Abstract
Purpose:
To report the long-term outcome of Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) for delivery of bevacizumab in the treatment of corneal neovascularization (KNV).
Methods:
Retrospective, non-comparative, interventional case series of 13 sequential patients treated for KNV at the BostonSight between 2006 and 2017. In all cases, PROSE treatment was initiated for management of ocular surface disease and patients wore PROSE consistently on a daily wear basis prior to bevacizumab treatment. Patients applied a drop of 1% preservative free bevacizumab to the reservoir of PROSE device twice daily. Patients continued with daily wear of the device during treatment and afterwards.
Results:
13 patients (8 female and mean age of 45 years) are included with a mean follow-up of 5.1 years (range 6 months to 11 years). Underlying ocular diagnoses included Stevens- Johnson syndrome (7), ocular chronic graft-versus-host disease (2), corneal transplant (2), contact lens-related corneal ulcer and limbal stem cell deficiency (1), and familial dysautonomia (1). Median duration of bevacizumab use was 6 months (range 3 months to 10 years). Twelve cases (92%) had regression of KNV and 10 cases (77%) had improved best-corrected visual acuity (BCVA) with treatment. Median BCVA improved from −1.1 (LogMAR) at baseline, to −0.66 at end of bevacizumab treatment, and remained −0.63 at last follow-up (P=0.047). KNV progressed in one eye after discontinuation of bevacizumab. There were no ophthalmic or systemic complications.
Conclusions:
Topical bevacizumab used in PROSE is effective in treating KNV and improving vision. Long-term follow-up reveals durable response and no complications.
Keywords: Prosthetic Replacement of Ocular Surface Ecosystem (PROSE), scleral lens, corneal neovascularization, vascular endothelial growth factor (VEGF), bevacizumab, ocular surface disease
Introduction:
The healthy cornea is transparent and avascular. Many corneal and ocular surface diseases can cause corneal neovascularization (KNV), which may in turn impair vision, promote opacification and increase risks associated with corneal transplantation. 1 Vascular endothelial growth factor (VEGF) is considered the most prominent pro-angiogenic factor in many conditions involving angiogenesis in the eye. 2 Anti-VEGF therapy is now the first-line treatment for retinal vascular diseases, and its use in treating KNV has been reviewed. 3 A 2013 meta-analysis showed that both topical and subconjunctival applications of VEGF inhibitor bevacizumab effectively reduce the area of KNV. 4
Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) treatment involves the use of a custom-designed and -fabricated prosthetic device developed by the BostonSight that is available at over a dozen centers in the U.S. and worldwide. The device is a gas-permeable lens with a diameter of 17.5–23.0 mm that rests on the bulbar conjunctiva and vaults over the entire cornea and bathes the cornea in artificial tears. PROSE treatment has been used in the management of distorted cornea as well as complex ocular surface diseases such as Stevens-Johnson Syndrome (SJS) and ocular graft-versus-host disease (GVHD), with long-term benefits. 5–13
In addition to protecting the ocular surface, PROSE device can serve as a novel drug delivery system for the ocular surface. Application of topical non-preserved ophthalmic moxifloxacin in the PROSE reservoir prior to device insertion and continuous wear has been reported as part of the treatment of persistent corneal epithelial defect. 14,15 In addition, we previously reported the use of non-preserved bevacizumab in PROSE device in the treatment of KNV with midterm outcomes. 16,17 In this current study, we report the long-term outcome of this treatment approach in a larger case series and discuss the clinical characteristics and implications.
Methods:
Study design and patients
This retrospective, non-comparative interventional case series with review of medical records was conducted according to the Declaration of Helsinki. It was compliant with the Health Insurance Portability and Accountability Act (HIPAA) and was determined to be exempt from review by New England Independent Review Board. Details of PROSE device and wear have been reported previously. 10 Thirteen sequential patients treated for KNV at BostonSight between 2006 and 2017 were identified. All 13 patients were already fitted and wearing PROSE device and had either progressive KNV or KNV approaching the visual axis, defined as such: the tips of KNV have rounded loops, KNV is associated with adjacent haze, and KNV is growing in size with extension of leading edge approaching or into visual axis.
Use of bevacizumab in PROSE device
The details of delivery of bevacizumab via PROSE device were reported previously. 16 Briefly, the potential risks, benefits, alternatives and indications associated with bevacizumab and its off-label use were discussed with patients and informed consent was obtained. The patients were instructed to continue wearing the device as usual, on a full-time daily wear basis, with nightly removal and disinfection in a hydrogen peroxide system. One drop of 1% bevacizumab, non-preserved and compounded in sterile saline, was instilled in the device reservoir, followed by filling the reservoir with non-preserved, buffered, sterile saline. The device was inserted as usual and worn for 4–6 hours. Patients then removed, emptied, and cleaned the device, and the reservoir was reconstituted with a second dose of bevacizumab in the same fashion. The device was then worn for an additional 6 to 10 hours (total of 10 to 16 hours daily wear). One patient with familial dysautonomia applied bevacizumab to both eyes via the reservoir only once daily due to difficulty with device removal during the day without a caregiver. To reduce the potential for systemic absorption, punctal occlusion by plugs or cautery were performed on patients with patent puncta. Patients’ existing systemic and ophthalmic medications were continued. The twice daily regimen was continued for three months and then tapered based on clinical response at the discretion of the treating physician. Clinical response is defined as: clearing of corneal haze, drop-out or reduction of rounded loops at the tips of KNV, ‘ghosting’ (lack of active blood flow) in KNV, and reduction in vascularized area. An improvement in vision is also evidence of clinical response. All patients monitored blood pressure while using bevacizumab and the primary care physician was involved in decision to continue use in those who wished to do so.
Data Extraction
Medical records were reviewed and the following information extracted and analyzed: demographics, systemic medical history and medications, ocular history and medications, indications for treatment, treatment duration, follow-up duration, BCVA and intraocular pressure (IOP) at base line, at the end of treatment, and at last follow-up date, slit lam findings, and when available photo documentation.
