Abstract
Background:
Claudication is the most common manifestation of peripheral artery disease (PAD), producing significant ambulatory compromise. Limited information exists on the routine physical activity of claudicating patients. Our objective was to record the intensity/time profiles of physical activity and the timing and duration of sedentary behavior of a sample of community-dwelling claudicating patients.
Methods:
Forty-four claudicating patients referred to our vascular clinic were recruited. Physical activity was recorded using the ActiGraph GT1M activity monitor. The Actigraph monitor is a lightweight instrument designed to measure human movement through changes in acceleration, measured as counts over 1-minute time periods. Data from 7 consecutive days were used for the calculations. We processed the data using the ActiLife software program.
Results:
The average daily activity of the claudicating patients shows a steady increase beginning approximately 05:30 AM until a peak plateau from approximately 10:00 AM to 01:30 PM followed by a steady decrease until approximately 09:30 PM, when a sustained period of inactivity begins. The average claudicating patient takes 3586 steps per day at an average intensity of 1.77 metabolic equivalents of task (METs, a physiological measure expressing the energy cost of physical activities). Average physical activity intensity and peak intensity fluctuate very little during the day, and they rarely exceed the level of light activity (light = <3 METs maximum effort, such as casual walking or light housework). During awake time, approximately 7 hours are spent in sedentary behaviors (<1.5 METs), and sedentary time is spread throughout the day mostly in short intervals between periods of low-energy activity.
Conclusions:
Our study objectively demonstrates the reduced physical activity of claudicating patients and documents physical activity/duration profiles throughout the day. The intensity of the physical activity of the average claudicating patient fluctuates very little during the day and rarely exceeds a light intensity level. Claudicating patients spend approximately half of their awake time in sedentary behavior and when they walk they do it in short bursts followed by several minutes of rest. We anticipate that changes in routine physical activity/duration profiles of patients with PAD will provide relevant, sensitive, and direct measures of the effectiveness of therapeutic interventions.
INTRODUCTION
Epidemiological studies of peripheral artery disease (PAD) show a prevalence of 3% to 10% in the general population and as high as 20% in populations older than 70 years.1,2 PAD affects an estimated 27 million people in Europe and North America and is caused by atherosclerotic plaques that limit blood flow to the lower extremities.3,4 The most common manifestation of PAD is intermittent claudication, a severe functional impairment characterized as walking-induced discomfort in the muscles of the leg, relieved by rest.1 This impairment is of major importance primarily because it restricts the physical activity of PAD patients, leading to further adverse effects on their general health and quality of life.
Historically, daily physical activity of claudicating patients has been difficult to record accurately, with most investigators relying on surrogate assessments. Such assessments include questionnaires such as the Walking Impairment Questionnaire and the 36-Item Medical Outcomes Study Short Form Survey,5 and measures of maximum walking ability using a standardized treadmill test (Gardner test)6 and a maximum distance six-minute walk test.7 The limitations of these tests as surrogate measures of daily physical activity were shown in a study by Zwerink et al. measuring physical activity in patients with chronic obstructive pulmonary disease. The investigators demonstrated that after a structured exercise program, an increase in the maximum physical exercise capacity did not translate to increased daily physical activity.8 Owing to these results, some investigators have proposed that routine physical activity is an important measure of the physical ability of claudicating patients and should be evaluated in PAD studies.9
More recently, a number of investigators have used sophisticated instrumentation to measure the routine physical activity of claudicating patients.10–12 These studies have focused on the limitation of daily activity produced by claudication, as determined by total steps taken per day (summarized in Table I) and rate of steps taken. However, our knowledge of the timing, duration, and intensity of routine physical activity remains limited. The purpose of this study was to determine the minute-to-minute, duration and intensity of routine physical activity and the timing and duration of inactivity among community-dwelling claudicating patients.
Table I.
Summary of studies investigating monitored daily physical activity
| Study | No. of claudicating patients studied | Activity monitor used | Age | ABI | Steps/day |
|---|---|---|---|---|---|
| Gardner et al. (2007)13 | 98 | StepWatch 3 | 66 ± 12 | 0.71 ± 0.21 | 3149 ± 1557 |
| Gardner et al. (2010)14 | 40 | StepWatch 3 | 66 ± 10 | 0.75 ± 0.23 | 3144 ± 265 (mean ± standard error) |
| Lauret et al. (2014)11 | 30 with claudication and WIQ score < 0.4 26 with claudication and WIQ score > 0.4 |
DynaPort Movement Monitor | 69 ± 9 69 ± 8 |
0.6 ± 0.18 0.66 ± 0.18 |
4663 ± 3104 5761 ± 2371 |
| Gardner et al. (2014)15 | 134 | StepWatch 3 | 65 ± 10 | 0.72 ± 0.24 | 3275 ± 1743 |
| Gommans et al. (2016)9 | 55 | Dynaport Movement Monitor | 66 ± 9 | 0.63 + 0.17 | 6315 (range 3364–9207) |
| Hernandez et al. (current study) | 44 | Actigraph GT1M | 64 ± 6 | 0.46 ± 0.04 | 3586 ± 298 (mean ± standard error) |
Values are presented as mean ± standard deviation unless otherwise noted. SE, standard error; WIQ, Walking Impairment Questionnaire.
