Abstract
Introduction:
Electrophysiological measurements are used in longitudinal clinical studies to provide insight into the progression of amyotrophic lateral sclerosis (ALS) and the relationship between muscle weakness and motor unit degeneration. Here, we used a similar longitudinal approach in the SOD1(G93A) mouse model of ALS.
Methods:
In vivo muscle contractility and motor unit connectivity assays were assessed longitudinally in SOD1(G93A) and wildtype mice from post-natal day 35 to 119.
Results:
In SOD1(G93A) males, muscle contractility was reduced by day 35 and preceded motor unit loss. Muscle contractility and motor unit reduction were delayed in SOD1(G93A) females when compared with males, but as with males, muscle contractility reduction preceded motor unit loss.
Discussion:
The longitudinal contractility and connectivity paradigm employed here provides additional insight into the SOD1(G93A) mouse model and suggests that loss of muscle contractility is an early finding that may precede loss of motor units and motor neuron death.
Keywords: ALS, Motor unit, Electrophysiology, Connectivity, Contractility
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder affecting approximately 5 in 100,000 individuals [1, 2]. The disease is characterized by progressive loss of upper and lower motor neurons resulting in weakness, muscle denervation and atrophy, and eventual death, typically within 3–5 years of symptom onset.
Electrophysiological measures, including compound muscle action potential (CMAP) and motor unit number estimate (MUNE), allow in vivo assessment of the motor unit (MU) connectivity. Such measurements have been applied in clinical natural history studies of ALS to provide important insight into the interrelationship of muscle function and MU connectivity [3–7]. A powerful aspect of CMAP and MUNE measurements is that the functional status of the neuromuscular system can be tracked longitudinally to identify disease onset, severity, and rate of progression. Furthermore, MUNE can assess the response of the neuromuscular system to a therapeutic intervention, from the standpoint of preservation or regeneration of MU number as well as increased output from individual MUs (i.e., compensatory collateral reinnervation) [8–10]. As such, a number of clinical studies have leveraged electrophysiological measures to gain pathophysiological insight into longitudinal disease progression, including the timing and rate of MU loss, the ability of individual MUs to develop collateral sprouting, and the relationship between MU degeneration and onset of muscle weakness [4, 7, 11–13]. Dantes and McComas demonstrated ~50% losses of MUs six months after initial screening, followed by a rapid reduction in MUNE over one year—nearly halving every six months—before reduction slowed over the next 18 months [4]. This study also demonstrated a corresponding increase in the single motor unit potential (SMUP) amplitude, consistent with reinnervation by collateral axonal sprouting of surviving MUs—probably explaining why patients might present with absent or mild muscle weakness despite such a dramatic reduction in MUNE. Another early clinical study by Yuen and Olney measured CMAP and MUNE in addition to functional grip strength in ten ALS patients over the course of six months [7]. They reported a significant reduction in MUNE at three and six months after initial screening and single fiber EMG demonstrated increased mean fiber density of the abductor digiti minimi. During this time CMAP and grip strength did not reduce, which the authors argued as evidence for the compensatory ability of remaining motor neurons via collateral sprouting. Clinical studies that track the natural history of MU integrity during ALS, like the aforementioned, provide several useful insights, including potential treatment windows and underlying dynamic biological processes that could not otherwise be studied [14–17].
Overexpression of human SOD1 with a G93A mutation was used to develop the first mouse model of ALS in 1994 [18, 19]. A number of studies have investigated neuromuscular function in the SOD1(G93A) mouse model using either electrophysiological or physiological measures, [20–32]. Of particular interest are several cross-sectional disease progression studies of MU dysfunction [21–23, 32]. Hegedus and colleagues demonstrated fast-twitch MU loss as early as post-natal day 40 (P40) utilizing in situ muscle contractile measurements [21]. Mancuso and colleagues also demonstrated pre-symptomatic CMAP amplitude reduction by P56 [32]. One of the challenges to understanding the pre-symptomatic changes that occur in the MU has been the difficulty of following the relationships between physiological (muscle contractility; twitch torque and tetanic torque) and electrophysiological (MU connectivity; CMAP and MUNE) measures in individual animals over the course of the disease in a way that mimics the longitudinal clinical studies performed in humans. We have recently applied methodology to perform in vivo longitudinal assessments in mouse models of neuropathy [33], spinal muscular atrophy [34, 35], and aging [36] and, here, have applied these methods to the SOD1(G93A) mouse.
Materials and methods
Animals
All procedures were performed in accordance with NIH Guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of the Ohio State University. A total of 54 mice were used in this study for reliability testing and for the longitudinal analysis of disease progression in the SOD1(G93A) mouse model. Adult wildtype and SOD1(G93A) (C57BL/6xSJL/J; No: 002726) mice used for longitudinal studies and pathology were obtained from Jackson Laboratories (Bar Harbor, ME). Wildtype C57BL/6 mice used for intra-rater reliability testing were obtained from Taconic Biosciences (Albany, NY). All procedures were performed with blinded raters.
Procedures
Behavioral assessments.
Body weight was recorded prior to performing grip strength and rotarod [37, 38]. For grip strength, the average right hindlimb grip strength, measured in grams, was calculated from five measurements per assessment using a standard grip meter (DEFII-002, Chatillon, Largo, FL, USA). Mice were positioned to allow only the right hindpaw to grasp the grid and were pulled towards the evaluator for the length of the grip meter [39]. Attempts that were ≥ ±10g different than the other attempts were discarded and re-performed. The average of three motor coordination tests was measured using an accelerating rotarod (LE8205, Panlab Harvard Apparatus) starting at 5RPM. Trials were stopped if 120s passed without a fall.
Anesthesia and animal preparation.
Mice were anesthetized (isoflurane inhalation, 1.5–3%) during electrophysiological and muscle physiological recordings. Lubricant ointment was applied to the eyes to prevent corneal drying. All measurements were performed on the right hindlimb which was shaved with electric clippers prior to studies. Electrophysiological procedures were performed on a heated platform set at 37°C (World Precision Instruments, Sarasota, FL). During muscle contractility procedures, a warm water bath HTP-1500 Heat Therapy Pump set at 37°C was used to maintain temperature of the testing stage (Androit Medical Systems, Loudon, TN). Procedures under anesthesia were typically less than 20 minutes.
Electrophysiology.
CMAP and MUNE were recorded as previously described [34, 35, 40]. Briefly, an active ring electrode was placed superficially over the right triceps surae (gastrocnemius and soleus) and a reference ring electrode was placed superficially over the metatarsals of the right hindpaw (Alpine Biomed, Skovlunde, Denmark). A ground electrode was placed on the tail (Carefusion, Middleton, WI). The sciatic nerve was stimulated (0.1ms pulse, 1–10mA intensity) using two insulated monopolar needles (28G) (Teca, Oxford Instruments Medical, NY). CMAP amplitudes were recorded following supramaximal stimulation (20mV sensitivity, 10Hz low filter, 10kHz high filter). Baseline-to-peak amplitudes were used for comparison of CMAP amplitudes and peak-to-peak amplitudes were used for calculation of MUNE. SMUP was calculated by taking the average of 10 incremental submaximal responses (50µV-500µV sensitivity, 10Hz low filter, 10kHz high filter). MUNE was calculated by dividing the average SMUP amplitude (peak-to-peak) into the maximum peak-to-peak CMAP amplitude. Single fiber electromyography (SFEMG) was recorded, as previously described, in a separate cohort of SOD1(G93A) males (n=3 animals, 19 single muscle fiber action potentials) and wildtype males (n=3 animals, 13 single muscle fiber action potentials) at P35 [41].