Statistical analysis
BCVA was converted from Snellen notation to logarithm of the minimum angle of resolution (LogMAR) as previously described. 18 Continuous variables were reported as mean (Standard Deviation, SD) if normally distributed, otherwise as median, and categorical variables were reported in numbers (percentage). GraphPad Prism software version 5.00 for Windows was used to analyze BCVA in PROSE device at baseline (prior to initiation of topical bevacizumab treatment), after treatment, and last follow-up (Repeated Measures ANOVA). P value <0.05 was considered statistically significant.
Results:
A total of 13 consecutive patients with either progressive KNV or KNV approaching the visual axis were treated with bevacizumab in a PROSE device between 2006 and 2017. The mean age at time of treatment was 45 years and 8 were female. The mean follow-up duration was 5.1 years (range 6 months to 11 years). Underlying ocular diagnoses included SJS (7), ocular GVHD (2), corneal transplant (2:1 penetrating keratoplasty for scarring from herpes simplex keratitis, and 1 patch graft for perforation related to Sjogren syndrome and neurotrophic sterile melt), contact lens-related corneal ulcer and limbal stem cell deficiency (1), and familial dysautonomia (1). Ten of the 13 patients were on topical corticosteroid when bevacizumab treatment was initiated.
Median duration of bevacizumab treatment was 6 months (range 3 months −10 years). Two patients with SJS reported reduced debris in device and improved photophobia and discomfort while using bevacizumab, therefore continued with various frequencies (no more than twice daily) for 14 months and 10 years. A third patient with SJS continued bevacizumab use in the device for 19 months for its presumed anti-inflammatory and anti-leaking property. The remaining 10 patients used it for 3 to 7 months. Twelve patients had KNV regression on slit lamp examination after initial treatment and 10 had improvement in BCVA. The median BCVA of the cohort improved from −1.09 before treatment (already wearing PROSE) to −0.66 after treatment, and remained −0.63 at the last follow-up (Figure 1). Treatment response and improvement in vision was maintained in long-term follow-up, except for one patient (case 2) in which there was KNV progression after discontinuation of bevacizumab. All patients were monitored closely while on treatment; no ophthalmic or systemic adverse events associated with the treatment were noted.
Figure 1.
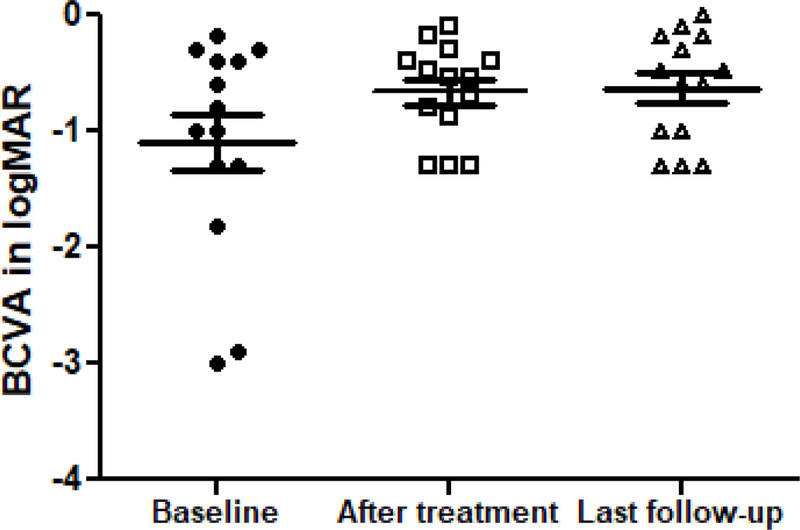
Change in BCVA with bevacizumab treatment in PROSE device. The BCVA improved from −1.09 in logMAR at baseline to −0.66 at the end of bevacizumab treatment and maintained at −0.63 at the last follow-up. P= 0.0473 (Repeated Measures ANOVA).
The clinical course of these 13 patients is summarized in Table 3. Several cases are discussed here in detail: Cases 1, 3, and 8 demonstrated rapid and sustained response to bevacizumab treatment and improved BCVA; case 2 showed KNV progression after discontinuation of bevacizumab; case 6 demonstrated the lack of clinical improvement after treatment, the only non-responder in this cohort; and case 11 showed rapid and sustained KNV regression, but BCVA eventually decreased due to progression of the underlying ocular surface disease.
Table 3.
