Abstract
Painful peripheral neuropathy is a severe and difficult-to-treat neurological complication associated with cancer chemotherapy. Although chemotherapeutic drugs such as paclitaxel are known to cause tonic activation of presynaptic NMDA receptors (NMDARs) to potentiate nociceptive input, the molecular mechanism involved in this effect is unclear. α2δ−1, commonly known as a voltage-activated calcium channel subunit, is a newly discovered NMDAR-interacting protein and plays a critical role in NMDAR-mediated synaptic plasticity. Here we show that paclitaxel treatment in rats increases the α2δ−1 expression level in the dorsal root ganglion and spinal cord and the mRNA levels of GluN1, GluN2A, and GluN2B in the spinal cord. Paclitaxel treatment also potentiates the α2δ−1–NMDAR interaction and synaptic trafficking in the spinal cord. Strikingly, inhibiting α2δ−1 trafficking with pregabalin, disrupting the α2δ−1–NMDAR interaction with an α2δ−1 C terminus–interfering peptide, or α2δ−1 genetic ablation fully reverses paclitaxel treatment-induced presynaptic NMDAR-mediated glutamate release from primary afferent terminals to spinal dorsal horn neurons. In addition, intrathecal injection of pregabalin or α2δ−1 C terminus–interfering peptide and α2δ−1 knockout in mice markedly attenuate paclitaxel-induced pain hypersensitivity. Our findings indicate that α2δ−1 is required for paclitaxel-induced tonic activation of presynaptic NMDARs at the spinal cord level. Targeting α2δ−1–bound NMDARs, not the physiological α2δ−1–free NMDARs, may be a new strategy for treating chemotherapy-induced neuropathic pain.
Keywords: NMDA receptor, primary afferent nerves, synaptic plasticity, gabapentin, gabapentinoids, microtubule, trafficking
Graphical Abstract

Although NMDA receptors are expressed presynaptically in the spinal dorsal horn, they are not bound to α2δ−1 proteins and are in a quiescent state under the normal condition. Treatment with paclitaxel increases the expression of α2δ−1 in primary sensory neurons and the interaction between α2δ−1 and NMDA receptors, which promote the synaptic trafficking of α2δ−1–bound NMDA receptor complexes and lead to presynaptic NMDA receptor hyperactivity in the spinal dorsal horn. The increased presynaptic NMDA receptor activity results in sustained potentiation in glutamate release to spinal dorsal horn neurons, which constitutes a critical mechanism of chemotherapy-induced chronic pain.
Introduction
Painful peripheral neuropathy is a major adverse effect of several chemotherapeutic agents used for treating cancer, including taxanes, bortezomib, platinum agents, and vinca alkaloids. The persistent pain caused by chemotherapy profoundly reduces patients’ quality of life and is often the reason for dose reduction or discontinuation of what is otherwise life-saving treatment (Grisold et al. 2012, Sisignano et al. 2014). Because chemotherapy-induced pain responds poorly to conventional analgesics, identifying its molecular determinants is critically important for the development of new mechanism-based therapies. Glutamate mediates the vast majority of fast excitatory synaptic transmissions in the central nervous system, and the glutamate N-methyl-D-aspartate receptor (NMDAR) is involved in many physiological functions, such as learning, memory, and the synaptic plasticity associated with chronic pain (Chaplan et al. 1997, Herron et al. 1986, Weisskopf & Nicoll 1995, Chen et al. 2014b, Zhou et al. 2012). Although conventional NMDARs are located postsynaptically, presynaptic NMDARs can also powerfully shape synaptic transmission and neuronal plasticity in the amygdala (Humeau et al. 2003), striatum (Park et al. 2014), hypothalamus (Ye et al. 2011), and hippocampus (McGuinness et al. 2010). NMDARs are present in primary sensory neurons and their the central terminals (Liu et al. 1994, McRoberts et al. 2011), but they are normally silent in the spinal dorsal horn (Li et al. 2016, Xie et al. 2016, Zhao et al. 2012, Zeng et al. 2006). Interestingly, both treatment with paclitaxel, a microtubule-stabilizing agent, and treatment with bortezomib, a proteasome inhibitor, have no effect on postsynaptic NMDAR activity but lead to tonic activation of presynaptic NMDARs in the spinal dorsal horn (Xie et al. 2017, Xie et al. 2016, Chen et al. 2014c). However, the molecular mechanism responsible for this chemotherapy-induced presynaptic NMDAR activation remains unclear.
α2δ−1 is commonly known as a subunit of voltage-activated calcium channels (VACCs) (Dolphin 2013). However, quantitative proteomic analysis shows that α2δ−1 has a weak interaction with VACC α1 subunits in the brain tissues (Muller et al. 2010), and VACC currents in brain neurons are similar in wild-type and α2δ−1 knockout mice (Felsted et al. 2017). α2δ−1 is a major binding site of gabapentinoids, including gabapentin and pregabalin (Fuller-Bicer et al. 2009, Gee et al. 1996, Field et al. 2006), which are used for treating patients with chronic pain, including chemotherapy-induced neuropathic pain (Tsavaris et al. 2008, Finnerup et al. 2015). But gabapentinoids have little effect on VACC, including N-type, currents (Chen et al. 2018, Rock et al. 1993, Schumacher et al. 1998) or VACC-mediated neurotransmitter release (Brown & Randall 2005, Hoppa et al. 2012). Thus, the relevant mechanism responsible for the therapeutic action of these drugs is uncertain. We showed recently that α2δ−1 can form a protein complex with NMDARs to regulate NMDAR activity in traumatic nerve injury-induced chronic pain (Chen et al. 2018). Nevertheless, chronic pain caused by chemotherapy involves different cellular and molecular mechanisms from those involved in chronic pain caused by traumatic nerve injury. For example, spinal nerve ligation impairs K+-Cl− cotransporter-2 (KCC2) activity (Zhou et al. 2012), whereas paclitaxel increases Na+-K+−2Cl− cotransporter-1 (NKCC1) activity, in the spinal dorsal horn (Chen et al. 2014c). Furthermore, spinal nerve ligation potentiates both presynaptic and postsynaptic NMDAR activity (Chen et al. 2014b, Li et al. 2016), whereas treatment with paclitaxel or bortezomib increases presynaptic, but not postsynaptic, NMDAR activity in the spinal cord (Chen et al. 2014c, Xie et al. 2017, Xie et al. 2016). Currently, little is known about the contribution of α2δ−1 to chemotherapy-induced tonic activation of presynaptic NMDARs.
The purpose of the present study was to determine the role of α2δ−1–NMDAR interaction in the potentiated synaptic glutamate release to dorsal horn neurons in paclitaxel-induced neuropathic pain. Our study shows for the first time that a switch from α2δ−1–free to α2δ−1–bound NMDARs is essential in paclitaxel-induced tonic activation of presynaptic NMDARs. This new information substantially extends our understanding of the molecular mechanism underlying chemotherapy-induced neuropathic pain and the therapeutic actions of gabapentinoids. Our findings also suggest that targeting the α2δ−1–NMDAR interaction site represents a new strategy for treating chemotherapy-induced chronic pain.
Materials and Methods
Animals and paclitaxel treatment –
All surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center (approval #1174-RN01) and complied with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. This study was not pre-registered. Adult male Sprague-Dawley rats (weight: 220–250 g; Harlan, Indianapolis, IN; RRID: RGD_5508397) were used for most experiments. All animals were housed (2–3 rats per cage) on a standard 12:12 light-dark cycle with normal illumination. They were housed with 3 animals per cage and had free access to food and water. All tests and assays were done between 9 am and 6 pm.
Generation of α2δ−1−/− (KO) mice has been described previously (Fuller-Bicer et al. 2009). Briefly, two breeding pairs of α2δ−1+/− mice (C57BL/6 genetic background) were purchased from Medical Research Council (Stock #6900; Harwell Didcot, Oxfordshire, UK). α2δ−1−/− and α2δ−1+/+ (WT) littermates were generated by breeding the α2δ−1+/− heterozygous mice. Animals were ear-marked at the time of weaning (3 weeks after birth), and tail biopsies were used for PCR genotyping. Both male and female adult mice were used for electrophysiological and behavioral experiments. Because we observed no differences in electrophysiological and behavioral data between male and female mice, these data were pooled.
To induce neuropathic pain, we intraperitoneally injected rats and mice with paclitaxel (2 mg/kg; TEVA Pharmaceuticals, North Wales, PA) every 2 days for a total of 4 injections (days 1, 3, 5 and 7; total dose, 8 mg/kg), as described previously (Polomano et al. 2001). Animals in the control group were intraperitoneally injected with the vehicle (Cremophor EL/ethanol, 1:1) on the same 4 days. The presence of tactile allodynia was confirmed 10 to 12 days after drug treatment. We implanted intrathecal catheters in some paclitaxel-treated rats during isoflurane-induced anesthesia. Briefly, we made a small puncture in the atlanto-occipital membrane of the cisterna magna and inserted a PE-10 catheter with the caudal tip reaching the lumbar enlargement of the spinal cord. To minimize animal’s suffering, 1% lidocaine was injected around the incision site during surgery. We allowed the animals to recover for at least 4 days before intrathecal injections. Rats displaying signs of motor or neurological dysfunction were immediately killed with an intraperitoneal injection of phenobarbital (200 mg/kg) or by CO2 inhalation. All final biochemical and electrophysiological recordings were performed 14 to 20 days after paclitaxel or vehicle injection.
Quantitative PCR –
Rats were deeply anesthetized with 5% isoflurane, and the DRG and dorsal half of spinal cord tissues at the L5 and L6 levels were removed. Total RNA was extracted from the DRG and spinal cord tissues using TRIsure (#BIO-38032, Bioline, Taunton, MA). After treatment with RNase-free DNase (#79254, QIAGEN, Hilden, Germany), 500 ng RNA was used for reverse transcription with a RevertAid RT Reverse Transcription Kit (#K1619, Thermo Fisher Scientific, Waltham, MA). One μL of 5-times diluted cDNA was added to a 10-μL reaction volume with SYBR Green Real-Time PCR Mix (#A25780, Thermo Fisher Scientific). Real-time PCR was performed using a QuantStudio 7 Flex Real-Time PCR System (Applied Biosystems, Waltham, MA). The thermal cycling conditions used were: 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s, and 60°C for 60 s. The following primers were used: α2δ−1 forward, GGACCTATTCAGTGGATGGCTTG; α2δ−1 reverse, CCATTGGTCTTCCCAGAACATCTAGA; α2δ−2 forward, CGGCTGCTACAAAAGGAGACACACT; α2δ−2 reverse, CGGCCACAGTCTGAGGTATCTTC; α2δ−3 forward, GGCTCAGAAGATCAGACGACGTC; α2δ−3 reverse, TTGAGAAGAGACTCGAAACCAGG; Gapdh forward, GACATGCCGCCTGGAGAAAC; Gapdh reverse, AGCCCAGGATGCCCTTTAGT; GluN1 forward, GGATAAGACATGGGTTCGGTATCAGG; GluN1 reverse, TGTGTCGCTTGTAGGCGATCTC; GluN2A forward, TCAGCCATTGCTGTCTTCGT; GluN2A reverse, TGATCTTGCTGGTTGTGCCT; GluN2B forward, TTTCTAGCGCAGAAGGGCTC; GluN2B reverse, ATGGCCTTCCTCAGAACACC. Relative mRNA levels were calculated using the 2−ΔΔCT method and normalized to the Gapdh level in the same sample.
