape from chemotherapy.
Activities of at least 2 functional MDR species were distinguished in the present studies based on differential sensitivity to inhibition with zosuquidar, a potent and selective P-gp-modulator (18–21). Earlier studies by Paietta et al. (29) and Leith et al. (30) provided evidence for P-gp and non-P-gp efflux activities by AML blasts from patients similar to those in the present study. However, this is the first study of its kind using zosuquidar as a means to allow non-P-gp efflux to be observed with full concurrent P-gp inhibition. AML blast samples identified in the present studies as P-gp-negative by either the zosuquidar accumulation or efflux ratio method often contained blasts capable of zosuquidar-resistant MDR efflux activities. Moreover, a significant fraction of samples that were identified as P-gp-positive by these screening bioassays also exhibited zosuquidar-resistant efflux. It would be reasonable to speculate that patients with a preponderance of leukemic blasts capable of zosuquidar-resistant MDR activity would not exhibit clinical efficacy from treatment with P-gp-modulators like zosuquidar in combination with P-gp substrate chemotherapeutic drugs (e.g., anthracyclines).
The identity of non-P-gp efflux molecule(s) documented by these studies remains a matter for speculation, especially considering the existence of ~48 ATP-binding cassette transporter genes in humans (6). However, known MDR specificities for zosuquidar and fluorescent probes offer clues concerning the more studied efflux pumps. MRP1, MRP2, and BCRP are not inhibited by zosuquidar (18–21), suggesting a potential role in the zosuquidar-resistant MDR activity observed in this study. It has been observed that BCRP does not substantially efflux DiOC2 (33, 34), ruling out that MDR molecule as the zosuquidar-resistant efflux pump documented by these studies. However, a contribution of BCRP to failed zosuquidar clinical trials has not been ruled out. The controversy remains whether MRP1 or MRP2 can efflux DiOC2. It has been observed that DiOC2 is not effluxed by cells expressing MPR-1 (33,34), but that Rh123, a known substrate for MRP1, was extruded by the cells (33). This observation was called into question more recently (35), where it was demonstrated that P-gp and MRP1 can exhibit very similar substrate specificities, including efflux of DiOC2. Thus, MRP1 and MRP2 (among other MDR species) remain candidates for the zosuquidar-resistant MDR efflux mediator(s) detected in the present experiments.
Additional studies will be needed using MDR modulators with specificities other than P-gp and more specific fluorescent dyes as proposed by others (e.g., 34, 35), to dissect and identify relevant transporter mechanisms. The advent of flow cytometry with mass spectrometry capacity offers the ability to directly monitor the intracellular fate of specific chemotherapeutic drugs in oncology vs. surrogate dyes. This should circumvent the problem of low fluorescence utility with many oncology drugs, which is the rationale for using the surrogate dyes in the MDR functional bioassays as used herein.
The collective clinical trial findings with P-gp modulators in AML indicate that other MDR species expressed by leukemic blasts will have to be addressed either pharmaceutically or diagnostically for the strategy to be successful (1, 2). This hypothesis reconciles the limited successes and failures of clinical trials involving P-gp modulation in AML.
The medicinal chemistry approach for addressing P-gp in oncology culminated in the development of third generation modulators like zosuquidar. The fact that zosuquidar minimally altered PK of concurrently administered chemotherapeutics (22–27) allowed for testing of P-gp modulation without the need to reduce chemotherapy dosages. Nevertheless, as demonstrated by AML trial E3999 (27), and corroborated by other studies with third generation P-gp modulators (1, 2, 4), zosuquidar administered in combination with standard induction therapy in elderly AML patients, failed to demonstrate efficacy in P-gp-positive and P-gp-negative patient populations. It was concluded from E3999 (27) that “Z did not improve outcome in older AML, in part, due to the presence P-gp independent mechanisms of resistance.” Targeting multiple MDR mechanisms with a broader-spectrum modulator has been suggested (1, 2).