Muscle contractility.
Following electrophysiological recordings, mice underwent triceps surae plantarflexion torque assessment using an in vivo muscle contractility apparatus (Model 1300A, Aurora Scientific Inc, Canada) (Supplemental Figure 1 and Supplemental Methods) as previously detailed [36]. Briefly, the right hindpaw was taped to the force sensor and positioned at 90°. The hindlimb was extended to position the knee in the locking position and secured at the femoral condyles. Two disposable monopolar electrodes were inserted near the tibial nerve, just posterior to the knee (Natus Neurology, Inc, Middleton, WI). Maximum plantarflexion twitch torque was recorded following a single, supramaximal stimulation (200µs square wave pulse). Maximum tetanic contraction torque was assessed following a train of supramaximal square wave stimulations at 200µs duration delivered at 125Hz stimulation frequency.
NMJ imaging and quantification.
To investigate the morphological correlates of loss of muscle contractility and reduced MU connectivity, the soleus muscle was collected from a separate cohort of SOD1(G93A) (3 males/3 females) and wildtype (3 males/3 females) mice for endpoint studies at P70 and fixed in 4% paraformaldehyde (PFA) at room temperature (RT) for 30min [42, 43]. Muscles were teased into fibers using size 55 forceps (Fine Science Tools) then incubated in blocking buffer (10% goat serum/4% BSA/3% triton-X 100/PBS) at RT for 2hr. An overnight (O/N) primary antibody (α-NF-200, Abcam, Ab72996, [1:5,000]) incubation at 4°C was performed followed by three 10min washes with PBS before receiving a 2hr incubation with secondary antibody (Alex 594 goat α-Chicken, Life Technologies, A11042, [1:1,000]) and α-α-Bungarotoxin-488 (Life Technologies, B13422, [1:1,000]) at RT. Samples then underwent three 10min washes with PBS at RT before being mounted onto Superfrost positively charged glass slides (Fisher Scientific) and sealed using Fluoromount-G (Southern Biotech). Samples were imaged at 20x and 40x magnification using a Leica confocal microscope (Leica DM IRE2) with Leica software (version 2.1). Images were viewed in FIJI (LOCI, University of Wisconsin-Madison) to quantify NMJ innervation, the co-labeling of NF-200 and α-Bungarotoxin. 95–120 NMJs per muscle sample (per mouse) were scored as fully innervated, partially innervated or denervated.
Experiments:
Intra-rater testing.
Intra-rater reliability was assessed for electrophysiology and muscle contractility. Sixteen wildtype mice (C57BL/6J; 7 males and 9 females) were assessed by an observer (CGW), with one day between trials. Reliability was reported using the intra-class correlation coefficient (ICC), which was defined as poor (ICC≤0.5), moderate (0.5<ICC≤0.75), good (0.75<ICC≤0.9) or excellent (ICC>0.9) [44].
Assessment of longitudinal SOD1(G93A) disease progression.
Raters (AEC, ABR) performed behavioral assessments weekly from P35 to P119 in a total of 20 mice [5 male/5female wildtype (C57BL/6J) and 5 male/5 female SOD1(G93A) (C57BL/6xSJL/J)]. Mice that displayed loss of the righting reflex for 30 seconds were euthanized for tissue harvesting and muscle wet weight recording [45]. Additional SOD1(G93A) (n=6) and wildtype (n=6) mice were sacrificed at P70, and right triceps surae muscles were collected, weighed and processed for NMJ quantification. A single evaluator (CGW) performed the longitudinal electrophysiological and muscle contractility.
Statistics
Intra-rater variability analyses were used to assess the degree of agreement between the electrophysiology and muscle contractility across all mice, over time. The R package irr (R version 3.3.2, The R Foundation for Statistical Computing) was used to perform these analyses.
A mixed effects model was used to model the mean of each of the six outcome variables for the longitudinal experiments. Fixed effects were included for the two groups, SOD1 mutants and wild type, and gender. A random intercept was used to account for repeated measures within mouse. Backward selection was used starting from an initial model with up to 3-way interactions. A square root transformation was used for CMAP, SMUP and MUNE. Furthermore, a quadratic time trend was included for CMAP, MUNE, and normalized twitch torque. Group means were compared at each time point at the 0.05 level using Holm’s Method to adjust for multiplicity within each outcome [46]. When group effects differed significantly by gender, comparisons were made within each gender. SAS 9.4 (Cary, NC) was used for the analysis. Unpaired t-test was performed using Graphpad Prism software (version 6) to compare jitter, muscle weight and NMJ quantification. Pearson’s correlation coefficients were calculated to determine correlations between: muscle contractility and MU connectivity, behavioral measurements and muscle contractility, and behavioral measurements and MU connectivity. Correlation coefficient strengths were interpreted using prior guidelines, negligible (0.0≤r≤0.3), weak (0.3<r≤0.5), moderate (0.5<r≤0.7), strong (0.7<r≤0.9) and very strong (0.9<r≤1.0) [47]. Statistical significance was set at p<0.05.
Results
Intra-rater reliability of CMAP, twitch torque and tetanic torque
CMAP and twitch measurements were moderately reliable with ICCs of 0.62 and 0.68, respectively, while tetanic measurements demonstrated good reliability with an ICC of 0.81.
Reduction of muscle contractility and of motor unit connectivity are early features in SOD1(G93A) mice
Muscle contractility and MU connectivity were assessed longitudinally from P35 to P119 in SOD1(G93A) (5 males/5 females) and wildtype (5 males/5 females) mice (Tables 1 and 2).
Table 1.
Longitudinal outcome measurements in SOD1(G93A) and wildtype male mice.