Coimmunoprecipitation using spinal cord tissue membrane extracts –
Tissues from the dorsal halves of the rat spinal cord at the L5 and L6 levels were collected under isoflurane anesthesia and homogenized in ice-cold hypotonic buffer (20 mM Tris [pH 7.4], 1 mM CaCl2, 1 mM MgCl2, and protease inhibitors) for extracting membrane proteins. The nuclei and unbroken cells were removed by centrifugation at 300 × g for 5 min, and the supernatant was centrifuged for 20 min at 21,000 × g. The pellets were re-suspended and solubilized in immunoprecipitation buffer (50 mM Tris [pH7.4], 250 mM NaCl, 10% glycerol, 0.5% NP-40, 20 mM NaF, 1 mM Na3VO4, 10 mM N-ethylmaleimide, 1 mM phenylmethylsulfonyl fluoride, 2 mM benzamide, and protease inhibitors), and the soluble fraction was incubated at 4°C overnight with Protein A/G beads (#16–266, Millipore, Darmstadt, Germany) prebound to mouse anti-GluN1 antibody (#75–272, 1:1000, NeuroMab, Davis, CA; RRID: AB_11000180). Protein A/G beads prebound to mouse IgG were uses as the control. All samples were washed 3 times with immunoprecipitation buffer and then immunoblotted with rabbit anti–α2δ−1 (#ACC-015, 1:500, Alomone Labs, Jerusalem, Israel; RRID: AB_2039785) and rabbit anti-GluN1 antibodies (#G8913, 1:1,000, Sigma-Aldrich, St. Louis, MO; RRID: AB_259978). The amount of the α2δ−1–GluN1 protein complex was calculated by normalizing the amount of α2δ−1 to that of GluN1 on the same gel.
Spinal cord synaptosome preparation –
The tissues from the dorsal half of the rat spinal cords were homogenized using a glass-Teflon homogenizer in 10 volumes of ice-cold HEPES-buffered sucrose (0.32 M sucrose, 1 mM EGTA, and 4 mM HEPES at pH 7.4) containing a protease inhibitor cocktail (Sigma-Aldrich). The homogenate was centrifuged at 1,000 × g for 10 min at 4 °C to remove the sediment, including nuclei and large debris. Then the supernatant was centrifuged at 10,000 × g for 15 min to obtain the crude synaptosomal fraction. The synaptosomal pellet was lysed in 9 volumes of ice-cold HEPES-buffer with the protease inhibitor cocktail for 30 min. The lysate was centrifuged at 25,000 × g for 20 min at 4 °C to obtain the synaptosomal fraction (Chen et al. 2018). After the protein concentration was measured, 30 μg of proteins were used for Western immunoblotting. The amount of synaptic α2δ−1 and GluN1 proteins was normalized to that of PSD-95, a synaptic protein marker, on the same gel (see below).
Western immunoblotting –
The rat DRG and dorsal spinal cord tissues at L5–L6 levels were homogenized in 300 μl radioimmunoprecipitation assay buffer containing 50 mM Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, and 1 mM NaF in the presence of a proteinase inhibitor cocktail (Sigma-Aldrich). The samples were homogenized with lysis buffer on ice for 30 min, and centrifuged at 13,000 × g for 30 min at 4°C. The supernatant was collected, and its protein concentration was measured using a DC Protein Assay Kit (Bio-Rad, Hercules, CA). Thirty μg of protein was loaded and separated by 4–15% Tris-HCl sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (#456–1086, Bio-Rad, Hercules, CA). The resolved proteins were transferred to a polyvinylidene difluoride membrane. The membrane was treated with 5% nonfat dry milk in Tris-buffered saline (TBS) at 25°C for 1 h and then incubated in TBS supplemented with 0.1% Triton X-100, 1% bovine serum albumin, and rabbit anti-α2δ−1 (#ACC-015, 1:500, Alomone Labs, Jerusalem, Israel), rabbit anti-GluN1 (#G8913, 1:1,000, Sigma-Aldrich), rabbit anti-GAPDH (#14C10, 1:5,000, Cell Signaling Technology, Danvers, MA), or mouse anti-PSD95 (#75–348, 1:1,000, NeuroMab, Davis, CA) antibodies overnight at 4°C. The membrane was washed 3 times and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:5,000; Jackson ImmunoResearch, West Grove, PA) for 1 h at 25°C. The protein bands were detected with an ECL kit (Thermo Fisher Scientific), and protein band intensity was visualized and quantified using an Odyssey Fc Imager (LI-COR Biosciences, Lincoln, NE).
Electrophysiological recording in spinal cord slices –
We anesthetized the rats and mice with isoflurane and rapidly removed the L3–L6 level of the lumbar spinal cord through laminectomy. The spinal cord tissues were immediately placed in ice-cold sucrose artificial cerebrospinal fluid (containing 234 mM sucrose, 3.6 mM KCl, 1.2 mM NaH2PO4, 12.2 mM glucose, 25.0 mM NaHCO3, 1.2 mM MgCl2, and 2.5 mM CaCl2) presaturated with 95% O2 and 5% CO2. Then the spinal cord tissue was placed onto the stage of a vibratome (Leica, Buffalo Grove, NY) and sliced transversely into 400 μm sections, which were preincubated in Krebs solution (117.0 mM NaCl, 3.6 mM KCl, 1.2 mM NaH2PO4, 11.0 mM glucose, 25.0 mM NaHCO3, 1.2 mM MgCl2, and 2.5 mM CaCl2) oxygenated with 95% O2 and 5% CO2 at 34℃ for at least 1 h before being transferred to recording chamber. The spinal cord slices were placed in a glass-bottomed recording chamber and continuously perfused with Krebs solution at 5.0 ml/min at 34℃, maintained by an inline solution heater and a temperature controller.
The lamina II outer neurons were identified for recording because they are mostly glutamate-releasing excitatory neurons and involved in nociceptive transmission (Pan et al. 2002, Santos et al. 2007, Wang et al. 2018). Excitatory postsynaptic currents (EPSCs) were recorded using whole-cell voltage-clamp techniques. The impedance of the glass electrode was 4 to 7 MΩ when the pipette was filled with the internal solution containing 135 mM potassium gluconate, 5 mM KCl, 2.0 mM MgCl2, 0.5 mM CaCl2, 5.0 mM HEPES, 5.0 mM EGTA, 5.0 mM ATP-Mg, 0.5 mM Na-GTP, and 10 mM lidocaine N-ethyl bromide (QX314, 280–300 mOsm, pH: 7.25). To measure glutamate release from primary afferent nerves, EPSCs were evoked from the dorsal root using a bipolar tungsten electrode connected to a stimulator (0.5 ms, 0.6 mA, and 0.1 Hz) and were recorded at a holding potential of –60 mV. Monosynaptic EPSCs were identified on the basis of the constant latency and absence of conduction failure of evoked EPSCs in response to a 20-Hz electrical stimulation. We have shown that bath application of an AMPA receptor antagonist can abolish all evoked monosynaptic EPSCs (elicited by glutamate release) in our preparation (Li et al. 2002, Pan et al. 2002) and that manipulating primary afferent nerves predictably alters glutamatergic input to lamina II neurons (Zhou et al. 2008, Zhou et al. 2010).
The paired-pulse ratio (PPR) was evoked by a pair of stimuli given at 50-ms intervals; the PPR was defined as the ratio of the amplitude of the second synaptic response to the amplitude of the first synaptic response. Miniature EPSCs (mEPSCs) were recorded at a holding potential of –60 mV in the presence of 2 μM strychnine, 10 μM bicuculline, and 1 μM tetrodotoxin. The input resistance was monitored, and the recording was abandoned if the input resistance changed by more than 15%. All signals were recorded using an amplifier (MultiClamp 700B; Axon Instruments Inc., Union City, CA), filtered at 1 to 2 kHz, and digitized at 10 kHz.
All drugs were prepared in artificial cerebrospinal fluid before the recording and delivered via syringe pumps at their final concentrations. 2-Amino-5-phosphonopentanoic acid (AP5) and tetrodotoxin citrate were purchased from Hello Bio Inc. (Princeton, NJ). Pregabalin was obtained from Tocris Bioscience (Bristol, UK). A peptide mimicking the C-terminal domain (VSGLNPSLWSIFGLQFILLWLVSGSRHYLW) of α2δ−1 fused with Tat protein (YGRKKRRQRRR) and a scrambled control peptide ((FGLGWQPWSLSFYLVWSGLILSVLHLIRSN) fused with Tat were synthesized by Bio Basic Inc. (Marham, Ontario, Canada). Pregabalin, α2δ−1Tat peptide, and control peptide were dissolved in artificial cerebrospinal fluid immediately before use.
Behavioral assessment of nociception –
To measure tactile allodynia, mice or rats were individually placed on a mesh floor within a suspended chamber. We applied a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) perpendicularly to the plantar surface of both hindpaws with sufficient force to bend the filament for 6 s. Brisk withdrawal of the paw or flinching was considered a positive response. When a response was observed, we applied the filament of the next lower force. In the absence of a response, we applied the filament of the next greater force. We used the “up-down” method (Chaplan et al. 1994) to calculate the tactile stimulus that produced a 50% likelihood of a withdrawal response.
To quantify the mechanical nociceptive threshold in rats, we conducted the paw pressure (Randall-Selitto) test on the left hindpaw using an analgesiometer (Ugo Basile, Varese, Italy). To activate the device, we pressed a foot pedal, which then applied a constantly increasing force on a linear scale. When the rat withdrew its paw or vocalized, the pedal was immediately released, and the withdrawal threshold was recorded (Chen et al. 2014a). We did not conduct the paw pressure test in mice because it is difficult to determine the mouse’s hindpaw withdrawal threshold using this analgesiometer.
To assess the thermal sensitivity of rats, we placed them individually on the glass surface of a thermal testing apparatus (IITC Life Sciences, Woodland Hills, CA). The rats were allowed to acclimate for 30 min before testing. The temperature of the glass surface was maintained at a constant 30°C. A mobile radiant heat source located under the glass was focused onto the hindpaw of each rat (Chen & Pan 2006). The paw withdrawal latency was recorded with a timer, and the hindpaw was tested twice to obtain an average time.
