Abstract
Purpose:
Our aim was to correlate cardiovascular risk factor estimation with bulbar conjunctival blood flow metrics as measured through Functional Slit Lamp Biomicroscopy (FSLB).
Methods:
Cross-sectional study of individuals with otherwise healthy eyelid and corneal anatomy recruited from the Miami Veterans Affairs (VA) Healthcare System eye clinic. We measured conjunctival microvascular hemodynamics by mounting a camera on a slit lamp and cardiovascular risk using the Framingham risk score. Our main outcome measures were correlations between conjunctival vessel parameters (axial and cross-sectional blood flow velocity, blood flow rate) and Framingham score.
Results:
We included 84 patients who underwent FSLB. The mean age was 60 years, the majority were male (88%) and approximately half the patients were black (54%). Mean vessel diameter was similar between all Framingham score categories. Axial and cross-sectional blood flow velocities and blood flow rate were lower in individuals with higher Framingham risk score. Specifically, mean cross-sectional blood flow velocity in individuals with a low Framingham risk score was 0.37±0.0.9 mm/s, with an intermediate score was 0.30±0.09 mm/s, and with a high score was 0.29±0.10 mm/s, p=0.04. Mean blood flow rate in individuals with a low Framingham risk score was 133.4±59.6 pl/s, with an intermediate score was 123.6±39.3 pl/s, and with a high score was 121.9±52.6 pl/s, p=0.04. The beta coefficient of the blood flow rate for change in Framingham score was −0.73; 95% CI-1.34—0.13, p=0.02, adjusted for race.
Conclusion:
FSLB correlates with cardiovascular risk estimation. Future studies should evaluate if FSLB can predict cardiovascular outcomes.
Keywords: Conjunctival blood vessels, functional slit lamp imaging, cardiovascular risk estimation
Introduction
The prevalence of cardiovascular risk factors continues to increase.1 This increase has catapulted cardiovascular diseases (CVD) to be the most common cause of death in the United States.2 Both cardiovascular risk factors and diseases are also related to an important burden of disability and health care costs.2 Estimating cardiovascular risk requires a history, physical exam and laboratory evaluation. The need for laboratory results is a significant limitation for in person and point of care estimation.3 There is a need for simpler cardiovascular risk estimation.
The Framingham score is the most common cardiovascular risk estimator used in clinical practice.4 Newer biomarkers, like E-selectin, Leptin, osteoprotegerin and oxidized low-density lipoprotein improve the predictability of the score.5 The only available point of care estimators rely on obtaining capillary blood to measure total and HDL cholesterol and glycosylated hemoglobin at the time of physician consultation.6 Resistance to the adoption of these miniaturized, portable point-of-care tests is in part explained by the challenges of cost, calibration requirements, maintenance of equipment, and others detailed by King et. al.7 Therefore, there is a need for low cost and easy to use CVD risk stratification.
Functional Slit Lamp Biomicroscopy (FSLB) is a reliable, cost-effective, and low-maintenance way to identify microvascular hemodynamics in the conjunctiva.8 Imaging via this technique is fast and easy, while processing of the images is a lengthy process which could be sped up with more automation, as demonstrated by Khansari et al.9 Several studies have found correlations between conjunctival microcirculation and the vessels in the rest of the body.3, 8, 10–13 For example, individuals with type 2 diabetes were found to have wider conjunctival vessel diameters and more abnormal microvascular distribution than healthy controls.12, 13
Based on the premise that cardiovascular risk estimation is cumbersome, that we need newer ways to identify patients at risk of CVD, and that bulbar conjunctival micro-circulation relates to vessels in other parts of the body, we hypothesize that directly visualizing conjunctival vessels may provide a direct window into cardiovascular health. Therefore, the aim of this study was to evaluate the correlation between cardiovascular risk factor estimation and bulbar conjunctival blood flow metrics as measured through FSLB, while considering the effects of race and ethnicity.