| Age (PND) |
CMAP (mV) (±StDv) |
MUNE (±StDv) |
SMUP (µV) (±StDv) |
Normalized Twitch (mN-m/g) (±StDv) |
Normalized Tetanic (mN-m/g) (±StDv) |
|---|---|---|---|---|---|
| 35 | 45.0 (8.0) | 383 (109) | 250.4 (26.6) | 0.127 (0.02) | 0.59 (0.064) |
| 34.02 (8.3) | 323 (119) | 258.7 (73.2) | 0.093 (0.027) | 0.48 (0.12) | |
| 42 | 41.7 (4.7) | 376 (118) | 233.8 (73.3) | 0.096 (0.014) | 0.57 (0.089) |
| 30.46 (3.8) | 359 (127) | 184.2 (49.3) | 0.083 (0.018) | 0.46 (0.17) | |
| 49 | 46.8 (13.1) | 433 (158) | 208.2 (49.4) | 0.123 (0.018) | 0.57 (0.080) |
| 45.38 (5.0) | 367 (70) | 236.7 (46.2) | 0.079 (0.011) | 0.38 (0.054) | |
| 56 | 43.8 (6.9) | 390 (47) | 204.3 (34.2) | 0.142 (0.016) | 0.59 (0.06) |
| 34.28 (2.8) | 317 (78) | 201.2 (12.7) | 0.083 (0.016) | 0.34 (0.046) | |
| 63 | 30.0 (16.5) | 239 (106) | 211.8 (29.7) | 0.089 (0.043) | 0.40 (0.21) |
| 25.84 (13.9) | 200 (113) | 226.3 (56.1) | 0.057 (0.035) | 0.26 (0.15) | |
| 70 | 29.4 (9.1) | 293 (148) | 213.0 (69.9) | 0.102 (0.039) | 0.45 (0.21) |
| 23.9 (17.7) | 147 (132) | 318.1 (87.6) | 0.074 (0.029) | 0.38 (0.14) | |
| 77 | 31.5 (9.0) | 255 (118) | 230.4 (61.7) | 0.146 (0.011) | 0.59 (0.088) |
| 17.46 (15.1) | 105 (81) | 302.5 (68.0) | 0.057 (0.034) | 0.25 (0.14) | |
| 84 | 43.3 (11.4) | 481 (150) | 170.8 (51.4) | 0.138 (0.012) | 0.58 (0.093) |
| 14.2 (8.9) | 105 (87) | 240.2 (63.0) | 0.060 (0.032) | 0.21 (0.049) | |
| 91 | 42.3 (12.7) | 390 (99) | 199.9 (50.0) | 0.144 (0.011) | 0.59 (0.080) |
| 12.46 (7.3) | 102 (103) | 227.9 (107.8) | 0.046 (0.027) | 0.19 (0.12) | |
| 98 | 44.9 (3.6) | 397 (88) | 184.9 (45.8) | 0.123 (0.01) | 0.57 (0.036) |
| 11.06 (8.7) | 78 (73) | 291.4 (108.2) | 0.037 (0.014) | 0.16 (0.038) | |
| 105 | 49.9 (9.8) | 385 (86) | 249.0 (43.4) | 0.135 (0.019) | 0.60 (0.090) |
| 9.72 (10.4) | 77 (107) | 228.5 (55.6) | 0.031 (0.020) | 0.14 (0.081) | |
| 112 | 48.5 (9.3) | 409 (100) | 205.5 (75.2) | 0.118 (0.01) | 0.58 (0.029) |
| 9.95 (12.3) | 59 (64) | 293.0 (50.3) | 0.031 (0.015) | 0.17 (0.072) | |
| 119 | 42.1 (5.0) | 407 (145) | 200.1 (59.1) | 0.131 (0.001) | 0.60 (0.050) |
| 9.0 (8.4) | 45 (42) | 351.3 (100.0) | 0.037 (0.023) | 0.18 (0.074) |
SOD1(G93A) male mice (shaded, n=5); wildtype male mice (unshaded, n=5). Mean outcome measurements with standard deviation (StDv) in brackets. Twitch and tetanic outcome measurements were normalized to mouse body mass. Abbreviations: Compound muscle action potential (CMAP), motor unit number estimation (MUNE), single motor unit potential (SMUP), and post-natal day (PND).
Table 2.
Longitudinal outcome measurements in SOD1(G93A) and wildtype female mice.
| Age (PND) |
CMAP (mV) (±StDv) |
MUNE (±StDv) |
SMUP (µV) (±StDv) |
Normalized Twitch (mN-m/g) (±StDv) |
Normalized Tetanic (mN-m/g) (±StDv) |
|---|---|---|---|---|---|
| 35 | 50.6 (9.4) | 373 (75) | 249.3 (50.1) | 0.112 (0.005) | 0.53 (0.017) |
| 45.8 (11.8) | 387 (32) | 219.7 (45.1) | 0.109 (0.018) | 0.50 (0.081) | |
| 42 | 33.4 (16.3) | 262 (121) | 245.7 (70.5) | 0.097(0.036) | 0.41 (0.17) |
| 38.0 (21.4) | 275 (123) | 233.5 (54.7) | 0.079 (0.014) | 0.38 (0.12) | |
| 49 | 41.7 (16.6) | 287 (110) | 253.7 (55.7) | 0.113 (0.023) | 0.57 (0.10) |
| 34.1 (12.9) | 291 (173) | 211.9 (43.8) | 0.096 (0.031) | 0.42 (0.097) | |
| 56 | 37.3 (14.3) | 309 (108) | 211.2 (26.2) | 0.119 (0.020) | 0.52 (0.088) |
| 33.5 (11.0) | 236 (101) | 248.8 (52.0) | 0.097 (0.019) | 0.44 (0.074) | |
| 63 | 38.4 (8.2) | 291 (55) | 252.5 (46.5) | 0.119 (0.019) | 0.51 (0.095) |
| 31.9 (8.3) | 203 (76) | 257.7 (68.1) | 0.092 (0.030) | 0.40 (0.081) | |
| 70 | 40.1 (9.0) | 311 (117) | 240.4 (62.9) | 0.123 (0.036) | 0.62 (0.11) |
| 34.5 (13.8) | 219 (58) | 256.5 (86.3) | 0.102 (0.026) | 0.45 (0.12) | |
| 77 | 44.6 (6.8) | 277 (30) | 260.9 (61.2) | 0.127 (0.021) | 0.60 (0.068) |
| 33.3 (10.1) | 174 (77) | 333.3 (90.9) | 0.094 (0.013) | 0.40 (0.14) | |
| 84 | 41.5 (6.8) | 339 (141) | 231.5 (67.1) | 0.134 (0.014) | 0.62 (0.11) |
| 32.0 (8.9) | 196 (40) | 266.4 (52.6) | 0.106 (0.019) | 0.47 (0.11) | |
| 91 | 38.5 (9.5) | 407 (94) | 176.53 (28.3) | 0.135 (0.012) | 0.58 (0.062) |
| 31.2 (14.9) | 194 (90) | 301.5 (110.6) | 0.090 (0.014) | 0.43 (0.079) | |
| 98 | 42.4 (8.8) | 400 (91) | 172.5 (26.3) | 0.119 (0.016) | 0.55 (0.059) |
| 21.2 (9.5) | 156 (76) | 264.7 (73.3) | 0.081 (0.017) | 0.36 (0.086) | |
| 105 | 39.6 (5.8) | 391 (103) | 178.8 (37.3) | 0.119 (0.015) | 0.56 (0.11) |
| 21.7 (8.9) | 125 (23) | 291.6 (77.9) | 0.072 (0.034) | 0.28 (0.14) | |
| 112 | 45.5 (14.7) | 319 (35) | 218.9 (48.8) | 0.115 (0.010) | 0.55 (0.022) |
| 14.7 (4.0) | 83 (17) | 329.8 (118.2) | 0.062 (0.023) | 0.26 (0.061) | |
| 119 | 44.9 (14.3) | 347 (47) | 225.0 (30.4) | 0.134 (0.014) | 0.64 (0.043) |
| 13.8 (1.9) | 70 (14) | 328.0 (82.9) | 0.052 (0.022) | 0.23 (0.081) |
SOD1(G93A) female mice (shaded, n=5); wildtype female mice (unshaded, n=5). Mean outcome measurements with standard deviation (StDv) in brackets. Twitch and tetanic outcome measurements were normalized to mouse body mass. Abbreviations: Compound muscle action potential (CMAP), motor unit number estimation (MUNE), single motor unit potential (SMUP), and post-natal day (PND).
