Precis:
This study highlights that the initial treatment that men with advanced prostate cancer receive may vary based on race/ethnicity. Therefore this work suggests that racial variation based on treatment received may be explained in part by cost and setting of diagnosis.
Keywords: Metastatic prostate cancer, orchiectomy, androgen deprivation therapy, cancer disparities
Abstract
Objective:
Surgical and medical androgen deprivation therapy (ADT) strategies are comparable in their ability to suppress serum testosterone levels as treatment in metastatic prostate cancer but differ in cost and impact on quality of life. Medical ADT is associated with better long-term quality of life due to the flexibility of possible therapy interruption but comes with a higher cumulative cost. We examined if surgical ADT (i.e., bilateral orchiectomy) was differentially utilized by race/ethnicity and other social factors.
Methods
We identified patients with metastatic disease at diagnosis through the California Cancer Registry. The association of race/ethnicity with receipt of surgical ADT was modeled using multivariable Firth logistic regression adjusting for age, Gleason score, prostate specific antigen, clinical tumor and lymph node stage, neighborhood socioeconomic status (nSES), insurance, marital status, comorbidities, initial treatment (radiation, chemotherapy), location of care, rural/urban area, and year of diagnosis.
Results
We examined 10,675 patients with metastatic prostate cancer: Non-Hispanic (NH) Black (11.4%), Asian/Pacific Islander (8.4%), Hispanic/Latino (18.5%), and NH White (60.4%). In the multivariable model, patients more likely to receive surgical ADT were Hispanic/Latino (OR=1.32, 95% CI 1.01–1.72), from a low nSES (OR=1.96, 95% CI 1.34–2.89) or rural area (OR=1.49, 95% CI 1.15–1.92), and had Medicaid/public insurance (OR=2.21, 95% CI 1.58–3.10). Patients with Military/Veterans Administration insurance were significantly less likely to receive surgical ADT than patients with private insurance (OR=0.34, 95% CI 0.13–0.88).
Conclusion:
Race/ethnicity, neighborhood SES, and insurance are significantly associated with receipt of surgical ADT. Future research will need to characterize other differences in initial treatments among men with advanced prostate cancer based on race/ethnicity and aim to better understand what factors drive the association between surgical ADT among men of Hispanic origin or from low nSES.
Introduction
Prostate cancer is the most common non-cutaneous malignancy among men in the United States [1, 2]. While the majority of men present with localized disease, the incidence of distant metastatic disease at diagnosis has increased [3]. It is well established that prostate cancer affects men differentially based on race/ethnicity, with African American men being more likely to present with and die from advanced disease compared to their white counterparts [4].
While the treatment paradigm for treating advanced prostate cancer is rapidly evolving, androgen deprivation therapy (ADT) remains the backbone of therapy [5, 6]. Charles Huggins first recognized the role of serum testosterone in mediating the progression of prostate cancer which led to the utility of surgical ADT, or bilateral orchiectomy, as a treatment for advanced disease [7]. Surgical ADT was perceived as a technically minor procedure associated with a one-time cost [1]. However, hormonal analogues were developed and quickly adopted that interfere with the hypothalamic-pituitary axis pathway for gonadal production of serum testosterone [8, 9]. The development of these medical ADT approaches allowed for the interruption of therapy, which has proven to be the optimal strategy for non-metastatic disease [10]. However, for metastatic prostate cancer, continuous, life-long ADT, in any form, remains the standard of care [11].
Surgical and medical ADT strategies are comparable in their ability to achieve suppressed serum testosterone levels but differ in cost [12, 13]. While medical ADT, which can be interrupted, is associated with improved quality of life [14], it is also associated with a greater cost overall and a need for close clinical follow-up for serial drug administration [15]. Alternatively, bilateral orchiectomy is a one-time intervention [13, 15]. Due to differential costs and the need for more regular clinical follow-up in medical versus surgical ADT, it is possible that the type of ADT that patients receive is a reflection of disparities in access to consistent treatment as well as physician bias in anticipated patient adherence [16].
There is limited knowledge about the differences in ADT approaches obtained among men with metastatic prostate cancer. This study aims to utilize a large population level dataset to more deeply understand the differences in treatments obtained among men with advanced disease. Given the increasing evidence of disparities in receipt of treatment, access to care, and quality of life outcomes among men with prostate cancer [4, 17, 18], this study sought to examine if orchiectomy was differentially utilized as the primary approach for ADT among men with de novo metastatic prostate cancer by race/ethnicity and other patient social factors including socioeconomic status, marital status, and health insurance status.
Methods
Data Collection
This is an observational cohort study within the population-based California Cancer Registry (CCR), a state-mandated registry that has collected data on all cancers diagnosed in residents of California since 1988. The CCR, comprising three regional registries that are members of the National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) program, abstracts information on patient demographics, tumor characteristics, and initial treatment. Data were obtained on all men with de novo metastatic prostate cancer at diagnosis in California from 2004–2015, regardless of histologic subtype (N=13,875). Men diagnosed on death certificate or autopsy only (N=21) and those who did not receive any form of ADT (N=3,179) were excluded from analysis.
This study was included under the approved Institutional Review Board protocol for the Greater Bay Area Cancer Registries (SEER San Francisco/Oakland and San Jose/Monterey registries).