Methods
Study design, setting and population
We conducted a cross-sectional study of patients with otherwise healthy eyelid and corneal anatomy who were prospectively recruited from the Miami Veterans Affairs (VA) Healthcare System eye clinic between October 2014 and February 2017 and underwent a complete ocular surface examination. Patients were excluded from participation if they wore contact lenses, underwent refractive surgery, used ocular medications with the exception of artificial tears, had ocular co-morbidities (pterygium, glaucoma, infection), had HIV, sarcoidosis, graft-versus host disease or a collagen vascular disease, or had cataract surgery within the last 6 months or any glaucoma or retinal surgery. The study was approved by the Miami VA Institutional Review Board and all subjects signed informed consent.
Cardiovascular risk estimation
We used the Framingham risk equation to quantify the estimated 10-year absolute cardiovascular risk for each individual. The score used was the adaptation of the original score developed on Framingham participants 30–74 years of age without overt heart disease at baseline. We calculated the Framingham risk score using age, sex, smoking status, prevalent diabetes, use of anti-hypertensive drugs, and systolic blood pressure and total and HDL cholesterol.14 This score has been found to be a better predictor of events than other older versions of the Framigham score.15 We defined groups of CV risk as: low risk (<10%), moderate (10–20%), and high (>20%). The investigator imaging the patients was blinded to the risk scores.
Other variables collected
Before having the FSLB, research participants were given a questionnaire to collect demographics. We collected race and ethnicity using the census definition of race and ethnicity. We also collected co-morbidity information including information on diabetes and hypertension. The Charlson comorbidity weighted index was calculated based on patient self-report and review of the medical record.16
Functional slit lamp biomicroscopy
The FSLB imaging system is composed of a digital camera (Canon) and a traditional slit-lamp. As described previously17, the camera has a Movie Crop Function (MCF) that uses a center portion of pixels on the camera chip to generate an equivalent of ~7× magnification for high speed video recording at 60 frames per second (fps) without the loss of image quality. With the built-in optical magnifications of up to 25× in the slit-lamp, the total magnification can be set up to ~175×. This imaging system is capable of capturing the movement of red blood cells (RBC) clusters which allows the blood flow velocity and vessel diameter to be measured.18, 19 We previously found that a sample size of 15 venules produced an acceptable standard error of 15%. For this study, the images and videos were captured under green light, as the red blood cells absorb green light more than surrounding tissues. We asked the patient to focus on a point in the far left field of vision. The images and videos were taken only of the right eye. The images of conjunctival vessels were first taken with a slit lamp magnification of 16x and a field of view of about 15.70 × 10.47 mm2.17 The size of the slit lamp light was at the maximum, set at half brightness, and placed at 20 degrees nasally. The images were followed by a video at a slit lamp magnification of 24x. This provides more than the suggested minimum of 1 pixel for one red blood cell. The slit lamp light size was reduced to 3 × 3mm2, placed 45 degrees temporally, and brightness was increased to the maximum. The video was taken of six separate fields of view of the temporal bulbar conjunctival vessels for a couple seconds each to assure that more than 15 venules were recorded and analyzed per subject. The International Standards Organization (ISO) sensitivity was set at 200 for pictures and 400 for video.
Imaging bulbar conjunctival nMPMs and fractal analysis
Custom software was used for the quantification of microvascular morphology and hemodynamics as described previously.17–19 The complexity of the microvascular morphology was represented as the fractional, or monofractal, dimension and the multifractal dimension. Both strategies measure the irregularity and complexity of a geometric structure, with the multifractal dimension being a more sensitive way of measuring vascular complexity due to the retina’s multifractal nature.20, 21
Imaging bulbar conjunctival hemodynamics
The software was also used to semi-automatically process the video clips to yield vessel diameters, lengths, vessel covering areas, blood flow rate, and cross-sectional and axial velocities for all measurable venules. Only conjunctival venules were analyzed for blood flow velocity because the conjunctival pre-capillary arteriole blood flow velocity can be impacted by the pulse. Venules were distinguished from arterioles based on their diameter (bulbar conjunctiva arterioles are smaller than venules) and by the direction of flow (venule collected flow from branches in the bifurcation). The average diameter of vessels was calculated based on images converted from the video clips in which the velocity was measured.