An incremental hot/cold plate analgesia meter (IITC Life Sciences) was used to determine the thermal sensitivity of mice. Mice were placed individually in the observation chamber on the plate, which had been preheated to 30℃ for 60 min. To measure heat sensitivity, the plate was heated at a rate of 6℃/min until the mice showed nocifensive behaviors such as paw shaking and jumping (Laumet et al. 2015). After the threshold temperature was recorded, the mice were immediately removed from the plate. The heat threshold measurement was repeated 30 min later, and the average of the 2 recorded temperatures was considered as the heat threshold. The cold threshold was measured by cooling the plate from 30℃ at a rate of 6℃/min until nocifensive behaviors were observed. The investigators performing the behavioral experiments were blinded to the treatment.
Study design and statistical analysis –
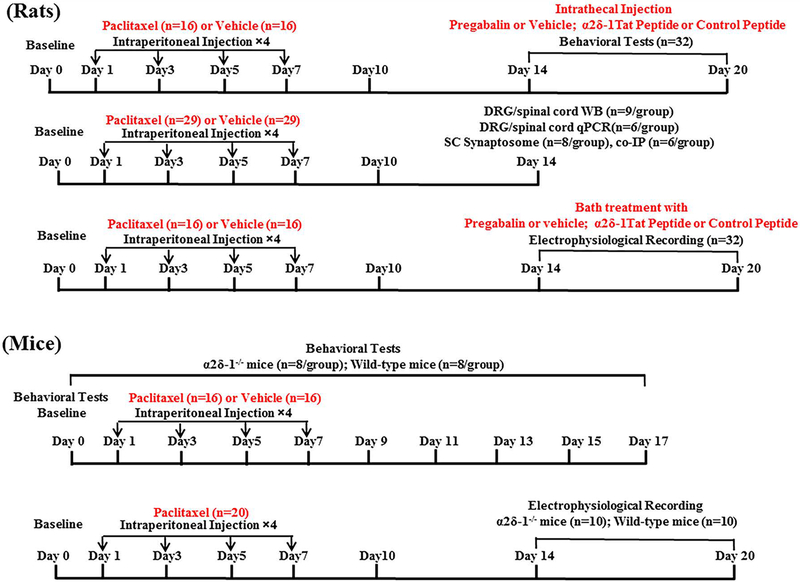
The timeline of experimental procedures is shown in Fig. 1. Although no statistical methods were used to predetermine sample sizes for the studies, our sample sizes were similar to those generally employed in the field. The animals were assigned (1:1 allocation) to the control and treatment groups, and no randomization methods were used. We did not exclude any animals from data analysis and did not use any test for outliers on the data. For electrophysiological experiments, at least 3 animals were used for each recording protocol, and only 1 neuron was recorded from each spinal cord slice. The amplitude of evoked EPSCs was quantified by averaging 10 consecutive EPSCs measured using Clampfit 10.0 software (Axon Instruments). mEPSCs were analyzed off-line using the peak detection program (MiniAnalysis, Synaptosoft, Leonia, NJ). We monitored cell capacitance, input resistance, and baseline holding current; the recording was discontinued if these parameters changed >15%. D’Agostino-Pearson normality test was used to assess the normality of data. The biochemical and electrophysiological data were presented in box-whisker plots, which show the minimum, 25th percentile, median, 75th percentile, and maximum. Two-tailed Student’s t tests were used to compare 2 groups, and one-way analysis of the variance (ANOVA) followed by Dunnett’s or Tukey’s post hoc test was used to determine the differences between more than 2 groups. Two-way ANOVA followed by Tukey’s post hoc test was performed to compare the withdrawal thresholds in WT mice and KO mice. All statistical analyses were performed using Prism software (version 7, GraphPad Software Inc., La Jolla, CA). P values of less than 0.05 were considered to indicate statistically significant differences.
Figure 1. Flowchart diagrams show the timeline of experimental procedures used in the study.
Rats were treated with either paclitaxel or vehicle (top panels) and then used for behavioral, biochemical or electrophysiological experiments. Wild-type and α2δ−1 knockout mice were treated with paclitaxel or vehicle (lower panels), and nociceptive tests or electrophysiological recordings were performed at the time indicated. The number of animals used for each group was indicated in parenthesis.
Results
Paclitaxel treatment increases α2δ−1 protein and mRNA levels in the DRG and spinal cord –
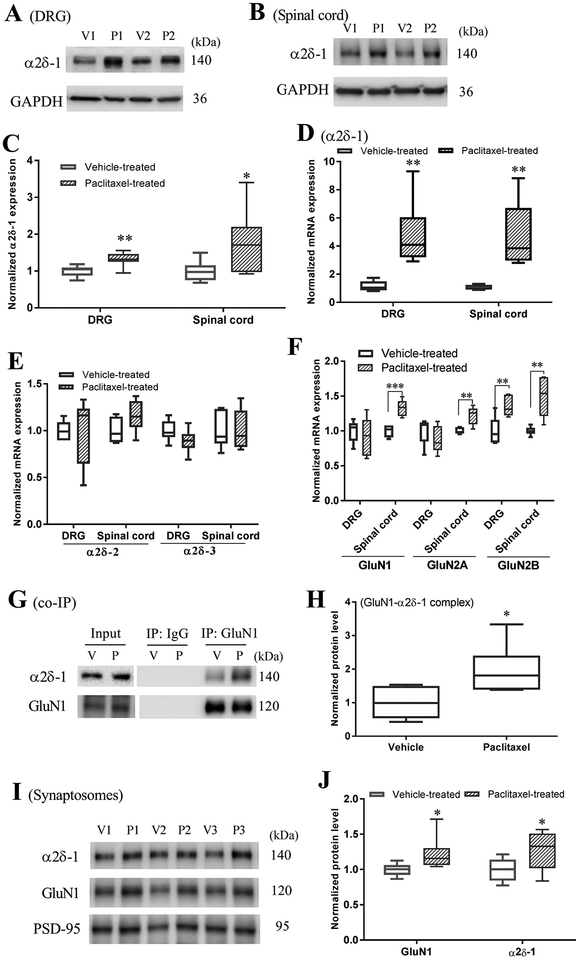
α2δ−1 is expressed in dorsal root ganglion (DRG) neurons and primary afferent nerve terminals in the superficial dorsal horn of the spinal cord. Peripheral nerve injury causes a large increase in the α2δ−1 expression level in the DRG and spinal cord (Luo et al. 2001, Newton et al. 2001). We used Western immunoblotting and real-time PCR to determine whether paclitaxel treatment affects the α2δ−1 expression level in the DRG and dorsal spinal cord tissues of rats. Immunoblotting detected a protein band corresponding to the molecular mass of α2δ−1 (~140 kDa). The α2δ−1 protein levels in the DRG (P = 0.0022, t(8) = 4.43; n = 9 rats in each group) and spinal cord tissues (P = 0.026, t(8) = 2.73; n = 9 rats in each group) were significantly higher in paclitaxel-treated than in vehicle-treated rats (Fig. 2, A–C).
Figure 2. Paclitaxel increases α2δ−1 expression levels and synaptic trafficking of α2δ−1–bound NMDARs in the spinal cord.
(A–C) Original gel images (A and B, 2 pairs of samples) and quantification (C) of the α2δ−1 protein level in the DRG and dorsal spinal cord tissues of paclitaxel-treated (P) and vehicle-treated (V) rats (n = 9 rats per group). The amount of α2δ−1 proteins was normalized to that of GAPDH on the same blot. (D–F) Quantification of the mRNA level of α2δ−1, α2δ−2, α2δ−3, GluN1 (GluN1), GluN2A, and GluN2B in the DRG and dorsal spinal cord tissues of paclitaxel-treated (P) and vehicle-treated (V) rats (n = 6 rats per group). (G,H) co-IP analysis showing the interaction between α2δ−1 and GluN1 in the membrane extracts of dorsal spinal cord tissues from rats treated with paclitaxel (P) or vehicle (V) (n = 6 rats per group). Proteins were immunoprecipitated initially with a mouse anti-GluN1 or anti-IgG antibody. Immunoblotting was performed by using rabbit anti-α2δ−1 and anti-GluN1 antibodies. The amount of α2δ−1 proteins was normalized to that of GluN1 on the same blot. (I,J) Representative gel images (I, 3 pairs of samples) and quantification (J) of the protein levels of α2δ−1, GluN1, and PSD-95 (a synaptic marker) in synaptosomes isolated from dorsal spinal cord tissues of paclitaxel-treated (P) and vehicle-treated (V) rats (n = 8 rats per group). The amount of α2δ−1 and GluN1 proteins was normalized to that of PSD-95 on the same blot. Values in C-F, H, and J are normalized to expression levels in vehicle-treated rats. *P < 0.05, **P < 0.01 compared with the vehicle-treated group (paired Student’s t-test).
Real-time PCR analysis showed that the α2δ−1 mRNA levels in the DRG (P = 0.0039, t(10) = 3.73; n = 6 rats in each group) and dorsal spinal cord (P = 0.0031, t(10) = 3.89; n = 6 rats in each group) were significantly higher in paclitaxel-treated than in vehicle-treated rats (Fig 2D). However, the mRNA level of α2δ−2 or α2δ−3 in the DRG and dorsal spinal cord was similar in paclitaxel-treated and vehicle-treated rats (n = 6 rats in each group, Fig. 2E). Furthermore, the mRNA levels of GluN1, GluN2A, and GluN2B in the dorsal spinal cord were significantly higher in paclitaxel-treated than in vehicle-treated rats (n = 6 rats in each group, Fig 2F). But in the DRG, only the mRNA level of GluN2B was significantly higher in paclitaxel-treated than in vehicle-treated rats (n = 6 rats in each group, Fig 2F). These results indicate that paclitaxel treatment increases the expression of α2δ−1 in the DRG and spinal cord and NMDAR expression level in the spinal cord.
Paclitaxel treatment increases the interaction between α2δ−1 and NMDARs and the synaptic targeting of NMDARs in the spinal cord –
Coimmunoprecipitation (co-IP) was performed to determine whether paclitaxel treatment affects the interaction of α2δ−1 and GluN1 (an obligatory subunit of NMDARs) in the dorsal spinal cord. Co-IP assays showed that a mouse anti-GluN1 antibody, but not an irrelevant mouse anti-immunoglobulin G (anti-IgG) antibody, coprecipitated with α2δ−1 in spinal cord membrane extracts (Fig. 2G). In addition, the expression level of the α2δ−1–GluN1 complex in the dorsal spinal cord was significantly higher in paclitaxel-treated than in vehicle-treated rats (P = 0.0197, t(10) = 2.77; n = 6 rats in each group; Fig. 2, G and H).
To determine whether paclitaxel treatment increases synaptic trafficking of NMDARs, we quantified α2δ−1 and GluN1 proteins in synaptosomes isolated from dorsal spinal cord tissues. Immunoblotting showed that both α2δ−1 and GluN1 protein levels in spinal synaptosomes were significantly higher in paclitaxel-treated than in vehicle-treated rats (n = 8 rats in each group; Fig. 2, I and J). These data suggest that paclitaxel treatment promotes the α2δ−1–GluN1 interaction and the synaptic trafficking of α2δ−1-bound NMDARs at the spinal cord level.