Clinical characteristics and course of 13 subjects
| Subject | Age | Sex | Eye | Ocular history | Treatment Frequency | Treatment Duration | Follow-up duration | Relevant systemi conditions | Ophthalmic medications at baseline | Indication for bevacizumab use | BCVA * at baseline | Disease course | BCVA * after treatment | BCVA *at last visit | After bevacizumab treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | male | OS | Penetrating keratoplasty secondary to herpes simplex keratitis and neurotrophic keratopathy | Twice daily | 7 months | 11 years | Human immunodeficiency virus, hypertension, and diabetes | Prednisolone, Restasis, Vigamox, and oral acyclovir | KNV approaching visual axis | Count Fingers at 3 inches | At 1 mo, KNV regressed and BCVA improved to 20/100 | 20/50 | 20/25 | Inactive KNV |
| 2 | 30 | female | OS | Stevens-Johnson syndrome with vascularized nodule | Twice daily | 10 years | 10 years | Hypertension | Acular, Alomide, Zymar, Pataday, serum tears, and oral doxycycline | Progression of vascularized nodule | 20/30 | At 1 mo, KNV regressed and BCVA improved to 20/25; subject continued bevacizumab to reduce photophobia and debris in PROSE with various frequency for 10 years | 20/25 * | 20/40 | Patient underwent laser treatment for KNV elsewhere after discontinuation of bevacizumab |
| 3 | 35 | male | OD | Contact lens-related ulcer and limbal stem cell deficiency | Twice daily | 4 months | 7 years | None | Lotemax, Zymar, Timolol, and oral doxycycline | KNV approaching visual axis | 20/400 | At 1 mo, KNV regressed and BCVA improved to 20/70 | 20/50 | 20/30 | Inactive KNV and ghost vessels |
| 4 | 61 | female | OD | Patch graft for perforation related to Sjogren syndrome and neurotrophic sterile melt; history of retinal detachment and repair | Twice daily | 7 months | 3 years | Mixed connect tissue disease and Sjogren syndrome | Prednisolone and erythromycin ointment | KNV in patch graft | Count Fingers at 3 feet | At 1 mo, KNV regressed and BCVA improved to 20/400 | 20/400 | 20/400 | Inactive KNV, underwent PKP, vision limited by history of retinal detachment |
| 5 | 26 | male | OD | Corneal pannus and scarring secondary to familial dysautonomia; history of amblyopia and nystagmus | Once daily both eyes | 6 months | 6 years | Familial dysautonomia, hypertension, and anxiety | Artificial tears | Old KNV and scar in visual axis | 20/200 | At 1 mo, KNV regressed and BCVA improved to 20/80; subject reported reduction in conjunctival injection | 20/100 | 20/400 | Dense scarring and deep KNV. Ocular surface disease progressed. |
| 6 | 52 | female | OD | Stevens-Johnson syndrome; history of rotational nystagmus and plaquenil toxicity | Twice daily | 6 months | 6 months | Hypertension, Chronic inflammatory demyelinating polyneuropathy | Prednisolone and Muro 128 | Longstanding KNV approaching visual axis | 20/50 | No response and subject reported no change in visual quality or symptoms | 20/60 | 20/60 | Lost to follow-up |
| 7 | 22 | female | OD | Graft-versus-host disease and limbal stem cell deficiency; history of nanophthalmia | Twice daily | 3 months | 3 years | Fanconi anemia s/p bone marrow transplant | Prednisolone | KNV in visual axis after recent persistent epithelial defect | Hand Motion | At 3 mo, fine KNV and haze decreased and BCVA improved to 20/400; subject reported reduced photophobia | 20/400 | 20/200 | Inactive KNV, underwent cataract extraction |
| 8 | 36 | male | OD | Stevens-Johnson syndrome with atypical mucous membrane pemphigoid and symblepharon | Twice daily | 3 months | 9 years | Ulcerative colitis anddepression | Restasis | Active KNV | 20/40 | At 1 mo, KNV regressed and BCVA improved to 20/30 | 20/40 | 20/20 | Inactive KNV |
| 9 | 23 | male | OD | Stevens-Johnson syndrome; history of glaucoma | Twice daily | 19 months | 3 years | Stevens-Johnson syndrome | Fluorometholone, Restasis, Combigan, and Vitamine A ointment | Active KNV | 20/400 | At 3 mo, no active KNV or haze, BCVA 20/200; Continued bevacizumab for presumed anti-inflammatory and anti-leaking properties with various frequency for 19 months | 20/70 | 20/60 | Inactive KNV |
| 10 | 29 | female | OS | Stevens-Johnson syndrome | Twice daily | 3 months | 6 years | Stevens-Johnson syndrome | Lotemax | Longstanding inferior KNV approaching visual axis after recent exposure | 20/50 | At 4 mo, KNV regressed | 20/30 | 20/30 | No KNV progression; underwent MMG |
| 11 | 59 | female | OS | Stevens-Johnson syndrome | Twice daily | 14 months | 4 years | Stevens-Johnson syndrome | Lotemax and oral doxycycline | KNV approaching visual axis | 20/40 | At 1 mo, vessels less congested and subject reported reduced photophobia, BCVA unchanged; subject continued to use bevacizumab for comfort for 14 months | 20/125 | 20/80 | No KNV progression; underwent MMG. Ocular surface disease progressed. |
| 12 | 72 | female | OS | Stevens-Johnson syndrome | Twice daily | 6 months | 3 years | Asthma and Stevens- Johnson syndrome | Prednisolone and Restasis | KNV approaching visual axis | 20/80 | Wearing PROSE around the clock for exposure; at 3 mo, KNV and haze improved | 20/70 | 20/200 | Deep KNV. Ocular surface disease progressed. |
| 13 | 79 | female | OS | Graft-versus-host disease; history of strabismus and amblyopia | Twice daily | 3 months | 1 year | Polycythemia vera s/p bone marrow transplant and nephrotic syndrome | Lotemax | KNV in visual axis | 20/125 | At 3 mo, haze and vessel congestion reduced | 20/150 | 20/80 | No KNV progression; underwent cataract extraction; vision limited by amblyopia |
3mo since initiation of treatment
All patients used artificial tears as needed
BCVA was recorded with PROSE device. BCVA at baseline was measured before initiation of bevacizumab treatment.
Abbreviations. KNV: corneal neovascularization; BCVA: best-corrected visual acuity; OD: right eye; OS: left eye; PKP: penetrating keratoplasty; MMG: mucous membrane graft.
Case 1 (Figure 2)
Figure 2.
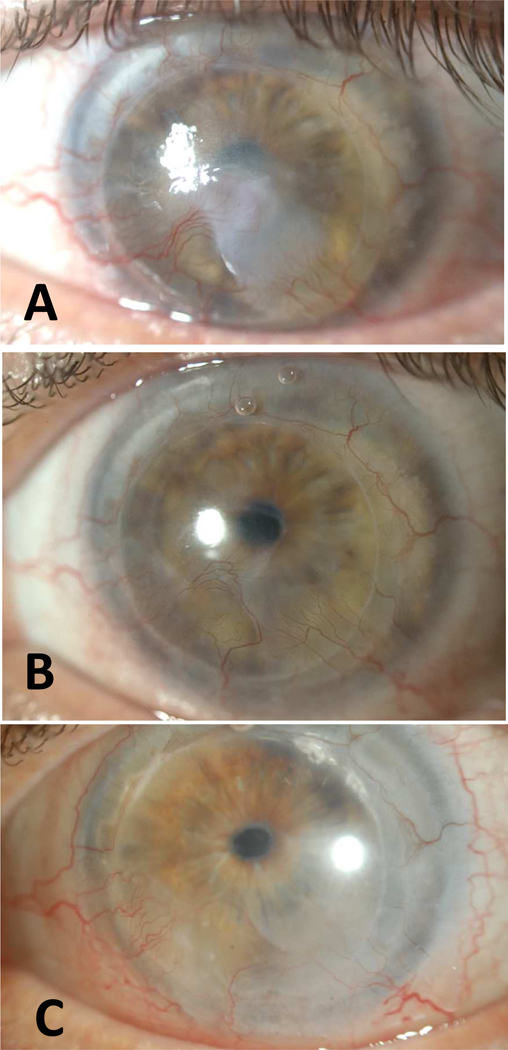
Slit lamp photographs of Case 1 with KNV regression and improvement in vision after bevacizumab treatment. (A) BCVA was count fingers at 3 feet at baseline. Note the active KNV and central haze. (B) BCVA was 20/50 after treatment with significant reduction in haze. (C) BCVA was 20/25 at last follow-up (11.5 years since treatment) with no recurrence.
