Since Garrod deduced the existence of inborn errors in 1901,a vast array of metabolic diseases has been identified and characterized in molecular terms. Anno 2018 it is hard to imagine that there is any uncharted backyard left in the metabolic disease landscape. Nevertheless, it took until 2013 to identify the cause of a relatively frequent inborn error, Pseudoxanthoma elasticum (PXE) a disorder resulting in aberrant calcification. The mechanism found was not only biochemically interesting, but also points to possible new treatments for PXE, a disease that has remained untreatable. In this review we sketch the tortuous road that led to the biochemical understanding of PXE and to new ideas for treatment. We also discuss some of the controversies still haunting the field.
PXE, the disease
PXE is caused by a late-onset generalized calcification of the body, most prominently affecting skin, blood vessels and the eye, as illustrated in Fig. 1. The symptoms result from the precipitation of amorphous calcium phosphate, gradually remodeled into hydroxyapatite. Usually the disease develops slowly, but the clinical course is highly variable. Some patients are spared the vision defects and cardiovascular symptoms until they pass 60, but there are also rare cases with fulminant calcification and cardiac infarction in young infants [1,2]. Attempts to slow down the disease process with dietary or pharmacological interventions have been unsuccessful until very recently [2,3].
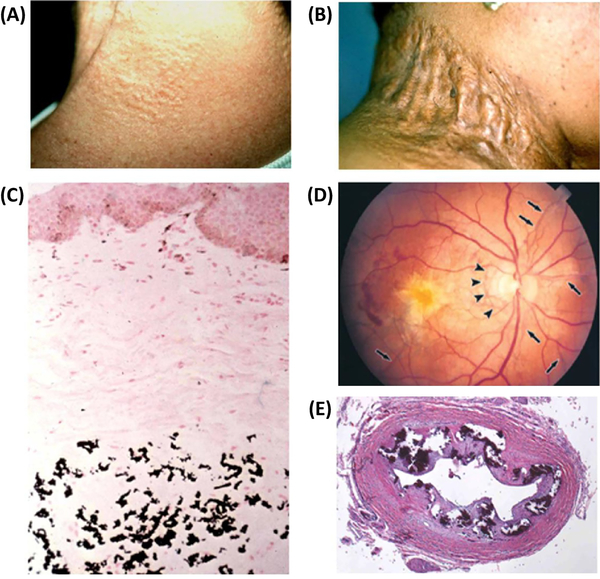
Figure 1.
Clinical features and histopathology of ectopic mineralization in pseudoxanthoma elasticum (PXE). (A) Characteristic early cutaneous signs of PXE consist of discrete yellowish papules at predilection sites, such as sides of the neck. (B) These early lesions coalesce into plaques of inelastic, leathery skin. (C) Staining of a parallel section with von Kossa stain reveals mineralization of the elastotic structures. (D) Characteristic ocular findings consist of angioid streaks, breaks in Bruch’s membrane behind the retina (arrows), which allow neovascularization (arrowheads) of the retina leading to loss of visual acuity and blindness. (E) Histopathology of the left renal artery in a GACI patient with ABCC6 mutations reveals extensive mineralization (Hematoxylin-Eosin stain). Adapted from Li et al (2018 Am. J. Pathol accepted for publication)
PXE was long known to be an autosomal, recessive inborn error with a frequency of 1:50,000. A search for the gene defect(s) responsible for the disease, vigorously supported by the active PXE patient association PXE International, led in 2000 to the unexpected discovery that PXE is caused by defects in the gene encoding the ABC-transporter Multidrug Resistance Protein 6 (MRP6), now known as ABCC6 [4–6]. This was a surprise, because it was not obvious how a drug transporter, mainly found in liver and kidney [7], could affect calcification of peripheral tissues. This paradox initiated the first PXE controversy: some investigators found small amounts of ABCC6 in peripheral tissues [8] and postulated that this peripheral ABCC6 was crucial for explaining the disease, the “ PXE cell” hypothesis; others focused on the liver, where the bulk of the ABCC6 is, and proposed that PXE is a metabolic disease [9]. This would be caused by the absence of an unknown compound secreted by ABCC6 from the liver into the circulation and counteracting peripheral calcification, as depicted in Fig. 2.
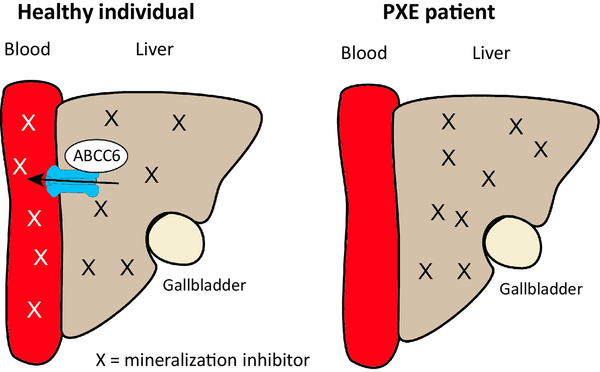
Figure 2.
The “metabolic” hypothesis for PXE. ABCC6 is located in the baso-lateral membrane of the hepatocyte where it secretes a compound X into the blood circulation. X is desributed throughout the body via the circulation and hypothesised to counteract the ectopic peripheral calcification that occurs in PXE patients with non-functioning ABCC6.
PXE is a metabolic disease
As in many inborn errors, Abcc6 KO mouse models that faithfully reproduce essential features of the human disease have been important for detailed mechanistic studies on the biochemical basis of PXE [10,11]. We mention here only a few key experiments:
Jiang et al used an in vitro calcification assay of cultured smooth muscle cells to demonstrate that serum from PXE patients and mice was unable to prevent calcium-phosphate precipitation, whereas normal serum did [12]. This suggested that normal serum contains an anti-calcification factor diminished in patients. This was confirmed in experiments with parabiotic mice. Linking the circulation of a wild-type mouse with that of an Abcc6−/− mouse, counteracted the peripheral calcification in the PXE mouse, whereas linking two Abcc6−/− mice had no effect on disease symptoms [13]. Jiang et al then used tissue transplantation experiments to test whether local tissue factors contributed to the calcification in Abcc6−/− mice. One of the first tissues to be affected by calcification is the connective tissue capsule of the vibrissae (whiskers) in the Abcc6−/− mice [11]. This is easily detected already at 12 weeks after birth. When Jiang et al transplanted muzzle skin of Abcc6−/− mice onto the skin of wild-type mice, the calcification was strongly diminished. Conversely, transplantation of the muzzle skin of wild-type mice onto Abcc6−/− mice resulted in calcification of the normal muzzle transplant [9]. To most investigators these experiments suggested that the absence of circulating serum factors in PXE was far more important for the peripheral calcification than local factors in the calcifying tissues. Hence, these experiments strengthened the basis for the “metabolic hypothesis “ for PXE (Fig. 2) and led to a search for the putative factor X secreted by ABCC6 from the liver into the circulation. The liver contains the bulk of ABCC6 in the body and its importance in PXE was also suggested by incidental observations on human liver transplantations resulting in PXE. Transplanted livers thought to be devoid of ABCC6 resulted in full-blown PXE in the recipients, even though the ABCC6 genes in the recipients were normal [14]. Unfortunately these intriguing observations were not conclusive, as data showing that the transplanted livers were deficient in ABCC6 were not obtained, nor the genotype of the liver donors, who were surmised to be ABCC6-deficient.
Not everybody was convinced by the evidence for the “metabolic hypothesis”, however, and we return to the arguments of these dissenters in the section on controversies.
Will the real substrate of ABCC6 please stand up?