Outcome Variable
The primary outcome of this analysis was receipt of bilateral orchiectomy (surgical ADT) as part of initial treatment among men with metastatic prostate cancer at time of diagnosis.
Demographic Characteristics
The CCR collects race and ethnicity data from medical records and additionally applies an established algorithm [19] based on surname and birthplace to establish Hispanic ethnicity. Patients are categorized by race and ethnicity into the following groups: Non Hispanic (NH) White, NH Black, Hispanic, Asian/Pacific Islander, and other/unknown. Marital status was categorized as married, unmarried, or unknown. Insurance status was categorized as uninsured/unknown, private, Medicaid, Medicare, or Military/Veterans Affairs.
The CCR does not collect data on individual-level measures of socioeconomic status (SES). However, as patients’ addresses at diagnosis are routinely geocoded by the CCR, we used a previously described composite measure of SES and assigned each case to a quintile of neighborhood SES (nSES) based on the Census block group [20, 21].
The Office of Statewide Health Planning and Development (OSHPD) uses population, demographic, and physician data to define medical service study areas (MSSAs) - sub-county and sub-city geographic areas composed of at least one census tract, that can be used to characterize health professional shortage areas, medically underserved areas, and medically underserved populations [22]. These MSSAs are characterized as urban, rural, or frontier based on population density. Each patient’s residence at diagnosis was classified as being either in an urban or rural/frontier MSSA. Additionally we identified patients who received care at a National Cancer Institute (NCI)-designated cancer center.
Clinical Characteristics
We grouped Gleason score into Gleason score less than or equal to 6, Gleason score 7, Gleason score 8–10, or unknown. We described tumor size using clinical T stage categories of less than or equal to T2a, T2b-T2c/T2NOS, greater than or equal to T3a, or Tx. We summarized lymph node status using clinical N stage and grouped as N0, N1, or Nx. We categorized PSA values as less than 10ng/mL, 10–20ng/mL, greater than 20ng/mL, or unknown.
We measured comorbidity burden using the Charlson comorbidity score derived from linking CCR data with the OSHPD discharge data [23]. The Charlson score is a weighted score categorized as 0 (no comorbidity), 1, 2, or 3 or more.
Type of androgen deprivation therapy received as part of initial treatment was obtained from CCR and categorized as no ADT, orchiectomy only, medical ADT only, both orchiectomy and medical ADT, or unknown. Both receipt of radiation and chemotherapy as part of initial treatment were dichotomized as yes or no.
Statistical Analysis
The utilization of ADT in men diagnosed with metastatic prostate cancer was characterized. Differences in characteristics between men who received surgical ADT and those who received medical ADT were compared using chi square tests. The association of race/ethnicity with receipt of surgical ADT was modeled using Firth logistic regression (penalized likelihood method) to reduce small sample bias [24, 25]. Men who received both medical and surgical ADT were included with those who received only surgical ADT; men who had not received ADT (N=3,179) were excluded from this analysis. Covariates considered for inclusion in the multivariable model were age at diagnosis, Gleason score, prostate specific antigen (PSA) value at diagnosis, tumor size (T stage), lymph node involvement (N stage), quintile of nSES, primary healthcare payer, marital status, Charlson comorbidity score, receipt of other primary treatments (radiation, chemotherapy), receipt of care at an NCI-designated cancer center, urban/rural designation of the OSHPD MSSA of the patient’s residence at diagnosis, and year of diagnosis. Age at diagnosis and year of diagnosis were included as continuous variables; the remainder were included as categorical variables. A purposeful selection strategy [26] was used to select variables for inclusion in the final multivariable model. Initially all variables that were significant at the p≤0.25 level on univariate analysis were included in the multivariable model. The model was iteratively reduced retaining only those variables with p<0.10. Variables that were not initially included in the model were then added back and retained as significant confounders in the final model if they changed effect estimates by more than 20%. First order interactions between race/ethnicity, nSES, insurance, and rural/urban status were tested and found not to be significant.
We conducted sensitivity analyses to assess the effect of potential under-ascertainment of systemic hormone therapy, in which men (N=1,838) for whom hormonal therapy was coded as “None”, “Recommended, unknown if given,” or “Unknown” were considered as having received hormone therapy.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Statistical tests were two-sided, and values with p<0.05 were considered statistically significant.
Results
Patient Characteristics
Overall, we identified 10,675 men with metastatic prostate cancer at diagnosis in California from 2004–2015. The patient characteristics of the study sample are summarized in Table 1. The study consisted of 60.5% non-Hispanic White (White), 11.5% non-Hispanic Black (Black), 18.7% Hispanic/Latino (Hispanic), and 8.1% Asian/Pacific Islander (Asian) men. The majority of the sample were 65 years or older (72.7%), married (56.1%) and resided in middle or high SES neighborhoods (63.1%). The majority of the sample were insured (94.1%) and almost half were covered by Medicare (47%). The median age of the study sample was 73 years old (range 29 to 105). The majority of the sample had a prostate biopsy performed (58.4%), histology type of adenocarcinoma (78.7%), Gleason grade of 7 or greater (57.1%) and a PSA level greater than 20 ng/mL at diagnosis (70.2%). Additionally, the majority received initial medical ADT (74.2%), without initial radiation therapy (80.2%) or initial chemotherapy (91.7%). A minority of the sample received care at a NCI-Designated Cancer Center (11.7%) and in a rural/frontier medical service setting (15.5%). The study sample was relatively equally distributed by year or diagnosis.