A 65-year-old man was initially fitted with PROSE in 1997 for persistent epithelial defect in a repeat penetrating keratoplasty (PKP) for scarring related to herpes simplex virus infection and neurotrophic keratitis. He healed with pannus and haze. A third PKP was performed in 2005 for visual rehabilitation and he continued PROSE wear. The patient had a stable ocular surface and vision of 20/25 until 20 months after the third PKP when rapid progression of inferior KNV was noted. The vascular pannus and haze progressed to the visual axis, reducing acuity to count fingers. Patient was then started on twice daily bevacizumab treatment in the PROSE device. Marked regression of pannus and improved vision (20/100) were note at 1 month. He continued bevacizumab treatment twice daily for a total of 3 months and then tapered to once daily and discontinued after a total of 7 months. His vision was 20/50 at the end of treatment. The stromal haze continued to improve after discontinuation of bevacizumab. BCVA at last follow-up (11.5 years since initiation of treatment) was 20/25. Slit lamp photos show inactive ghost vessels in the cornea.
Case 2 (Figure 3)
Figure 3.
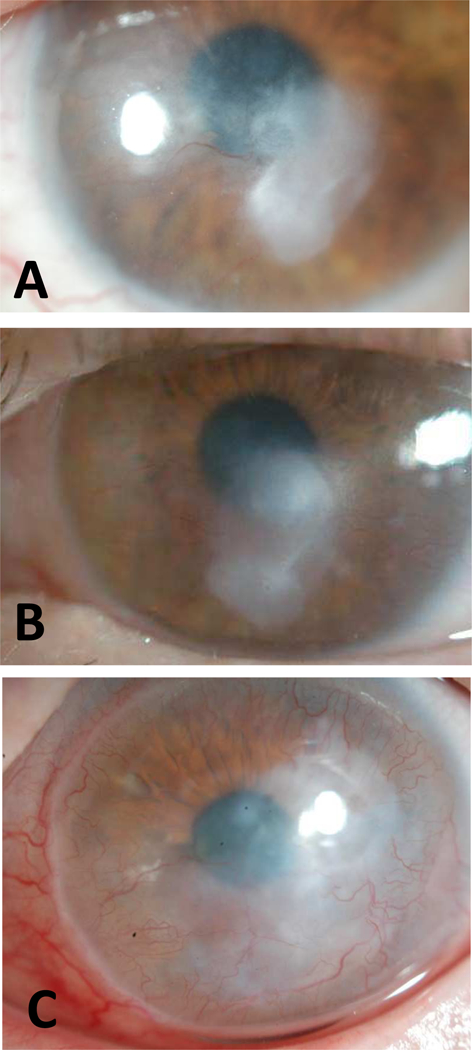
Slit lamp photographs of Case 2. (A) BCVA was 20/30 at baseline with a central vascularized corneal nodule. (B) BCVA was 20/25 at 7 months since initiation of treatment. Subject continued to use bevacizumab for a total of 10 years. (C) BCVA was 20/40 shortly after discontinuation of bevacizumab and KNV progressed with increased paracentral haze.
A 30-year-old woman with SJS at age 12 associated with carbamazepine had right eye vision reduced to light perception from microbial keratitis, corneal perforation, and glaucoma. She developed fungal keratitis in the left eye that eventually resolved leaving a vascularized paraxial subepithelial nodule. She had been wearing PROSE device for comfort and maintenance of ocular surface with no further episodes of microbial keratitis. The vascularized nodule increased in size requiring increased vault of her PROSE device. Vision at that time was 20/30. Bevacizumab treatment was then undertaken to reduce KNV and the size of the nodule. At 1 month, there was KNV regression and vision improved to 20/25. Moreover, she subjectively noted improved comfort and a decrease in ocular surface debris while on the bevacizumab. She continued with use of bevacizumab at various frequencies (never more than twice daily) for a total of 10 years. After discontinuation of bevacizumab, her KNV progressed and she underwent laser treatment with physicians elsewhere.
Case 3 (Figure 4)
Figure 4.

Slit lamp photographs of Case 3 with KNV regression and improvement in vision after bevacizumab treatment. (A) BCVA was 20/400 at baseline with active sea fan-shaped KNV extending into paracentral cornea. (B) BCVA was 20/50 after treatment. Note significant reduction in KNV. (C) BCVA was 20/30 at last follow-up (7 years since treatment) and no recurrence.
A 35-year-old male with soft contact lens-related corneal ulcer and limbal stem cell deficiency (LSCD) was referred to BostonSight for persistent epithelial defect, which healed with PROSE treatment. Meanwhile, he developed diffuse and active KNV and haze with a BCVA of 20/400. He started twice daily bevacizumab use in the PROSE device and KNV regressed quickly. He was treated for a total of 4 months and vision was 20/50 at the end of treatment. The KNV and haze continued to regress and there was no apparent central/paracentral KNV at his last follow-up (7 years since initiation of therapy) and BCVA was 20/30.
Case 6 (Figure 5)
Figure 5.