METHODS
Patient Recruitment
Forty-four patients were recruited from the Vascular Surgery Clinic at the Veterans Affairs Nebraska-Western Iowa HealthCare System (VA-NWIHCS) in Omaha, Nebraska. Patients were recruited after consultation for intermittent claudication and before any sort of intervention for their PAD. PAD was confirmed by history, physical examination, noninvasive testing, and standard or computerized tomo-graphic angiography. To determine that it was indeed intermittent claudication and not some other disorder that was affecting ambulation, each participant walked a standard, predetermined lap around the clinic floor accompanied by a vascular surgeon. The diagnosis was established based on description and location of symptoms. Patients were excluded if the primary factor limiting exercise tolerance was something other than claudication (e.g., shortness of breath, arthritis, chest pain, back pain, and so forth). The procedures used in this study were approved by the institutional review board at the VA-NWIHCS. Written informed consent was obtained from each patient before investigation.
Demographics
All study patient demographics are reported in Table II.
Table II.
Demographics of study participants
| Demographics | |
|---|---|
| Gender | |
| Male | 97.7% |
| Age | 64.16 ± 6.55 |
| Race | |
| White | 93.1% |
| Black | 4.5% |
| Hispanic | 2.3% |
| Ankle-brachial index | 0.46 ± 0.04 |
| BMI | 28.59 ± 4.725 |
| CAD | 41% |
| HTN | 82% |
| Obesity | 45% |
| HLD | 86% |
| Family history | 55% |
| Tobacco users | |
| Current | 52% |
| Never | 7% |
| Former | 41% |
| DM | 43% |
| Renal insufficiency | 9% |
| Statin use | 86% |
Ankle-brachial index figures are from the more affected leg in cases of bilateral disease. All disease diagnoses are from the patient’s history and electronic medical record review.
BMI, body mass index; CAD, coronary artery disease; DM, diabetes mellitus; HTN, hypertension; HLD, hyperlipidemia; Family history, family history of vascular disease.
Daily Physical Activity Monitoring
Physical activity was recorded using the Actigraph (Actigraph, LLC; Fort Walton Beach, FL) GT1M accelerometer. The Actigraph GT1M uses a capacitive accelerometer capable of detecting both static(i.e., gravity detected when stationary) and dynamic accelerations. Actigraph specifies restrictions that allow the detection of only those accelerations between 0.05–2G. This way it only records movements in the human spectrum of physical activity and avoids erroneous overestimates.16 As a result, when the wearer’s acceleration is detected, the Acti-graph accelerometer is able to determine and record the force and direction of that acceleration. The Acti-graph GT1M is a small, lightweight device (weight: 19 grams and dimensions: 4.6 × 3.3 × 1.5 cm) that can worn on a waistband or belt loop and has minimal, if any, effect on our patient’s activity level.17,18 Actigraph technology is validated for quantifying physical activity, activity-related energy expenditure, and assessment of reproducibility.17,18
Monitoring Protocol
The study participants were instructed to wear the accelerometer for 7 consecutive days. They were instructed to wear the monitor all day and could take it off to sleep and must take it off before showering or submerging in water. The monitor was placed on an elastic waistband worn on the hip that the participants could wear either over or under a shirt. Patients returned the monitor at their next follow-up appointment in our clinic. The participants were instructed to perform their daily physical activities as they normally would.