The “metabolic hypothesis” depicted in Fig. 2 predicts that ABCC6 transports an unknown anti-calcification compound X. What might X be? The known properties of ABCC6 provided clues. ABCC6 belongs to the large ABCC family of drug transporters [15,16]. All family members are promiscuous organic anion transporters of some sort and there was no reason to assume that ABCC6 would be wildly different from the others. ABCC6 is directly adjacent to ABCC1 on chromosome 6p13 and looks like its non-identical twin-brother created in evolution by gene duplication. Both genes have the same exon-intron structure with 31 introns, even though their amino acid sequences are only 54% identical [17]. In vitro vesicular transport experiments provided the first list of substrates transported by ABCC6. The principle of the assay is depicted in Fig. 3 and the substrates found were an odd and uninspiring lot: BQ-123, an anionic cyclopentapeptide and endothelin receptor antagonist [18]; leukotriene C4 (LTC4); N-ethylmaleimide S-glutathione (NEM-GS) [19] and S-(2, 4-dinitrophenyl)glutathione [20]. All organic anions of some sort, as expected for an authentic ABCC-type transporter. These transport experiments were useful to prove that PXE was connected to the ATP-dependent transport function of ABCC6, as disease-causing mutants inactivating the ATP-binding catalytic site were found to be transport-inactive [19]. However, none of the substrates looked as if it could help prevent calcification. Moreover, none of these compounds was transported at the rates obtained with physiological substrates of other ABCC transporters. Hence, most investigators remained unconvinced that the real physiological substrate of ABCC6 had been found.
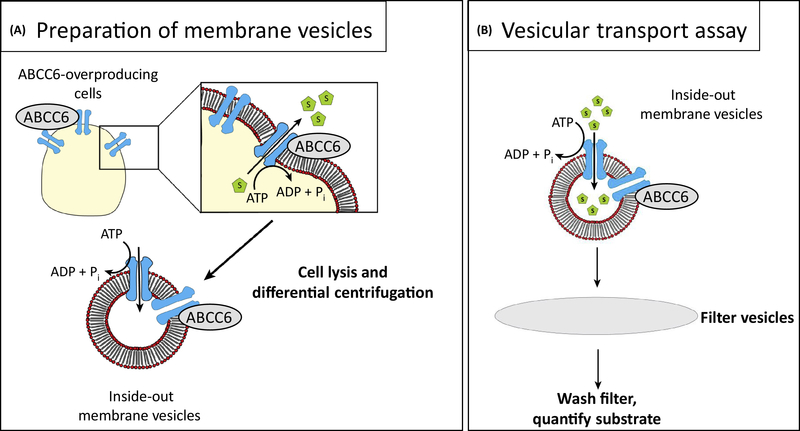
Figure 3.
How membrane vesicles can be used to study transport of substrates by ABC-transporters. When cells are disrupted, the plasma membrane forms two types of vesicles, inside-in vesicles in which the transporter has its normal orientation pumping substrates out; and inside-out vesicles in which the orientation is inverted, the transporter pumping substrates into the vesicle, if ATP is provided to energise the pump. Following the incubation of the vesicles with radioactive substrate and ATP, the vesicles are washed on filters and counted. The inside-in vesicles do not contribute to the reaction, as under these conditions no ATP is present inside the vesicles to support ATP hydrolysis at the ABCC6 nucleotide-binding domains.
This conundrum came to the attention of the entire transporter field at the ABC-transporter meeting in Innsbruck in 2008. In a session dedicated to the mystery of PXE Jouni Uitto presented his persuasive evidence that PXE is due to the absence of functional ABCC6 from the liver, whereas the other speakers were unable to come up with a plausible candidate for the ABCC6 substrate able to counteract calcification. This enticing discrepancy induced a brain wave in one of us (PB), as an interested outsider sitting in the audience: “The unknown substrate X is a vitamin K derivative!”. Biochemical brain waves are usually wrong and this one was no exception. The hypothesis was plausible enough to get it published [21] and it made sense to investigators in the field, as there are plasma proteins known that bind calcium and that require vitamin K for their post-translational modification. Indeed, several labs started to test the vitamin K hypothesis and proved it conclusively incorrect [22–25]. Nevertheless, vitamin K has remained an interesting player in the background, as vitamin K is an essential cofactor of the posttranslational gamma carboxylation of several Ca-binding proteins [26]. Moreover, the disease known as PXE-like calcification syndrome is caused by mutations in the GGCX gene (gamma-glutamyl carboxylase) and not in the ABCC6 gene [27].
Fortunately, incorrect hypotheses can sometimes start a detour on the garden path leading to the correct answer. KvdW, the postdoc involved in the ABCC6 experiments in Amsterdam, had set up a new line of research in the Borst lab,-a search for physiological substrates of transporters using metabolomics-, and this had resulted in a large catalogue of new substrates for ABCC2 and 3 [28,29]. It seemed probable that metabolomics would also be able to unearth the elusive ABCC6 substrate, factor X. And so it did, but it was no plain sailing.
Finding the physiological substrate of ABCC6
ABCC6 is located in the basolateral membrane of the hepatocyte [7,30], where it secretes its substrate(s) into the circulation [9]. To find these substrates, the obvious experiment was to compare all compounds in plasma from PXE patients and their non-affected controls. Experiments with humans are complicated and Jansen and vdW [31] therefore used one of the mouse PXE models [10] for a systematic metabolomics analysis of Abcc6+/+ and Abcc6−/− plasma. No differences were found.
After 2 years of negative experiments with increasingly sophisticated metabolomics methods, a change of tactics finally led to positive results. HEK cells were transfected with constructs encoding human or rat ABCC6 and the supernatants of the transfected cells were compared with supernatants of cultured untransfected cells. The first indication that this approach would work came when the supernatants of the transfected cells were shown to inhibit calcification in the in vitro calcification assay, whereas the supernatants of control cells did not. Metabolic analysis showed that a range of ABCC6-specific compounds were coming out of the transfected cells, most prominently AMP and GMP [31]. None of the extruded compounds could easily be imagined to inhibit calcification. We quickly realised, however, that cells often contain powerful ectonucleotidases that convert nucleosidetriphosphates (NTPs) into the corresponding nucleosidemonophosphates (NMPs) and pyrophosphate (PPi), a known inhibitor of calcification [32]. PPi does not show up in the metabolomics analysis, but a dedicated PPi assay confirmed that the transfected HEK cells produced large amounts of PPi. If an inhibitor of the ectonucleotidase was added to the transfected cells, the levels of PPi went down and ATP appeared in the cell supernatant [31]. This proved that ABCC6 mediates the extrusion of ATP.