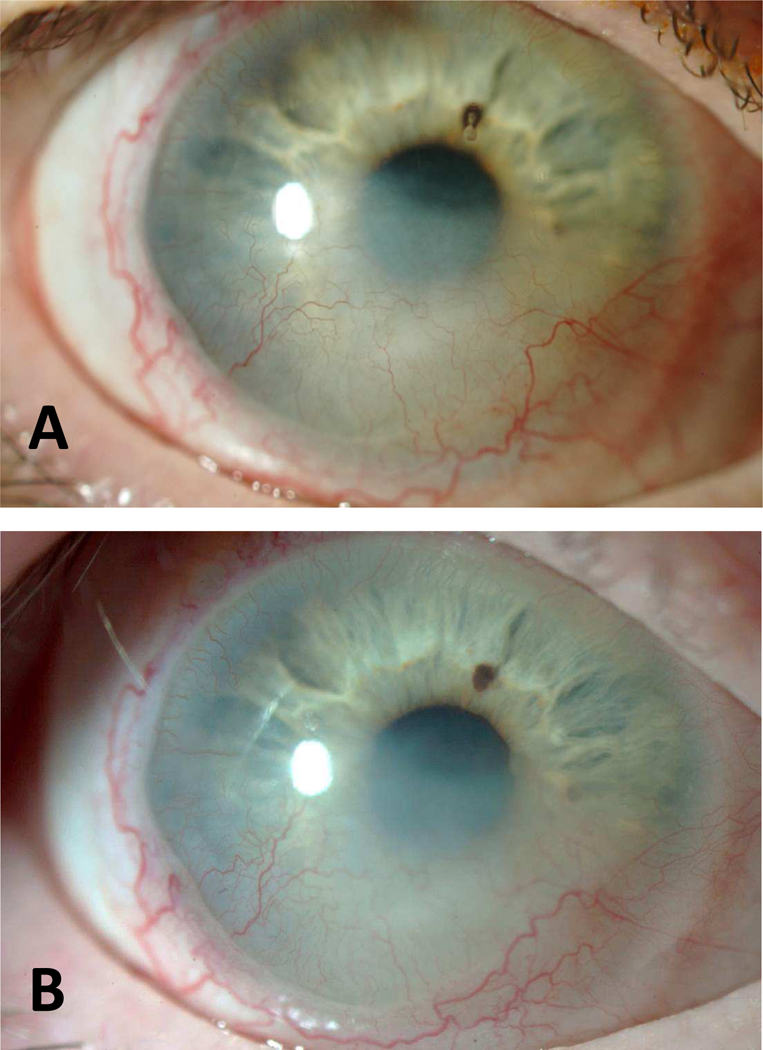
Slit lamp photographs of Case 6. (A) BCVA was 20/50 at baseline with long-standing KNV and haze extending into central cornea. (B) BCVA was 20/60 after 6-month treatment with bevacizumab and there was no regression or progression of the existing KNV.
A 52-year-old woman with a longstanding history of SJS and KNV was treated with bevacizumab twice daily in the PROSE device for extension of KNV into the visual axis. She reported no improvement in vision or symptoms after 6 months of use during which time vision decreased slightly from 20/50 to 20/60. She reported no adverse events and was then lost to follow-up.
Case 8 (Figure 6)
Figure 6.
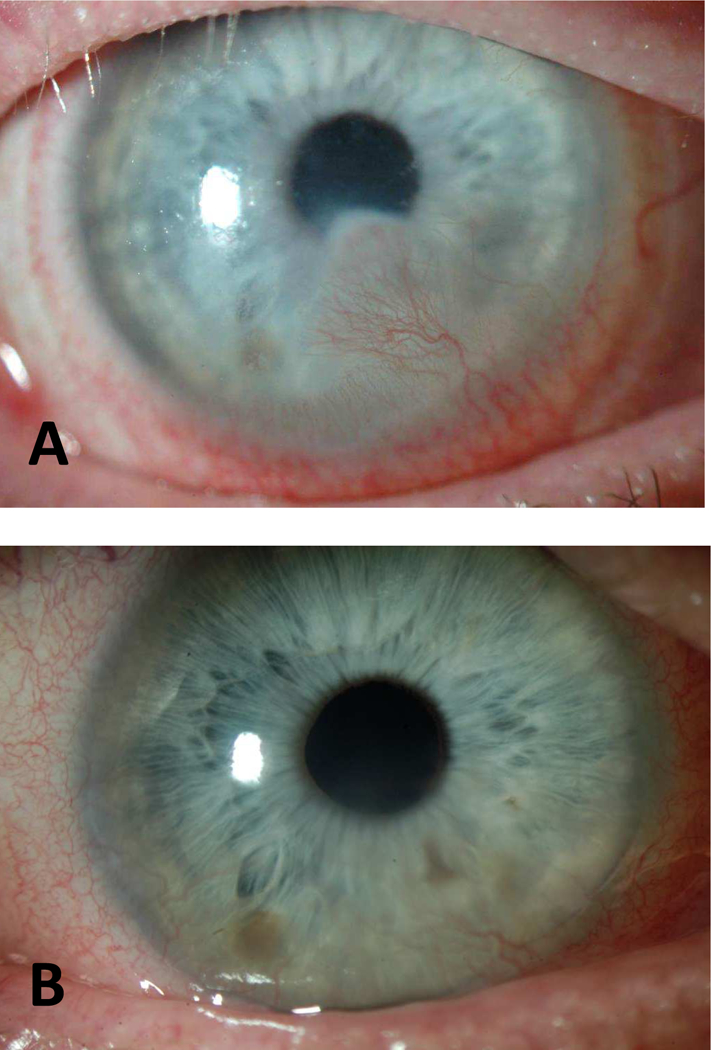
Slit lamp photographs of Case 8 with KNV regression and improvement in vision after treatment. (A) BCVA was 20/40 at baseline with active KNV and haze extending into paracentral cornea. (B) BCVA was 20/20 at last follow-up (9 years since treatment) with inactive peripheral KNV.