α2δ−1 mediates paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal dorsal horn –
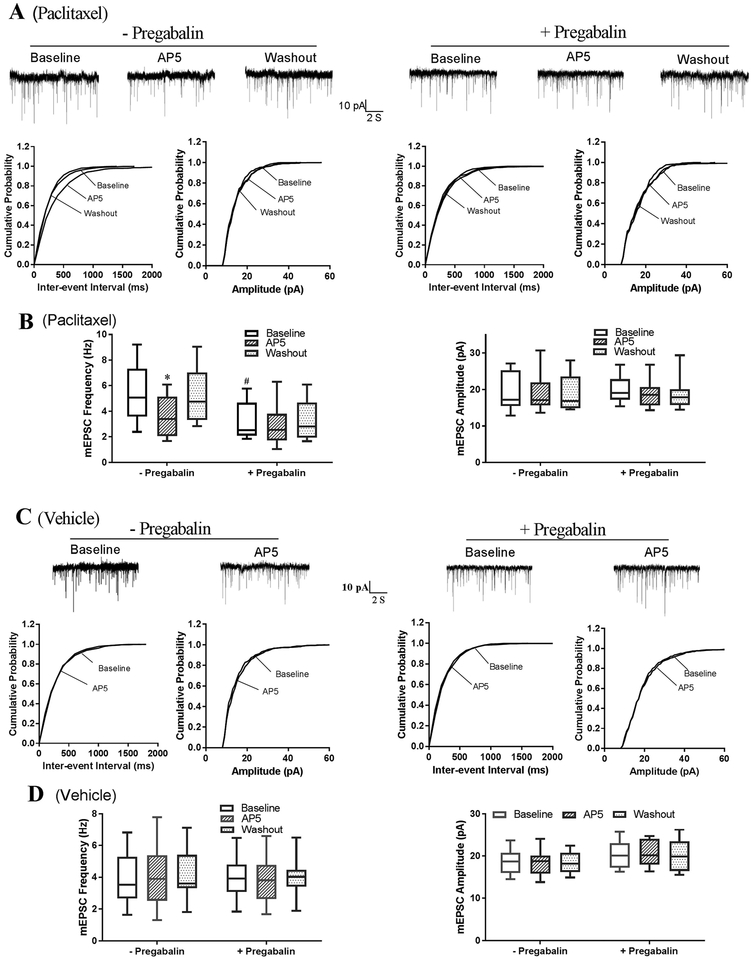
Chemotherapy-induced neuropathic pain is associated with tonic activation of presynaptic NMDARs, which promotes nociceptive glutamatergic input to spinal dorsal horn neurons (Xie et al. 2017, Xie et al. 2016). To determine the contribution of α2δ−1 to the increase in presynaptic NMDAR activity caused by paclitaxel treatment, we examined the effect of pregabalin, a clinically used α2δ−1 inhibitory ligand (Li et al. 2011, Patel et al. 2013), on glutamatergic miniature excitatory postsynaptic currents (mEPSCs), which represent spontaneous quantal glutamate release from presynaptic terminals of spinal dorsal horn neurons. Both gabapentin and pregabalin can inhibit synaptic trafficking and activity of α2δ−1–bound NMDARs (Chen et al. 2018, Ma et al. 2018). The baseline frequency, but not the amplitude, of mEPSCs in lamina II neurons was significantly higher in paclitaxel-treated rats than in vehicle-treated rats (5.48 ± 0.52 Hz vs. 3.86 ± 0.43 Hz, P = 0.028; n = 12 neurons in each group; Fig. 3). Bath application of the specific NMDAR antagonist 2-amino-5-phosphonopentanoate (AP5, 50 μM) rapidly reversed the increase in the frequency of mEPSCs in paclitaxel-treated rats but had no effect in vehicle-treated rats (Fig. 3), confirming that presynaptic NMDARs in the spinal dorsal horn are tonically activated by paclitaxel treatment (Xie et al. 2016). Treatment with pregabalin (20 μM for 30–60 min) completely normalized the baseline frequency of mEPSCs of lamina II neurons, which had been increased by paclitaxel treatment (n = 10 neurons, Fig. 3, A,B). In these pregabalin-treated neurons from paclitaxel-treated rats, subsequent bath application of 50 μM AP5 no longer had any effect on the frequency of mEPSCs. In contrast, pregabalin had no effect on the baseline frequency of mEPSCs in lamina II neurons from vehicle-treated rats (n = 10 neurons; Fig. 3, C,D). These data suggest that α2δ−1 mediates paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal cord.
Figure 3. Inhibiting α2δ−1 with pregabalin reverses paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal cord.
(A,B) Representative recording traces and cumulative plots (A) and box-and-whisker plots (B) show the effect of bath application of 50 μM AP5 on the frequency and amplitude of mEPSCs of lamina II neurons from paclitaxel-treated rats. Neurons were treated with 20 μM pregabalin (+pregabalin, n = 10 neurons from 4 rats) or untreated (–pregabalin, n = 12 neurons from 4 rats). (C,D) Original recording traces and cumulative plots (C) and box-and-whisker plots (D) show the effect of bath application of AP5 on the frequency and amplitude of mEPSCs of lamina II neurons from vehicle-treated rats. Neurons were treated with 20 μM pregabalin (+pregabalin, n = 10 neurons from 4 rats) or untreated (–pregabalin, n = 12 neurons from 4 rats). *P < 0.05 compared with the respective baseline control (one-way ANOVA followed by Dunnett’s post hoc test). #P < 0.05 compared with the baseline level in the group without pregabalin treatment (one-way ANOVA followed by Tukey’s post hoc test).
α2δ−1 is critical for paclitaxel-induced activation of NMDARs at primary afferent terminals –
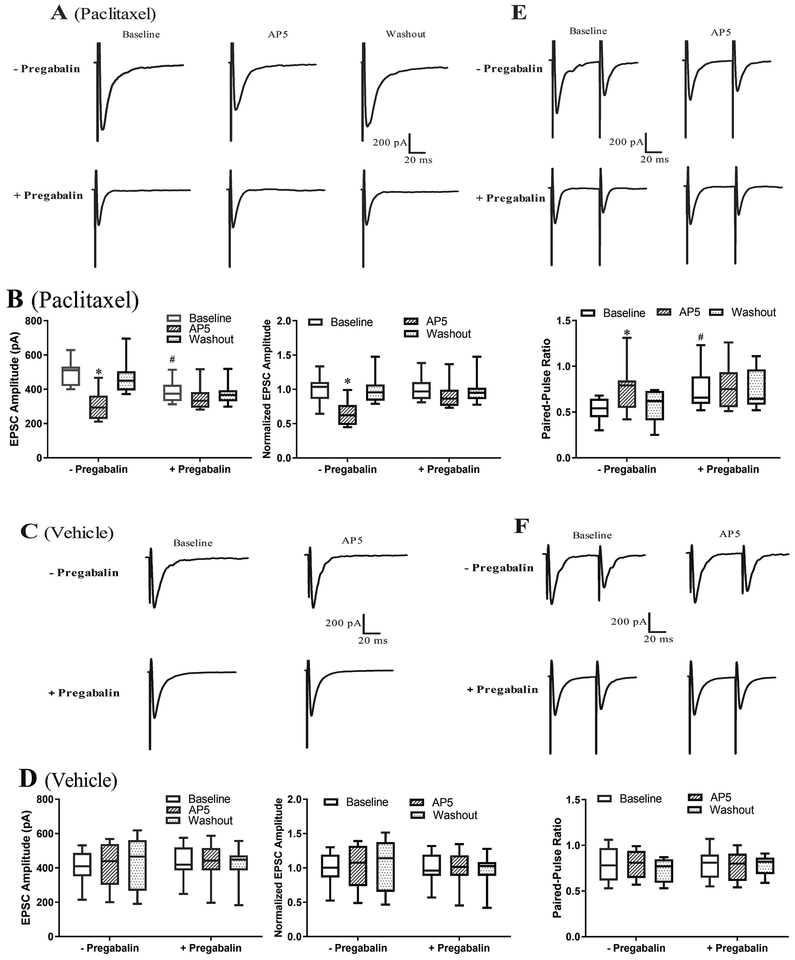
NMDARs are expressed at primary afferent terminals in the superficial dorsal horn of the spinal cord (Liu et al. 1994), where they mediate chemotherapy-induced potentiation of glutamatergic input to the spinal dorsal horn (Xie et al. 2017, Xie et al. 2016). To determine the role of α2δ−1 in the increased activity of NMDARs at primary afferent terminals caused by paclitaxel treatment, we recorded EPSCs of lamina II neurons monosynaptically evoked from the dorsal root, which correspond to synaptic glutamate release elicited from primary afferent nerve terminals. The baseline amplitude of evoked monosynaptic EPSCs was significantly larger in paclitaxel-treated rats (n = 12 neurons) than in vehicle-treated rats (516.83 ± 34.16 pA vs. 408.25 ± 25.49 pA, P = 0.031; n = 8 neurons) (Fig. 4). Bath application of 50 μM AP5 rapidly reversed the increased amplitude of monosynaptic EPSCs of lamina II neurons from paclitaxel-treated rats but had no effect in vehicle-treated rats (Fig. 4). In spinal cord slices from paclitaxel-treated rats, treatment with 20 μM pregabalin for 30 to 60 min returned the amplitude of evoked monosynaptic EPSCs of lamina II neurons to the baseline level observed in vehicle-treated rats (n = 8 neurons, Fig. 4, A,B). However, pregabalin treatment had no effect on the amplitude of evoked monosynaptic EPSCs of lamina II neurons from vehicle-treated rats (n = 9 neurons, Fig. 4, C,D).
Figure 4. Inhibiting α2δ−1 with pregabalin normalizes paclitaxel-induced activation of presynaptic NMDARs at primary afferent terminals.
(A,B). Original recording traces (A) and box-and-whisker plots (B) show the effect of bath application of 50 μM AP5 on evoked monosynaptic EPSCs of lamina II neurons from paclitaxel-treated rats. Neurons were treated with 20 μM pregabalin (+pregabalin, n = 8 neurons from 4 rats) or untreated (–pregabalin, n = 12 neurons from 4 rats). (C,D). Representative recording traces (C) and box-and-whisker plots (D) shows the effect of bath application of 50 μM AP5 on evoked monosynaptic EPSCs of lamina II neurons from vehicle-treated rats. Neurons treated with 20 μM pregabalin (+pregabalin, n = 9 neurons from 4 rats) or untreated (–pregabalin, n = 8 neurons from 4 rats). In B and D (right panels), values are normalized to their respective baselines. (E,F). Original recording traces (E) and box-and-whisker plots (F) shows the effect of bath application of AP5 on the paired-pulse ratio (PPR) of lamina II neurons treated with pregabalin (+pregabalin) and of untreated neurons (–pregabalin) from vehicle-treated (n = 9 neurons with pregabalin from 4 rats; n = 8 neurons without pregabalin from 4 rats) and paclitaxel-treated rats (n = 8 neurons with pregabalin from 4 rats; n = 9 neurons without pregabalin from 4 rats). *P < 0.05 compared with the respective baseline control (one-way ANOVA followed by Dunnett’s post hoc test). #P < 0.05 compared with the baseline level in the group without pregabalin treatment (one-way ANOVA followed by Tukey’s post hoc test).