Outcomes
The Actigraph monitor quantifies accelerations and filters the raw data, assigning each acceleration a value measure in “counts” over a specified amount of time. We programmed the monitors to record data as “counts per minute” over the entire data-recording period. Recording accelerations in this way allows interpretation of a person’s activity and distinguishes between dynamic movements such as walking and sedentary movements such as adjusting postural position while seated in a chair. Counts per minute can then be used to convert physical activity data into steps taken and energy expenditure. Energy expenditure from the physical activity data is measured in metabolic equivalents of task (METs), a physiological measure expressing the energy cost of physical activity as a ratio to resting metabolic rate. Energy expenditure expressed in METs is a description of energy intensity; the Acti-graph monitor is able to determine a baseline resting energy expenditure for each patient while they are sedentary and that is then used to determine the METs performed during physical activity. The Acti-graph monitors were set to sample data at a rate of 60 samples per minute; the average of the 60 samples for each minute was used to determine the average value over that minute. Raw data from the Actigraph monitors were used to determine the following endpoints:
Average total steps taken per day: Using ActiLife (Actigraph, LLC; Fort Walton Beach, FL) software and algorithms, counts per minute were recorded for each minute of the day for a 7-day period for each of the 44 participants. Counts per minute were then converted to steps taken each minute of the day for each participant. For each participant, total steps per day over a 7-day period, and average steps taken per day were computed. The averages for each participant were then used to compute the average steps taken per day for the group of claudicating patients.
Average intensity and average peak intensity per day for only awake time: The ActiLife software and algorithms were used to calculate the average intensity and the average peak intensity of physical activity during minutes of recorded physical activity for each participant. For these calculations, we did not include data from minutes during which participants were either asleep/napping or possibly had removed the accelerometer for whatever reason. This method ensures that the averages are not weighed down by zero counts from asleep or nonwear time during the day. Awake time was defined as any time of recordable activity. Periods lasting 10 minutes or more with consecutive zero counts per minute were counted as asleep or nonwear time. Asleep or nonwear time is over when there are at least 3 consecutive minutes with nonzero counts per minute or 1-minute reading ≥100 counts per minute. The intensity is expressed in METs and is calculated by taking the average intensity recorded by the Actigraph.
Average total sedentary minutes per day during awake time: Using validated algorithms, ActiLife software determines sedentary minutes as minutes with <100 counts per minute that is separate from nonwear time. Total sedentary minutes per day, during awake time, were recorded for each participant and were used to calculate individual and group averages.
Average number of sedentary periods per day of >10 consecutive minutes during awake time: For each participant, we recorded all sedentary periods lasting ≥ 10 consecutive minutes, during awake time. We used the data to calculate the average number of sedentary periods per day of ≥10 consecutive minutes for each individual and for the group.
Average length in minutes of the longest sedentary period during awake time: We recorded the longest sedentary period each day for each participant and computed the average for the group of patients.
Average intensity of only nonsedentary minutes during awake time: Using minutes with <100 counts per minute to define sedentary minutes, we excluded all sedentary minutes during each day and only used nonsedentary minutes (minutes with ≥ 100 CPM) to determine the intensity of physical activity. This method is intended to show the intensity level of the group during only nonsedentary time and gives the best picture of the level of activity patients with PAD can achieve during the day.
RESULTS
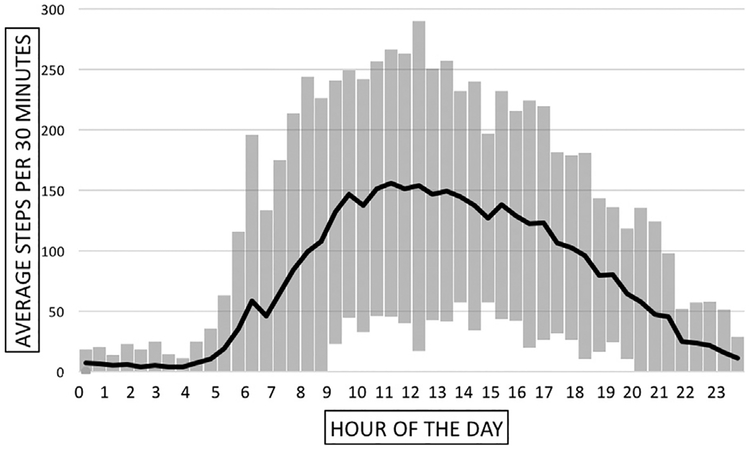
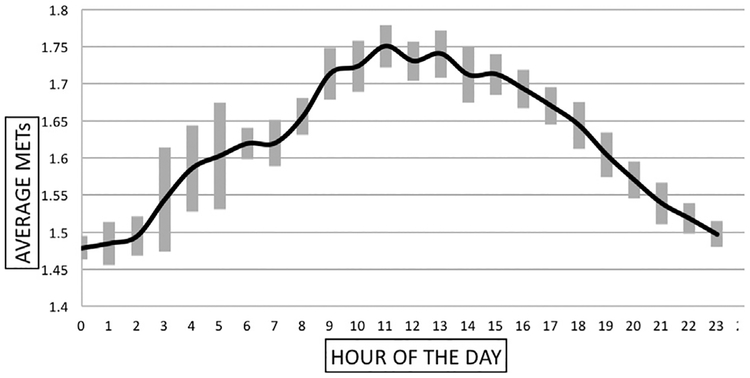
This study showed that, on average, patients with claudication took 3586 total steps per day (Table III). The average steps per 30 minutes ranged from an average of 10 to just over 150, with the most steps occurring between the hours of 8:00 AM–06:00 PM, and the highest step counts occurring from 10:00 AM–01:00 PM (Fig. 1). During awake time, the average intensity for our group of PAD patients was 1.62 METs, and the average peak intensity was 2.83 METs. Figure 2 shows our patients overall daily intensity level.