Table 1.
Characteristics of men diagnosed with de novo metastatic (M1) prostate cancer California Cancer Registry, 2004–2015
| N | ||
| Age at diagnosis | ||
| <55 | 858 (6.2%) | |
| 55–64 | 2,929 (21.1%) | |
| 65–74 | 3,809 (27.5%) | |
| 75+ | 6,258 (45.2%) | |
| Median age at diagnosis (range) | 73 (29–105) | |
| Gleason score | ||
| Unknown | 5,550 (40.1%) | |
| Gleason <=6 | 395 (2.9%) | |
| Gleason 7 | 1,629 (11.8%) | |
| Gleason 8–10 | 6,280 (45.3%) | |
| Clinical T stage | ||
| ≤T2a | 2,766 (20.0%) | |
| T2b-T2c, T2NOS | 3,722 (26.9%) | |
| ≥T3a | 2,630 (19.0%) | |
| TX | 4,736 (34.2%) | |
| N stage | ||
| N0 | 6,317 (45.6%) | |
| N1 | 3,033 (21.9%) | |
| NX | 4,504 (32.5%) | |
| PSA value | ||
| <10 ng/ml | 1,165 (8.4%) | |
| 10–20 ng/ml | 1,210 (8.7%) | |
| >20 ng/ml | 9,728 (70.2%) | |
| Unknown | 1,751 (12.6%) | |
| Prostate biopsy performed | ||
| Yes | 8,085 (58.4%) | |
| No | 5,769 (41.6%) | |
| Histology | ||
| Adenocarcinoma | 10,903 (78.7%) | |
| Carcinoma NOS | 2,625 (18.9%) | |
| Neuroendocrine carcinoma | 62 (0.4%) | |
| Other | 190 (1.4%) | |
| Small cell carcinoma | 74 (0.5%) | |
| Race/ethnicity | ||
| NH White | 8,375 (60.5%) | |
| NH Black | 1,600 (11.5%) | |
| Hispanic | 2,587 (18.7%) | |
| Asian/PI | 1,121 (8.1%) | |
| other/unknown | 171 (1.2%) | |
| Neighborhood SES quintile | ||
| 1 - lowest | 2,402 (17.3%) | |
| 2 | 2,704 (19.5%) | |
| 3 | 3,033 (21.9%) | |
| 4 | 2,965 (21.4%) | |
| 5 - highest | 2,750 (19.8%) | |
| Marital status | ||
| Married | 7,772 (56.1%) | |
| Unmarried | 5,204 (37.6%) | |
| Unknown | 878 (6.3%) | |
| Primary payer | ||
| Uninsured/Unknown | 811 (5.9%) | |
| Private or insurance NOS | 4,725 (34.1%) | |
| Medicaid/public | 1,259 (9.1%) | |
| Medicare | 6,516 (47.0%) | |
| Military, VA | 543 (3.9%) | |
| Charlson comorbidity score | ||
| 0 | 4,720 (34.1%) | |
| 1 | 1,752 (12.6%) | |
| 2 | 1,246 (9.0%) | |
| 3 or higher | 1,648 (11.9%) | |
| Unknown | 4,488 (32.4%) | |
| Type of androgen deprivation (ADT) | ||
| No ADT | 3,049 (22.0%) | |
| Orchiectomy only | 221 (1.6%) | |
| Medical ADT only | 10,273 (74.2%) | |
| Both orchiectomy and medical ADT | 181 (1.3%) | |
| Unknown | 130 (0.9%) | |
| Radiation | ||
| No | 11,116 (80.2%) | |
| Yes | 2,738 (19.8%) | |
| Chemotherapy | ||
| No | 12,707 (91.7%) | |
| Yes | 1,147 (8.3%) | |
| NCI-designated cancer center | ||
| No | 12,232 (88.3%) | |
| Yes | 1,622 (11.7%) | |
| Designation of medical service study area | ||
| Rural or Frontier | 2,149 (15.5%) | |
| Urban | 11,705 (84.5%) | |
| Year of diagnosis | ||
| 2004–2007 | 4,084 (29.5%) | |
| 2008–2011 | 4,349 (31.4%) | |
| 2012–2015 | 5,421 (39.1%) | |
The overwhelming majority of patients received medical ADT. However, differences based on type of ADT received were noted across several demographic characteristics. In Table 2, the patient characteristics were stratified by type of ADT (surgery versus medical). Compared to the group receiving medical ADT, there were higher proportions of Hispanic men, men living in lower SES neighborhoods or rural medical service areas, with Medicare or Medicaid/public insurance, unmarried men, men with >=cT3a disease, unknown lymph node status, and PSA levels of 20 ng/ml or greater in the group that received surgical ADT. The proportion of men with unknown Charlson comorbidity score was significantly lower in the surgical ADT group. No differences in age at diagnosis were noted by type of ADT received with the median age being 73 years old in both groups. In figure 1a–b, we observed that the percentage of patients who received medical ADT increased over time across all insurance types, race/ethnicity, nSES, and rural versus urban settings.
Table 2.