The crucial importance of PPi in PXE
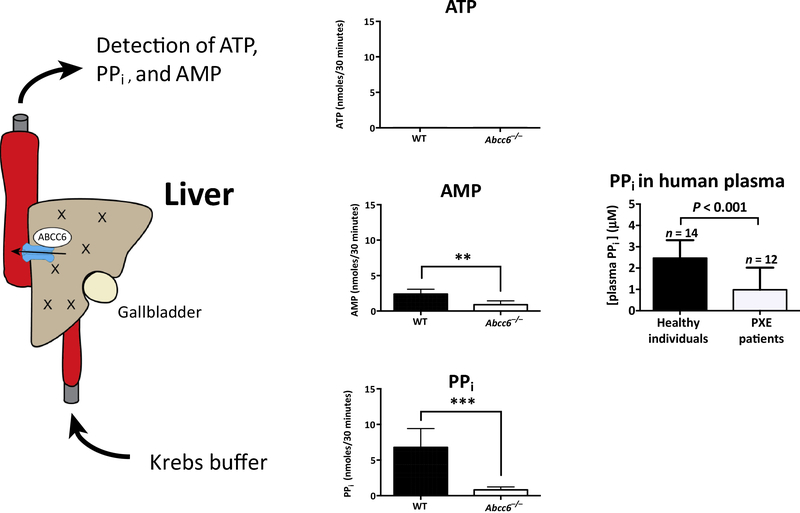
The Amsterdam group backed up their initial findings by showing that plasma PPi was only about 40% of normal in the Abcc6−/− mice [31], as well as in PXE patients [33]. With liver perfusion experiments Jansen et al. showed that mouse liver secretes substantial amounts of PPi into the circulation, but not in the Abcc6−/− mice (Fig. 4). Virtually no ATP was detected in the perfusate of the wild-type livers, but Jansen et al. attribute this to a rapid conversion of ATP into AMP and PPi once it enters the circulation and meets the ecto-nucleotidase in the endothelial lining [33]. The results of Jansen et al in the mouse have fully been duplicated in the recently produced Abcc6 KO rat [34]. In addition, Li et al. showed that the rat liver produced the bulk of the plasma PPi. The kidney contains only 5% of the Abcc6 RNA of the liver and a corresponding small amount of PPi came out of the kidney in perfusion experiments. A crucial role for PPi is plausible, as plasma PPi is normally kept in a narrow range, whereas elevated or abnormally low PPi can result in serious disease, as shown by inborn errors leading either to elevated or lowered plasma PPi [35]. The near complete absence of PPi in the plasma of GACI patients [36], usually caused by homozygous loss of the gene for ENPP1, leads to devastating calcification already early in life [37]. This disease also shows that ATP is by far the most important source of PPi in plasma both in humans and in mice [36,38]. Other sources exist, as discussed below, but the near-complete loss of PPi in ENPP1 deficiency shows that hydrolysis of NTPs by ENPP1 is essential for generating plasma PPi. The crucial role of PPi in PXE is also supported by animal experiments showing that supplementation of PPi can delay the appearance of calcification in Abcc6 mice (see below) [39,40].
Figure 4.
Liver perfusion experiments showing that mouse liver produces large amounts of AMP and PPi, but not in mice without functional ABCC6. The very low levels of ATP in the perfusates are contributed to the rapid conversion of any ATP released into the perfusion buffer into AMP and PPi. In PXE patients reduced ABCC6-mediated ATP release results in low plasma PPi concentrations.
Although the available evidence shows that PPi is crucial in calcium-phosphate homeostasis, PPi is clearly not the only player. The hydrolysis of ATP also yields AMP, which contributes to maintaining plasma PPi as well. We return to an overview of all players below.
Doubts and controversies
From the start the ABCC6 field has been plagued by errors of experiment and interpretation. The ABCC6 gene entered the lime-light as the anthracycline resistance-associated gene (ARA), a factor associated with multidrug resistance of cancer cells [41]. Because the cDNAs found to be associated with resistance did not encode a complete ABC-transporter, the association looked suspicious from the start. Experiments in Amsterdam then showed that the ABCC6 gene is directly adjacent to the ABCC1 gene and that its 3’-end was often coamplified with ABCC1 in cell lines made resistant to doxorubicin [17]. Since the complete ABCC1 gene was co-amplified with the truncated ABCC6 gene in the amplicons and since ABCC1 was known to confer resistance to doxorubicin, it was obvious that the MDR connection of ABCC6 was a spurious one, a conclusion confirmed in later work.
A second controversy emerged when the results of Jansen et al. [31,33] on diminished PPi in PXE patients and mice, were initially not reproduced in other labs. This became clear in heated discussions during a PXE meeting in 2014. In the end this controversy boiled down to a technical problem. PPi determinations are complicated and there are many pitfalls. For instance, minor damage to platelets will swamp the plasma with PPi [42,43]. Indeed, published plasma values for PPi can be off by an order of magnitude [44,45]. Once KvdW had gone round the world to teach the proper way to determine PPi, full agreement was reached.
Some investigators questioned whether a relatively minor decrease in plasma PPi, to about 40% of normal both in PXE mice and PXE patients [31,33], was sufficient to explain calcification of peripheral tissues. The plasma PPi concentration is micromolar, whereas Ca2+ and phosphate are in the millimolar range. How can a little more or less PPi make a difference? The explanation of this paradox was found long ago: PPi coats hydroxyapatite crystals and prevents their further growth [35]. Apparently, micromolar PPi is sufficient to accomplish this feat, 40% of normal levels are not. This delicate balance may also explain why the symptoms of PXE are so variable. There are several sources of plasma PPi besides ABCC6 (see below), and the rate of PPi degradation could also vary (normal t1/2 about 0.5 hour), depending on the genetic background.
A long-standing controversy in the PXE field concerns the potential contribution of peripheral ABCC6 to the prevention of symptoms [9,46]. Extensive data support the activity of some ABCC6 in cultured fibroblasts [47,48], but it has not been shown conclusively that fibroblasts in tissues also contain ABCC6. Culture can induce artifactual gene expression and most experiments with tissue RNA or protein have given negative results. The issue is complicated by a commercial antibody against ABCC6, used by some labs as the only antibody to detect the protein [49]. Detailed analysis of this antibody by one of us (AV) has shown that it does not react with ABCC6 at all (Report available on request). On the basis of this work the company has withdrawn the antibody.
Another controversy came with the publication of a high-profile paper in Science Translational Medicine [50], challenging the central role of PPi in the circulation and of ABCC6 in the liver in PXE. The authors find that knocking out Abcc6 in the Enpp1 KO mice has no effect on the (severe) calcification or on plasma PPi and they conclude that ABCC6 must therefore be DOWNSTREAM of ENPP1. We find this conclusion incomprehensible. Plasma PPi virtually disappears in the Enpp1 KO mouse [38], as this enzyme is the only significant contributor to plasma PPi. If ATP cannot be converted into PPi, providing more ATP through ABCC6, whether upstream (where it is), or downstream of ENPP1, cannot have any influence on plasma PPi.
The second argument presented by Ziegler et al. [50] rests on experiments in which functional ABCC6 is eliminated by disruption of the gene by the Cre recombinase. When the Cre construct is expressed throughout the body, the mice develop full-blown PXE. If a liver-specific albumin gene promoter is used, the mice are initially fine and only develop more calcification than WT mice after one year. The authors conclude that this experiment “excluded the prevailing pathogenic hypothesis that singularly invokes failure of hepatic secretion of an endocrine inhibitor of calcification”(i.e. PPi). It is notoriously difficult, however, to get complete gene disruption in all cells of a tissue with promotor-driven Cre and the CMV promotor, used for body-wide gene disruption, is far more active than the albumin promotor [51]. We therefore expect that the putative liver-specific KO of Abcc6 produced by Ziegler et al [50] is incomplete. It is known that the PXE symptoms in the Abcc6−/− mouse can be mitigated by a rather inefficient transfection of Abcc6 cDNA into the liver, resulting in a very limited restoration of liver Abcc6 expression [52,53]. Obviously, results of a liver-specific KO of Abcc6 can only be interpreted if the KO is shown to be near-complete. The issue will eventually be settled by liver transplantation experiments. These are hard to do in mice, but should be doable in the recently produced Abcc6 rat KO [34].
Another curious controversy erupted with the publication of a high-profile paper in Circulation Research [54]. This paper reported an unusual localisation of ABCC6 in heart in an ill-defined para-mitochondrial structure and the authors presented imaginative speculations about the possible function of ABCC6 in this new location. In the early days of ABCC6 research there was some controversy about its cellular location [18], but with the generation of mono-specific, high-affinity MABs the transporter was conclusively localised in the apical membrane of liver and kidney cells [7]. The intracellular location of ABCC6 reported by Martin et al. [54] in heart was therefore completely unexpected. A detailed reinvestigation of the issue with multiple MABs, confirmed, however, that ABCC6 is only in the plasma membrane [30]. Martin et al were not convinced [55], but they are probably the only investigators in the field who still believe in para-mitochondrial ABCC6.