A 36-year-old male with SJS and atypical mucous membrane pemphigoid was referred to BostonSight for reduced vision and intense photophobia. He presented with KNV, symblepharon, trichiasis, and keratinization of his posterior lid margins. His vision improved with PROSE wear but active KNV approached visual axis. Bevacizumab twice daily in the device was started. KNV regressed one month after treatment and vision improved from 20/40 to 20/30. The total duration of bevacizumab treatment was 3 months. He later underwent cataract surgery and BCVA was 20/20 at last follow-up in 2017 (9 years since initiation of bevacizumab treatment) with inactive KNV.
Case 11(Figure 7)
Figure 7.
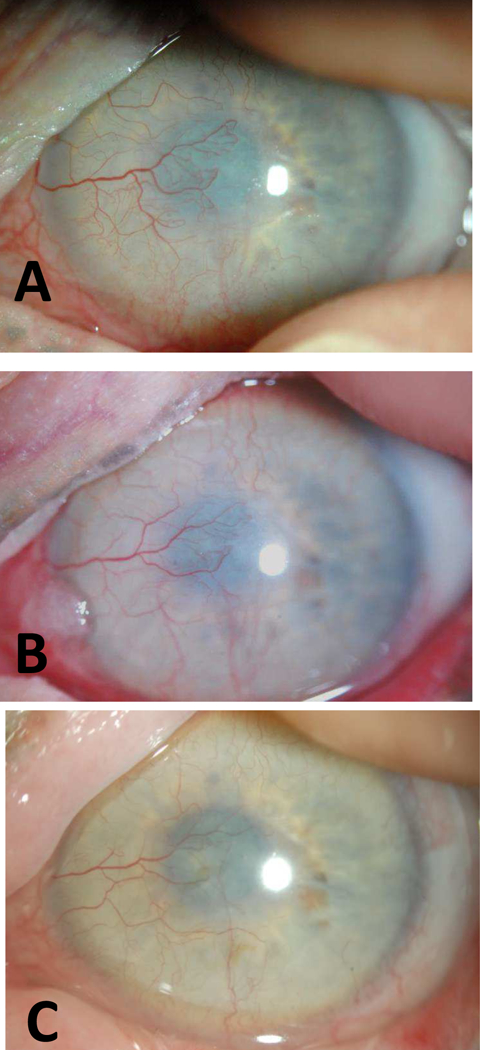
Slit lamp photographs of Case 11. (A) BCVA was 20/40 at baseline with active KNV extending into central cornea. (B) BCVA was 20/60 at 11 months since initiation of treatment. Subject used bevacizumab for a total of 14 months. BCVA was 20/80 at last follow-up (5 years since treatment). KNV regressed and remained inactive. The conjunctivalization of the cornea continued despite the inactive KNV and accounted for the reduced BCVA.
A 59-year-old female with a history of SJS since childhood and significant lid abnormality status post-surgical correction had been wearing PROSE for improved vision and comfort. She was noted to have active KNV approaching visual axis in the left eye and started on bevacizumab in PROSE device twice daily. BCVA was 20/40. At 1 month, patient reported decreased photophobia and the vessels were less congested. Patient continued to use bevacizumab due to improved visual quality for a total of 14 months. KNV continued to regress but conjunctivalization of the ocular surface progressed and her BCVA declined to 20/125. After discontinuation of bevacizumab, there was no KNV regression and she underwent mucous membrane grafting with a last recorded BCVA of 20/80.
Discussion:
In the current study, we evaluated the long-term outcome of PROSE for delivery of bevacizumab in the treatment of KNV in 13 consecutive patients at the BostonSight between 2006 and 2017. All patients wore a PROSE device regularly for ocular surface disease and were treated with 1% preservative free bevacizumab in the reservoir of a PROSE device for active or vision-threatening KNV. Patients used bevacizumab for a median duration of 6 months and were followed for an average of 5 years (longest follow-up 11 years). With bevacizumab treatment, 12 out of the 13 patients had KNV regression and 10 had improvement in visual acuity, which was sustained over the duration of follow-up. Only 1 case had KNV progression after discontinuation of bevacizumab. There were no ophthalmic or systemic complications over the duration of follow-up; the cohort included a patient with familial dysautonomia. We are not aware of any other report on the long term impact of topical VEGF inhibitors on the ocular surface in patients with ocular surface disease.
Corneal neovascularization results from inflammatory and infectious etiologies, reduces vision and increases risks associated with corneal transplantation. 19,20 Medical management targets the underlying cause of KNV; topical corticosteroids are often utilized to reduce ocular surface inflammation. 19,20 Anti-VEGF agents, delivered topically or subconjunctivally, have been explored for the treatment of KNV since 2007. 4,21–23 A 2013 meta-analysis demonstrated that anti-VEGF agents are effective in reducing neovascularized area in the cornea and appeared to be more effective against ‘active’ KNV.4 Meanwhile, side effects associated with bevacizumab use at the ocular surface have been reported, including persistent epithelial defect 24–26, corneal melt 27,28, and progression upon discontinuation 29
In this study, bevacizumab delivered in a PROSE device had a durable effect on reducing KNV in a group of patients with significant pre-existing ocular surface disease. Yet, no ophthalmic side effects were observed, even in a patient using bevacizumab for 10 years. We speculate that the lack of ophthalmic adverse events or KNV recurrence may be due to the following reasons: first, the ocular surface is protected and nourished underneath the PROSE device; second, the PROSE device provides a preservative-free, and pH-balanced microenvironment to deliver the bevacizumab; third, retention of bevacizumab, a large molecule, in the reservoir of PROSE may increase its bio-availability to the cornea; last but not least, the PROSE device reduces the cornea’s susceptibility to the various factors that may cause KNV, thus breaking the vicious cycle between KNV and heightened inflammation.