Receipt of orchiectomy as form of androgen deprivation for de novo metastatic (M1) prostate cancer California Cancer Registry, 2004–2015
|
Total N= 10,675 N |
Surgical ADT N= 402 N |
Medical ADT N= 10,273 N |
Chi square p value |
||
|---|---|---|---|---|---|
| Age at diagnosis | <55 | 755 ( 7.1%) | 25 ( 6.2%) | 730 ( 7.1%) | 0.1467 |
| 55–64 | 2,413 (22.6%) | 79 (19.7%) | 2,334 (22.7%) | ||
| 65–74 | 3,041 (28.5%) | 108 (26.9%) | 2,933 (28.6%) | ||
| 75+ | 4,466 (41.8%) | 190 (47.3%) | 4,276 (41.6%) | ||
| Median (Range) | 73 (29 – 105) | 73 (41 – 96) | 73 (29 – 105) | ||
| Gleason score | Gleason ≤6 | 249 ( 2.3%) | 13 ( 3.2%) | 236 ( 2.3%) | 0.0588 |
| Gleason 7 | 1,343 (12.6%) | 64 (15.9%) | 1,279 (12.5%) | ||
| Gleason 8–10 | 5,519 (51.7%) | 208 (51.7%) | 5,311 (51.7%) | ||
| Unknown | 3,564 (33.4%) | 117 (29.1%) | 3,447 (33.6%) | ||
| Clinical T stage | ≤T2a | 2,233 (20.9%) | 56 (13.9%) | 2,177 (21.2%) | 0.0010 |
| T2b-T2c, T2NOS | 3,153 (29.5%) | 113 (28.1%) | 3,040 (29.6%) | ||
| ≥T3a | 2,250 (21.1%) | 103 (25.6%) | 2,147 (20.9%) | ||
| TX | 3,039 (28.5%) | 130 (32.3%) | 2,909 (28.3%) | ||
| N stage | N0 | 5,094 (47.7%) | 156 (38.8%) | 4,938 (48.1%) | <.0001 |
| N1 | 2,567 (24.0%) | 70 (17.4%) | 2,497 (24.3%) | ||
| NX | 3,014 (28.2%) | 176 (43.8%) | 2,838 (27.6%) | ||
| PSA at diagnosis | <10 ng/ml | 850 ( 8.0%) | 21 ( 5.2%) | 829 ( 8.1%) | 0.0032 |
| 10–20 ng/ml | 960 ( 9.0%) | 20 ( 5.0%) | 940 ( 9.2%) | ||
| >20 ng/ml | 8,030 (75.2%) | 326 (81.1%) | 7,704 (75.0%) | ||
| Unknown | 835 ( 7.8%) | 35 ( 8.7%) | 800 ( 7.8%) | ||
| Prostate biopsy performed | Yes | 6,925 (64.9%) | 276 (68.7%) | 6,649 (64.7%) | 0.1051 |
| No | 3,750 (35.1%) | 126 (31.3%) | 3,624 (35.3%) | ||
| Race/ethnicity* | NH White | 6,443 (60.4%) | 216 (53.7%) | 6,227 (60.6%) | 0.0032 |
| NH Black | 1,222 (11.4%) | 42 (10.4%) | 1,180 (11.5%) | ||
| Hispanic | 1,979 (18.5%) | 104 (25.9%) | 1,875 (18.3%) | ||
| Asian/PI | 900 ( 8.4%) | 36 ( 9.0%) | 864 ( 8.4%) | ||
| Neighborhood SES quintile | 1 - lowest | 1,758 (16.5%) | 93 (23.1%) | 1,665 (16.2%) | <.0001 |
| 2 | 2,045 (19.2%) | 103 (25.6%) | 1,942 (18.9%) | ||
| 3 | 2,342 (21.9%) | 94 (23.4%) | 2,248 (21.9%) | ||
| 4 | 2,347 (22.0%) | 67 (16.7%) | 2,280 (22.2%) | ||
| 5 - highest | 2,183 (20.4%) | 45 (11.2%) | 2,138 (20.8%) | ||
| Marital Status | Married | 6,112 (57.3%) | 221 (55.0%) | 5,891 (57.3%) | 0.0031 |
| Unmarried | 3,851 (36.1%) | 168 (41.8%) | 3,683 (35.9%) | ||
| Unknown | 712 ( 6.7%) | 13 ( 3.2%) | 699 ( 6.8%) | ||
| Primary Payer* | Private or insurance NOS | 3,841 (36.0%) | 116 (28.9%) | 3,725 (36.3%) | <.0001 |
| Medicaid/public | 1,007 ( 9.4%) | 67 (16.7%) | 940 ( 9.2%) | ||