Finally, there is the ongoing controversy, already mentioned, about the possible contribution of peripheral ABCC6 to the prevention of PXE. There is no doubt that cultured PXE fibroblasts behave differently from their normal cultured counterparts but there is also no unambiguous evidence for the presence of ABCC6 or ABCC6 RNA in connective tissue. The question remains therefore whether CULTURED PXE fibroblasts differ from fibroblasts in situ, for instance, because the culture conditions induce ABCC6 expression; or alternatively, whether available methods fail to detect ABCC6 (RNA) in situ. The issue was re-investigated recently by Li et al. [34] in their rat Abcc6 KO, using a sensitive RT-PCR method. Abcc6 RNA was abundant in liver, much less in kidney (5% of liver) and undetectable in peripheral tissues, including tissues affected by calcification in PXE patients, rats and mice [34]. Unfortunately, the authors did not titrate in decreasing amounts of liver RNA in the tissue samples to determine how low undetectable was, 1%, 0.1%, or even lower than liver RNA. Rats are no humans, but we think that the results of Li et al. further detract from the concept that peripheral ABCC6 exists and is significant in preventing PXE.
Known players in calcium phosphate homeostasis that might affect the severity of symptoms in PXE patients.
PPi homeostasis
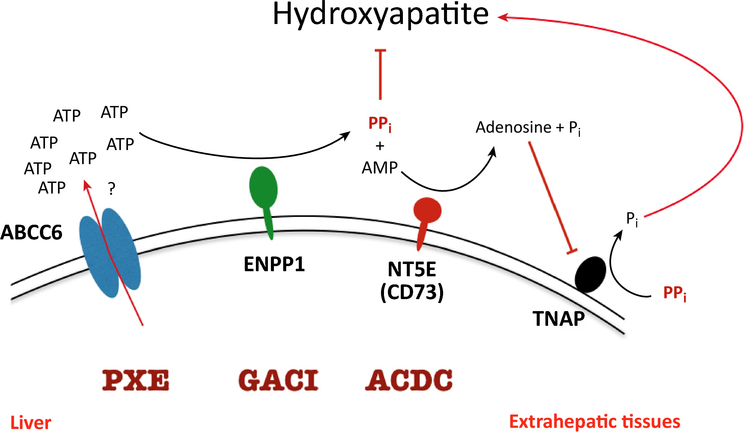
ABCC6 is part of a network keeping plasma PPi levels within the physiological range of 1.5–3 μM in healthy individuals. Fig. 5 shows the major participants in this network. The crucial role of ENPP1 in PPi homeostasis was already stressed. Enpp1−/− mice virtually lack PPi in their blood circulation [38]. All PPi present in the circulation must therefore be produced by ENPP1 from NTPs, predominantly ATP, released into the extracellular milieu and converted into their respective monophosphate and PPi. In contrast, PPi plasma levels in PXE patients are about 40% of those found in healthy individuals [33]. This remaining plasma PPi must therefore come from sources of extracellular NTPs that are independent of ABCC6 activity. Other pathways used by cells to get ATP out of the cell include a range of non-specific ion channels like pannexins, connexin hemichannels and maxi-anion channels, in addition to exocytosis of ATP-loaded vesicles [56]. The relative contributions of each of the alternative ATP conduits to plasma PPi concentrations is unknown and warrants further study, as ABCC6-independent ATP release pathways might represent potential pharmacological targets to increase ATP release and PPi formation in PXE patients. Differential activity of these alternative ATP conduits might also contribute to the variation in plasma PPi concentrations seen in PXE patients [33].
Figure 5.
The known major mechanisms regulating plasma pyrophosphate level. Quantitatively the most important source PPi is the ABCC6-mediated ATP-release from hepatocytes. This ATP is rapidly converted into AMP and to PPi in the liver vasculature by ENPP1. The most important enzyme degrading PPi is TNAP. The activity of this enzyme is regulated by NT5E, which cleaves AMP to adenine and phosphate (Pi); the AMP generated inhibits TNAP, suppressing PPi hydrolysis. Mutations in the ABCC6, the ENPP1 and the NT5E genes cause various calcification diseases. (For more details see text).
PPi is predominantly degraded by Tissue Non-specific Alkaline Phosphatase (TNAP). This enzyme is absent in hypophosphatasia patients and consequently these have high levels of PPi in their plasma and suffer from life-threatening hypomineralization of their bones [57,58]. People with only one functional allele of the gene encoding TNAP do not have clinical manifestations of hypophosphatasia. Inhibition of TNAP represents therefore a potential way to increase plasma PPi in PXE patients.
A third layer of regulation of plasma PPi is formed by CD73, an ecto-enzyme that converts adenosine monophosphate (AMP) into adenosine and Pi [59]. Adenosine is an endogenous TNAP inhibitor and absence of CD73 results in reduced local adenosine formation, higher plasma TNAP activity, lower PPi plasma levels and, consequently, mineralization of arteria, especially in the legs, a disease known as Arterial Calcification Due to the Absence of CD73 (ACDC).
Fetuin-A
Fetuin-A is a major liver-derived plasma protein with an important function in mineral homeostasis. Monomeric fetuin-A binds to amorphous calcium phosphate micro-precipitates, thereby forming colloidal protein-mineral clusters that are also known as “calciprotein particles (CPPs)”. These CCPs prevent microcrystals from further growth and keep them in solution till they are filtered out of the circulation by macrophages [60]. PXE patients have reduced serum Fetuin-A concentrations [61]. This might be caused by increased consumption of Fetuin-A to deal with the increased formation of calcium phosphate micro-precipitates due to reduced PPi. In line with this hypothesis the overproduction of fetuin-A in livers of Abcc6−/− mice substantially reduced the ectopic mineralization phenotype [62]. The severity of the calcification phenotype seen in fetuin-A−/− mice depends to a large degree on the genetic background: On a C57C6 background there is no substantial calcification, whereas in DBA/2 mice, which harbor an inactivating mutation in both Abcc6 alleles, absence of Fetuin-A results in massive calcification [60]. This further demonstrates the interaction between the different systems that are in place to prevent soft tissue mineralization.
Matrix-Gla protein (MGP) and Gamma-glutamyl-carboxylase (GGCX)
Vascular smooth muscle cells (VSMCs) produce and secrete MGP. Patients lacking MGP suffer from the Keutel syndrome, a mineralization disorder characterized by calcification of arteries and cartilage [63]. This phenotype is reproduced in Mgp−/− mice [64]. How MGP exactly prevents calcification is unknown. It needs to undergo two posttranslational modifications, phosphorylation and glutamate carboxylation to become fully active. Interaction via these negatively charged ligands with micro-crystals might prevent hydroxyapatite formation, but inactivation of the osteogenic growth factor bone-morphogenic protein 2 (BMP2) has also been proposed to explain MGP action [65]. The carboxylation of MGP is catalyzed by gamma-glutamyl-carboxylase (GGCX). Patients with defects in the gene encoding this GCCX present with mild connective tissue calcification, in addition to clotting defects [27]. As GCCX needs vitamin K as a cofactor, insufficient intake of vitamin K results in undercarboxylation of MGP and increased vascular calcification [26]. Inhibition of vitamin K dependent carboxylation with warfarin similarly induces vascular calcification [66], but the partial inhibition achieved in patients on coumarin-type anti-coagulants does not seem to contribute to arterial calcification.