The measurement of blood flow velocity was based on the space-time image technique. The videos are first cropped into 6 separate videos for each of the 6 fields of view and then run through custom software developed on Matlab to convert each video into individual images. At least 30 consecutive images were selected from each video and the centerlines of each of the vessels were automatically determined. The new images were then run through custom Matlab software to yield spatial-temporal images (Figure 1). The y-axis of the image is the vessel path length, while the x-axis is the time elapsed in the video. Slope of the bands on the images were manually indicated to yield axial blood flow velocity (mm/s).
Figure 1.
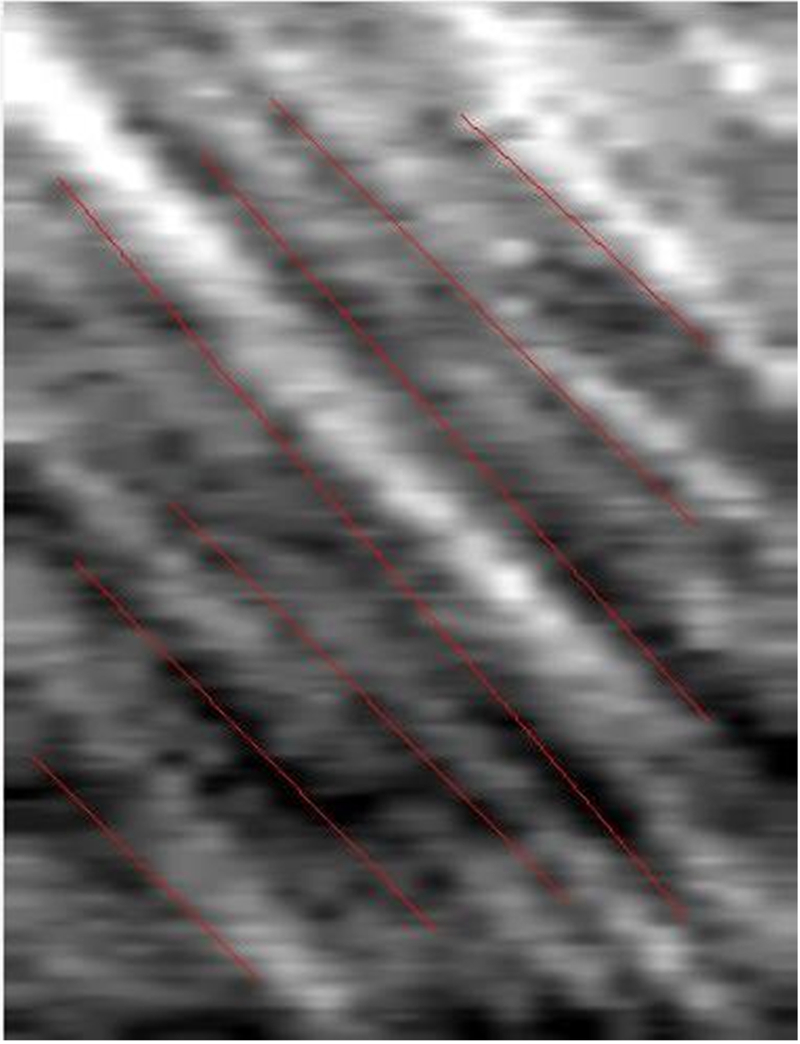
Spatial-temporal Image Generated to Determine Axial Blood Flow Velocity. A space time image is generated by custom Matlab software using at least 30 consecutive frames from a video of bulbar conjunctival blood flow. The vessel path length is represented on the Y axis and the time in the video is represented on the X axis. The slope is manually outlined as the pictured red lines and automatically calculated as the axial blood velocity.