Table III.
Results of physical activity measures
| Parameter | Mean ± standard error | |
|---|---|---|
| a | Average total steps taken per day | 3586.44 ± 298.02 |
| b | Average intensity (METs) and peak intensity per day (METs) | 1.62 ± 0.002 2.83 ± 0.07 |
| c | Average total sedentary minutes per day | 433.45 ± 29.94 |
| d | Average number of sedentary periods per day of >10 consecutive minutes | 9.50 ± 0.90 |
| e | Average length of the longest sedentary period > 10 consecutive minutes (minutes) | 45.14 ± 5.01 |
| f | Average intensity of only nonsedentary minutes (METs) | 1.77 ± 0.01 |
Fig. 1.
Average steps per 30 minutes taken by our group of claudicating patients during an average day. Patients with PAD take 3586 total steps per day. The bars at every time point represent standard deviation.
Fig. 2.
The average daily intensity level of patients with PAD. The bars at every time point represent standard deviation. METs were sampled every second and expressed as an average for each 1-minute interval.
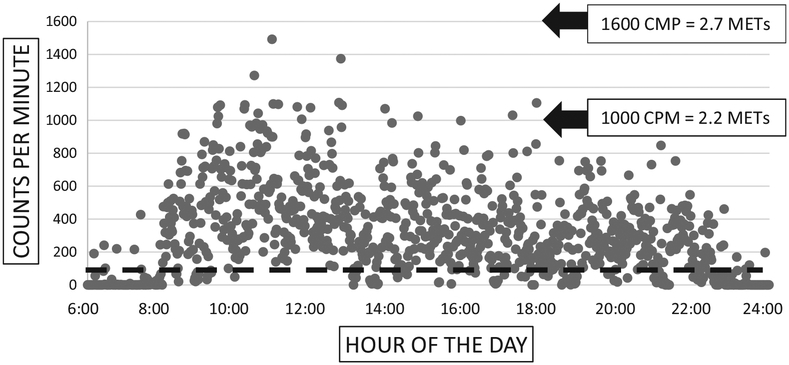
Analysis of sedentary time, defined as time spent with exertion levels below 100 counts per minute on the accelerometer,17,18 shows that the average total sedentary time of our patients with PAD was 433.45 minutes during awake time. Figure 3 presents both sedentary and nonsedentary time points of 1 representative patient (has activity i.e. close to the average for our group of patients) during his awake hours. To better represent the daily pattern of patient inactivity during awake time, we identified sedentary periods lasting longer than 10 consecutive minutes. We found that, on average, patients with PAD had 9 of these sedentary periods per day. We also calculated that, on average, the longest sedentary period for PAD patients, during awake time, lasted 45 minutes. Finally, after determining each patient’s sedentary time, we excluded this from intensity calculations and determined that on average, during their nonsedentary time, patients with PAD had an average intensity of1.77 METs.
Fig. 3.
The counts per minute recorded during awake hours of one median-achieving patient. The black dashed line designates 100 counts per minute; any marks below the black dashed line indicate minutes spent in sedentary activity (defined as <100 count/minute). Counts per minute can be converted into metabolic equivalents of tasks, a physiological measure expressing the energy cost of physical activity as a ratio to resting metabolic rate. One hundred counts/minute corresponds to 1.52 METs, which is less than the energy expenditure needed to sit at a desk and type. For reference, we show 1000 counts/minute roughly equal to 2.2 METs, or the energy needed to make the bed or wash dishes and 1600 counts/minute roughly equal to 2.7 METs, or the energy needed to play billiards or darts.