In addition, we determined the effect of pregabalin on the paired-pulse ratio (PPR), a commonly used measure of the probability of neurotransmitter release from the presynaptic neuron, of monosynaptically evoked EPSCs of spinal lamina II neurons. Paclitaxel treatment significantly reduced the PPR in lamina II neurons (n = 9) compared with that in neurons (n = 8) from vehicle-treated rats (0.53 ± 0.04 vs. 0.78 ± 0.06, P = 0.028; Fig. 4, E,F). Bath application of AP5 reversed the reduction in the PPR of lamina II neurons from paclitaxel-treated rats but had no effect on the PPR in neurons from vehicle-treated rats (Fig. 4, E,F). Treatment with pregabalin completely normalized the PPR that had been reduced by paclitaxel treatment (n = 8 neurons) but had no effect on the PPR in neurons from vehicle-treated rats (n = 9 neurons) (Fig. 4, E,F). These results suggest that α2δ−1 is critically involved in paclitaxel-induced activation of NMDARs at primary afferent terminals.
α2δ−1–bound NMDARs are essential for paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal dorsal horn –
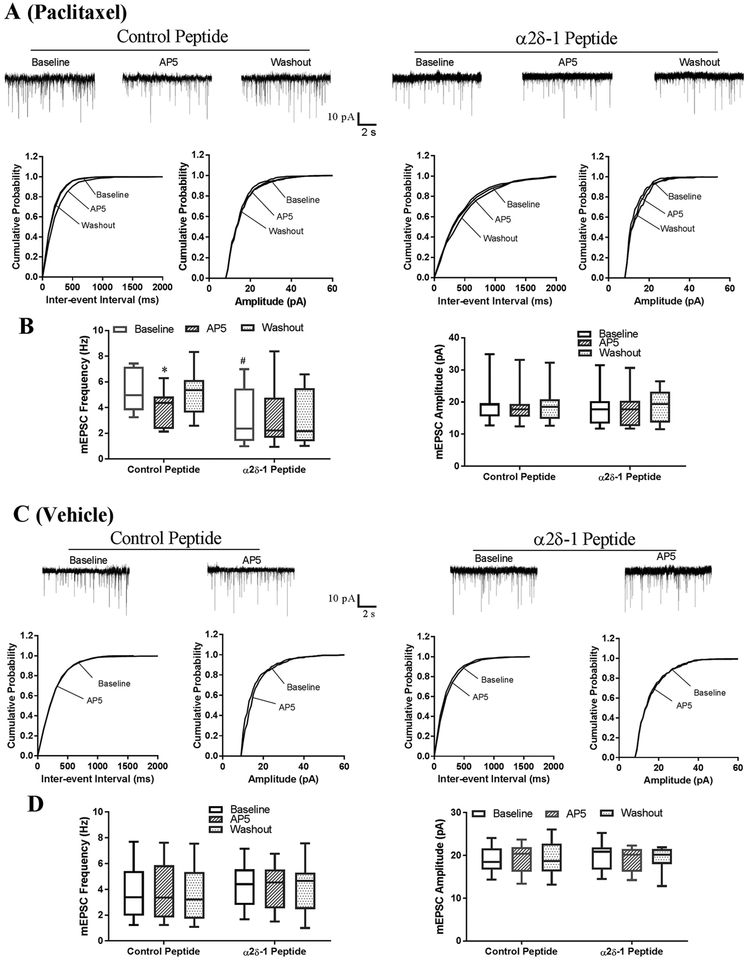
We next determined whether the α2δ−1–NMDAR interaction is involved in the paclitaxel-induced activation of presynaptic NMDARs in the spinal dorsal horn. Because the C-terminus of α2δ−1 is essential for its interaction with NMDARs, we developed a 30-amino-acid peptide (VSGLNPSLWSIFGLQFILLWLVSGSRHYLW) mimicking the C-terminal domain of α2δ−1 fused with Tat protein (YGRKKRRQRRR) termed as α2δ−1Tat peptide. We have shown that α2δ−1Tat peptide effectively interrupts the α2δ−1–NMDAR interaction in vitro and in vivo (Chen et al. 2018, Luo et al. 2018). Treatment with this α2δ−1Tat peptide (1 μM for 30–60 min) completely normalized the increased baseline frequency of mEPSCs in lamina II neurons from paclitaxel-treated rats (n = 10 neurons) to the level seen in vehicle-treated rats (Fig. 5, A,B). Subsequent bath application of AP5 had no effect on the frequency of mEPSCs in α2δ−1Tat peptide-treated spinal cord slices from paclitaxel-treated rats. However, in spinal cord slices from vehicle-treated rats, neither incubation with α2δ−1Tat peptide nor bath application of AP5 had any effect on the baseline frequency of mEPSCs in lamina II neurons (n = 11 neurons; Fig. 5, C,D).
Figure 5. Disrupting the α2δ−1–NMDAR interaction using α2δ−1Tat peptide reverses paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal cord.
(A,B). Representative recording traces and cumulative plots (A) and box-and-whisker plots (B) show the effect of bath application of 50 μM AP5 on the frequency and amplitude of mEPSCs of lamina II neurons from paclitaxel-treated rats, Neurons were treated with 1 μM α2δ−1Tat peptide (n = 10 neurons from 5 rats) or 1 μM control peptide (n = 11 neurons from 5 rats). (C,D) Original recording traces and cumulative plots (C) and box-and-whisker plots (D) show the effect of bath application of AP5 on the frequency and amplitude of mEPSCs of lamina II neurons from vehicle-treated rats. Neurons were treated with 1 μM α2δ−1Tat peptide (n = 11 neurons from 5 rats) or 1 μM control peptide (n = 12 neurons from 5 rats). *P < 0.05 compared with the respective baseline control (one-way ANOVA followed by Dunnett’s post hoc test). #P < 0.05 compared with the baseline level in the group treated with control peptide (one-way ANOVA followed by Tukey’s post hoc test).
In contrast, treatment with a Tat-fused scrambled control peptide (FGLGWQPWSLSFYLVWSGLILSVLHLIRSN; 1 μM for 30–60 min) did not affect the mEPSC frequency of lamina II neurons from paclitaxel-treated rats (n = 11 neurons) or vehicle-treated rats (n = 12 neurons) (Fig. 5, A,B). These findings indicate that α2δ−1–bound NMDARs are responsible for the paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal dorsal horn.
α2δ−1–bound NMDARs are crucial for paclitaxel-induced activation of NMDARs at primary afferent terminals –
To determine whether α2δ−1–bound NMDARs contribute to the paclitaxel-induced increase in NMDAR activity at primary afferent terminals, we examined the effect of α2δ−1Tat peptide on EPSCs of lamina II neurons monosynaptically evoked from the dorsal root. In spinal cord slices from paclitaxel-treated rats (n = 9 neurons), treatment with 1 μM α2δ−1Tat peptide for 30 to 60 min completely reversed the increased amplitude of evoked EPSCs to the level found in vehicle-treated rats (Fig. 6, A–D). Subsequent bath application of AP5 had no effect on the amplitude of evoked EPSCs in neurons from paclitaxel-treated rats. Also, α2δ−1Tat peptide did not affect the amplitude of evoked EPSCs in neurons from vehicle-treated rats (n = 10 neurons). In contrast, treatment with Tat-fused control peptide (1 μM for 30–60 min) did not significantly alter the amplitude of evoked EPSCs of lamina II neurons from paclitaxel-treated rats (n = 12 neurons) or vehicle-treated rats (n = 9 neurons) (Fig. 6, A–D). In control peptide-incubated neurons from paclitaxel-treated rats, bath application of AP5 returned the amplitude of evoked EPSCs to the level observed in vehicle-treated rats.
Figure 6. Disrupting the α2δ−1–NMDAR interaction using α2δ−1Tat peptide abrogates paclitaxel-induced activation of presynaptic NMDARs at primary afferent terminals.
(A,B). Original recording traces (A) and box-and-whisker plots (B) show the effect of bath application of 50 μM AP5 on evoked monosynaptic EPSCs of lamina II neurons from paclitaxel-treated rats. Neurons were treated with 1 μM α2δ−1Tat peptide (n = 9 neurons from 4 rats) or 1 μM control peptide (n = 12 neurons from 4 rats). (C,D). Representative recording traces (C) and box-and-whisker plots (D) show the effect of bath application of 50 μM AP5 on evoked monosynaptic EPSCs of lamina II neurons from vehicle-treated rats. Neurons were treated with 1 μM α2δ−1Tat peptide (n = 10 neurons from 4 rats) or 1 μM control peptide (n = 9 neurons from 4 rats). In B and D (right panels), values are normalized to their respective baselines. (E,F). Original recording traces (E) and box-and-whisker plots (F) shows the effect of bath application of 50 μM AP5 on the paired-pulse ratio (PPR) of lamina II neurons treated with α2δ−1Tat peptide or control peptide from vehicle-treated (n = 10 neurons with α2δ−1Tat peptide from 4 rats; n = 9 neurons with control peptide from 4 rats) and paclitaxel-treated rats (n = 9 neurons with α2δ−1Tat peptide from 4 rats; n = 9 neurons with control peptide from 4 rats). *P < 0.05 compared with the respective baseline control (one-way ANOVA followed by Dunnett’s post hoc test). #P < 0.05 compared with the baseline level in the group treated with control peptide (one-way ANOVA followed by Tukey’s post hoc test).
In addition, treatment with α2δ−1Tat peptide, but not Tat-fused control peptide, fully reversed the reduction in the baseline PPR of evoked EPSCs in neurons from paclitaxel-treated rats (n = 9 neurons in each group; Fig. 6E). Bath application of AP5 reversed the reduced PPR in control peptide-treated neurons from paclitaxel-treated rats but had no effect on the PPR in α2δ−1Tat peptide-treated neurons from paclitaxel-treated rats. In spinal cord slices from vehicle-treated rats, treatment with α2δ−1Tat peptide (n = 10 neurons) or control peptide (n = 9 neurons) had no effect on the baseline PPR of evoked EPSCs (Fig. 6F). These results indicate that α2δ−1–bound NMDARs are critically involved in paclitaxel-induced activation of NMDARs at primary afferent terminals in the spinal dorsal horn.