A prevailing hypothesis relates heterogeneity in MDR mechanisms to the ineffectiveness of P-gp modulators like zosuquidar to significantly influence P-gp-positive AML as used in E3999 and KAN-979 (1, 2), as reviewed in the Introduction. The substantial utility of P-gp as a prognostic indicator for response to therapy strongly suggests that neutralization of P-gp function should have a positive influence on AML outcomes. Pharmacodynamic studies with autologous plasma bioassay cultures established that P-gp function can be > 90% neutralized in P-gp-positive NK cells and leukemic blasts in zosuquidar-infused AML patients concurrent with chemotherapy (26,31). These observations of apparently strong P-gp involvement in AML outcomes, but lack of obvious effect with P-gp neutralization by zosuquidar, indicates a possible role for other MDR mechanisms in escape from therapy. The studies presented herein have identified heretofore undescribed, zosuquidar-resistant MDR efflux activities by leukemic blasts in samples from study E3999, suggesting a plausible relationship between zosuquidar-resistant MDR blast function with the lack of observed efficacy for the drug in that study. Further analyses of results and cryopreserved specimens from clinical studies such as E3999 and KAN-979 could identify processes that promote AML drug-resistance when P-gp has been apparently neutralized.
Conclusions
A challenge has emerged for developing effective oncology predictive biomarkers, using methods to detect multiple, and interrelated resistance mechanisms. The results reported herein illustrate this concept of redundant or compensatory drug resistance mechanisms in leukemic blasts, and an approach to incorporating detection of such resistance mechanisms in the design of companion diagnostics.
Acknowledgements
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health (NIH) under the following award numbers: CA180820, CA180794, CA180795, CA180816, and CA189859. Additional funding was provided by the NIH, including R01 CA114037 and R01 CA184968 (Branimir I. Sikic), and U24-CA196172 (Elizabeth Paietta). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. There are no conflict of interest to report.
References
- 1.Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18(7):1863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, Gottesman MM. Drug resistance: still a daunting challenge to the successful treatment of AML. Drug Resist Update. 2012;15(1-20):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khamisipour G, Jadidi-Niaragh F, Jahromi AS, Zandi K, Hojjat-Farsangi M. Mechanisms of tumor cell resistance to the current targeted-therapy agents. Tumour Biol. 2016;37(8):10021–39. DOI: 10.1007/s13277-016-5059-1 [DOI] [PubMed] [Google Scholar]
- 4.Chung FS, Santiago JS, De Jesus FM, et al. Disrupting P-glycoprotein function in clinical settings: what can we learn from the fundamental aspects of this transporter. Am J Cancer Res. 2016;6(8):1583–1598. [PMC free article] [PubMed] [Google Scholar]
- 5.Bartholomae S, Gruhn B, Debatin KM, Zimmermann M, Creutzig U, Reinhardt D, Steinbach D. Coexpression of multiple ABC-transporters is strongly associated with treatment response in childhood acute myeloid leukemia. Pediatr Blood Cancer. 2016:63(2):242–247. DOI: 10.1002/pbc.25785 [DOI] [PubMed] [Google Scholar]
- 6.Fukuda Y, Lian S, Schuetz JD. Leukemia and ABC transporters. Adv. Cancer Res. 2015;125:171–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos L, Guyotat D, Archimbaud E, et al. Clinical significance of multidrug resistance P-glycoprotein expression on acute nonlymphoblastic leukemia cells at diagnosis. Blood . 1992;79(2):473–476. [PubMed] [Google Scholar]