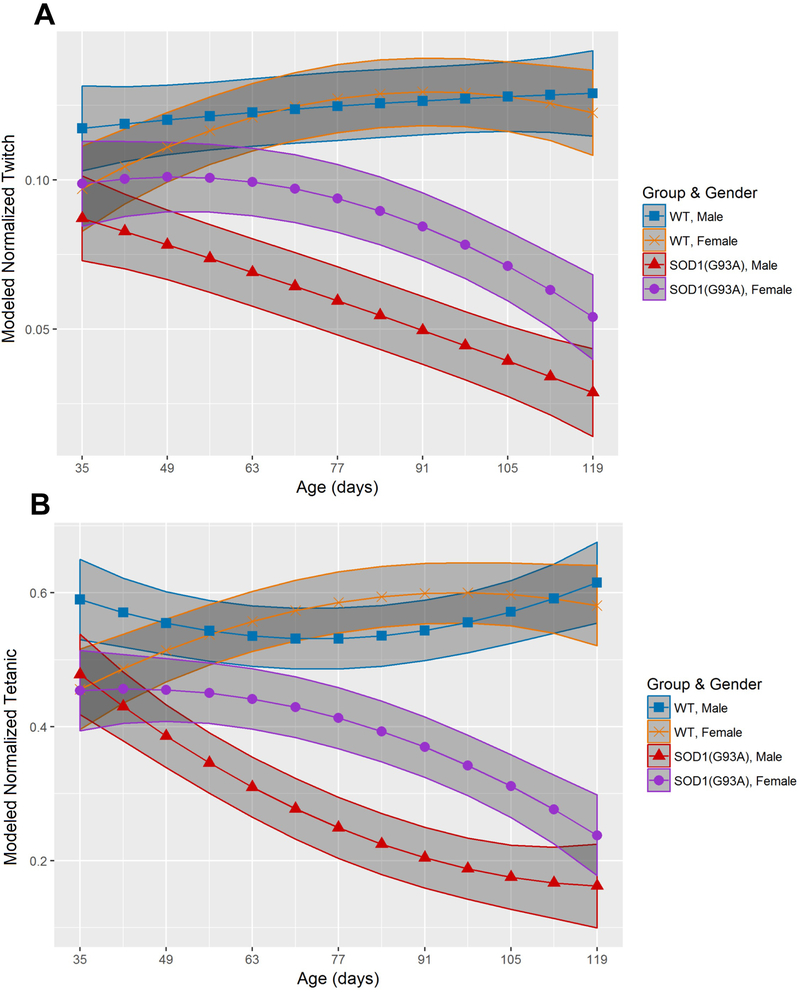
Mixed effects models for longitudinal muscle contractility and MU connectivity reduction were used from age P35 to P119 in separate cohorts of male and female mice (Figure 1 and Figure 2, Supplemental tables 1 and 2). Reduced muscle contractility preceded MU connectivity reduction in both male and female SOD1(G93A) mice. Normalized twitch torque in SOD1(G93A) males (0.087mN-m/g, 95% CI: 0.073–0.101mN-m/g) was reduced compared to wildtype males (0.117mN-m/g, 95% CI: 0.103–0.131mN-m/g) at the start of the study (P35) (Figure 1A). Normalized tetanic torque was also reduced at P35 in SOD1(G93A) males (0.48mN-m/g, 95% CI: 0.42–0.54mN-m/g) compared to wildtype males (0.59mN-m/g, 95% CI:0.53–0.65mN-m/g) (Figure 1B). Normalized twitch torque was reduced at P63 in SOD1(G93A) female mice (0.099mN-m/g, 95% CI: 0.088–0.111mN-m/g) compared to wildtype female mice (0.121mN-m/g, 95% CI: 0.110–0.132mN-m/g), whereas normalized tetanic torque was reduced by P56 in SOD1(G03A) females (0.45mN-m/g, 95% CI: 0.41–0.50mN-m/g) relative to wildtype females (0.54mN-m/g, 95% CI: 0.49–0.58mN-m/g) (Figure 1A-B).
Figure 1.
SOD1(G93A) males demonstrate earlier muscle contractility reduction than SOD1(G93A) females. (A) Modeled twitch torque (normalized to body mass) (mN-m/g) outcomes of wildtype (WT) male mice (blue square, n=5), wildtype female mice (orange x, n=5), SOD1(G93A) male mice (red triangle, n=5) and SOD1 female mice (purple circle, n=5). (B) Modeled tetanic torque (normalized to body mass) (mN-m/g) outcomes in wildtype and SOD1(G93A) cohorts, organized by gender. Shaded regions depict 95% confidence interval.
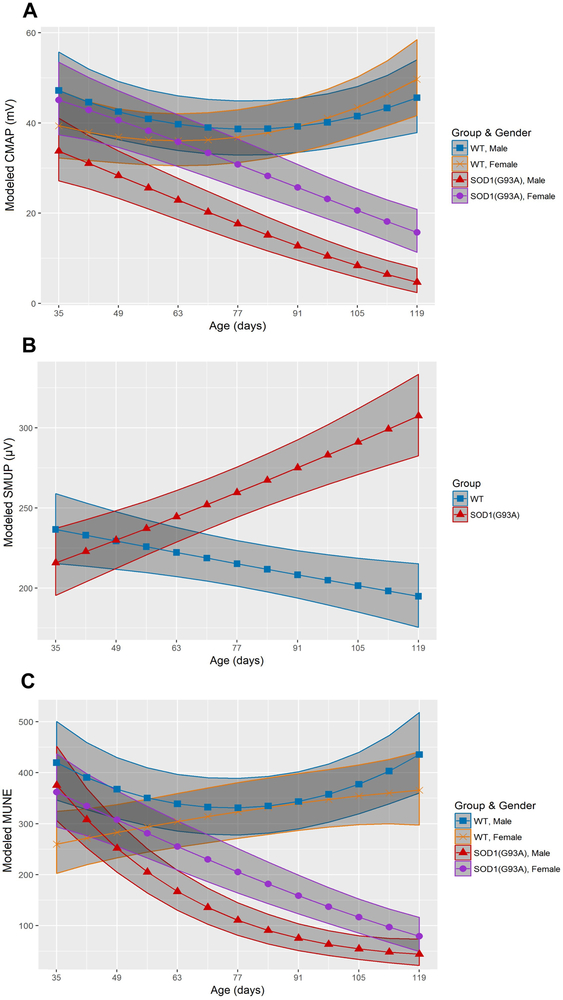
Figure 2.
SOD1(G93A) males demonstrate earlier MU connectivity reduction than SOD1(G93A) females. (A) Longitudinal modeled CMAP (mV) of wildtype male mice (blue square, n=5), wildtype female mice (orange x, n=5), SOD1(G93A) male mice (red triangle, n=5) and SOD1 female mice (purple circle, n=5). (B) Modeled SMUP (µV) of wildtype mice (blue square, n=10) and SOD1(G93A) mutants (red triangle, n=10). (C) Modeled MUNE of wildtype male mice, wildtype female mice, SOD1(G93A) male mice and SOD1(G93A) female mice. Shaded regions depict 95% confidence interval. Abbreviations: compound muscle action potential (CMAP), single motor unit potential (SMUP), motor unit number estimation (MUNE), and wildtype (WT).