In addition to clinically evident KNV regression, our study also observed improved visual acuity in 10 out of 13 patients. The median BCVA of the cohort improved and such improvement was sustained throughout the duration of follow-up. We have previously reported that initiation of PROSE treatment improved visual acuity in ocular surface diseases and such improvement sustained over time. 5,10,11 In the current study, all our patients were wearing a PROSE device on a daily basis before the initiation of bevacizumab treatment, so the ocular surface was already ‘optimized’ by PROSE device as far as optical neutralization of refractive error and/or surface irregularity. Therefore any residual deficit in visual acuity can be attributed to corneal opacity and we believe that bevacizumab treatment reduced such opacity and led to an improvement in vision. Bachmann et al systemically reviewed the associated between KNV regression and visual acuity in studies examining anti-VEGF treatment and found there was eak evidence to support that KNV reduction correlated with an increase in visual acuity. 30 In our study, the decision to start bevacizumab was made when the KNV was progressive, active, approaching visual axis, and/or vision threatening, which may explain the significant improvement in visual acuity. That being said, we acknowledge that continued wear of PROSE (after bevacizumab treatment) may result in continued support of ocular surface, contributing to maintenance of transparency of the ocular surface.
One patient (Case 6) with longstanding SJS since childhood and KNV had no clinical or visual improvement with bevacizumab treatment, which may be explained by the chronicity and maturity of her KNV. Indeed experimental studies in animal models demonstrate that anti-VEGF therapy is effective in reducing KNV if employed early, and that KNV becomes refractory to VEGF deprivation over time when there is pericyte coverage and vessel maturation. 31–33 This is in line with the aforementioned meta-analysis that topical and subconjunctival anti-VEGF is more effective against ‘active’ KNV.4 In addition to the objective improvement noted, bevacizumab treatment also alleviated subjective symptoms in 3 patients with SJS. They reported reduced photophobia, discomfort, and debris when using bevacizumab and continued to use it beyond 1 year with no adverse events.
The current study has several limitations. The study is retrospective and non-comparative in nature. The true bioavailability of bevacizumab in the device and to the cornea was unknown. KNV extent or severity was not rated before and after treatment based on any pre-specified classification system, and although clinical photographs were available for all cases, objective quantification of vessels was not undertaken due to varied quality of photographs. We believe, however, these limitations are mitigated by the objective and sustained improvement in BCVA, which highlights the overall efficacy of the treatment. Although our study observed no adverse events, the majority of our patients used bevacizumab no longer than several months and our sample size is small. Given the potential systemic side effects associated ophthalmic bevacizumab use 34, it is critical to inform patients of such effects and monitor them closely in conjunction with primary care physicians.
In conclusion, the current study of the long-term outcome of using PROSE as a drug delivery system for bevacizumab in the treatment of corneal neovascularization demonstrates the safety, efficacy and durability of this approach to treat KNV and improve vision. The use of PROSE treatment as a drug delivery system to the ocular surface warrants further investigation.
Table 1.
Baseline characteristics of 13 subjects
| Age in years | Mean (SD) | 45 (20) |
| Range | 22–79 | |
| Sex | Female | 8 |
| Male | 5 | |
| Laterality * | OD | 7 |
| OS | 7 | |
| Diagnosis | Stevens-Johnson Syndrome | 7 |
| Ocular graft-versus-host disease | 2 | |
| Corneal transplant ** | 2 | |
| Limbal stem cell deficiency | 1 | |
| Familial dysautonomia | 1 | |
| Use of topical corticosteroid | 10 | |
| BCVA *** | Median (LogMAR) | −1.1 |
| Range | 20/30 - Hand Motion | |
| Duration of follow-up | Mean (SD) | 5.1 (3.4) years |
| Range | 6 months-11 years | |
Subject #5 underwent bilateral treatment
1 penetrating keratoplasty for scarring from herpes simplex keratitis, and 1 patch graft for perforation related to Sjogren syndrome and neurotrophic sterile melt
BCVA was recorded with PROSE device
Table 2.
Outcomes of bevacizumab treatment in PROSE
| Median duration of bevacizumab use (range) | 6 months (3 months-10 years) |
| KNV regression after treatment, Number (%) | 12 (92%) |
| Improvement in BCVA, Number (%) | 10 (77%) |
| Median BCVA in LogMAR before treatment (range in Snellen) * | −1.09 (20/30 to Hand Motion) |
| Median BCVA in LogMAR at end of treatment (range in Snellen) * | −0.66 (20/25 to 20/400) |
| Median BCVA in LogMAR at last follow-up (range in Snellen) * | −0.63 (20/20 to 20/400) |
| KNV progression after treatment discontinuation, Number (%) | 1 (8%) |
| Complications, Number (%) | 0 (0%) |
BCVA was recorded with PROSE device
Acknowledgments:
The authors wish to thank BostonSight, Needham MA, for permission to present the data and images in this study.
This work was supported by the National Eye Institute 5K12EY016335 (JY, trainee). The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflicts of Interest: DJ was an employee of BostonSight, 501(c), during the time that the patients reported here were treated. DJ has no financial or proprietary interest in any contact lens or prosthetic device.