- 8.Del Poeta G, Stasi R, Aronica G, et al. Clinical relevance of P-glycoprotein expression in de novo acute myeloid leukemia. Blood. 1996;87(5):1997–2004. [PubMed] [Google Scholar]
- 9.Leith CP, Kopecky KJ, Godwin J, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. A Southwest Oncology Group study. Blood. 1997;89(9):3323–3329. [PubMed] [Google Scholar]
- 10.Stasi R, Del Poeta G, Venditti A, et al. Prognostic value of cytogenetics and multidrug resistance (MDR1) in elderly patients with acute myeloid leukemia. Blood. 1998;92(2):695–697. [PubMed] [Google Scholar]
- 11.van den Heuvel-Eibrink MM, van der Holt B, te Boekhorst PA, et al. MDR 1 expression is an independent prognostic factor for response and survival in de novo acute myeloid leukaemia. Br J Haematol. 1997;99(1):76–83. [DOI] [PubMed] [Google Scholar]
- 12.Vezmar M1, Georges E. Reversal of MRP-mediated doxorubicin resistance with quinoline-based drugs. Biochem Pharmacol. 2000. May 15;59(10):1245–52. [DOI] [PubMed] [Google Scholar]
- 13.Qadir M, O’Loughlin KL, Fricke SM, et al. Cyclosporin A is a broad-spectrum multidrug resistance modulator. Clin Cancer Res. 2005;11:2320–2326. [DOI] [PubMed] [Google Scholar]
- 14.Wattel E, Salary E, Hecquet B, et al. Quinine improves the results of intensive chemotherapy in myelodysplastic syndromes expressing P glycoprotein: results of a randomized study. Br J Haematol. 1998;102(4):1015–24. [DOI] [PubMed] [Google Scholar]
- 15.Wu CP, Calcagno AM, Ambudkar SV. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr Mol Pharmacol. 2008;1(2):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.List AF, Kopecky KJ, Willman CL, et al. Benefit of cyclosporine modulation of drug resistance in patients with poor-risk acute myeloid leukemia: a Southwest Oncology Group study. Blood 2001;98:3212–20. [DOI] [PubMed] [Google Scholar]
- 17.Liu-Yin JA, Wheatley K, Rees JK, Burnett AK, Party UMALW. Comparison of ‘sequential’ versus ‘standard’ chemotherapy as re-induction treatment, with or without cyclosporine, in refractory/relapsed acute myeloid leukaemia (AML): results of the UK Medical Research Council AML-R trial. Br J Haematol . 2001;113(3):713–726. [DOI] [PubMed] [Google Scholar]
- 18.Dantzig AH, Shepard RL, Law KL, et al. Selectivity of the multidrug resistance modulator, LY335979, for P-glycoprotein and effect on cytochrome P-450 activities. J Pharmacol Exp Ther 1999;290:854–62. [PubMed] [Google Scholar]
- 19.Shepard RL, Cao J, Starling JJ, Dantzig AH. Modulation of P-glycoprotein but not MRP1- or BCRP-mediated drug resistance by LY335979. Int J Cancer 2003;103:121–5. [DOI] [PubMed] [Google Scholar]
- 20.Dantzig AH, Law KL, Cao J, Starling JJ. Reversal of multidrug resistance by the P-glycoprotein modulator, LY335979, from the bench to the clinic. Curr Med Chem. 2001;8(1):39–50. [DOI] [PubMed] [Google Scholar]
- 21.Green LJ, Marder P, Slapak CA. Modulation by LY335979 of P-glycoprotein function in multidrug-resistant cell lines and human natural killer cells. Biochem Pharmacol 2001;61:1393–9. [DOI] [PubMed] [Google Scholar]
- 22.Sandler A, Gordon M, De Alwis DP, et al. A Phase I trial of a potent P-glycoprotein inhibitor, zosuquidar trihydrochloride (LY335979), administered intravenously in combination with doxorubicin in patients with advanced malignancy. Clin Cancer Res 2004;10:3265–72. [DOI] [PubMed] [Google Scholar]