The cross-sectional velocity (mm/s) and blood flow rate (pl/s) were calculated from the axial blood velocity based on an equation previously published to better represent blood flow velocity in vessels with diameter smaller than approximately 20 μm.22, 23 The accuracy and repeatability of this method has been reported in one of our previous studies.19
Statistical analysis
We reported baseline characteristics and blood flow metrics using percentages, means and standard deviations and compared the distribution of variables between groups using analysis of variance (ANOVA) and Chi Square methodology, as appropriate. We next examined blood flow parameters by race, while adjusting for age.
To determine the effect of cardiovascular risk on blood flow, we used linear regression to calculate the beta-coefficient of the 10-year risk and the corresponding 95% confidence interval (CI). We included race as the main adjusting variable and could not adjust for age, gender or the Charlson score because they are components of the cardiovascular risk scores. We further assessed the isolated impact of hypertension and diabetes on mean blood flow via a stratified analysis. Analyses were performed using STATA 14.0 (College Station, Texas), all significance tests were two-tailed, and a p value <0.05 was considered significant.
Results
Baseline characteristics
Table 1 reports the baseline characteristics of 84 patients who underwent FSLB. The mean age was 60.8±9.4 years old, the majority were male (88%) and approximately half of the patients were black (54%). Table 1 also reports the 84 patients stratified by race and ethnicity. Black patients were younger (58 years) and had a higher Charlson score while Hispanics were more likely to have diabetes and less likely to have hyper-tension.
Table 1:
Baseline characteristics by race and ethnicity
| Characteristic | White | Black | Hispanic | p-value |
|---|---|---|---|---|
| Number | 20 | 43 | 21 | |
| Age, years, mean±SD | 65.8±8.3 | 57.6±9.6 | 62.5±7.7 | 0.02 |
| Male gender, % | 95 | 84 | 90 | 0.40 |
| Diabetes, % | 20 | 27 | 33 | 0.62 |
| Hypertension, % | 75 | 72 | 57 | 0.38 |
| Charlson comorbidity score, mean±SD | 0.6±0.94 | 1.18±1.09 | 1.04±0.92 | 0.12 |
SD=standard deviation
Effect of age, race and ethnicity over blood flow
Table 2 reports FSLB measures by race and ethnicity, adjusted for age. Mean vessel diameter was similar between all races. Axial and cross-sectional blood flow velocities and blood flow rate were higher in blacks when compared to other races (p<0.05).
Table 2:
Age adjusted Functional slit lamp biomicroscopy (FSLB) measures by race and ethnicity
| FSLB measure mean ± SD | White | Black | Hispanic | p-value |
| Number | 20 | 43 | 21 | |
| Mean vessel diameter μm) | 21.0±0.19 | 21.3±0.04 | 20.3±0.04 | 0.08 |
| Mean vessel length (μm) | 211.3±.6 | 218.8±0.22 | 254.0±3.2 | 0.15 |
| Axial blood flow velocity (mm/s) | 0.41±0.003 | 0.52±0.001 | 0.42±0.002 | 0.03 |
| Cross-sectional blood flow velocity (mm/s) | 0.29±0.001 | 0.36±0.0007 | 0.29±0.001 | 0.02 |
| Blood flow rate (pl/s) | 109.2±3.32 | 148.1±0.02 | 107.5±1.51 | 0.03 |
| Fractional dimension | 1.61 ±0.0003 | 1.64±0.0001 | 1.60±0.0003 | 0.16 |
| Multi-fractal dimension | 1.66±0.0001 | 1.68±0.0003 | 1.64±0.004 | 0.32 |
SE=standard error
Effect of Framingham risk score over blood flow
The majority of patients had a Framingham score of greater than 20%. Patients who had a high Framingham score were older, more likely to be male, and had more prevalent cardiovascular risk factors and comorbidities (all p<0.05). (Table 3)
Table 3:
Baseline characteristics by Framingham score
| Characteristic | Low Framingham score (<10%) | Intermediate Framingham score (10–20%) | High Framingham score (>20%) | p-value |
|---|---|---|---|---|
| Number | 11 | 14 | 59 | |
| Age, years, mean±SD | 47.4±8.3 | 56.5±3.7 | 64.3±7.8 | <0.01 |
| Male gender, % | 36 | 86 | 98 | <0.01 |
| Black, % | 64 | 64 | 49 | 0.45 |
| Hispanic, % | 18 | 14 | 29 | 0.45 |
| Diabetes, % | 9 | 7 | 36 | 0.03 |
| Hypertension, % | 27 | 36 | 69 | <0.01 |
| Charlson comorbidity score, mean±SD | 0.54±0.82 | 0.85±1.09 | 1.13±1.09 | 0.66 |
SD=standard deviation
Table 4 reports the FSLB measures by Framingham risk score. Mean vessel diameter was similar between all Framingham score categories. Cross-sectional flow velocity and blood flow rate were lower in the high Framingham risk score group (p<0.05).The beta coefficient of the blood flow for the change in Framingham score was −0.73; 95% CI −1.34 to −0.13 p=0.02 adjusted for race (Table 5).