CMAP reduction occurred at P42 in SOD1(G93A) males (31.0mV, 95% CI: 25.4–37.2mV) compared to wildtype male mice (44.6mV, 95% CI: 37.8–51.9mV) and at P91 in SOD1(G93A) females (25.7mV, 95% CI: 21.1–30.8mV) compared to wildtype females (39.2mV, 95% CI: 33.4–45.5mV) (Figure 2A). MUNE reduction in SOD1(G93A) males (252, 95% CI: 205–304) relative to wildtype males 368, 95% CI: 310–430) occurred at P49 and at P77 in SOD1(G93A) females (205, 95% CI: 164–251) compared to wildtype females (323, 95% CI: 271–381) (Figure 2B). There was no observed sexual dimorphism in SMUP, with increases occurring at P70 for both SOD1(G93A) males and females (252.0µV, 95% CI: 236.6–267.9µV) relative to wildtype males and females (218.7µV, 95% CI: 204.3–233.5µV) (Figure 2C). Single fiber electromyography (SFEMG) was performed to exclude the possibility that failure of NMJ transmission could be the explanation for early contractility reduction in SOD1 mice. Separate cohorts of SOD1(G93A) males and wildtype males were studied with SFEMG in the gastrocnemius at P35 to assess NMJ integrity. There was no difference in jitter between SOD1(G93A) males (8.02±2.29µs; range: 4.01–12.27µs and wildtype males (8.38±2.45µs; range 5.07–12.57µs) (p=0.669). Table 3 summarizes and compares onset of MU degeneration and reduced muscle contractility.
Table 3.
Onset of muscle contractility and MU connectivity reduction
| Post-natal Day | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOD1(G93A) vs WT |
35 | 42 | 49 | 56 | 63 | 70 | 77 | 84 | 91 | 98 | 105 | 112 | 119 | |
| Twitch | Males | 0.0079→ | ||||||||||||
| Females | - | - | - | - | 0.0427→ | |||||||||
| Tetanic | Males | 0.0119→ | ||||||||||||
| Females | - | - | - | 0.0326→ | ||||||||||
| CMAP | Males | - | 0.04→ | |||||||||||
| Females | - | - | - | - | - | - | - | - | 0.018→ | |||||
| SMUP | Males | - | - | - | - | 0.0244→ | ||||||||
| Females | - | - | - | - | 0.0244→ | |||||||||
| MUNE | Males | - | - | 0.0382→ | ||||||||||
| Females | - | - | - | - | - | - | 0.0157→ | |||||||
Abbreviations: wildtype (WT), compound muscle action potential (CMAP), single motor unit potential (SMUP), motor unit number estimation (MUNE). Dashes represent no significant difference, arrows indicate that difference was significant for duration of study.
Muscle atrophy occurs after loss of muscle contractility
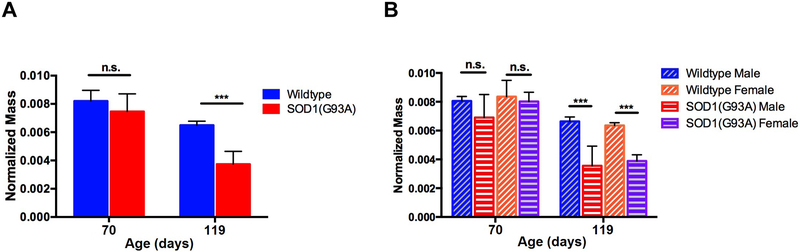
We assessed wet triceps surae muscle mass (normalized to body mass) to examine whether the early findings of reduced muscle contractility and loss of MU connectivity were associated with coexistent loss of muscle mass. Separate groups of wildtype and SOD1(G93A) were sacrificed at P70, in addition to the longitudinal cohorts at P119, and their right triceps surae muscles were harvested and weighed (Figure 3). There was no change in the relative percentage of denervated soleus NMJs between SOD1(G93A) and wildtype mice at P70 (Supplemental Figure 2). Despite reduction of both muscle contractility and MU connectivity there were no overt differences in normalized triceps surae wet mass at P70 between wildtype and SOD1(G93A) mice (Figure 3A). At P119, normalized triceps surae wet mass was reduced in SOD1(G93A) mice compared to wildtype mice (Figure 3A). When we organized normalized triceps surae wet mass by sex at P70, there was no difference in normalized triceps surae mass between SOD1(G93A) males relative to wildtype males nor was there difference between SOD1(G93A) female and wildtype female (Figure 3B). At P119, normalized triceps surae mass was reduced in SOD1(G93A) males compared to wildtype males as well as in SOD1(G93A) females compared to wildtype females (Figure 3B).
Figure 3.
Normalized triceps surae wet mass at P70 and P119. (A) Normalized triceps surae mass at P70 (wildtype: 0.0082g±0.0008g, n=6 vs SOD1(G93A): 0.0075g±0.00130g, n=6; p=0.123) and P119 (wildtype: 0.0065g±0.0003g, n=10 vs SOD1(G93A): 0.0037g±0.0009g, n=9). (B) Normalized triceps surae wet mass by gender at P70 (wildtype males: 0.0081g±0.0003g, n=3 vs SOD1(G93A) males: 0.0069g±0.0016g n=3, p=0.19; wildtype females: 0.0084g±0.0011g, n=3 vs SOD1(G93A) females: 0.0083g±0.0006g, n=3, p=0.52) and P119 (wildtype males: 0.0066g±0.0003g, n=5 vs SOD1(G93A) males: 0.0035g±0.0014g, n=4; wildtype females: 0.0064g±0.0002g, n=5 vs SOD1(G93A) females: 0.0039g±0.0004g, n=5). Error bars denote standard deviation. n.s.=no significance, *** = p<0.001.
Correlations between MU connectivity, muscle contractility, and behavioral assessments
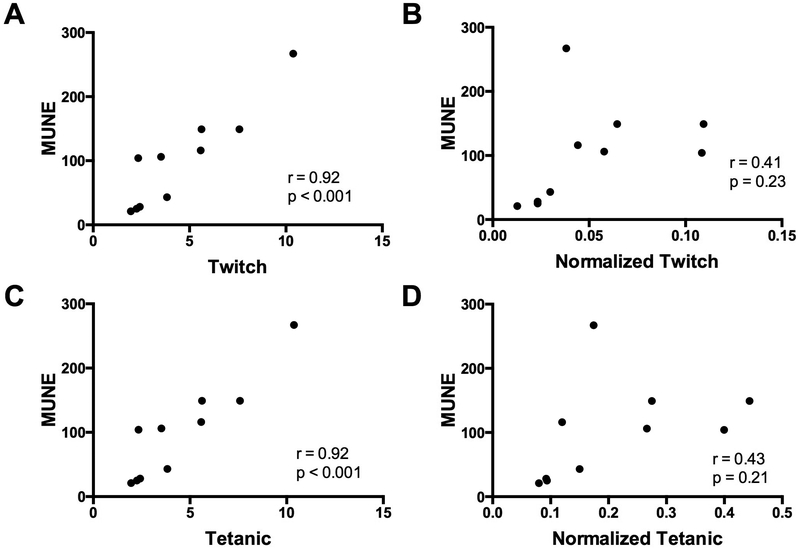
Correlations were analyzed between electrophysiological measures, muscle contractility, and grip strength at week 15. MUNE showed a very strong positive correlation with absolute twitch torque and absolute tetanic torque (Figure 4A and 4C), but MUNE showed no significant correlation with normalized twitch and tetanic torque (Figure 4B and 4D). CMAP demonstrated a strong positive correlation with both absolute twitch torque (r=0.84, p<0.01) and absolute tetanic torque (r=0.76, p<0.05) and moderately positive correlated with both normalized twitch torque (r=0.68, p<0.05) and normalized tetanic torque (r=0.65, p<0.05) (not shown). Grip strength was strongly correlated with CMAP (r=0.82, p<0.01) and MUNE (r=0.71, p<0.05) and moderately correlated with absolute twitch torque (r=0.66, p<0.05) (not shown). There was no significant correlation for grip compared with absolute tetanic torque (r=0.579, p=0.079), normalized twitch torque (r=0.513, p=0.130) and normalized tetanic torque (r=0.516, p=0.127) (not shown).