| Medicare | 4,854 (45.5%) | 194 (48.3%) | 4,660 (45.4%) | ||
| Uninsured/Unknown | 506 ( 4.7%) | 21 ( 5.2%) | 485 ( 4.7%) | ||
| Charlson Comorbidity score | 0 | 3,662 (34.3%) | 179 (44.5%) | 3,483 (33.9%) | <.0001 |
| 1 | 1,286 (12.0%) | 64 (15.9%) | 1,222 (11.9%) | ||
| 2 | 890 ( 8.3%) | 38 ( 9.5%) | 852 ( 8.3%) | ||
| 3 or higher | 1,140 (10.7%) | 50 (12.4%) | 1,090 (10.6%) | ||
| Unknown | 3,697 (34.6%) | 71 (17.7%) | 3,626 (35.3%) | ||
| Medical hormone therapy | No | 221 ( 2.1%) | 221 (55.0%) | 0 ( 0.0%) | <.0001 |
| Yes | 10,454 (97.9%) | 181 (45.0%) | 10,273 ( 100%) | ||
| Radiation | No | 8,339 (78.1%) | 325 (80.8%) | 8,014 (78.0%) | 0.1774 |
| Yes | 2,336 (21.9%) | 77 (19.2%) | 2,259 (22.0%) | ||
| Chemotherapy | No | 9,743 (91.3%) | 380 (94.5%) | 9,363 (91.1%) | 0.0183 |
| Yes | 932 ( 8.7%) | 22 ( 5.5%) | 910 ( 8.9%) | ||
| NCI-designated cancer center | No | 9,258 (86.7%) | 386 (96.0%) | 8,872 (86.4%) | <.0001 |
| Yes | 1,417 (13.3%) | 16 ( 4.0%) | 1,401 (13.6%) | ||
| MSSA designation | Rural or Frontier | 1,593 (14.9%) | 89 (22.1%) | 1,504 (14.6%) | <.0001 |
| Urban | 9,082 (85.1%) | 313 (77.9%) | 8,769 (85.4%) | ||
| Year of diagnosis | 2004–2007 | 3,135 (29.4%) | 193 (48.0%) | 2,942 (28.6%) | <.0001 |
| 2008–2011 | 3,277 (30.7%) | 119 (29.6%) | 3,158 (30.7%) | ||
| 2012–2015 | 4,263 (39.9%) | 90 (22.4%) | 4,173 (40.6%) |
Data for men with military or VA insurance (N=476) and other or unknown race (N=131) not shown per CCR confidentiality policy
Figure 1:
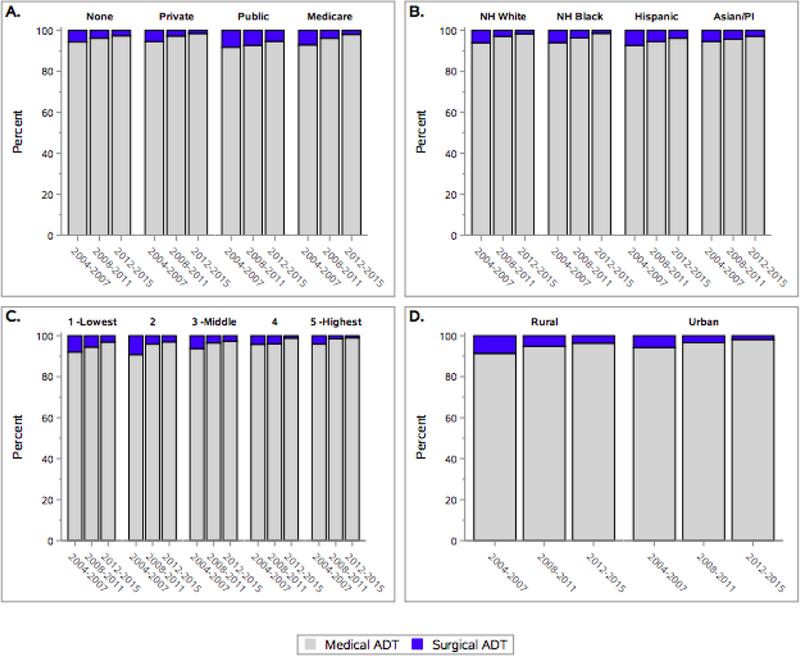
1a. Percent receipt of medical versus surgical ADT by healthcare payer over time*
1b. Percent receipt of medical versus surgical ADT by race/ethnicity over time*
1c. Percent receipt of medical versus surgical ADT by neighborhood socioeconomic status over time*
1d. Percent receipt of medical versus surgical ADT by rural versus urban setting over time*
*Men who did not receive any form of ADT were excluded.
Multivariable Analysis
In the overall model presented in Table 3 for the outcome receipt of bilateral orchiectomy as form of ADT (medical ADT as reference group), Hispanic ethnicity, older age, lower neighborhood SES, Medicaid/public insurance, residing in a rural MSSA, and unknown lymph node status were associated with an increased likelihood of having bilateral orchiectomy. Conversely, having organ confined disease (<T3), PSA 10–20 ng/ml, unknown Gleason score, military or VA insurance, unknown marital status, unknown comorbidity score, receipt of care at an NCI-designated cancer center, and later years of diagnosis were inversely associated with bilateral orchiectomy.
Table 3.