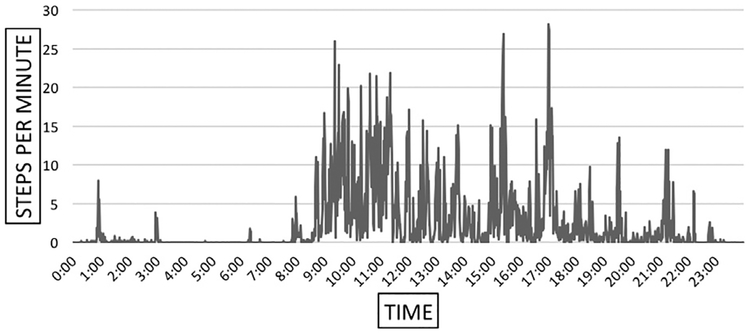
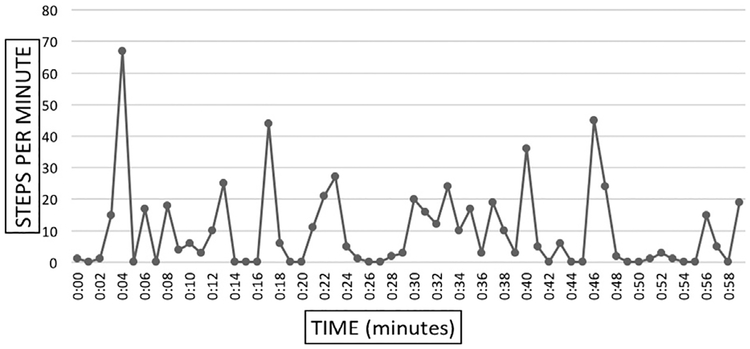
Figure 4 demonstrates that some participants can achieve close to 30 steps per minute during some minutes of the day. Patients with intermittent claudication can achieve relatively higher levels of activity and yet most of the day is spent at very low activity, below 5 steps per minute. The frequency of low/inactive minutes are responsible for the very low average activity of our patients. Figure 5 further breaks down the activity of a median-achieving patient and shows that in a representative hour of the day our patients can reach 30–60 + steps per minute, but 37 of the 60 minutes are spent below 5 steps per minute and 29 of those minutes are spent at or near zero steps per minute.
Fig. 4.
Twenty-four—hour profile of total steps per minute taken in a day by one representative patient.
Fig. 5.
Recorded steps per minute of an actual hour of activity of one particular patient from our study.
We analyzed the correlations between our physical activity measurements (total daily steps, average METs/min, average peak METs/min, total daily sedentary minutes, total number of sedentary bouts lasting >10 minutes, average longest sedentary bout, and average nonsedentary time METs/min) and our recorded patient demographics (age, body mass index, coronary artery disease, hypertension, obesity, hyperlipidemia, family history of vascular disease, smoking status, diabetes mellitus, and ankle-brachial index). To analyze correlations, we calculated the Pearson correlation (r) and determined significance to any correlation with a P value < 0.05. Significant correlations included the following: (1) body mass index with average nonsedentary time METs/min; (2) obesity with total daily sedentary minutes; (3) obesity with total number of sedentary bouts lasting >10 minutes; and (4) diabetes mellitus with total daily steps. Table IV demonstrates the specific findings.
Table IV.
Significant correlations between patient demographics and physical activity measures
| Demographic and physical activity measure | Pearson correlation (r) | P value |
|---|---|---|
| BMI—average nonsedentary time METs/min | −0.3239 | 0.0320 |
| Obesity—total daily sedentary minutes | 0.3306 | 0.0284 |
| Obesity—total sedentary bouts longer than 10 minutes | 0.3124 | 0.0390 |
| DM—total daily steps | −0.3326 | 0.0274 |
BMI, body mass index; DM, diabetes mellitus.
We divided our patients based on the anatomic location of their occlusive disease to patients with aortoiliac, femoropopliteal, and combined aortoiliac/femoropopliteal disease. We then compared the average performance of claudicating patients in each group using independent t-test analysis and determined significance to any comparison with a P value < 0.05. Our analysis did not show any significant difference between the different anatomic groups in any of the following areas: ankle-brachial index, total daily steps, average METs/min, average peak METs/min, total daily sedentary minutes, total number of sedentary bouts lasting >10 minutes, average longest sedentary bout, and average nonsedentary time METs/min.
DISCUSSION
This study builds on the work of several other groups that evaluated the limitation of daily activity caused by PAD and claudication.5,6,8–11,13,19–21 Our findings agree with previous reports showing that patients with intermittent claudication are severely limited in their physical activity, walking on average 3586 steps/day. In our analysis, we expanded on published work by determining step count, intensity and duration of physical activity, and pattern and duration of inactivity, on a minute-to-minute basis. Our data demonstrate that the average (1.62 METs) and peak intensity (2.83 METs) of physical activity of our claudicating patients fluctuated very little during the day and that the patients rarely exceeded a light intensity level. Furthermore, we found that claudicating patients spent more than 7 hours per day in sedentary behavior and that when they walked they did so in short bursts followed by 2–3 minutes of rest. These findings have significant implications for our understanding of the physiology of PAD and the limitations PAD introduces in the daily activity and the general state of health of claudicating patients and can form the basis for the design and evaluation of interventions to increase activity of patients with PAD.