Figure 4.
Correlations of muscle contractility with MUNE. (A-B) Correlation of Motor Unit Number (MUNE) with (A) absolute twitch torque and (B) normalized twitch torque. (C-D) Correlation of MUNE with (C) absolute twitch torque and (D) normalized tetanic torque. Abbreviations: motor unit number estimation (MUNE).
Discussion
In the SOD1(G93A) mouse model, overt behavioral disease onset is typically observed at approximately P90 [18, 48]. However, behavioral measurements used in SOD1(G93A) mice are limited in their ability to directly and accurately measure MU integrity. A number of physiological and pathological cross-sectional studies that directly assess the MU demonstrated earlier phenotypic features relative to typical behavioral disease onset [20, 21, 23, 32, 49]. Fast fatigable muscles have increased vulnerability to denervation and are less efficient at maintaining NMJ collateral reinnervation [49]. Accordingly, in situ contractility analyses of isolated fast fatigable muscles by Hegedus and colleagues demonstrated MU loss and corresponding contractile weakness in male mice as early as P40 [21]. Similarly, our results identified reduction of muscle contractility as early as P35 using non-invasive muscle contractility measurements. In contrast to prior studies, muscle contractility was assessed in the intact triceps surae muscle, which includes the gastrocnemius, a muscle predominantly comprised of fast-fatigable muscle fibers, and the soleus, predominantly composed of slow-fatigue resistant muscle fibers. Using intact muscle groups with mixed muscle fiber types closely mirrors clinical muscle testing in patients in which multiple muscles are tested together [50].
Our results show that reduced contractility occurs before reduction of MU connectivity in both SOD1(G93A) males and females. Importantly, in vivo muscle contractility measurements require intact and functioning motor axons, neuromuscular junctions and muscle excitability contraction coupling. Therefore, it was important to consider whether early NMJ degeneration might explain early twitch and tetanic muscle contraction torque losses. We found several results in our study arguing against this possibility. First, we assessed NMJ transmission using SFEMG which is the most sensitive measure of NMJ transmission in vivo [41]. SFEMG at P35 (when the twitch and tetanic muscle contractions were reduced) was unchanged in mutant versus wildtype male mice. Furthermore, twitch and CMAP responses are both measured following a single supramaximal nerve stimulation, and our results showed that reduced twitch occurred prior to CMAP was reduced in both SOD1(G93A) males and females by 7 days and 28 days respectively. Together, the discrepant twitch and CMAP findings, along with our SFEMG results, support excitation-contraction decoupling, and not NMJ transmission failure, as the cause of early contractility reduction. Lastly, if muscle contractility precedes MU connectivity reduction, we would expect to also see no muscle atrophy related to denervation. In our studies, wet muscle weight did not reveal triceps surae (gastrocnemius and soleus) atrophy at P70 when muscle contractility was already reduced, suggesting that loss of muscle contractility was not simply related to muscle size.
Our findings of early reduction of muscle contractility (prior to SFEMG abnormalities and CMAP and MUNE reduction) suggest early subsarcolemmal abnormalities resulting in excitation-contraction decoupling. Prior studies have suggested muscle specific defects in ALS patients and SOD1(G93A) mice [51–54]. Increased oxidative stress in the sarcolemma and the sarcoplasmic reticulum have also been observed in SOD1(G93A) mice [54, 55]. The increased oxidative stress may result in muscle excitation-contraction decoupling, possibly through abnormalities of calcium regulation, myofilament function or ATP production [55, 56]. One protein critical for excitation-contraction coupling which may be negatively impacted to produce early contractile reduction is sarcoplasmic reticulum Ca2+ ATPase (SERCA). SERCA pump activity is diminished following increased oxidative stress, with the intracellular Ca2+ imbalances resulting in muscle contractile weakness [57, 58].
Sexual dimorphism observed in our studies is consistent with previous reports that SOD1(G93A) males exhibit earlier disease onset compared to their female counterparts in C57BL/6xSJL/J mice [59, 60]. Sex differences appear to be background strain specific. Male SOD1(G93A) mice on the strain used in this work (C57BL/6xSJL/J) as well as SJL have been shown to present earlier symptom onset compared to females, whereas male SOD1(G93A) on C57BL/6 exhibit no sexual differences [59]. A previous study utilized mice generated from SOD1(G93A) C57BL/6xSJL/6 crossed with wildtype C57BL/6 mice and demonstrated no sex-specific differences in MU number loss or in muscle contractile force deficits [22]. The potential differences in the background strains of SOD1(G93A) mice used may account for the discrepant results.
Our paradigm is strengthened by the capacity for repeat measures of muscle contractility and MU connectivity in individual animals. Previous studies of MU reduction in SOD1(G93A) mice were performed via a cross-sectional paradigm [21–23, 32]. While such cross-sectional analyses allow examination of aggregate differences over time, these do not permit consideration of disease trajectories of individual mice. Utilizing a longitudinal approach allowed us to assess disease progression in individual mice over time and legitimize the approach to use statistical modeling of the disease time course to properly account for heterogeneity between mice. Our results suggest that longitudinal assessments of in vivo muscle contractility in conjunction with MU connectivity may be a powerful readout for pre-clinical drug testing in this model as well as other models of neuromuscular disease.
The development of meaningful outcome measures for ALS is critically important. The methodology for measuring muscle contractility presented herein as a physiological outcome measure shares qualities of a good physiological biomarker, in that it is reproducible, minimally-invasive, easy to obtain and has the capacity to make longitudinal measures [61]. Rater reliability results are in line with intra-rater reliabilities of muscle function and MU connectivity measurements in clinical studies (ICCs ranging from 0.55 to 0.99) [62–67] and pre-clinical studies utilizing MU connectivity measurements in mice (ICCs ranging from 0.56 to 0.76) [68]. Muscle contractility measurements following nerve stimulation could be performed alongside current strength tests in people with ALS, such as hand-held dynamometers, with the added benefit of not being impacted by the strength of the evaluator [69].
In conclusion, when combined with longitudinal MU connectivity measurements, muscle contractility measurements allow a more complete analysis of MU innervation and functional status in degenerative models. Early muscle contractility dysfunction occurred prior to MU connectivity deficits and may precede neuronal death in SOD1(G93A) mice. Thus, the design and execution of pre-clinical studies using SOD1(G93A) mice can be enhanced through this paradigm. Moreover, we believe that muscle contractility outcome measures have the potential to be directly applied in patients to detect early changes in contractility.