New (and old) attempts to treat PXE
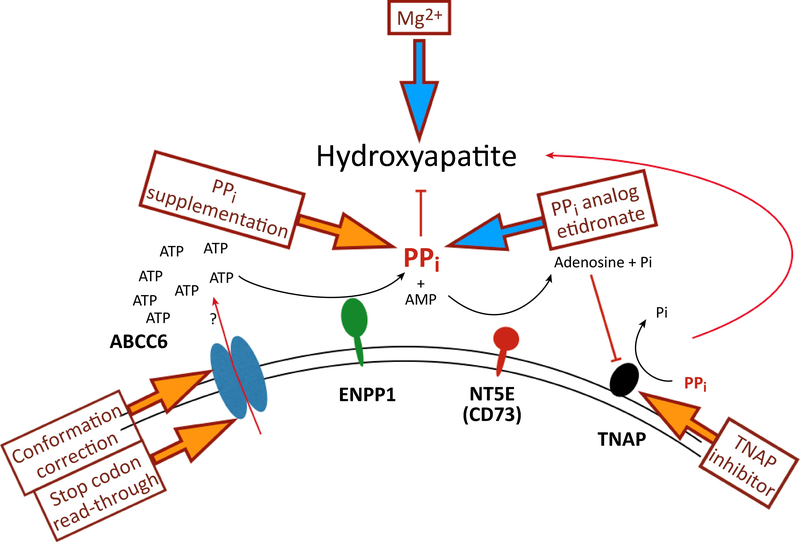
With the discovery that low plasma PPi plays a central role in PXE, new therapeutic interventions came in focus. Fig. 6 summarizes the main players now known to affect plasma PPi and possible ways to alter these. The most obvious one is supplementation of PPi itself. Indeed, intraperitoneal administration of PPi to Abcc6−/− mice fully inhibited acute dystrophic cardiac calcification [39], proving that PPi supplementation therapy could work. Intraperitoneal administration is not practical in human PXE patients that need lifelong treatment. The alternative, oral administration of PPi, did not seem to make any sense, as the oral availability of PPi was thought to be zero [35]. The highly charged PPi cannot be expected to pass through membranes and is susceptible to prompt hydrolysis by intestinal phosphatases. Nevertheless, large doses of oral PPi in drinking water were found to raise plasma PPi and to result in significant attenuation of calcification in both the PXE-and in the GACI KO mice. Elevation of plasma PPi was also achieved in humans, even though only 0.3 % of the ingested dose reached the circulation [40]. This tiny fraction substantially increased plasma PPi concentrations, however, to levels that are expected to inhibit the soft tissue mineralization seen in PXE patients and in several other hereditary ectopic calcification disorders. This encouraging result points the way to an effective, simple, and low-cost medication for these patients. In fact, clinical trials to test oral PPi are already planned in different countries.
Figure 6.
Current therapeutic attempts to counteract the ectopic calcification in PXE patients. Interventions targeting different steps in the regulation of ectopic mineralization are indicated by arrows. Yellow block arrows indicate preclinical interventions, blue arrows ongoing or recently completed clinical trials. (For more details see text).
The acute risk of oral administration PPi to patients is probably negligible as the compound is abundant in our food and considered safe by the FDA. However, there is no experience yet with hefty oral doses of PPi taken for years. Abnormally elevated plasma PPi levels are known to induce defects in bone calcification and very high doses of PPi also yield lots of Pi, which promotes calcification. The therapeutic window for PPi is therefore not unlimited. It is not known either during which fraction of the day the PPi should be normalized to get an optimal therapeutic effect. Is once in 24 hours sufficient to prevent calcification (as animal experiments suggest)? Moreover, more attractive ways of administrating PPi should be found than the large volumes of an unpleasantly tasting solution used thus far, if kids need to be seduced to take their daily PPi. We are enthusiastic about this new therapy, but it is early days and oral PPi still needs to prove its long-term usefulness.
An obvious alternative to PPi are the bisphosphonates, non-hydrolysable PPi analogues developed long ago for the treatment of osteoporosis and bone metastases (for a review, see [67]). The potential of these drugs to prevent PXE-related calcification was addressed by feeding Abcc6−/− mice with chow containing two different bisphoshonates, etidronate disodium (ETD) or alendronate sodium trihydrate (AST) [68]. High doses of ETD significantly reduced mineralization, while AST had no effect. These encouraging results have led to a prospective clinical trial of etidronate treatment in adult PXE patients [3]. After one year the treated patients had moderately less calcification symptoms than the controls. This is encouraging in the absence of other treatment options, but it remains to be seen whether the improvement can be maintained over longer periods and whether the (modest) side–effects [69,70] of the drug remain acceptable.
It is obvious from Fig. 6 that another way to increase plasma PPi is the inhibition of PPi degradation by TNAP. Indeed, recent papers reported that an orally bioavailable TNAP inhibitor attenuated both the development and progression of calcification in Abcc6−/− mice in vivo [50,71]. Ziegler et al. [50] found that plasma PPi level did not increase upon TNAP inhibition treatment, although plasma TNAP activity was decreased. This is surprising in the light of previous results demonstrating profound effects of TNAP activity on plasma PPi. The absence of NT5E results in increased TNAP activity and in Nt5e–/– mice plasma PPi concentrations were indeed reduced [45]. In contrast, the absence of TNAP in the inborn error hypophosphatasia leads to high plasma PPi concentrations and a (lethal) block in bone calcification [57,72]. One would therefore expect that inhibiting the PPi hydrolytic activity of TNAP should increase plasma PPi concentrations. This was not found in the mice treated with a TNAP inhibitor. To substantially increase plasma PPi concentrations TNAP most likely needs to be inhibited more than the 50–60% achieved by Li et al. [71]. In Enpp1−/− mice subcutaneous administration of ENPP1 dramatically increases plasma PPi concentrations [73]. As there is competition between various ectonucleotidases for available ATP [74], increasing ENPP1 activity in plasma could result in a larger fraction of released ATP being converted into PPi. Studies are now ongoing to find out if ENPP1 enzyme substitution in Abcc6−/− mice prevents disease progression. Indications that this approach might actually work came from a recent study showing that ectopic mineralization was greatly reduced in Abcc6−/− mice expressing high levels of human ENPP1 [75].
Several attempts have been made to modify the clinical progression of PXE by changing the mineral content of the diet. Variations in dietary calcium levels do not affect calcification in Abcc6−/− mice [76], possibly because plasma calcium levels are very tightly regulated. Plasma phosphate concentrations are more variable and increasing phosphate in the diet of Abcc6−/− mice accelerated ectopic calcification [77]. Despite this clear effect in mice, phosphate binders do not prevent disease progression in mice or humans [78,79]. Why, is unclear. In contrast, increasing the magnesium content of the diet does have an effect, at least in mice. It has long been known that Mg2+ counteracts soft tissue mineralization (for a recent review, see Braake et al, 2017 [80]), and increasing Mg2+ in the diet completely prevented ectopic mineralization in Abcc6−/− mice [76,81]. A prospective Mg-suplementation clinical trial in PXE patients (https://clinicaltrials.gov/ct2/show/NCT01525875) was completed in March 2015. The results have still not been published and it seems therefore unlikely that spectacular improvements can be obtained in PXE patient with dietary magnesium supplementation.
A different route to elevate plasma PPi concentration, indicated in Fig. 6, is to increase ABCC6 activity in the liver. Most mutations in PXE are missense, and many of the mutant proteins preserve transport activity, but cause intracellular retention [52]. It has been shown for other large transport proteins that the chemical chaperone 4-phenylbutyrate (4-PBA) promotes the routing of missense mutant proteins to the plasma membrane [82]. Pomozi et al. [83] found that this is also the case for ABCC6 mutant proteins. In a humanized mouse model of PXE, transiently expressing these human ABCC6 trafficking mutants, 4-PBA administration was able to inhibit calcification [53]. Therapeutic use of 4-PBA therefore looks like a promising strategy for allele-specific therapy of ABCC6-associated calcification disorders [53]. The studies with other defective transporters have already shown that long-term therapy with 4-PBA is well tolerated, even though huge doses of 4-PBA were needed for correction [82,84]. Nevertheless, other pharmacological correctors are needed because 4-PBA was found to be active only in certain missense mutants (in 3 out of randomly selected 7). Correctors with different selectivities would be required, since there are a large array of disease-causing mutations, resulting in intra-cellular retention.