α2δ−1 ablation prevents paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal dorsal horn –
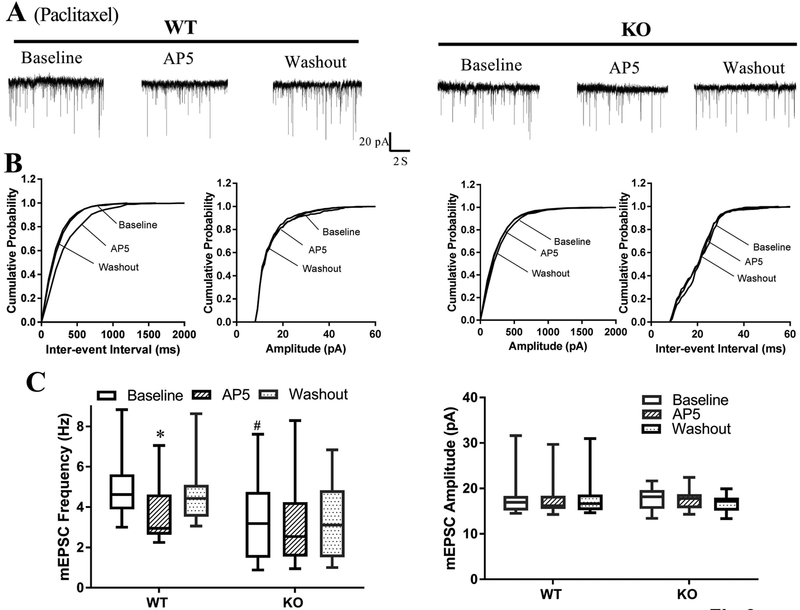
To further validate the critical role of α2δ−1 in paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal dorsal horn, we performed electrophysiological recordings in spinal cord slices from wild-type (WT) and α2δ−1 knockout (KO) mice that had been treated with paclitaxel. The baseline frequency, but not the amplitude, of mEPSCs was significantly greater in lamina II neurons from WT mice (n = 11 neurons) than in those from α2δ−1 KO mice (n = 16 neurons) (4.92 ± 0.48 Hz vs. 3.13 ± 0.46 Hz, P = 0.018). Also, bath application of AP5 normalized the paclitaxel-induced increase in the frequency of mEPSCs in WT mice but had no effect in α2δ−1 KO mice (Fig. 7, A–C).
Figure 7. Ablation of α2δ−1 prevents paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal cord in mice.
(A–C). Representative recording traces (A), cumulative plots (B), and box-and-whisker plots (C) show the effect of bath application of 50 μM AP5 on the frequency and amplitude of mEPSCs of lamina II neurons from wild-type (WT, n = 11 neurons from 5 mice) and α2δ−1 knockout (KO, n = 16 neurons from 5 mice) mice treated with paclitaxel. *P < 0.05 compared with the respective baseline control (one-way ANOVA followed by Dunnett’s post hoc test). #P < 0.05 compared with the baseline level in WT mice (one-way ANOVA followed by Tukey’s post hoc test).
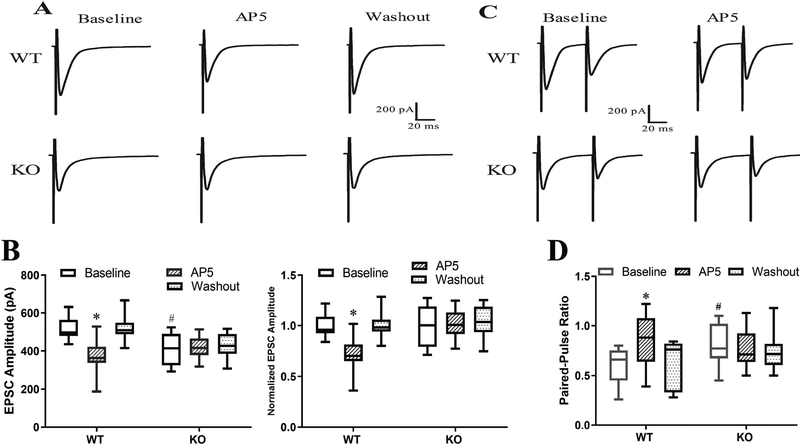
The amplitude of monosynaptic EPSCs of spinal lamina II neurons evoked from the dorsal root in paclitaxel-treated WT mice (n = 13 neurons) was significantly higher than that in paclitaxel-treated α2δ−1 KO mice (n = 11 neurons) (518.68 ± 15.06 pA vs. 412.14 ± 21.82 pA, P < 0.016). Bath application of AP5 normalized the increased baseline amplitude of evoked EPSCs in WT mice but had no effect in α2δ−1 KO mice (Fig. 8, A,B). Furthermore, the PPR of evoked EPSCs was significantly lower in neurons from paclitaxel-treated WT mice (n = 13 neurons) than in those from paclitaxel-treated α2δ−1 KO mice (n = 10 neurons) (0.60 ± 0.05 vs. 0.80 ± 0.07, P = 0.029). Bath application of AP5 restored the baseline PPR that had been decreased by paclitaxel in WT mice but had no effect in paclitaxel-treated α2δ−1 KO mice (Fig. 8, C,D). These data provide unequivocal evidence of the obligatory role of α2δ−1 in paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal dorsal horn.
Figure 8. Ablation of α2δ−1 abolishes paclitaxel-induced activation of presynaptic NMDARs at primary afferent terminals in mice.
(A,B). Original recording traces (A) and box-and-whisker plots (B) shows the effect of bath application of 50 μM AP5 on evoked monosynaptic EPSCs of lamina II neurons from wild-type (WT, n = 13 neurons from 5 mice) and α2δ−1 knockout (KO, n = 11 neurons from 5 mice) mice treated with paclitaxel. In B (right panel), values are normalized to the respective baselines. (C,D). Original recording traces (C) and box-and-whisker plots (D) shows the effect of bath application of AP5 on the paired-pulse ratio (PPR) of lamina II neurons from WT (n = 13 neurons from 5 mice) and α2δ−1 KO (n = 10 neurons from 5 mice) mice treated with paclitaxel. *P < 0.05 compared with the respective baseline control (one-way ANOVA followed by Dunnett’s post hoc test). #P < 0.05 compared with the baseline level in WT mice (one-way ANOVA followed by Tukey’s post hoc test).
Inhibiting α2δ−1 or interrupting the α2δ−1–NMDAR interaction at the spinal cord level reverses paclitaxel-induced pain hypersensitivity –
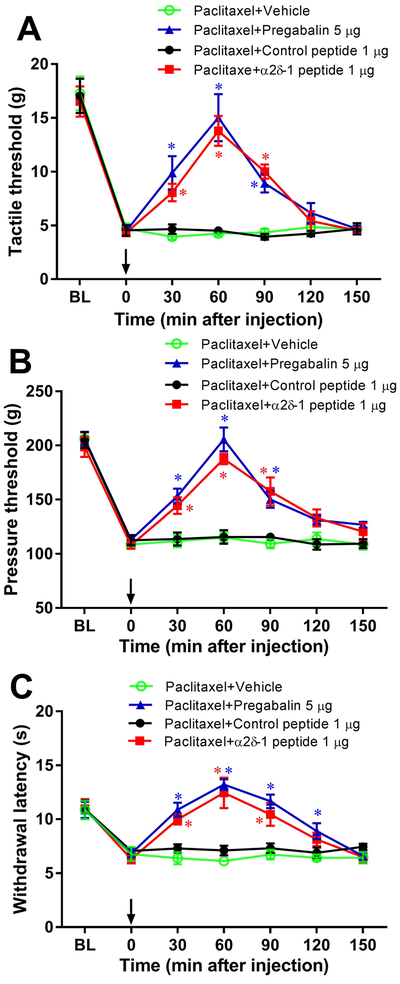
Blocking NMDARs at the spinal cord level can rapidly reverse pain hypersensitivity induced by chemotherapeutic agents (Xie et al. 2017, Xie et al. 2016). We next determined specifically whether α2δ−1–bound NMDARs at the spinal cord level contribute to paclitaxel-induced neuropathic pain. Rats were injected intraperitoneally with 2 mg/kg of paclitaxel every 2 days for a total of 4 injections. The behavioral tests were conducted 14 days after treatment with paclitaxel. Paclitaxel-treated rats had lower withdrawal thresholds in response to application of a tactile stimulus (von Frey filaments), a noxious pressure stimulus, and a noxious heat stimulus to the hindpaw than did vehicle-treated rats, indicating the presence of pain hypersensitivity. Intrathecal injection of 5 μg pregabalin readily reversed tactile allodynia and mechanical and thermal hyperalgesia in paclitaxel-treated rats (n = 8 rats per group, Fig. 9, A–C). Similarly, intrathecal injection of 1 μg α2δ−1Tat peptide, but not 1 μg control peptide, markedly attenuated the pain hypersensitivity induced by paclitaxel (n = 8 rats per group; Fig. 9, A–C). These data suggest that α2δ−1 and α2δ−1–bound NMDARs at the spinal cord level play a prominent role in paclitaxel-induced neuropathic pain.
Figure 9. α2δ−1 at the spinal cord level is involved in paclitaxel-induced pain hypersensitivity.
(A–C). Time course of the effect of intrathecal injection with vehicle, 5 μg pregabalin, 1 μg α2δ−1Tat peptide, or 1 μg control peptide on the paw withdrawal thresholds measured with von Frey filaments (A), a pressure stimulus (B), and a heat stimulus (C) in paclitaxel-treated rats (n = 8 rats per group). Data are expressed as means ± SEM. *P < 0.05, compared with the control (time 0) before intrathecal injection. BL, baseline before paclitaxel treatment. Vehicle or agent injections are indicated by the arrows. (D–F). Time course of changes in the paw withdrawal thresholds tested with von Frey filaments (D), a heat stimulus (E), and a cold stimulus (F) in wild-type (WT) and α2δ−1 knockout (KO) mice treated with paclitaxel (n = 8 mice per group). Data are expressed as means ± SEM. *P < 0.05, compared with respective control (time 0) before vehicle or agent injection (one-way ANOVA followed by Dunnett’s post hoc test).
α2δ−1 ablation attenuates the development of pain hypersesitivity caused by paclitaxel treatment –
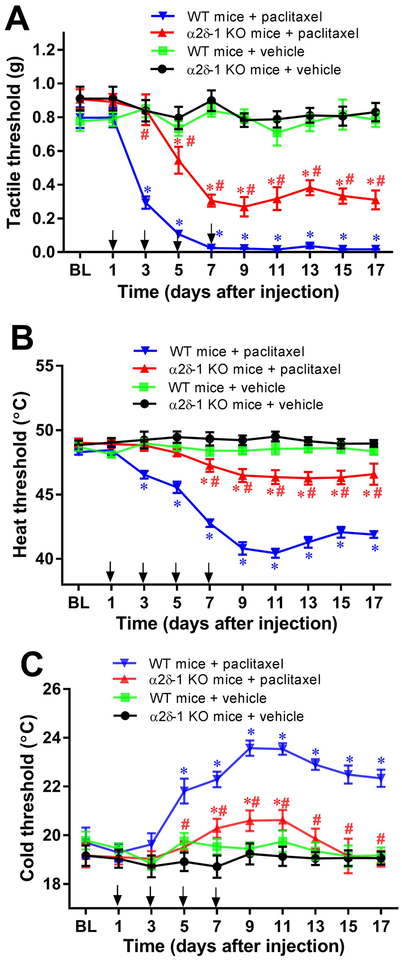
Next, we used α2δ−1 KO mice to confirm the contribution of α2δ−1 to the development of pain hypersensitivity after paclitaxel treatment (2 mg/kg, 4 injections on days 1, 3, 5 and 7). Systemic treatment with paclitaxel in WT mice induced tactile allodynia and heat and cold hyperalgesia, which began after the second paclitaxel injection and lasted for at least 10 days after the last injection (n = 8 mice per group; Fig. 10, A–C). The withdrawal thresholds in response to von Frey filaments and heat/cold stimuli at baseline (before paclitaxel treatment) did not differ significantly between WT and α2δ−1 KO mice. However, the degree of tactile allodynia and heat/cold hyperalgesia induced by paclitaxel was profoundly attenuated in α2δ−1 KO mice (n = 8 mice per group; Fig. 10, A–C). Treatment with vehicle had no effects on the tactile and thermal withdrawal thresholds in either WT or α2δ−1 KO mice (n = 8 mice per group). These results provide substantial evidence that α2δ−1 contributes to the development of paclitaxel-induced neuropathic pain.