A major finding in this study is that the average total steps/day of claudicating patients (3586 steps) is much lower than the recent recommendations of 6500 steps/day for individuals living with disability/chronic illness.19 These recommendations were introduced by Tudor-Locke et al. and were based on their review of data on patients undergoing cardiac rehabilitation. The investigators recommended that these 6500 steps/day should include 3000 steps at a cadence of 100 steps/min which for PAD patients is roughly equal to 4.2 METs. In separate studies, Gardner et al. and Montgomery et al. determined that patients with PAD and claudication are usually not able to reach a cadence of 100 steps/min showing that maximum efforts during a 6-minute walk test yield results of 96 steps/min.13,20 This suggests that even if our patients are somehow able to increase their total steps/day to the recommended level (this would require addition of another 3,000 steps/day), they likely would be unable to perform enough physical activity in the moderate-vigorous range. Our findings indicate that patients with PAD and claudication are severely limited in their physical activity being unable to achieve even minimum recommended daily physical activity of healthy counterparts or adults over age 65 years with disabilities/chronic illnesses.
Similar studies of physical activity among patients with medical conditions other than PAD have been reviewed and summarized by Tudor-Locke et al.21 Their review concluded that, on average, patients with chronic obstructive pulmonary disease take 2237 steps/day, patients with arthritis affecting the hips or knees take 4086 steps/day and patients with joint (hip/knee) arthroplasty took 4892 steps/day, patients with heart/vascular disease take 4695 steps/day, Parkinson’s disease) take 5887 steps/day, patients with type II diabetes mellitus took 6342 steps/day, breast cancer survivors take 7409 steps/day, and patients with neuromuscular disease (e.g., multiple sclerosis, muscular dystrophy) and patients with HIV take 7545 steps/day. In comparison, our group of participants average 3586 steps/day. These data underscore the severity of the limitation of physical activity among patients with PAD.
Our study also included measurement of energy expenditure by our patients. The energy required to take a certain number of steps is a function of both body weight and rate of taking steps and will be different for different participants. Thus, in addition to expressing physical activity as the number of steps taken, we expressed physical activity in terms of energy intensity, or METs. Intensity was calculated for awake time physical activity on the basis of recorded counts (steps) per minute obtained from the accelerometer and the wearer’s weight. On doing this, we found that the average intensity for our patients was 1.62 METs and that the average peak intensity was 2.83 METs. In the Compendium of Physical Activities, Ainsworth et al. define one MET as equal to the energy cost of resting or sitting quietly; 1.5 METs is similar to driving or doing desk work such as writing, typing, or using the computer; 2 METs is similar to level walking at 2 miles per hour or standing and doing light work such as cooking, washing the dishes, or making the bed; 3 METs is similar to sweeping, mopping, or vacuuming the floor or gardening; 4 METs is similar to slow step dancing or walking and playing golf; and 5 METs is similar to square dancing or performing household chores such as mowing the lawn.22 This means that our group’s average and peak daily intensities are at the same level as driving or doing desk work such as writing, typing, or using the computer. This provides a clearer picture of the severity of physical limitation experienced by our participants.
Sedentary behavior defined as minutes with <100 CPM, that is, roughly the energy required to sit and watch television, was also of interest to us. Extended periods of sedentary behavior have also been shown to be associated with adverse health risks, even among those who reach recommended levels of physical activity.23−25 Our data showed that during awake time, on average, our patients had 433.45 total minutes of sedentary time per day. Just over 7 hours were spent in sedentary behavior, and on average, participants had 9.5 sedentary periods lasting longer than 10 consecutive minutes with the longest daily sedentary period lasting 45.14 minutes. Looking at the raw data, we saw many instances where the patient took a few steps followed by a 2- to 3-minute period of sedentary activity. These activity patterns were present throughout the day and accounted for approximately 2 hours per day. We believe that these findings can help explain the mechanisms operating to produce the physical disability of claudication. We offer 2 possible interpretations for these findings. First, claudicating patients experience leg ischemia and associated claudication symptoms many times throughout the day possibly with much less activity than we thought, and because of this, they can only be active in very short bursts that require subsequent short periods of rest. An additional component of the symptoms associated with claudication includes possible systemic effects of leg ischemia mediated through metabolic, neural or inflammatory pathways. Of note, the group of Sinoway and Cauffman have shown in several elegant studies that it takes a few, low-intensity, contractions of the posterior calf muscles of patients with PAD to produce significant sympathetic system activation and adverse effects on physiologic parameters such as the heart rate, blood pressure, and coronary and renal artery blood flow.26−28 Second, claudicating patients have adapted to the leg symptoms produced by longer periods of activity by performing low-intensity activities that are terminated before they experience pain and therefore rest many times per day. Interestingly, work by Najafi et al. has shown that patients with dementia exhibit a similar walking pattern taking few steps and then resting. These findings suggest that a failure of the neuromusculoskeletal system at any level may be responsible for the pattern of activity/inactivity seen in both PAD and dementia patient populations.29,30 Future work should evaluate the type of activities in these short periods of walking in association with symptoms and muscle oxygenation and blood flow. This type of work can provide insight into the pathophysiologic mechanisms operating in PAD and claudication and the way they operate to produce the symptoms and limitations that are well known for our patients. In addition, this work may provide guidance for improved diagnostic and therapeutic approaches for the care of patients with PAD.