Another allele-specific approach is to “read through” premature termination codons (PTCs) present in some ABCC6 gene mutants. The R1141X (a PTC-type) mutation is the most frequent disease-causing variant in PXE [85] and in vitro studies demonstrated low level production of full-length protein upon treatment with PTC124, a nonsense codon read-through-inducing drug [86]. More recent studies have utilized another drug, amlexanox, which also counteracts nonsense- mediated mRNA decay, in addition to promoting PTC read-through, suggesting its potential usefulness for a subset of patients with PXE caused by PTC mutations [87].
It should be clear from this brief overview that the number of potential treatment options for PXE has considerably increased in the past years through the basic science that has elucidated the molecular defects underlying PXE. The treatment problem has not been solved yet, but the future for patients has certainly brightened.
Open questions and future directions
How ABCC6 mediates ATP secretion is the most pressing question that remains to be answered, biochemically speaking. Is ATP a substrate of ABCC6, or is ABCC6 an activator of another transporter/channel? This looks like a simple question, easily answered by vesicular transport experiments, but it is not. Enzymes using ATP as substrate generally have a low affinity for ATP, as this substrate is present in millimolar concentrations in normal cells [88]. ABC-transporters are no exception to this elementary principle [15]. This makes it hard to find the minute amounts of ATP that might be transported by ABCC6 in the presence of the vast amounts of ATP required to drive transport. As the members of the ABCC family of drug transporters prefer organic anions [15], ATP is not an implausible substrate for ABCC6. There are also ABCC family members, however, that regulate channels [89]. Since there are several channels mediating export of ATP from cells, such an indirect effect of ABCC6 is not a priori improbable, although a channel specific for large molecules, such as NTPs, would be a novelty. Experiments are in progress to find out how ABCC6 actually works.
Unsolved is also how plasma PPi is controlled. The study of inborn errors has shown that production of PPi cannot be drastically decreased if degradation is severely diminished, nor can degradation be slowed down if supply fails. Nevertheless, we find it hard to believe that there is not some sort of feed-back control to fine-tune the plasma level in the narrow range found (and required to avoid disease). Both experiments on humans and mice would greatly benefit from a robust micro-determination for PPi, as the presently available assays are cumbersome and need relatively large volumes of plasma.
A further open question concerns the variability of symptoms in PXE patients [90]. This is not attributable to the degree of remaining ABCC6 activity, as zero activity may be associated with widely variable symptoms. Genetic background must play a role given the large range of players involved in PPi metabolism (see Fig. 5).There is also experimental proof for this: several mouse strains are available with exactly the same Abcc6 inactivating mutation. Disease severity in these animals very much depends on the genetic background of the mouse strain [91]. The intriguing recent finding that variations in dietary PPi can affect the severity of symptoms in KO mice [40] suggests that dietary PPi could also have this effect in humans. As pointed out in the Therapy Section, PPi is used for preserving food and is even present in tooth paste. Intake could therefore vary between patients depending on food composition. Plasma PPi levels are probably also not the only factor affecting disease in PXE patients. Although PPi is clearly essential, the correlation between PPi and symptoms is not a simple one and other factors could therefore affect the severity of the disease, such as Mg2+ and phosphate intake.
A major task for the near future is the translation of recent basic insights in PXE pathogenesis into better treatments of patients. We expect much from the optimization of oral PPi treatment, but several other options look promising as well. We hope that in the end the PXE patients’ association PXE International may see the practical fruits of its persistent support for research into the molecular basis of PXE.
Highlights (box 2).
The hereditary mineralization disorder Pseudoxanthoma elasticum (PXE) is due to inactivating mutations in the gene encoding the hepatic efflux transporter ABCC6.
Recent work has shown that ABCC6 in the liver mediates the secretion of ATP into the circulation, where it is rapidly converted into AMP and the calcification inhibitor pyrophosphate (PPi).
In PXE patients, absence of functional ABCC6 leads to a substantial decrease of plasma PPi, providing a plausible explanation for their ectopic calcification.
The discovery of the lowered plasma PPi in PXE patients has led to new initiatives to treat the disease, such as oral PPi supplementation and first generation bisphosphonates, ultra-cheap and relatively simple treatment options.
The new treatment initiatives for PXE also offer new perspectives for the many other disturbances in calcium-phosphate metabolism for which no treatment exists.
Open questions (box 3).
How does ABCC6 mediate ATP secretion? Is it an ATP transporter, or does it activate another transporter/channel?
How is the fine-control of plasma PPi concentrations regulated?
What are the main reasons for the variability in symptoms and progression in PXE patients?
What is the function of ABCC6 in the kidney?
Can oral PPi supplementation in PXE patients be turned into an effective and patient-friendly treatment?
Can oral PPi also benefit patients with other inborn errors of calcium phosphate metabolism?
Can an effective, safe genetic modification protocol be developed to repair the defective ABCC6 gene in the livers of PXE patients or to replace it with a functional copy?
Acknowledgements:
We thank our colleagues Jouni Uitto, Robert Jansen, Gergely Szakacs and Balazs Sarkadi for their helpful comments. All three authors are indebted to PXE International for continued support; experimental work in the lab of KvdW is supported by an NIH/NIAMS grant R01AR072695; AV is supported by grants of the Hungarian Academy of Sciences OTKA 104227, OTKA 114336 and VKSz14‐1‐2015‐0155
Abbreviations(box 1)
- ABCC1
ABC-transporter C1, also known as Multidrug Resistance Protein 1 (MRP1) ABCC6, idem C6 (MRP6)
- Abcc6−/−
KO mice (or rats) with disrupted Abcc6 genes
- ACDC
Arterial Calcification Due to the absence of CD73
- ARA
the Antracycline Resistance-Associated gene
- CD73
ecto-5’-nucleotidase
- CMV
Cytomegalo Virus
- ENPP1
ecto-nucleotide pyrophosphatase phosphodiesterase 1
- GACI
Generalised Arterial Calcification of Infancy
- GGCX
gamma-glutamyl carboxylase
- HEK
Human Embryonic Kidney cells
- Hepatocyte
main (epithelial) cell of the liver, in which ABCC6 is located in the baso-lateral membrane
- LC-MS
Liquid Chromatography-Mass Spectrometry
- MAB
mono-clonal antibody
- MGP
Matrix-Gla Protein
- NT5E
Ecto-5’Nucleotidase, also known as CD73
- NTP
nucleoside triphosphate
- Parabiotic mice
mice joined together and sharing one blood circulation
- 4-PBA
4-phenylbutyrate
- Pi
inorganic phosphate
- PPi
inorganic pyrophosphate
- PTC
premature termination codon
- PXE
Pseudoxanthoma elasticum, an inborn error of calcium-phosphate metabolism
- TNAP
Tissue Non-specific Alkaline Phosphatase
- Vibrissae
mouse/rat whiskers
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Boulanger G et al. (2009) An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am. J. Med. Genet 152, 118–123 [DOI] [PubMed] [Google Scholar]
- 2.Uitto J et al. (2017) Insights into Pathomechanisms and Treatment Development in Heritable Ectopic Mineralization Disorders: Summary of the PXE International Biennial Research Symposium-2016. J. Invest. Dermatol 137, 790–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kranenburg G et al. (2018) Etidronate for Prevention of Ectopic Mineralization in Patients With Pseudoxanthoma Elasticum. J. Am. Coll. Cardiol 71, 1117–1126 [DOI] [PubMed] [Google Scholar]