Figure 10. α2δ−1 contributes to paclitaxel-induced pain hypersensitivity.
(A–C). Time course of changes in the paw withdrawal thresholds tested with von Frey filaments (A), a heat stimulus (B), and a cold stimulus (C) in wild-type (WT) and α2δ−1 knockout (KO) mice treated with paclitaxel (n = 8 mice per group). BL, baseline before paclitaxel treatment. The 4 paclitaxel injections are indicated by the arrows. Data are expressed as means ± SEM. *P < 0.05, compared with respective baseline (BL) value before paclitaxel treatment (one-way ANOVA followed by Dunnett’s post hoc test). #P < 0.05 compared with the corresponding value at the same time point in WT mice (two-way ANOVA followed by Tukey’s post hoc test).
Discussion
The most striking finding of our study is that α2δ−1 is essential for paclitaxel-induced tonic activation of presynaptic NMDARs in the spinal dorsal horn. We have shown that postsynaptic NMDAR activity in the spinal dorsal horn is not affected by paclitaxel treatment (Chen et al. 2014c). In our recording condition, the postsynaptic NMDARs were minimally open because the cell was voltage-clamped at –60 mV and recorded in the presence of 1.2 mM Mg2+ in the extracellular solution. Presynaptic NMDARs are emerging as important regulators of neuronal signaling in processes such as long-term potentiation (Park et al. 2014, Zhou et al. 2010) and spinal synaptic plasticity in various chronic pain conditions (Chen et al. 2018, Li et al. 2016, Xie et al. 2017, Xie et al. 2016). α2δ−1 is expressed in the DRG and spinal cord laminae I and II (Newton et al. 2001, Cole et al. 2005, Taylor & Garrido 2008), and the obligatory NMDAR subunit GluN1 is present at primary afferent nerve terminals near the active zone in the rat spinal dorsal horn (Liu et al. 1994). However, the role of α2δ−1 in regulating presynaptic NMDARs has been generally overlooked until recently. We showed in this study that inhibiting α2δ−1 trafficking with pregabalin or disrupting the α2δ−1–NMDAR interaction with α2δ−1Tat peptide completely normalized paclitaxel-induced, NMDAR-mediated, increases in synaptic glutamate release to dorsal horn neurons. Also, inhibiting α2δ−1 or disrupting the α2δ−1–NMDAR interaction reversed the paclitaxel-induced increase in evoked glutamate release from primary afferent terminals. Furthermore, our results from α2δ−1 KO mice indicate that presynaptic NMDAR activation in paclitaxel-treated animals fully depends on α2δ−1. Paclitaxel is mostly accumulated in the DRG and peripheral nerves upon systemic administration (Cavaletti et al. 2000), which may explain its preferential toxic effect on primary afferent nerves. As a whole, our study provides unambiguous evidence that paclitaxel-induced activation of presynaptic NMDARs is predominantly mediated by α2δ−1–bound NMDARs.
Our study provides new evidence about the molecular mechanism through which paclitaxel-induced α2δ−1 upregulation leads to activation of presynaptic NMDARs in the spinal dorsal horn. Under physiological conditions, presynaptic NMDARs in the spinal cord are not functionally active. However, these receptors become tonically activated by endogenous glutamate in painful conditions caused by paclitaxel, bortezomib, and calcineurin inhibitors (Chen et al. 2018, Li et al. 2016, Xie et al. 2017, Xie et al. 2016). We found that paclitaxel treatment caused α2δ−1 upregulation in the DRG and the dorsal spinal cord, which is consistent with other reports (Gauchan et al. 2009, Xiao et al. 2007, Matsumoto et al. 2006). Because α2δ−1 is a highly glycosylated protein involved in surface trafficking (Tetreault et al. 2016), the paclitaxel-induced excess of α2δ−1 protein at primary afferent terminals may promote its interaction with NMDARs and facilitate synaptic expression of α2δ−1–NMDAR complexes. In support of this notion, we found that paclitaxel increased the association between α2δ−1 and NMDARs in the spinal cord and the prevalence of α2δ−1–bound NMDARs in the spinal cord synaptosomes. It should be noted that dorsal spinal cord synaptosomes do not necessarily represent the central terminals of primary afferents, and this synaptosomal preparation can underestimate the changes in the protein complexes at the presynaptic terminals of primary afferents. Paclitaxel-induced presynaptic NMDAR activation involves primarily GluN2A-containing NMDARs (Xie et al. 2016). Although these receptors are normally blocked by Mg2+ at negative membrane potentials, their association with α2δ−1 can markedly reduce the voltage-dependent Mg2+ block of NMDARs (Chen et al. 2018). This distinct effect of α2δ−1 on GluN2A-containing NMDARs also likely accounts for the paclitaxel-induced emergence of functionally active NMDARs at primary afferent terminals. Thus, the switch from α2δ−1–free to α2δ−1–bound NMDARs constitutes a crucially important molecular mechanism for tonic activation of presynaptic NMDARs, which potentiates glutamate release from primary afferent nerves through Ca2+ influx.
We showed that neither pregabalin nor α2δ−1Tat peptide had any effect on synaptic glutamate release to spinal dorsal horn neurons in rats without paclitaxel-induced neuropathic pain. The finding that α2δ−1 interacts with NMDARs and plays a key role in activating presynaptic NMDARs under chronic pain conditions could explain why gabapentinoids reduce the release of neurotransmitters including calcitonin gene-related peptide, substance P, and glutamate in the spinal cord under persistent painful, but not normal, conditions (Chen et al. 2018, Fehrenbacher et al. 2003). In a heterologous expression system, gabapentinoids reduce NMDAR activity only when α2δ−1 is co-expressed (Chen et al. 2018), indicating that α2δ−1–bound NMDARs are the predominant NMDARs involved in the effect of gabapentinoids. The role of α2δ−1 in the activation of presynaptic NMDARs caused by paclitaxel treatment is likely independent of VACCs, because mEPSCs of dorsal horn neurons were recorded in the presence of tetrodotoxin (a voltage-activated sodium channel blocker), which prevents neuronal depolarization and VACC activation. Also, α2δ−1Tat peptide specifically targets the C-terminus of α2δ−1, which is required for the α2δ−1–NMDAR interaction, whereas the VWA domain (near the N-terminus) of α2δ−1 mediates the interaction between α2δ−1 and VACCs (Canti et al. 2005, Hoppa et al. 2012). Furthermore, the α2δ−1Tat peptide and gabapentinoids have no effect on VACC activity or on the interaction between α2δ−1 and the VACC α1 subunit. In addition, α2δ−1 physically interacts with NMDARs in HEK293 cells, which do not express VACC α1 subunits (Chen et al. 2018).
Our findings suggest that paclitaxel treatment increases both the expression and synaptic localization of NMDARs at the spinal cord level. However, our electrophysiological data from treatment with pregabalin and α2δ−1Tat peptide as well as α2δ−1 KO mice indicate that α2δ−1 is critically involved in the paclitaxel treatment-induced increase in presynaptic NMDAR activity at the spinal cord level. Although it has been reported that α2δ−1 may interact with thrombospondins to promote synaptogenesis (Eroglu et al. 2009), a recent study showed that the α2δ−1–thrombospondin interaction is very weak and that no α2δ−1–thrombospondin interaction takes place on the membrane surface (Lana et al. 2016). In this study, we showed that the paclitaxel-induced increase in the mEPSC frequency of dorsal horn neurons was rapidly reversed by blocking NMDARs, indicating that augmented glutamatergic input is mediated by NMDARs, but not by increased synaptogenesis.
Our study provides new evidence for the pivotal role of α2δ−1–bound NMDARs at the spinal cord level in the development of chronic pain after paclitaxel treatment. We showed that inhibiting α2δ−1 or disrupting the α2δ−1–NMDAR interaction at the spinal cord level readily reversed the pain hypersensitivity caused by paclitaxel treatment. In addition, the development of paclitaxel-induced pain hypersensitivity was profoundly blunted in α2δ−1 KO mice. Although blocking NMDARs at the spinal cord level effectively reverses persistent pain caused by paclitaxel and bortezomib (Xie et al. 2017, Xie et al. 2016), conventional NMDAR antagonists, such as ketamine, often produce intolerable adverse effects because they inhibit normal NMDAR function. It is conceivable that gabapentinoids or compounds specifically targeting the α2δ−1–NMDAR interaction site may have more favorable therapeutic profiles than general NMDAR antagonists.
In summary, our study reveals that a switch from α2δ−1–free to α2δ−1–bound NMDARs serves as a key molecular mechanism by which presynaptic NMDARs are activated in chemotherapy-induced pain and that this switch also mediates the therapeutic effect of gabapentinoids. Chemotherapy induces dominance of α2δ−1–bound NMDARs at primary afferent terminals, causing long-lasting increases in glutamatergic nociceptive input to spinal dorsal horn neurons and thereby maintaining chronic pain. This new information not only extends our understanding of the fundamental molecular mechanism underlying chemotherapy-induced neuropathic pain but also provides a basis for the rational use of gabapentinoids. Targeting the α2δ−1–NMDAR interaction site may be a new strategy for specifically inhibiting abnormally increased NMDAR activity (without interfering with normal, α2δ−1–free NMDARs) to treat this painful condition in cancer patients and survivors.
Open Science Badges.
This article has received a badge for *Open Materials* because it provided all relevant information to reproduce the study in the manuscript. The complete Open Science Disclosure form for this article can be found at the end of the article. More information about the Open Practices badges can be found at https://cos.io/our-services/open-science-badges/.
Acknowledgements
This work was supported by grants from the National Institutes of Health (GM120844 and NS101880) and by the N.G. and Helen T. Hawkins Endowment (to H.-L.P.).