Table 4:
Functional slit lamp biomicroscopy (FSLB) by Framingham score
| Low Framingham score Mean ± SD | Intermediate Framingham score Mean ± SD | High Framingham score Mean ± SD | P-value | |
|---|---|---|---|---|
| Number | 11 | 14 | 59 | |
| Mean vessel diameter μm) | 19.5±3.2 | 21.5±2.0 | 21.3±2.8 | 0.90 |
| Mean vessel length (μm) | 246.6±85.7 | 227.8±45.9 | 224.4±51.0 | <0.01 |
| Axial blood flow velocity (mm/s) | 0.54±0.13 | 0.44±0.13 | 0.42±0.15 | 0.05 |
| Cross-sectional blood flow velocity (mm/s) | 0.37±0.0.9 | 0.30±0.09 | 0.29±0.10 | 0.04 |
| Blood flow rate (pl/s) | 133.4±59.6 | 123.6±39.3 | 121.9±52.6 | 0.04 |
| Fractional dimension | 1.64±0.45 | 1.64±0.03 | 1.63±0.05 | 0.65 |
| Multi-fractal dimension | 1.67±0.02 | 1.67±0.03 | 1.66±0.04 | 0.78 |
SD=standard deviation
Table 5:
Race adjusted beta coefficients for the Framingham risk score on each FSLB measure
| Beta coefficient (95% CI) p-value | |
|---|---|
| Mean vessel diameter | 0.11 (−2.39–2.61) 0.92 |
| Mean vessel length | −28.4 (−80.8–23.9) 0.27 |
| Axial blood flow velocity | −0.07 (−0.22–0.07) 0.30 |
| Cross-sectional blood flow velocity | −0.03 (−0.16–0.10) 0.61 |
| Blood flow rate | −0.73 (−1.34–0.13) 0.02 |
| Fractional dimension | 0.008 (−0.043–0.060) 0.74 |
| Multi-fractal dimension | −0.016 (−0.10–0.06) 0.69 |
CI=confidence interval
To evaluate the isolated effects of diabetes and/or hypertension on mean blood flow rate, we next grouped subjects into categories based on the presence or absence of these co-morbidities. Individuals without diabetes or hypertension had higher blood flow rates (n=22,138.1 mm/s ±47.7) compared to those with hypertension alone (n=39, 136.1 mm/s ± 56.6), diabetes alone (n=4,122.1 mm/s ± 55.7) and both hypertension and diabetes (n=19,105.1 mm/s ± 67), but the difference was not significant p>0.05.
Discussion
Our results report two important findings. First, that conjunctival blood flow differs by race and ethnicity and second that slower bulbar conjunctival blood flow rates are associated with higher cardiovascular risk. Our study also showed that this lower blood flow was independent of race and ethnicity.
The use of retinal microvasculature in estimating cardiovascular risk24, 25 and the use of bulbar conjunctival blood flow to assess the effects of sickle cell disease and diabetes have been investigated in numerous studies.3, 12, 13, 26, 27 The use of the easily accessible bulbar conjunctival flow, however, has not yet been assessed as a means to understand cardiovascular risk in patients.