Our evaluation of correlations between parameters of physical activity and demographic, clinical and hemodynamic parameters demonstrated no association with ankle-brachial index or level of disease. This finding is in agreement with prior works from our31−33 and other groups34−36 showing poor or no relationship between functional parameters and burden of occlusive disease. The finding suggests that, in addition to occlusive disease, several parameters, the well-described ischemic myopathy and neuropathy of PAD legs, microvascular disease, patient motivation and comorbidities, play a significant role in the daily activity levels of claudicating patients.33,37−40 The potential contribution of comorbidities to the functional decline of patients with PAD was further substantiated by the association we identified between parameters such as body mass index (BMI), obesity, and diabetes and physical activity in our patients. More specifically, increasing BMI correlated with lower average nonsedentary METs/min, meaning that the higher a patient’s BMI then the lower the intensity level when that person is active. The presence of obesity (described as BMI > 30) correlated with total daily sedentary minutes and with total number of sedentary bouts lasting longer than 10 consecutive minutes, meaning that the presence of obesity predicted greater overall sedentary time and longer bouts of sedentary time. The presence of diabetes mellitus correlated with less total daily steps, meaning that claudicating patients with diabetes walked less than nondiabetic claudicating patients.
A limitation of our study is the lack of a control group. It would be informative to have physical activity data for patients without claudication but our protocol was designed to focus solely on the description of physical activity and sedentary behavior of a sample of community-dwelling claudicating patients. An additional limitation of our work is that our data are produced from the evaluation of a rather homogenous group of patients. Our study involved patients seen in a vascular surgery clinic of a Veterans hospital where most subjects with claudication are Caucasian males over 60 years of age. This may limit the generalization of our findings across genders, race, and age groups.
CONCLUSION
We quantified the reduced physical activity of claudicating patients and detailed their daily activity patterns. Average intensity and average peak intensity of the physical activity of the claudicating patients fluctuated very little during the day, and average intensity rarely exceeded the level of light intensity. Claudicating patients spent close to 7 hours per day in sedentary behavior and when they walked, they usually did so for short periods of only a few steps followed by 2 to 3 minutes of rest. Our objective measures of physical activity and sedentary behavior establish sensitive and relevant physical performance parameters that may be correlated with biochemical, histological, and physiological parameters of the legs of patients with PAD. Furthermore, these objective measures may help us improve the diagnosis and treatment of these patients.
Acknowledgments
This work was supported by the NIH R01 grants AG034995, AG049868, and HD090333, NIH P20GM109090, and by the Charles and the Mary Heider Fund for Excellence in Vascular Surgery. Furthermore, this material is the result of work supported with resources and the use of facilities at the VA Nebraska-Western Iowa Health Care System.
Footnotes
Presented as an oral presentation at the Midwestern Vascular Surgical Society 2016 Annual Meeting, Columbus, OH.