- 4.Le Saux O et al. (2000) Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat. Genet 25, 223–227 [DOI] [PubMed] [Google Scholar]
- 5.Bergen AA et al. (2000) Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat. Genet 25, 228–231 [DOI] [PubMed] [Google Scholar]
- 6.Ringpfeil F et al. (2000) Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc. Natl. Acad. Sci. U.S.A 97, 6001–6006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheffer GL et al. (2002) MRP6 (ABCC6) detection in normal human tissues and tumors. Lab. Invest 82, 515–518 [DOI] [PubMed] [Google Scholar]
- 8.Hendig D et al. (2008) Gene expression profiling of ABC transporters in dermal fibroblasts of pseudoxanthoma elasticum patients identifies new candidates involved in PXE pathogenesis. Lab. Invest 88, 1303–1315 [DOI] [PubMed] [Google Scholar]
- 9.Jiang Q et al. (2009) Pseudoxanthoma elasticum is a metabolic disease. J. Invest. Dermatol 129, 348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorgels TGMF et al. (2005) Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum. Mol. Genet 14, 1763–1773 [DOI] [PubMed] [Google Scholar]
- 11.Klement JF et al. (2005) Targeted ablation of the abcc6 gene results in ectopic mineralization of connective tissues. Mol. Cell. Biol 25, 8299–8310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Q et al. (2007) Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: systemic and local regulatory factors. J. Invest. Dermatol 127, 1392–1402 [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q et al. (2010) Parabiotic heterogenetic pairing of Abcc6−/−/Rag1−/− mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am. J. Pathol 176, 1855–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bercovitch L et al. (2011) Acquired pseudoxanthoma elasticum presenting after liver transplantation. J. Am. Acad. Dermatol 64, 873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borst P et al. (1999) The multidrug resistance protein family. Biochim. Biophys. Acta 1461, 347–357 [DOI] [PubMed] [Google Scholar]
- 16.Deeley RG et al. (2006) Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev 86, 849–899 [DOI] [PubMed] [Google Scholar]
- 17.Kool M et al. (1999) Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 59, 175–182 [PubMed] [Google Scholar]
- 18.Madon J et al. (2000) Transport function and hepatocellular localization of mrp6 in rat liver. Mol. Pharmacol. 57, 634–641 [DOI] [PubMed] [Google Scholar]
- 19.Iliás A et al. (2002) Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J. Biol. Chem. 277, 16860–16867 [DOI] [PubMed] [Google Scholar]
- 20.Belinsky MG et al. (2002) Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res. 62, 6172–6177 [PubMed] [Google Scholar]
- 21.Borst P et al. (2008) Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle 7, 1575–1579 [DOI] [PubMed] [Google Scholar]
- 22.Gorgels TGMF et al. (2011) Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic calcification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med 89, 1125–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Q et al. (2011) Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6−/−mice, a model for Pseudoxanthoma elasticum. Cell Cycle 10, 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brampton C et al. (2011) Vitamin K does not prevent soft tissue mineralization in a mouse model of pseudoxanthoma elasticum. Cell Cycle 10, 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fülöp K et al. (2011) ABCC6 does not transport vitamin K3-glutathione conjugate from the liver: relevance to pathomechanisms of pseudoxanthoma elasticum. Biochem. Biophys. Res. Commun 415, 468–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schurgers LJ et al. (2013) Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol. Med 19, 217–226 [DOI] [PubMed] [Google Scholar]
- 27.Vanakker OM et al. (2007) Pseudoxanthoma elasticum-like phenotype with cutis laxa and multiple coagulation factor deficiency represents a separate genetic entity. J. Invest. Dermatol 127, 581–587 [DOI] [PubMed] [Google Scholar]
- 28.van de Wetering K et al. (2009) Targeted metabolomics identifies glucuronides of dietary phytoestrogens as a major class of MRP3 substrates in vivo. Gastroenterology 137, 1725–1735 [DOI] [PubMed] [Google Scholar]
- 29.Krumpochova P et al. (2012) Transportomics: screening for substrates of ABC transporters in body fluids using vesicular transport assays. FASEB J. 26, 738–747 [DOI] [PubMed] [Google Scholar]
- 30.Pomozi V et al. (2013) ABCC6 Is a Basolateral Plasma Membrane Protein. Circ. Res 112, e148–e151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen RS et al. (2013) ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. U.S.A 110, 20206–20211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleisch H et al. (1966) Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature 212, 901–903 [DOI] [PubMed] [Google Scholar]
- 33.Jansen RS et al. (2014) ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler. Thromb. Vasc. Biol 34, 1985–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q et al. (2017) Abcc6 Knockout Rat Model Highlights the Role of Liver in PPi Homeostasis in Pseudoxanthoma Elasticum. J. Invest. Dermatol 137, 1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orriss IR et al. (2016) Pyrophosphate: a key inhibitor of mineralisation. Curr. Opin. Pharmacol 28, 57–68 [DOI] [PubMed] [Google Scholar]
- 36.Rutsch F et al. (2001) PC-1 Nucleoside Triphosphate Pyrophosphohydrolase Deficiency in Idiopathic Infantile Arterial Calcification. Am. J. Pathol 158, 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutsch F et al. (2003) Mutations in ENPP1 are associated with “idiopathic” infantile arterial calcification. Nat. Genet 34, 379–381 [DOI] [PubMed] [Google Scholar]
- 38.Lomashvili KA et al. (2014) Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int 85, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomozi V et al. (2017) Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am. J. Pathol 187, 1258–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dedinszki D et al. (2017) Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol. Med 9, 1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longhurst TJ et al. (1996) The anthracycline resistance-associated (ara) gene, a novel gene associated with multidrug resistance in a human leukaemia cell line. Br. J. Cancer 74, 1331–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill WC (2006) Pyrophosphate, Alkaline Phosphatase, and Vascular Calcification. Circ. Res. 99, e2–e2 [DOI] [PubMed] [Google Scholar]
- 43.Tolouian R et al. (2012) Using a filtration technique to isolate platelet free plasma for assaying pyrophosphate. Clin. Lab 58, 1129–1134 [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q et al. (2013) Mutant Enpp1asj mice as a model for generalized arterial calcification of infancy. Dis. Models Mech 6, 1227–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q et al. (2014) Juxta-articular joint-capsule mineralization in CD73 deficient mice: Similarities to patients with NT5Emutations. Cell Cycle 13, 2609–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boraldi F et al. (2003) Multidrug resistance protein-6 (MRP6) in human dermal fibroblasts. Comparison between cells from normal subjects and from Pseudoxanthoma elasticum patients. Matrix Biol. 22, 491–500 [DOI] [PubMed] [Google Scholar]
- 47.Boraldi F et al. (2014) Changes in dermal fibroblasts from Abcc6(−/−) mice are present before and after the onset of ectopic tissue mineralization. J. Invest. Dermatol 134, 1855–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dabisch-Ruthe M et al. (2014) Variants in genes encoding pyrophosphate metabolizing enzymes are associated with Pseudoxanthoma elasticum. Clin. Biochem 47, 60–67 [DOI] [PubMed] [Google Scholar]