The gradual decrease of the blood flow rate with the incremental increase of Framingham score mirrors a pattern found in a study where the combined severity of several bulbar conjunctival microvascular abnormalities corresponded with the severity of type 2 diabetes mellitus.13 This suggests the bulbar conjunctival blood flow may be used to determine the extent of cardiovascular risk rather than merely the presence of it. In another study, an improvement in bulbar conjunctival flow stoppage and a noticeable but not significant increase in blood flow velocity was seen after a simultaneous pancreas-kidney transplantation in a type 1 diabetics.11 This suggests that the decreased flow rate seen in our study may be reversed by proper management of cardiovascular risk. A similar decrease in axial blood flow velocity was also reported in individuals with sickle cell disease.26 In conjunction, our findings suggest that factors determining high cardiovascular risk such as hypertension, smoking, obesity and diabetes can be considered systemic hematological abnormalities and may be causing altered conjunctival micro-vascular hemodynamics, perhaps through microvascular occlusion or endothelial dys-function. Several previous studies have shown that hypertension, dyslipidaemia, and insulin resistance predict worse microvascular endothelial function.28, 29 Our findings that conjunctival microvascular hemodynamics, specifically blood flow rate, are affected by changes in CVD risk factors, raises the possibility that endothelial dysfunction is directly affecting hemodynamics.
While the blood vessel diameter in our study did not significantly differ between patients with low, middle, and high Framingham scores, other diseases have shown variability in vessel diameter. Diabetics were found to have larger conjunctival vessel diameters11, 13 while no significant difference in conjunctival vessel diameter were found among those with and without sickle cell disease.26, 27 As our differences in diameter did not significantly change based on cardiovascular risk, we can assume that the significant difference in blood flow is attributed to cardiovascular risk and not just diameter.
While narrowing of the retinal microvasculature has been used as a surrogate marker of cardiovascular risk, it is interesting that examination of retinal blood velocities showed an inverse relationship with coronary flow.30 As slow coronary flow is related to a high risk of coronary atherosclerosis, Arbel et al. considered higher retinal blood flow velocities to be an indicator of coronary atherosclerosis risk. Coronary atherosclerosis has been linked with a higher Framingham score and is considered to have added value in predicting cardiovascular disease occurrence.31, 32 In our study, lower conjunctival microvascular flow (and not higher flow as seen in the retina) associated with CVD. Other forms of microcirculation have shown similar patterns as bulbar conjunctival flow. Sublingual and bulbar conjunctival flow have been found to be substitutable in a rat model33, and sublingual flow has been shown to have a reduced index in another CVD, pulmonary arterial hypertension.34
Our study examined the relationship between bulbar conjunctival hemodynamics and race, and saw a significantly heightened axial and cross-sectional blood flow velocity and blood flow rate in the black population. While we do not have an explanation for this finding, it is well known that blacks have a different cardiovascular risk profile than whites. Even in black patients free of traditional cardiovascular risk factors, poor micro-vascular endothelial function and arterial stiffness were observed.35 The black population has been widely found to have higher hypertension, to be more aware of it, and to be more likely to be treated than whites, but to have less success in controlling their blood pressure during treatment.36−38 The black population also has been found to experience a significantly higher rate of comorbidities, including dyslipidemia, obesity, diabetes mellitus, chronic kidney disease, coronary artery disease, and transient ischemic attack, than other races in America.39
The limitations of our study should be taken into account when interpreting our results. The cross-sectional nature of our study limits our bulbar conjunctival blood flow measurements and the measurement of some of our cardiovascular risk factors to a very small time period. The information requested from the patients was gathered in the form of self-reported questionnaires. We did not standardize patients as far as comorbidities, so because we focused on Framingham scores, any comorbidities that fall outside of that calculation may have affected bulbar conjunctival hemodynamics despite the significant relationship between cardiovascular risk and conjunctival blood flow. Also the Framingham risk score does not have the same predictability in minorities because it was developed in white middle age men.