References:
- 1.Maddula S, Davis DK, Maddula S, Burrow MK, Ambati BK. Horizons in therapy for corneal angiogenesis. Ophthalmology 2011;118(3):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Vascular endothelial growth factor in eye disease. Prog Retin Eye Res 2008;27(4):331–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roshandel D, Eslani M, Baradaran-Rafii A, et al. Current and upcoming therapies for corneal neovascularization. Ocul Surf 2018. [DOI] [PMC free article] [PubMed]
- 4.Papathanassiou M, Theodoropoulou S, Analitis A, Tzonou A, Theodossiadis PG. Vascular endothelial growth factor inhibitors for treatment of corneal neovascularization: a meta-analysis. Cornea 2013;32(4):435–444. [DOI] [PubMed] [Google Scholar]
- 5.Stason WB, Razavi M, Jacobs DS, et al. Clinical benefits of the Boston Ocular Surface Prosthesis. Am J Ophthalmol 2010;149(1):54–61. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, Chiu GB, Bach D, Bababeygy SR, Irvine J, Heur M. Functional and visual improvement with prosthetic replacement of the ocular surface ecosystem scleral lenses for irregular corneas. Cornea 2013;32(12):1540–1543. [DOI] [PubMed] [Google Scholar]
- 7.DeLoss KS, Fatteh NH, Hood CT. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) scleral device compared to keratoplasty for the treatment of corneal ectasia. Am J Ophthalmol 2014;158(5):974–982. [DOI] [PubMed] [Google Scholar]
- 8.Heur M, Bach D, Theophanous C, Chiu GB. Prosthetic replacement of the ocular surface ecosystem scleral lens therapy for patients with ocular symptoms of chronic Stevens-Johnson syndrome. Am J Ophthalmol 2014;158(1):49–54. [DOI] [PubMed] [Google Scholar]
- 9.Theophanous C, Irvine JA, Parker P, Chiu GB. Use of Prosthetic Replacement of the Ocular Surface Ecosystem Scleral Lenses in Patients with Ocular Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant 2015;21(12):2180–2184. [DOI] [PubMed] [Google Scholar]
- 10.Papakostas TD, Le HG, Chodosh J, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem as treatment for ocular surface disease in patients with a history of Stevens-Johnson syndrome/toxic epidermal necrolysis. Ophthalmology 2015;122(2):248–253. [DOI] [PubMed] [Google Scholar]
- 11.Agranat JS, Kitos NR, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem: impact at 5 years. Br J Ophthalmol 2016;100(9):1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahal JS, Heur M, Chiu GB. Prosthetic Replacement of the Ocular Surface Ecosystem Scleral Lens Therapy for Exposure Keratopathy. Eye Contact Lens 2017;43(4):240–244. [DOI] [PubMed] [Google Scholar]
- 13.Parra AS, Roth BM, Nguyen TM, Wang L, Pflugfelder SC, Al-Mohtaseb Z. Assessment of the Prosthetic Replacement of Ocular Surface Ecosystem (PROSE) scleral lens on visual acuity for corneal irregularity and ocular surface disease. Ocul Surf 2018;16(2):254–258. [DOI] [PubMed] [Google Scholar]
- 14.Lim P, Ridges R, Jacobs DS, Rosenthal P. Treatment of persistent corneal epithelial defect with overnight wear of a prosthetic device for the ocular surface. Am J Ophthalmol 2013;156(6):1095–1101. [DOI] [PubMed] [Google Scholar]
- 15.Ciralsky JB, Chapman KO, Rosenblatt MI, et al. Treatment of Refractory Persistent Corneal Epithelial Defects: A Standardized Approach Using Continuous Wear PROSE Therapy. Ocul Immunol Inflamm 2015;23(3):219–224. [DOI] [PubMed] [Google Scholar]
- 16.Lim M, Jacobs DS, Rosenthal P, Carrasquillo KG. The Boston Ocular Surface Prosthesis as a novel drug delivery system for bevacizumab. Semin Ophthalmol 2009;24(3):149–155. [DOI] [PubMed] [Google Scholar]
- 17.Keating AM, Jacobs DS. Anti-VEGF Treatment of Corneal Neovascularization. Ocul Surf 2011;9(4):227–237. [DOI] [PubMed] [Google Scholar]
- 18.Holladay JT. Visual acuity measurements. J Cataract Refract Surg 2004;30(2):287–290. [DOI] [PubMed] [Google Scholar]
- 19.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol 2001;12(4):242–249. [DOI] [PubMed] [Google Scholar]
- 20.Roshandel D, Eslani M, Baradaran-Rafii A, et al. Current and upcoming therapies for corneal neovascularization. Ocular surf 2018;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol 2007;125(6):834–836. [DOI] [PubMed] [Google Scholar]
- 22.Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: an anti-VEGF therapy review. Surv Ophthalmol 2012;57(5):415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosseini H, Nowroozzadeh MH, Salouti R, Nejabat M. Anti-VEGF therapy with bevacizumab for anterior segment eye disease. Cornea 2012;31(3):322–334. [DOI] [PubMed] [Google Scholar]
- 24.Kim SW, Ha BJ, Kim EK, Tchah H, Kim TI. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology 2008;115(6):e33–38. [DOI] [PubMed] [Google Scholar]
- 25.Koenig Y, Bock F, Horn F, Kruse F, Straub K, Cursiefen C. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 2009;247(10):1375–1382. [DOI] [PubMed] [Google Scholar]
- 26.Gueudry J, Richez F, Tougeron-Brousseau B, Genevois O, Muraine M. [Subconjunctival bevacizumab for corneal neovascularization]. J Fr Ophtalmol 2010;33(9):630–636. [DOI] [PubMed] [Google Scholar]
- 27.Galor A, Yoo SH. Corneal Melt While Using Topical Bevacizumab Eye Drops. Ophthalmic Surg Lasers Imaging 2010:1–3. [DOI] [PubMed]
- 28.Bhasin P, Gujar P, Bhasin P. A case of recipient bed melt and wound dehiscence after penetrating keratoplasty and subconjunctival injection of bevacizumab. Cornea 2012;31(11):1342–1343. [DOI] [PubMed] [Google Scholar]
- 29.Chu HS, Chen TC, Hu FR, Chen WL. Recurrence of corneal neovascularization associated with lipid deposition after subconjunctival injection of bevacizumab. Cornea 2013;32(11):1446–1453. [DOI] [PubMed] [Google Scholar]
- 30.Bachmann B, Taylor RS, Cursiefen C. The association between corneal neovascularization and visual acuity: a systematic review. Acta Ophthalmol (Oxf) 2013;91(1):12–19. [DOI] [PubMed] [Google Scholar]
- 31.Jo N, Mailhos C, Ju M, et al. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol 2006;168(6):2036–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaoran Z, Zhirong L, Gezhi X. Combination of vascular endothelial growth factor receptor/platelet-derived growth factor receptor inhibition markedly improves the antiangiogenic efficacy for advanced stage mouse corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 2011;249(10):1493–1501. [DOI] [PubMed] [Google Scholar]
- 33.Chen WL, Chen YM, Chu HS, et al. Mechanisms controlling the effects of bevacizumab (avastin) on the inhibition of early but not late formed corneal neovascularization. PLoS ONE 2014;9(4):e94205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 2013;27(7):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]