- 49.Kuzaj P et al. (2014) Large-Scaled Metabolic Profiling of Human Dermal Fibroblasts Derived from Pseudoxanthoma Elasticum Patients and Healthy Controls. PLoS ONE 9, e108336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegler SG et al. (2017) Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci. Transl. Med 9, eaal1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer MG et al. (2003) In vitro and in vivo comparative study of chimeric liver-specific promoters. Mol. Ther 7, 375–385 [DOI] [PubMed] [Google Scholar]
- 52.Le Saux O et al. (2011) Expression and In Vivo Rescue of Human ABCC6 Disease-Causing Mutants in Mouse Liver. PLoS ONE 6, e24738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pomozi V et al. (2017) Functional Rescue of ABCC6 Deficiency by 4-Phenylbutyrate Therapy Reduces Dystrophic Calcification in Abcc6−/− Mice. J. Invest. Dermatol 137, 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin LJ et al. (2012) ABCC6 localizes to the mitochondria-associated membrane. Circ. Res 111, 516–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin LJ et al. (2013) Response to Pomozi et al’s Research Commentary. Circ. Res 112, e152–e153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazarowski ER (2012) Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal 8, 359–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell RG et al. (1971) Inorganic pyrophosphate in plasma in normal persons and in patients with hypophosphatasia, osteogenesis imperfecta, and other disorders of bone. J. Clin. Invest 50, 961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mornet E (2000) Hypophosphatasia: the mutations in the tissue-nonspecific alkaline phosphatase gene. Hum. Mutat 15, 309–315 [DOI] [PubMed] [Google Scholar]
- 59.St Hilaire C et al. (2011) NT5E mutations and arterial calcifications. N. Engl. J. Med 364, 432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herrmann M et al. (2012) Fetuin-A Function in Systemic Mineral Metabolism. TCM 22, 197–201 [DOI] [PubMed] [Google Scholar]
- 61.Hendig D et al. (2006) Role of serum fetuin-A, a major inhibitor of systemic calcification, in pseudoxanthoma elasticum. Clin. Chem 52, 227–234 [DOI] [PubMed] [Google Scholar]
- 62.Jiang Q et al. (2010) Overexpression of Fetuin-A Counteracts Ectopic Mineralization in a Mouse Model of Pseudoxanthoma Elasticum (Abcc6−/−). J. Invest. Dermatol 130, 1288–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Young J et al. (2011) Matrix Gla Protein Inhibits Ectopic Calcification by a Direct Interaction with Hydroxyapatite Crystals. J. Am. Chem. Soc 133, 18406–18412 [DOI] [PubMed] [Google Scholar]
- 64.Kornak U (2011) Animal models with pathological mineralization phenotypes. Joint Bone Spine 78, 561–567 [DOI] [PubMed] [Google Scholar]
- 65.Schurgers LJ et al. (2017) Matrix Gla-protein: The calcification inhibitor in need of vitamin K. Thromb. Haemost 100, 593–603 [PubMed] [Google Scholar]
- 66.Li Q et al. (2013) Warfarin accelerates ectopic mineralization in Abcc6(−/−) mice: clinical relevance to pseudoxanthoma elasticum. Am. J. Pathol 182, 1139–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodan GA and Fleisch HA (1996) Bisphosphonates: mechanisms of action. J. Clin. Invest 97, 2692–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q et al. (2015) The effects of bisphosphonates on ectopic soft tissue mineralization caused by mutations in the ABCC6gene. Cell Cycle 14, 1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cortet B et al. (2001) Comparative efficacy and safety study of etidronate and alendronate in postmenopausal osteoporosis. effect of adding hormone replacement therapy. Joint Bone Spine 68, 410–415 [DOI] [PubMed] [Google Scholar]
- 70.Oizumi T et al. (2016) A Strategy against the Osteonecrosis of the Jaw Associated with Nitrogen-Containing Bisphosphonates (N-BPs): Attempts to Replace N-BPs with the Non-N-BP Etidronate. Biol. Pharm. Bull 39, 1549–1554 [DOI] [PubMed] [Google Scholar]
- 71.Li Q et al. (2018) Inhibition of Tissue-Nonspecific Alkaline Phosphatase Attenuates Ectopic Mineralization in the Abcc6−/− Mouse Model of PXE but not in the Enpp1 Mutant Mouse Models of GACI. J. Invest. Dermatol DOI: 10.1016/j.jid.2018.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henthorn PS and Whyte MP (1992) Missense mutations of the tissue-nonspecific alkaline phosphatase gene in hypophosphatasia. Clin. Chem 38, 2501–2505 [PubMed] [Google Scholar]
- 73.Albright RA et al. (2015) ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nature Communications 6, 10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Villa-Bellosta R (2018) Synthesis of Extracellular Pyrophosphate Increases in Vascular Smooth Muscle Cells During Phosphate-Induced Calcification. Arterioscler. Thromb. Vasc. Biol DOI: 10.1161/ATVBAHA.118.311444 [DOI] [PubMed] [Google Scholar]
- 75.Zhao J et al. (2017) Plasma PPi Deficiency Is the Major, but Not the Exclusive, Cause of Ectopic Mineralization in an Abcc6−/− Mouse Model of PXE. J. Invest. Dermatol 137, 2336–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorgels TGMF et al. (2010) Dietary magnesium, not calcium, prevents vascular calcification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med 88, 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang Q and Uitto J (2012) Restricting dietary magnesium accelerates ectopic connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6−/−). Exp. Dermatol 21, 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Q et al. (2009) Magnesium carbonate-containing phosphate binder prevents connective tissue mineralization in Abcc6(−/−) mice-potential for treatment of pseudoxanthoma elasticum. Clin. Transl. Sci 2, 398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoo JY et al. (2011) A randomized controlled trial of oral phosphate binders in the treatment of pseudoxanthoma elasticum. J. Am. Acad. Dermatol 65, 341–348 [DOI] [PubMed] [Google Scholar]
- 80.Braake, ter AD et al. (2017) Magnesium Counteracts Vascular Calcification: Passive Interference or Active Modulation? Arterioscler. Thromb. Vasc. Biol 37, 1431–1445 [DOI] [PubMed] [Google Scholar]
- 81.LaRusso J et al. (2009) Elevated dietary magnesium prevents connective tissue mineralization in a mouse model of pseudoxanthoma elasticum (Abcc6(−/−)). J. Invest. Dermatol 129, 1388–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gonzales E et al. (2012) Successful mutation-specific chaperone therapy with 4-phenylbutyrate in a child with progressive familialintrahepatic cholestasis type 2. J. Hepatol 57, 695–698 [DOI] [PubMed] [Google Scholar]
- 83.Pomozi V et al. (2014) Analysis of pseudoxanthoma elasticum-causing missense mutants of ABCC6 in vivo; pharmacological correction of the mislocalized proteins. J. Invest. Dermatol 134, 946–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzales E et al. (2015) Targeted pharmacotherapy in progressive familial intrahepatic cholestasis type 2: Evidence for improvement of cholestasis with 4-phenylbutyrate. Hepatology 62, 558–566 [DOI] [PubMed] [Google Scholar]
- 85.Trip MD et al. (2002) Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circulation 106, 773–775 [DOI] [PubMed] [Google Scholar]
- 86.Zhou Y et al. (2013) Premature termination codon read-through in the ABCC6 gene: potential treatment for pseudoxanthoma elasticum. J. Invest. Dermatol 133, 2672–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atanasova VS et al. (2017) Amlexanox Enhances Premature Termination Codon Read-Through in COL7A1 and Expression of Full Length Type VII Collagen: Potential Therapy for Recessive Dystrophic Epidermolysis Bullosa. J. Invest. Dermatol 137, 1842–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel A et al. (2017) ATP as a biological hydrotrope. Science 356, 753–756 [DOI] [PubMed] [Google Scholar]
- 89.Bryan J et al. (2006) ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch 453, 703–718 [DOI] [PubMed] [Google Scholar]
- 90.Nitschke Y and Rutsch F (2012) Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front. Genet 3, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Q and Uitto J (2010) The mineralization phenotype in Abcc6 (−/−) mice is affected by Ggcx gene deficiency and genetic background--a model for pseudoxanthoma elasticum. J. Mol. Med 88, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]