Supplementary Material
Acknowledgements:
We would like to thank the Neuroscience Imaging Core at Ohio State University and Paula Monsma for their technical expertise in acquiring NMJ images. We would also like to thank Matthew Borkowski and the technical support staff at Aurora Scientific, Inc for their assistance in setting up the muscle contractility rig.
This project was funded by the National Institutes of Health/National Institute on Aging [R03AG050877], The Neurological Research Institute at The Ohio State Wexner Medical Center, the Julie Bonasera Fund for ALS and Neuromuscular Diseases and the Fred F. and Herman M. Dreier ALS Fund.
Abbreviations:
- ALS
amyotrophic lateral sclerosis
- CMAP
compound muscle action potentia
- ICC
intra-class coefficient
- MU
motor unit
- MUNE
motor unit number estimation
- O/N
overnight
- PFA
paraformaldehyde
- RT
room temperature
- SFEMG
single fiber electromyography
- SMUP
single motor unit potential
- SOD1
Cu/Zn superoxide dismutase
Footnotes
Ethical Publication Statement:
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Disclosure of Conflicts of Interest:
None of the authors has any conflict of interest to disclose
References
- 1.Al-Chalabi A and Hardiman O, The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol, 2013. 9(11): p. 617–28. [DOI] [PubMed] [Google Scholar]
- 2.Talbot K, Clinical tool for predicting survival in ALS: do we need one? J Neurol Neurosurg Psychiatry, 2016. 87(12): p. 1275. [DOI] [PubMed] [Google Scholar]
- 3.Arasaki K, et al. , Longitudinal study of functional spinal alpha motor neuron loss in amyotrophic lateral sclerosis. Muscle Nerve, 2002. 25(4): p. 520–6. [DOI] [PubMed] [Google Scholar]
- 4.Dantes M and McComas A, The extent and time course of motoneuron involvement in amyotrophic lateral sclerosis. Muscle Nerve, 1991. 14(5): p. 416–21. [DOI] [PubMed] [Google Scholar]
- 5.Felice KJ, Thenar Motor Unit Number Estimates Using the Multiple Point Stimulation Technique - Reproducibility Studies in Als Patients and Normal Subjects. Muscle & Nerve, 1995. 18(12): p. 1412–1416. [DOI] [PubMed] [Google Scholar]
- 6.Kelly JJ Jr., et al. , Use of electrophysiologic tests to measure disease progression in ALS therapeutic trials. Muscle Nerve, 1990. 13(6): p. 471–9. [DOI] [PubMed] [Google Scholar]
- 7.Yuen EC and Olney RK, Longitudinal study of fiber density and motor unit number estimate in patients with amyotrophic lateral sclerosis. Neurology, 1997. 49(2): p. 573–8. [DOI] [PubMed] [Google Scholar]
- 8.Gooch CL, The canaries in the coal mine: mune and munix in amyotrophic lateral sclerosis. Muscle Nerve, 2017. 56(2): p. 183–184. [DOI] [PubMed] [Google Scholar]
- 9.Gooch CL, et al. , Motor unit number estimation: a technology and literature review. Muscle Nerve, 2014. 50(6): p. 884–93. [DOI] [PubMed] [Google Scholar]
- 10.Gooch CL and Shefner JM, ALS surrogate markers. MUNE. Amyotroph Lateral Scler Other Motor Neuron Disord, 2004. 5 Suppl 1: p. 104–7. [DOI] [PubMed] [Google Scholar]
- 11.Armon C and Brandstater ME, Motor unit number estimate-based rates of progression of ALS predict patient survival. Muscle Nerve, 1999. 22(11): p. 1571–5. [DOI] [PubMed] [Google Scholar]
- 12.Carleton SA and Brown WF, Changes in motor unit populations in motor neurone disease. J Neurol Neurosurg Psychiatry, 1979. 42(1): p. 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen S and Ballantyne JP, A quantitative electrophysiological study of motor neurone disease. J Neurol Neurosurg Psychiatry, 1978. 41(9): p. 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutkove SB, Clinical Measures of Disease Progression in Amyotrophic Lateral Sclerosis. Neurotherapeutics, 2015. 12(2): p. 384–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shefner JM, et al. , Quantitative strength testing in ALS clinical trials. Neurology, 2016. 87(6): p. 617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon NG, et al. , Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol, 2014. 76(5): p. 643–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Eijk RPA, et al. , Monitoring disease progression with plasma creatinine in amyotrophic lateral sclerosis clinical trials. J Neurol Neurosurg Psychiatry, 2018. 89(2): p. 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurney ME, Transgenic-mouse model of amyotrophic lateral sclerosis. N Engl J Med, 1994. 331(25): p. 1721–2. [DOI] [PubMed] [Google Scholar]
- 19.Zou ZY, et al. , Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry, 2017. 88(7): p. 540–549. [DOI] [PubMed] [Google Scholar]
- 20.Dibaj P, Schomburg ED, and Steffens H, Contractile characteristics of gastrocnemius-soleus muscle in the SOD1G93A ALS mouse model. Neurol Res, 2015. 37(8): p. 693–702. [DOI] [PubMed] [Google Scholar]
- 21.Hegedus J, Putman CT, and Gordon T, Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis, 2007. 28(2): p. 154–64. [DOI] [PubMed] [Google Scholar]
- 22.Hegedus J, Putman CT, and Gordon T, Progressive motor unit loss in the G93A mouse model of amyotrophic lateral sclerosis is unaffected by gender. Muscle Nerve, 2009. 39(3): p. 318–27. [DOI] [PubMed] [Google Scholar]
- 23.Hegedus J, et al. , Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol, 2008. 586(14): p. 3337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shefner JM, et al. , Effect of neurophilin ligands on motor units in mice with SOD1 ALS mutations. Neurology, 2001. 57(10): p. 1857–61. [DOI] [PubMed] [Google Scholar]
- 25.Shefner JM, Cudkowicz ME, and Brown RH Jr., Comparison of incremental with multipoint MUNE methods in transgenic ALS mice. Muscle Nerve, 2002. 25(1): p. 39–42. [DOI] [PubMed] [Google Scholar]
- 26.Shefner JM, Cudkowicz M, and Brown RH Jr., Motor unit number estimation predicts disease onset and survival in a transgenic mouse model of amyotrophic lateral sclerosis. Muscle Nerve, 2006. 34(5): p. 603–7. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C, et al. , A method comparison in monitoring disease progression of G93A mouse model of ALS. Amyotroph Lateral Scler, 2007. 8(6): p. 366–72. [DOI] [PubMed] [Google Scholar]
- 28.Mancuso R, et al. , Electrophysiological analysis of a murine model of motoneuron disease. Clin Neurophysiol, 2011. 122(8): p. 1660–70. [DOI] [PubMed] [Google Scholar]