Association of patient, tumor, and neighborhood characteristics with receipt of orchiectomy as form of androgen deprivation for de novo M1 prostate cancer California Cancer Registry, 2004–2015
| Univariable model | Multivariable model | ||||
|---|---|---|---|---|---|
|
Odds ratio |
95% confidence interval |
Odds ratio |
95% confidence interval |
||
| Race/ethnicity | NH White | ref. | ref. | ||
| Asian/PI | 1.21 | ( 0.85 – 1.74) | 1.33 | ( 0.92 – 1.93) | |
| Hispanic | 1.60 | ( 1.26 – 2.04) | 1.32 | ( 1.02 – 1.72) | |
| NH Black | 1.04 | ( 0.74 – 1.45) | 0.96 | ( 0.67 – 1.37) | |
| other/unknown | 1.02 | ( 0.39 – 2.63) | 1.18 | ( 0.45 – 3.14) | |
| Age at diagnosis | Per year | 1.01 | ( 1.00 – 1.02) | 1.02 | ( 1.01 – 1.03) |
| Gleason score | Gleason ≤6 | 1.45 | ( 0.83 – 2.56) | 1.21 | ( 0.68 – 2.16) |
| Gleason 7 | 1.28 | ( 0.96 – 1.71) | 1.14 | ( 0.85 – 1.53) | |
| Gleason 8–10 | ref. | ref. | |||
| Unknown | 0.87 | ( 0.69 – 1.09) | 0.60 | ( 0.46 – 0.78) | |
| Clinical T stage | ≤T2a | 0.54 | ( 0.39 – 0.75) | 0.61 | ( 0.44 – 0.86) |
| T2b-T2c, T2NOS | 0.77 | ( 0.59 – 1.02) | 0.70 | ( 0.53 – 0.92) | |
| ≥T3a | ref. | ref. | |||
| TX | 0.93 | ( 0.71 – 1.21) | 0.89 | ( 0.66 – 1.20) | |
| N stage | N0 | 1.12 | ( 0.84 – 1.49) | 1.09 | ( 0.81 – 1.47) |
| N1 | ref. | ref. | |||
| NX | 2.20 | ( 1.66 – 2.92) | 1.63 | ( 1.21 – 2.20) | |
| PSA at diagnosis | <10 ng/ml | 0.61 | ( 0.39 – 0.95) | 0.76 | ( 0.48 – 1.19) |
| 10–20 ng/ml | 0.51 | ( 0.33 – 0.81) | 0.57 | ( 0.36 – 0.89) | |
| >20 ng/ml | ref. | ref. | |||
| Unknown | 1.05 | ( 0.73 – 1.49) | 0.94 | ( 0.66 – 1.36) | |
| Neighborhood SES quintile | 1 - lowest | 2.64 | ( 1.84 – 3.78) | 1.96 | ( 1.34 – 2.89) |
| 2 | 2.50 | ( 1.76 – 3.57) | 2.00 | ( 1.38 – 2.89) | |
| 3 | 1.98 | ( 1.38 – 2.83) | 1.70 | ( 1.18 – 2.46) | |
| 4 | 1.39 | ( 0.95 – 2.04) | 1.30 | ( 0.89 – 1.91) | |
| 5 - highest | ref. | ref. | |||
| Primary Payer | Private or insurance NOS | ref. | ref. | ||
| Medicaid/public | 2.30 | ( 1.69 – 3.12) | 2.21 | ( 1.58 – 3.10) | |
| Medicare | 1.33 | ( 1.06 – 1.69) | 1.09 | ( 0.85 – 1.39) | |
| Military, VA | 0.31 | ( 0.12 – 0.80) | 0.34 | ( 0.13 – 0.88) | |
| Uninsured/Unknown | 1.42 | ( 0.89 – 2.27) | 1.25 | ( 0.78 – 2.01) | |
| Marital Status | Married | ref. | ref. | ||
| Unmarried | 1.22 | ( 0.99 – 1.49) | 1.14 | ( 0.92 – 1.41) | |
| Unknown | 0.51 | ( 0.29 – 0.89) | 0.54 | ( 0.31 – 0.94) | |
| Charlson Comorbidity score | 0 | ref. | ref. | ||
| 1 | 1.02 | ( 0.76 – 1.37) | 0.98 | ( 0.73 – 1.32) | |
| 2 | 0.88 | ( 0.61 – 1.25) | 0.86 | ( 0.60 – 1.24) | |
| 3 or higher | 0.90 | ( 0.65 – 1.24) | 0.89 | ( 0.64 – 1.23) | |
| Unknown | 0.38 | ( 0.29 – 0.51) | 0.38 | ( 0.29 – 0.51) | |
| Radiation | Yes | 0.84 | ( 0.66 – 1.09) | 0.84 | ( 0.65 – 1.09) |
| No | ref. | ref. | |||
| MSSA designation | Urban | ref. | ref. | ||
| Rural or Frontier | 1.66 | ( 1.31 – 2.12) | 1.49 | ( 1.15 – 1.92) | |
| NCI-designated cancer center | Yes | 0.27 | ( 0.16 – 0.44) | 0.30 | ( 0.18 – 0.49) |
| No | ref. | ref. | |||
| Year of diagnosis | Per year | 0.86 | ( 0.84 – 0.89) | 0.87 | ( 0.85 – 0.90) |
Discussion
This study observed that race/ethnicity, neighborhood SES, and Medicaid/public insurance are significantly associated with utilization of orchiectomy over medical forms of ADT. These results are consistent with prior research suggesting that the choice of two treatments for prostate cancer that have identical efficacy may differ based on social factors such as SES, insurance, and race/ethnicity [27–29].
We observed in our univariable and multivariable models that Hispanic men with de novo metastatic disease were more likely to receive surgical ADT as type of initial therapy. While disparities in outcomes among Black men with advanced prostate cancer are well established [18], there is less known about Hispanic men with this lethal disease. Chinea and colleagues examined a large cohort of men with prostate cancer and observed that, even among Hispanic men, substantial ethnic heterogeneity exists and may influence prostate cancer specific mortality [30]. Unfortunately, in cancer registry data, more than 40% of Hispanic/Latino patients have unspecified Hispanic origin. Therefore future research will need to examine disaggregated race/ethnicity data to determine if differences in ADT strategy exist among different subgroups (e.g. Mexican American, Puerto Rican) and if cultural factors contribute to observed disparities.