List of abbreviations used:
- AP5
2-Amino-5-phosphonopentanoic acid
- co-IP
coimmunoprecipitation
- DRG
dorsal root ganglion
- EPSC
excitatory postsynaptic current
- KO
knockout
- mEPSC
miniature excitatory postsynaptic current
- NMDAR
N-methyl-D-aspartate receptor
- PPR
paired-pulse ratio
- RRID
Research Resource Identifiers
- VACC
voltage-activated calcium channels
Footnotes
Conflicts of Interest
The authors declare no competing interests.
References
- Brown JT and Randall A (2005) Gabapentin fails to alter P/Q-type Ca2+ channel-mediated synaptic transmission in the hippocampus in vitro. Synapse, 55, 262–269. [DOI] [PubMed] [Google Scholar]
- Canti C, Nieto-Rostro M, Foucault I et al. (2005) The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A, 102, 11230–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletti G, Cavalletti E, Oggioni N, Sottani C, Minoia C, D’Incalci M, Zucchetti M, Marmiroli P and Tredici G (2000) Distribution of paclitaxel within the nervous system of the rat after repeated intravenous administration. Neurotoxicology, 21, 389–393. [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM and Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods, 53, 55–63. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB and Yaksh TL (1997) Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. The Journal of pharmacology and experimental therapeutics, 280, 829–838. [PubMed] [Google Scholar]
- Chen J, Li L, Chen S, et al R. (2018) The α2δ 1–NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep, 22, 2307–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Hu YM, Chen H and Pan HL (2014a) Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. The Journal of physiology, 592, 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR and Pan HL (2006) Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. Journal of neurophysiology, 95, 3086–3096. [DOI] [PubMed] [Google Scholar]
- Chen SR, Zhou HY, Byun HS, Chen H and Pan HL (2014b) Casein kinase II regulates N-methyl-D-aspartate receptor activity in spinal cords and pain hypersensitivity induced by nerve injury. The Journal of pharmacology and experimental therapeutics, 350, 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Zhu L, Chen H, Wen L, Laumet G and Pan HL (2014c) Increased spinal cord Na(+)-K(+)-2Cl(−) cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J Biol Chem, 289, 31111–31120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA and Gu G (2005) Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol, 491, 246–269. [DOI] [PubMed] [Google Scholar]
- Dolphin AC (2013) The alpha2delta subunits of voltage-gated calcium channels. Biochim Biophys Acta, 1828, 1541–1549. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW et al. (2009) Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell, 139, 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP and Vasko MR (2003) Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain, 105, 133–141. [DOI] [PubMed] [Google Scholar]
- Felsted JA, Chien CH, Wang D, Panessiti M, Ameroso D, Greenberg A, Feng G, Kong D and Rios M (2017) Alpha2delta-1 in SF1(+) Neurons of the Ventromedial Hypothalamus Is an Essential Regulator of Glucose and Lipid Homeostasis. Cell reports, 21, 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Cox PJ, Stott E et al. (2006) Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A, 103, 17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S et al. (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol, 14, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Bicer GA, Varadi G, Koch SE et al. (2009) Targeted disruption of the voltage-dependent calcium channel alpha2/delta-1-subunit. Am J Physiol Heart Circ Physiol, 297, H117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchan P, Andoh T, Ikeda K, Fujita M, Sasaki A, Kato A and Kuraishi Y (2009) Mechanical allodynia induced by paclitaxel, oxaliplatin and vincristine: different effectiveness of gabapentin and different expression of voltage-dependent calcium channel alpha(2)delta-1 subunit. Biological & pharmaceutical bulletin, 32, 732–734. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R and Woodruff GN (1996) The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem, 271, 5768–5776. [DOI] [PubMed] [Google Scholar]
- Grisold W, Cavaletti G and Windebank AJ (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol, 14 Suppl 4, iv45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron CE, Lester RA, Coan EJ and Collingridge GL (1986) Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature, 322, 265–268. [DOI] [PubMed] [Google Scholar]
- Hoppa MB, Lana B, Margas W, Dolphin AC and Ryan TA (2012) alpha2delta expression sets presynaptic calcium channel abundance and release probability. Nature, 486, 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Shaban H, Bissiere S and Luthi A (2003) Presynaptic induction of heterosynaptic associative plasticity in the mammalian brain. Nature, 426, 841–845. [DOI] [PubMed] [Google Scholar]
- Lana B, Page KM, Kadurin I, Ho S, Nieto-Rostro M and Dolphin AC (2016) Thrombospondin-4 reduces binding affinity of [(3)H]-gabapentin to calcium-channel alpha2delta-1-subunit but does not interact with alpha2delta-1 on the cell-surface when co-expressed. Sci Rep, 6, 24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumet G, Garriga J, Chen SR et al. (2015) G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci, 18, 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI and Pan HL (2002) Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. The Journal of physiology, 543, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen SR, Chen H, Wen L, Hittelman WN, Xie JD and Pan HL (2016) Chloride Homeostasis Critically Regulates Synaptic NMDA Receptor Activity in Neuropathic Pain. Cell reports, 15, 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Taylor CP, Weber M, Piechan J, Prior F, Bian F, Cui M, Hoffman D and Donevan S (2011) Pregabalin is a potent and selective ligand for alpha(2)delta-1 and alpha(2)delta-2 calcium channel subunits. Eur J Pharmacol, 667, 80–90. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN and Basbaum AI (1994) Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci U S A, 91, 8383–8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Ma H, Zhou JJ, Li L, Chen SR, Zhang J, Chen L and Pan HL (2018) Focal cerebral ischemia and reperfusion induce brain injury through α2δ−1–bound NMDA receptors. Stroke, 49, 2464–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME and Yaksh TL (2001) Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. The Journal of neuroscience : the official journal of the Society for Neuroscience, 21, 1868–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Chen SR, Chen H, Li L, Li DP, Zhou JJ and Pan HL (2018) α2δ−1 is essential for sympathetic output and NMDA receptor activity potentiated by angiotensin II in the hypothalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience, 38, 6388–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Inoue M, Hald A, Xie W and Ueda H (2006) Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther, 318, 735–740. [DOI] [PubMed] [Google Scholar]
- McGuinness L, Taylor C, Taylor RD et al. (2010) Presynaptic NMDARs in the hippocampus facilitate transmitter release at theta frequency. Neuron, 68, 1109–1127. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Ennes HS, Marvizon JC, Fanselow MS, Mayer EA and Vissel B (2011) Selective knockdown of NMDA receptors in primary afferent neurons decreases pain during phase 2 of the formalin test. Neuroscience, 172, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CS, Haupt A, Bildl W et al. (2010) Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proceedings of the National Academy of Sciences of the United States of America, 107, 14950–14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RA, Bingham S, Case PC, Sanger GJ and Lawson SN (2001) Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res, 95, 1–8. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Li DP and Pan HL (2002) Inhibition of glutamatergic synaptic input to spinal lamina II(o) neurons by presynaptic alpha(2)-adrenergic receptors. J Neurophysiol, 87, 1938–1947. [DOI] [PubMed] [Google Scholar]
- Park H, Popescu A and Poo MM (2014) Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron, 84, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Bauer CS, Nieto-Rostro M et al. (2013) alpha2delta-1 gene deletion affects somatosensory neuron function and delays mechanical hypersensitivity in response to peripheral nerve damage. The Journal of neuroscience : the official journal of the Society for Neuroscience, 33, 16412–16426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polomano RC, Mannes AJ, Clark US and Bennett GJ (2001) A painful peripheral neuropathy in the rat produced by the chemotherapeutic drug, paclitaxel. Pain, 94, 293–304. [DOI] [PubMed] [Google Scholar]
- Rock DM, Kelly KM and Macdonald RL (1993) Gabapentin actions on ligand- and voltage-gated responses in cultured rodent neurons. Epilepsy Res, 16, 89–98. [DOI] [PubMed] [Google Scholar]
- Santos SF, Rebelo S, Derkach VA and Safronov BV (2007) Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. The Journal of physiology, 581, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher TB, Beck H, Steinhauser C, Schramm J and Elger CE (1998) Effects of phenytoin, carbamazepine, and gabapentin on calcium channels in hippocampal granule cells from patients with temporal lobe epilepsy. Epilepsia, 39, 355–363. [DOI] [PubMed] [Google Scholar]
- Sisignano M, Baron R, Scholich K and Geisslinger G (2014) Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol, 10, 694–707. [DOI] [PubMed] [Google Scholar]
- Taylor CP and Garrido R (2008) Immunostaining of rat brain, spinal cord, sensory neurons and skeletal muscle for calcium channel alpha2-delta (alpha2-delta) type 1 protein. Neuroscience, 155, 510–521. [DOI] [PubMed] [Google Scholar]
- Tetreault MP, Bourdin B, Briot J, Segura E, Lesage S, Fiset C and Parent L (2016) Identification of Glycosylation Sites Essential for Surface Expression of the CaValpha2delta1 Subunit and Modulation of the Cardiac CaV1.2 Channel Activity. J Biol Chem, 291, 4826–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavaris N, Kopterides P, Kosmas C et al. (2008) Gabapentin monotherapy for the treatment of chemotherapy-induced neuropathic pain: a pilot study. Pain Med, 9, 1209–1216. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen SR, Ma H, Chen H, Hittelman WN and Pan HL (2018) Regulating nociceptive transmission by VGluT2-expressing spinal dorsal horn neurons. Journal of neurochemistry, doi: 10.1111/jnc.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG and Nicoll RA (1995) Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature, 376, 256–259. [DOI] [PubMed] [Google Scholar]
- Xiao W, Boroujerdi A, Bennett GJ and Luo ZD (2007) Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience, 144, 714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JD, Chen SR, Chen H and Pan HL (2017) Bortezomib induces neuropathic pain through protein kinase C-mediated activation of presynaptic NMDA receptors in the spinal cord. Neuropharmacology, 123, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JD, Chen SR, Chen H, Zeng WA and Pan HL (2016) Presynaptic N-Methyl-d-aspartate (NMDA) Receptor Activity Is Increased Through Protein Kinase C in Paclitaxel-induced Neuropathic Pain. J Biol Chem, 291, 19364–19373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Li DP, Li L and Pan HL (2011) Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. The Journal of neuroscience : the official journal of the Society for Neuroscience, 31, 8271–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Thomson LM, Aicher SA and Terman GW (2006) Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. The Journal of neuroscience : the official journal of the Society for Neuroscience, 26, 12033–12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YL, Chen SR, Chen H and Pan HL (2012) Chronic opioid potentiates presynaptic but impairs postsynaptic N-methyl-D-aspartic acid receptor activity in spinal cords: implications for opioid hyperalgesia and tolerance. J Biol Chem, 287, 25073–25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Byun HS, Chen H, Li L, Han HD, Lopez-Berestein G, Sood AK and Pan HL (2012) N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem, 287, 33853–33864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H and Pan HL (2008) Sustained inhibition of neurotransmitter release from nontransient receptor potential vanilloid type 1-expressing primary afferents by mu-opioid receptor activation-enkephalin in the spinal cord. J Pharmacol Exp Ther, 327, 375–382. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Chen H and Pan HL (2010) Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. The Journal of neuroscience : the official journal of the Society for Neuroscience, 30, 4460–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]