- 23.Rubin EH, de Alwis DP, Pouliquen I, et al. A phase I trial of a potent Pglycoprotein inhibitor, Zosuquidar.3HCl trihydrochloride (LY335979), administered orally in combination with doxorubicin in patients with advanced malignancies. Clin Cancer Res. 2002;8(12):3710–3717. [PubMed] [Google Scholar]
- 24.Gerrard G, Payne E, Baker RJ, et al. Clinical effects and P-glycoprotein inhibition in patients with acute myeloid leukemia treated with zosuquidar trihydrochloride, daunorubicin and cytarabine. Haematologica . 2004;89(7):782–790. [PubMed] [Google Scholar]
- 25.Callies S, de Alwis DP, Mehta A, Burgess M, Aarons L. Population pharmacokinetic model for daunorubicin and daunorubicinol coadministered with zosuquidar.3HCl (LY335979). Cancer Chemo Pharmacol. 2004;54:39–48. [DOI] [PubMed] [Google Scholar]
- 26.Lancet JE, Baer MR, Duran GE, List AF, Fielding R, Marcelletti JF, Multani PS, Sikic BI. A Phase I Trial of Continuous Infusion of the Multidrug Resistance Inhibitor Zosuquidar with Daunorubicin and Cytarabine in Acute Myeloid Leukemia. Leuk Res. 2009;33:1055–1061. [DOI] [PubMed] [Google Scholar]
- 27.Cripe LD, Uno H, Paietta EM, et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled, trial of the Eastern Cooperative Oncology Group (ECOG 3999). Blood. 2010;116:4077–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Libby E, Hromas R. Dismounting the MDR Horse. Blood. 2010;116:4037–8. [DOI] [PubMed] [Google Scholar]
- 29.Paietta E, Anderson J, Racevskis M, Ashigbi M, Cassileth P, Wiernik PH, and the Eastern Cooperative Oncology Group. Modulation of multidrug resistance in de novo adult acute myeloid leukemia: variable efficacy of reverting agents in vitro. Eastern Cooperative Oncology Group. Blood Rev. 1995;9:47–52. [DOI] [PubMed] [Google Scholar]
- 30.Leith CP, Chen IM, Kopecky KJ, et al. Correlation of multidrug resistance (MDR1) protein expression with functional dye/drug efflux in acute myeloid leukemia by multiparameter flow cytometry: identification of discordant MDR-/efflux+ and MDR1+/efflux- cases. Blood 1995;86:2329–42. [PubMed] [Google Scholar]
- 31.Marcelletti JF, Multani PS, Lancet JE, Baer MR, Sikic BI. Leukemic blast and natural killer cell P-glycoprotein function and inhibition in a clinical trial of zosuquidar infusion in acute myeloid leukemia. Leuk Res. 2009;33:769–774. [DOI] [PubMed] [Google Scholar]
- 32.Paietta E, Andersen J, Racevskis J, et al. Significantly lower P-glycoprotein expression in acute promyelocytic leukemia than in other types of acute myeloid leukemia: immunological, molecular and functional analyses. Leukemia. 1994;8(6):968–973. [PubMed] [Google Scholar]
- 33.Minderman H, Vanhoefer U, Toth K, et al. DiOC2(3) is not a substrate for multidrug resistance protein (MRP)-mediated drug efflux. Cytometry 1996;25:14–20. [DOI] [PubMed] [Google Scholar]
- 34.Lebedeva IV, Pande P, Patton WF. Sensitive and specific fluorescent probes for functional analysis of the three major types of mammalian ABC transporters. PLoS One. 2011;6(7):e22429. doi: 10.1371/journal.pone.0022429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strousea JJ, Irena Ivnitski-Steelea I, Wallera A, et al. Fluorescent substrates for flow cytometric evaluation of efflux inhibition in ABCB1, ABCC1, and ABCG2 transporters. Anal Biochem. 2013;437(1): 77–87. doi: 10.1016/j.ab.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marzac C, Garrido E, Tang R, et al. ATP binding cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica. 2011;96(9):1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]