- 29.Ngo ST, et al. , The relationship between Bayesian motor unit number estimation and histological measurements of motor neurons in wild-type and SOD1(G93A) mice. Clin Neurophysiol, 2012. 123(10): p. 2080–91. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Sung M, and Rutkove SB, Electrophysiologic biomarkers for assessing disease progression and the effect of riluzole in SOD1 G93A ALS mice. PLoS One, 2013. 8(6): p. e65976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, et al. , Single and modeled multifrequency electrical impedance myography parameters and their relationship to force production in the ALS SOD1G93A mouse. Amyotroph Lateral Scler Frontotemporal Degener, 2016. 17(5–6): p. 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancuso R, Osta R, and Navarro X, Presymptomatic electrophysiological tests predict clinical onset and survival in SOD1(G93A) ALS mice. Muscle Nerve, 2014. 50(6): p. 943–9. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava AK, et al. , Mutant HSPB1 overexpression in neurons is sufficient to cause age-related motor neuronopathy in mice. Neurobiol Dis, 2012. 47(2): p. 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnold W, et al. , The neuromuscular impact of symptomatic SMN restoration in a mouse model of spinal muscular atrophy. Neurobiol Dis, 2016. 87: p. 116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold WD, et al. , Electrophysiological Biomarkers in Spinal Muscular Atrophy: Preclinical Proof of Concept. Ann Clin Transl Neurol, 2014. 1(1): p. 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheth KA, et al. , Muscle strength and size are associated with motor unit connectivity in aged mice. Neurobiol Aging, 2018. 67: p. 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frakes AE, et al. , Additive amelioration of ALS by co-targeting independent pathogenic mechanisms. Ann Clin Transl Neurol, 2017. 4(2): p. 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song S, et al. , Major histocompatibility complex class I molecules protect motor neurons from astrocyte-induced toxicity in amyotrophic lateral sclerosis. Nat Med, 2016. 22(4): p. 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller TM, et al. , Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A, 2006. 103(51): p. 19546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnold WD, et al. , Electrophysiological Motor Unit Number Estimation (MUNE) Measuring Compound Muscle Action Potential (CMAP) in Mouse Hindlimb Muscles. J Vis Exp, 2015(103). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gooch CL and Mosier DR, Stimulated single fiber electromyography in the mouse: techniques and normative data. Muscle Nerve, 2001. 24(7): p. 941–5. [DOI] [PubMed] [Google Scholar]
- 42.Chai RJ, et al. , Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One, 2011. 6(12): p. e28090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng A, et al. , Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLoS One, 2013. 8(7): p. e67970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koo TK and Li MY, A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med, 2016. 15(2): p. 155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foust KD, et al. , Therapeutic AAV9-mediated suppression of mutant SOD1 slows disease progression and extends survival in models of inherited ALS. Mol Ther, 2013. 21(12): p. 2148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holm S, A Simple Sequentially Rejective Multiple Test Procedure. Scandinavian Journal of Statistics, 1979. 6(2): p. 65–70. [Google Scholar]
- 47.Mukaka MM, Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J, 2012. 24(3): p. 69–71. [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu AY, et al. , Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci, 1995. 6(4): p. 349–62. [DOI] [PubMed] [Google Scholar]
- 49.Pun S, et al. , Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci, 2006. 9(3): p. 408–19. [DOI] [PubMed] [Google Scholar]
- 50.Johnson MA, et al. , A comparison of fibre size, fibre type constitution and spatial fibre type distribution in normal human muscle and in muscle from cases of spinal muscular atrophy and from other neuromuscular disorders. J Neurol Sci, 1973. 20(4): p. 345–61. [DOI] [PubMed] [Google Scholar]
- 51.Luo G, et al. , Defective mitochondrial dynamics is an early event in skeletal muscle of an amyotrophic lateral sclerosis mouse model. PLoS One, 2013. 8(12): p. e82112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe H, et al. , A rapid functional decline type of amyotrophic lateral sclerosis is linked to low expression of TTN. J Neurol Neurosurg Psychiatry, 2016. 87(8): p. 851–8. [DOI] [PubMed] [Google Scholar]
- 53.Yi J, et al. , Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (E-C) coupling. J Biol Chem, 2011. 286(37): p. 32436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dobrowolny G, et al. , Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab, 2008. 8(5): p. 425–36. [DOI] [PubMed] [Google Scholar]
- 55.Yamada T, et al. , Oxidation of myosin heavy chain and reduction in force production in hyperthyroid rat soleus. J Appl Physiol (1985), 2006. 100(5): p. 1520–6. [DOI] [PubMed] [Google Scholar]
- 56.Powers SK, et al. , Reactive oxygen species: impact on skeletal muscle. Compr Physiol, 2011. 1(2): p. 941–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arai M, et al. , Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca(2+)-ATPase gene transcription. Circ Res, 2000. 86(1): p. 8–14. [DOI] [PubMed] [Google Scholar]
- 58.Qaisar R, et al. , Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heiman-Patterson TD, et al. , Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci, 2005. 236(1–2): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 60.Veldink JH, et al. , Sexual differences in onset of disease and response to exercise in a transgenic model of ALS. Neuromuscul Disord, 2003. 13(9): p. 737–43. [DOI] [PubMed] [Google Scholar]
- 61.Bowser R, Turner MR, and Shefner J, Biomarkers in amyotrophic lateral sclerosis: opportunities and limitations. Nat Rev Neurol, 2011. 7(11): p. 631–8. [DOI] [PubMed] [Google Scholar]
- 62.Ives CT and Doherty TJ, Intra- and inter-rater reliability of motor unit number estimation and quantitative motor unit analysis in the upper trapezius. Clin Neurophysiol, 2012. 123(1): p. 200–5. [DOI] [PubMed] [Google Scholar]
- 63.Ives CT and Doherty TJ, Intra-rater reliability of motor unit number estimation and quantitative motor unit analysis in subjects with amyotrophic lateral sclerosis. Clin Neurophysiol, 2014. 125(1): p. 170–8. [DOI] [PubMed] [Google Scholar]
- 64.Neuwirth C, et al. , Motor Unit Number Index (MUNIX): a novel neurophysiological marker for neuromuscular disorders; test-retest reliability in healthy volunteers. Clin Neurophysiol, 2011. 122(9): p. 1867–72. [DOI] [PubMed] [Google Scholar]
- 65.Clark BC, Cook SB, and Ploutz-Snyder LL, Reliability of techniques to assess human neuromuscular function in vivo. J Electromyogr Kinesiol, 2007. 17(1): p. 90–101. [DOI] [PubMed] [Google Scholar]
- 66.Kaya RD, Hoffman RL, and Clark BC, Reliability of a modified motor unit number index (MUNIX) technique. J Electromyogr Kinesiol, 2014. 24(1): p. 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sleivert GG and Wenger HA, Reliability of measuring isometric and isokinetic peak torque, rate of torque development, integrated electromyography, and tibial nerve conduction velocity. Arch Phys Med Rehabil, 1994. 75(12): p. 1315–21. [PubMed] [Google Scholar]
- 68.Kasselman LJ, Shefner JM, and Rutkove SB, Motor unit number estimation in the rat tail using a modified multipoint stimulation technique. Muscle Nerve, 2009. 40(1): p. 115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beck M, et al. , Comparison of maximal voluntary isometric contraction and Drachman’s hand-held dynamometry in evaluating patients with amyotrophic lateral sclerosis. Muscle Nerve, 1999. 22(9): p. 1265–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.