Despite these limitations, our study demonstrated that bulbar conjunctival blood flow has a significant inverse relationship with the Framingham risk scores. The technique used to determine conjunctival hemodynamics is reliable, simple, inexpensive, and with some automation of image and video processing, very quick. The trend in microvascular blood flow rate indicates the possible utility of the bulbar conjunctival micro-vasculature in indicating cardiovascular risk. This study is a supplement to existing knowledge that microcirculation is a viable proxy for detecting systemic issues. It is worth looking further into the value of bulbar conjunctival hemodynamics in predicting risk of cardiac events and health outside the purview of the Framingham score, such as coronary atherosclerosis. The racial disparities in conjunctival hemodynamics are significant but must be taken into consideration with intrinsic differences in cardiac health of the different racial populations. Considering our findings, it is important that race should be accounted for when determining healthy blood flow rates. Further studies that are race-specific with larger sample sizes are needed to clarify the role of bulbar conjunctival blood flow rate as a diagnostic tool for cardiovascular risk.
Acknowledgements
Funding statement:
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006–15S (Dr. Galor), R01EY026174 (Dr. Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant.
Role of sponsors:
The above sponsors provided financial support to cover the researchers time but were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. We would like to note that the contents of this study do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Meeting Presentation:
This material has not been presented or been under consideration for presentation anywhere.
Access to data:
Anat Galor and Leonardo Tamariz had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Song Y, Liu X, Zhu X, et al. Increasing trend of diabetes combined with hypertension or hypercholesterolemia: NHANES data analysis 1999–2012. Sci Rep 2016;6:36093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018. [DOI] [PubMed] [Google Scholar]
- 3.Owen CG, Newsom RS, Rudnicka AR, et al. Vascular response of the bulbar conjunctiva to diabetes and elevated blood pressure. Ophthalmology 2005;112(10):1801–8. [DOI] [PubMed] [Google Scholar]
- 4.Sayin MR, Cetiner MA, Karabag T, et al. Framingham risk score and severity of coronary artery disease. Herz 2014;39(5):638–43. [DOI] [PubMed] [Google Scholar]
- 5.Reddy RK, Mahendra J, Gurumurthy P, et al. Identification of Predictable Biomarkers in Conjunction to Framingham Risk Score to Predict the Risk for Cardiovascular disease (CVD) in Non Cardiac Subjects. J Clin Diagn Res 2015;9(2):BC23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karmali KN, Brown T, Sanchez T, et al. Point-of-care testing to promote cardiovascular disease risk assessment: A proof of concept study. Prev Med Rep 2017;7:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King K, Grazette LP, Paltoo DN, et al. Point-of-Care Technologies for Precision Cardiovascular Care and Clinical Research: National Heart, Lung, and Blood Institute Working Group. JACC Basic Transl Sci 2016;1(1–2):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Jiang H, Tao A, et al. Measurement variability of the bulbar conjunctival microvasculature in healthy subjects using functional slit lamp biomicroscopy (FSLB). Microvasc Res 2015;101:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khansari MM, Wanek J, Felder AE, et al. Automated Assessment of Hemodynamics in the Conjunctival Microvasculature Network. IEEE Trans Med Imaging 2016;35(2):605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banaee T, Pourreza H, Doosti H, et al. Distribution of Different Sized Ocular Surface Vessels in Diabetics and Normal Individuals. J Ophthalmic Vis Res 2017;12(4):361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung AT, Chen PC, Leshchinsky TV, et al. Improvement in conjunctival microangiopathy after simultaneous pancreas-kidney transplants. Transplant Proc 1997;29(1–2):660–1. [DOI] [PubMed] [Google Scholar]
- 12.Owen CG, Newsom RS, Rudnicka AR, et al. Diabetes and the tortuosity of vessels of the bulbar conjunctiva. Ophthalmology 2008;115(6):e27–32. [DOI] [PubMed] [Google Scholar]