In our study, we observed that Medicaid insured men were more likely to receive surgical ADT as part of initial treatment. Prior research has described that uninsured or Medicaid insured cancer patients may have worse outcomes compared to patients with private health insurance [31, 32]. Further research is needed to understand if the cumulative cost differential between surgical and medical ADT is a driver of differences in type of ADT received by insurance.
We observed that men from lower SES neighborhoods were more likely to have surgical ADT. Overall, the results of this study add to the growing body of literature characterizing the influence of contextual-level SES on outcomes among men with prostate cancer [33]. In prior research, the burden of travel has been shown to affect the receipt of medical care [34], and specifically cancer care [35–37]. Since individuals diagnosed in rural settings were more likely to receive surgical ADT, these results support the notion that travel burden may affect cancer treatment plans even in advanced disease. These data highlight the need to clarify if receipt of surgical ADT among patients from lower nSES areas were driven by patient preference, physician bias, or cost. As we lacked information on patient-level SES factors such as education and income, the contextual level measure of nSES may also reflect residual confounding by patient SES. Additionally, it is possible that utilization of surgical ADT is an indicator of physician bias or expectation of patient non-adherence. Therefore future effort to better understand the physician perspective in selecting ADT strategies for patients is necessary.
There are a few limitations of this study that are worth noting. The primary limitation is secondary to this study utilizing cancer registry data that may have potential under-ascertainment of the initial treatment received by patients. Prior research comparing cancer registry data to Medicare claims data revealed that cancer registries may under-ascertain systemic therapy, particularly chemotherapy [38]. However, in a recently completed SEER study that compared California cancer registry treatment data to electronic health record data, we found that the sensitivity for capturing hormone therapy in the setting of distant stage prostate cancer was 89%. In our study, given that twenty-two percent of patients did not have androgen deprivation therapy in the registry, a sensitivity analysis was performed to determine if the results were different if we assumed patients without documentation of androgen deprivation therapy were given medical ADT. This sensitivity analysis did not demonstrate an appreciable difference in results (data not shown). Therefore this suspected under-ascertainment is unlikely to affect the conclusions of this study.
This study also observed a large number of unknown Charlson comorbidity scores. The comorbidity scores are specifically derived from linkage to OSHPD discharge data. The availability of OSHPD data sources was expanded from inpatient discharge data to also include ambulatory surgery and emergency department discharges starting in 2005. The inverse association we observed with unknown comorbidity score likely reflects the difference in treatment setting for medical ADT (office/clinic) vs. surgical ADT (ambulatory or inpatient surgery), as unknown comorbidity score indicates men who had no contact with the California hospital system. Additionally, as expected, a higher Charlson comorbidity score was not associated with physician utilization of bilateral orchiectomy, reflecting that this surgical procedure is indeed minor and well-tolerated even in context of increasing co-morbidity.
Despite these limitations, this study has several strengths. To our knowledge, this is the first contemporary analysis of the type of ADT received by patients in a large population based sample, since life-long, continuous ADT became the standard of care for metastatic prostate cancer [11]. Most importantly, this study adds to the growing literature to characterize the contextual attributes that may contribute to disparities in outcomes in advanced prostate cancer.
Conclusions
This study highlights that the initial treatment that men with advanced prostate cancer receive may vary based on race/ethnicity. This contemporary analysis suggests that racial variation based on treatment received may be explained in part by cost and setting of diagnosis. Therefore these results underscore the role of cost in shaping the type of initial treatments patients receive. Future research will need to further characterize racial/ethnic differences in initial treatments patients with advanced prostate cancer receive in order to disentangle how these differences contribute to disparities in outcomes.
Acknowledgement:
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862–01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Footnotes
Conflict of interest disclosure: There are no disclaimers for this manuscript. This work was supported in part by the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California.