Conflict of interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013;382:1329–40. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Aboyans V, Fowkes FJ, et al. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 2017;14:156–70. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statisticsd2016 update: a report from the American heart association. Circulation 2016;133:447–54. [DOI] [PubMed] [Google Scholar]
- 4.Weiss DJ, Casale GP, Koutakis P, et al. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J Translational Med 2013;11:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 2003;37: 197–206. discussion: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner AW, Skinner JS, Cantwell BWL, et al. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 1991;23:402–8. [PubMed] [Google Scholar]
- 7.Butland RJ, Pang J, Gross ER, et al. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J 1982;284: 1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwerink M, van der Palen J, van der Valk P, et al. Relationship between daily physical activity and exercise capacity in patients with COPD. Respir Med 2013;107:242–8. [DOI] [PubMed] [Google Scholar]
- 9.Gommans L, Hageman D, Jansen I, et al. Minimal correlation between physical exercise capacity and daily activity in patients with intermittent claudication. J Vasc Surg 2016;63:983–9. [DOI] [PubMed] [Google Scholar]
- 10.Fokkenrood HJP, Lauret GJ, Verhofstad N, et al. The effect of supervised exercise therapy on physical activity and ambulatory activities in patients with intermittent claudication. Eur J Vasc Endovasc Surg 2015;49: 184–91. [DOI] [PubMed] [Google Scholar]
- 11.Lauret GJ, Fokkenrood HJ, Bendermacher BL, et al. Physical activity monitoring in patients with intermittent claudication. Eur J Vasc Endovasc Surg 2014;47:656–63. [DOI] [PubMed] [Google Scholar]
- 12.Weitz JI, Byrne J, Clagett GP, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation 1996;94:3026–49. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Montgomery PS, Scott KJ, et al. Patterns of ambulatory activity in subjects with and without intermittent claudication. J Vasc Surg 2007;46:1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner AW, Parker DE, Montgomery PS, et al. Gender differences in daily ambulatory activity patterns in patients with intermittent claudication. J Vasc Surg 2010;52: 1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner AW, Parker DE, Montgomery PS, et al. Monitored daily ambulatory activity, inflammation, and oxidative stress in patients with claudication. Angiology 2014;65:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John D, Freedson P. Actigraph and actical physical activity monitors: a Peek under the Hood. Med Sci Sports Exerc 2012;44(1Suppl 1):S86–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evenson KR, Buchner DM, Morland KB. Objective measurement of physical activity and sedentary behavior among US adults aged 60 years or older. Prev Chronic Dis 2012;9: E26. [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol 2008;167:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 2011;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998;46:706–11. [DOI] [PubMed] [Google Scholar]
- 21.Tudor-Locke C, Washington TL, Hart TL. Expected values for steps/day in special populations. Prev Med 2009;49:3–11. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 23.Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 2012;55:2895–905. [DOI] [PubMed] [Google Scholar]
- 24.Thorp AA, Owen N, Neuhaus M, et al. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies, 1996–2011. Am J Prev Med 2011;41:207–15. [DOI] [PubMed] [Google Scholar]
- 25.Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults. Ann Intern Med 2015;162: 123–32. [DOI] [PubMed] [Google Scholar]
- 26.Muller MD, Reed AB, Leuenberger UA, et al. Physiology in medicine: peripheral arterial disease. J Appl Physiol 1985;115:1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross AJ, Gao Z, Blaha CA, et al. Coronary exercise hyperemia is impaired in patients with peripheral arterial disease. Ann Vasc Surg 2017;38:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drew RC, Muller MD, Blaha CA, et al. Renal vasoconstriction is augmented during exercise in patients with peripheral arterial disease. Physiol Rep 2013;1:eDD154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwenk M, Hauer K, Zieschang T, et al. Sensor-derived physical activity parameters can predict future falls in people with dementia. Gerontology 2014;60:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bahureksa L, Najafi B, Saleh A, et al. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology 2017;63:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myers SA, Johanning JM, Stergiou N, et al. Claudication distances and the Walking Impairment Questionnaire best describe the ambulatory limitations in patients with symptomatic peripheral artery disease. J Vasc Surg 2008;47:550–5. [DOI] [PubMed] [Google Scholar]
- 32.Wurdeman SR, Myers SA, Johanning JM, et al. External work is deficient in both limbs of patients with unilateral PAD. Med Eng Phys 2012;34:1421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg 2008;41:481–9. [DOI] [PubMed] [Google Scholar]
- 34.Izquierdo-Porrerea AM, Gardner AW, Bradham DD, et al. Relationship between objective measures of peripheral arterial disease severity to self-reported quality of life in older adults with intermittent claudication. J Vasc Surg 2005;41:625–30. [DOI] [PubMed] [Google Scholar]
- 35.Pernow B, Zetterquist S. Metabolic evaluation of the blood flow in claudicating patients with arterial obstructions at different levels. Scand J Lab Invest 1968;21:277–87. [DOI] [PubMed] [Google Scholar]
- 36.Hiatt WR, Armstrong EJ, Larson CJ, et al. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res 2015;116:1527–39. [DOI] [PubMed] [Google Scholar]
- 37.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral artery disease. Vas Med 2000;5:55–9. [DOI] [PubMed] [Google Scholar]
- 38.Pipinos II, Judge AR, Selsby JT, et al. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovasc Surg 2008;42:101–12. [DOI] [PubMed] [Google Scholar]
- 39.Hamburg NM, Creager MA. Pathophysiology of intermittent claudication in peripheral artery disease. Circ J 2017;81: 281–9. [DOI] [PubMed] [Google Scholar]
- 40.McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res 2015;116:1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]