- 13.Cheung AT, Ramanujam S, Greer DA, et al. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocr Pract 2001;7(5):358–63. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB, Vasan RS Sr., Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 15.Eichler K, Puhan MA, Steurer J, Bachmann LM. Prediction of first coronary events with the Framingham score: a systematic review. Am Heart J 2007;153(5):722-31, 31 e1–8. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Batawi HI, Alava JR, et al. Bulbar conjunctival microvascular responses in dry eye. Ocul Surf 2017;15(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Zhong J, DeBuc DC, et al. Functional slit lamp biomicroscopy for imaging bulbar conjunctival microvasculature in contact lens wearers. Microvasc Res 2014;92:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Yuan J, Jiang H, et al. Vessel Sampling and Blood Flow Velocity Distribution With Vessel Diameter for Characterizing the Human Bulbar Conjunctival Microvasculature. Eye Contact Lens 2016;42(2):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talu S The influence of the retinal blood vessels segmentation algoritm on the monofractal. Oftalmologia 2012;56(3):73–83. [PubMed] [Google Scholar]
- 21.Talu S Multifractal geometry in analysis and processing of digital retinal photographs for early diagnosis of human diabetic macular edema. Curr Eye Res 2013;38(7):781–92. [DOI] [PubMed] [Google Scholar]
- 22.Koutsiaris AG, Tachmitzi SV, Batis N. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc Res 2013;85:34–9. [DOI] [PubMed] [Google Scholar]
- 23.Koutsiaris AG, Tachmitzi SV, Papavasileiou P, et al. Blood velocity pulse quantification in the human conjunctival pre-capillary arterioles. Microvasc Res 2010;80(2):202–8. [DOI] [PubMed] [Google Scholar]
- 24.Bek T Diameter Changes of Retinal Vessels in Diabetic Retinopathy. Curr Diab Rep 2017;17(10):82. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Wong TY, Sharrett AR, et al. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension 2008;51(1):119–26. [DOI] [PubMed] [Google Scholar]
- 26.Kord Valeshabad A, Wanek J, Zelkha R, et al. Conjunctival microvascular haemodynamics in sickle cell retinopathy. Acta Ophthalmol 2015;93(4):e275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wanek J, Gaynes B, Lim JI, et al. Human bulbar conjunctival hemodynamics in hemoglobin SS and SC disease. Am J Hematol 2013;88(8):661–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005;1(3):183–98. [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoo A, Chanchlani N, Hodson J, et al. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: a six-year prospective study. Arthritis Res Ther 2013;15(6):R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbel Y, Sternfeld A, Barak A, et al. Inverse correlation between coronary and retinal blood flows in patients with normal coronary arteries and slow coronary blood flow. Atherosclerosis 2014;232(1):149–54. [DOI] [PubMed] [Google Scholar]
- 31.Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 2016;77:1–7. [DOI] [PubMed] [Google Scholar]
- 32.Peters SA, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012;98(3):177–84. [DOI] [PubMed] [Google Scholar]
- 33.Yin L, Yang Z, Yu H, et al. Changes in Sublingual Microcirculation Is Closely Related with That of Bulbar Conjunctival Microcirculation in a Rat Model of Cardiac Arrest. Shock 2016;45(4):428–33. [DOI] [PubMed] [Google Scholar]
- 34.Dababneh L, Cikach F, Alkukhun L, et al. Sublingual microcirculation in pulmonary arterial hypertension. Ann Am Thorac Soc 2014;11(4):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris AA, Patel RS, Binongo JN, et al. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc 2013;2(2):e002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease C, Prevention. Racial/Ethnic disparities in the awareness, treatment, and control of hypertension - United States, 2003–2010. MMWR Morb Mortal Wkly Rep 2013;62(18):351–5. [PMC free article] [PubMed] [Google Scholar]
- 37.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005;165(18):2098–104. [DOI] [PubMed] [Google Scholar]
- 38.Lackland DT. Racial disparities in hypertension. J Clin Hypertens (Greenwich) 2005;7(9):500–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvaraj S, Aguilar FG, Martinez EE, et al. Association of comorbidity burden with abnormal cardiac mechanics: findings from the HyperGEN study. J Am Heart Assoc 2014;3(3):e000631. [DOI] [PMC free article] [PubMed] [Google Scholar]