References
- 1.Sharifi N, Gulley JL, and Dahut WL, Androgen deprivation therapy for prostate cancer. JAMA, 2005. 294(2): p. 238–44. [DOI] [PubMed] [Google Scholar]
- 2.Lee DJ, et al. , Recent Changes in Prostate Cancer Screening Practices and Epidemiology. J Urol, 2017. 198(6): p. 1230–1240. [DOI] [PubMed] [Google Scholar]
- 3.Hu JC, et al. , Increase in Prostate Cancer Distant Metastases at Diagnosis in the United States. JAMA Oncol, 2017. 3(5): p. 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis L, et al. , Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol, 2018. 36(1): p. 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, et al. , Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med, 2017. 377(4): p. 352–360. [DOI] [PubMed] [Google Scholar]
- 6.Small EJ, Redefining Hormonal Therapy for Advanced Prostate Cancer: Results from the LATITUDE and STAMPEDE Studies. Cancer Cell, 2017. 32(3): p. 392. [DOI] [PubMed] [Google Scholar]
- 7.Huggins C, Effect of Orchiectomy and Irradiation on Cancer of the Prostate. Ann Surg, 1942. 115(6): p. 1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denmeade SR and Isaacs JT, A history of prostate cancer treatment. Nat Rev Cancer, 2002. 2(5): p. 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melton LJ 3rd, et al. , Decline in bilateral orchiectomy for prostate cancer in Olmsted county, Minnesota, 1956–2000. Mayo Clin Proc, 2001. 76(12): p. 1199–203. [DOI] [PubMed] [Google Scholar]
- 10.Crook J, The role of intermittent androgen suppression in biochemically recurrent or newly diagnosed metastatic prostate cancer. Curr Opin Support Palliat Care, 2013. 7(3): p. 258–64. [DOI] [PubMed] [Google Scholar]
- 11.Hussain M, et al. , Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med, 2013. 368(14): p. 1314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oefelein MG, et al. , Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology, 2000. 56(6): p. 1021–4. [DOI] [PubMed] [Google Scholar]
- 13.Nygard R, Norum J, and Due J, Goserelin (Zoladex) or orchiectomy in metastatic prostate cancer? A quality of life and cost-effectiveness analysis. Anticancer Res, 2001. 21(1B): p. 781–8. [PubMed] [Google Scholar]
- 14.Lucas MD, et al. , Quality of life, sexual functioning and sex role identity after surgical orchidectomy in patients with prostatic cancer. Scand J Urol Nephrol, 1995. 29(4): p. 497–500. [DOI] [PubMed] [Google Scholar]
- 15.Morrison BF, Aiken WD, and Reid ME, Impact of the National Health Fund policy on hormone treatment for prostate cancer in Jamaica. Rev Panam Salud Publica, 2011. 29(6): p. 404–8. [DOI] [PubMed] [Google Scholar]
- 16.Carson AP, et al. , Trends and racial differences in the use of androgen deprivation therapy for metastatic prostate cancer. J Pain Symptom Manage, 2010. 39(5): p. 872–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albain KS, et al. , Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst, 2009. 101(14): p. 984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antwi S, et al. , Racial disparities in survival after diagnosis of prostate cancer in Kentucky, 2001–2010. Am J Mens Health, 2013. 7(4): p. 306–16. [DOI] [PubMed] [Google Scholar]
- 19.NAACCR Race and Ethnicity Work Group, NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1] 2011, North American Association of Central Cancer Registries: Springfield (IL). [Google Scholar]
- 20.Yost K, et al. , Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control, 2001. 12(8): p. 703–11. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, et al. , Developing an area-based socioeconomic measure from American Community Survey data 2014, Cancer Prevention Institute of California: Fremont, CA. [Google Scholar]
- 22.Ca.gov. Medical Service Study Areas Medical Service Study Areas; 2018. March 28, 2018 [cited 2018 May 29, 2018]; Available from: https://www.oshpd.ca.gov/HWDD/MSSA.html. [Google Scholar]
- 23.Lichtensztajn DY, et al. , Comorbidity index in central cancer registries: the value of hospital discharge data. Clin Epidemiol, 2017. 9: p. 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firth D, Bias reduction of maximum likelihood estimates. Biometrika, 1993. 80(1): p. 27–38. [Google Scholar]
- 25.Heinze G and Schemper M, A solution to the problem of separation in logistic regression. Stat Med, 2002. 21(16): p. 2409–19. [DOI] [PubMed] [Google Scholar]
- 26.Bursac Z, et al. , Purposeful selection of variables in logistic regression. Source Code Biol Med, 2008. 3: p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moses KA, et al. , Racial/Ethnic Disparity in Treatment for Prostate Cancer: Does Cancer Severity Matter? Urology, 2017. 99: p. 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahal BA, et al. , Getting back to equal: The influence of insurance status on racial disparities in the treatment of African American men with high-risk prostate cancer. Urol Oncol, 2014. 32(8): p. 1285–1291. [DOI] [PubMed] [Google Scholar]
- 29.Ziehr DR, et al. , Income inequality and treatment of African American men with high-risk prostate cancer. Urol Oncol, 2015. 33(1): p. 18.e7–18.e13. [DOI] [PubMed] [Google Scholar]
- 30.Chinea FM, et al. , Ethnic heterogeneity and prostate cancer mortality in Hispanic/Latino men: a population-based study. Oncotarget, 2017. 8(41): p. 69709–69721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niu X, et al. , Cancer survival disparities by health insurance status. Cancer Med, 2013. 2(3): p. 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis L, et al. , Trends in Cancer Survival by Health Insurance Status in California From 1997 to 2014. JAMA Oncol, 2018. 4(3): p. 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeRouen MC, et al. , Impact of individual and neighborhood factors on disparities in prostate cancer survival. Cancer Epidemiol, 2018. 53: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probst JC, et al. , Effects of residence and race on burden of travel for care: cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res, 2007. 7: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onega T, et al. , Geographic access to cancer care in the U.S. Cancer, 2008. 112(4): p. 909–18. [DOI] [PubMed] [Google Scholar]
- 36.Schroen AT, et al. , Impact of patient distance to radiation therapy on mastectomy use in early-stage breast cancer patients. J Clin Oncol, 2005. 23(28): p. 7074–80. [DOI] [PubMed] [Google Scholar]
- 37.Borno HSA; Chang E; Zhang Li; Charles R, At what cost to clinical trial enrollment? A retrospective study of patient travel burden in cancer clinical trials. 2018: Under review, The Oncologist [DOI] [PMC free article] [PubMed]
- 38.Noone AM, et al. , Comparison of SEER Treatment Data With Medicare Claims. Med Care, 2016. 54(9): p. e55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


