Abstract
Background:
Platinum and etoposide with thoracic radiation followed by prophylactic cranial irradiation is the standard treatment for limited-stage small cell lung cancer (LS-SCLC). Many patients with LS-SCLC are elderly with co-morbidities.
Methods:
We collected Individual patient data (IPD) from 11 phase 2 or 3 trials for LS-SCLC conducted by the National Clinical Trials Network and activated from 1990 to 2010. The primary end-point was overall survival (OS); secondary end-points were progression-free survival (PFS), the rate of severe adverse events (AE’s), and off-treatment reasons. We compared the outcomes for patient’s age ≥ 70 years (elderly) and < 70 years (younger).
Results:
We analyzed IPD from 1,049 younger (81%) and 254 elderly patients (19%). In the multivariable model elderly compared to younger patients had a worse OS (HR=1.38; 95% CI: 1.18–1.63; median OS of 17.8 and 23.5 months, respectively), and a worse PFS (HR=1.19; 95% CI: 1.03– 1.39; median PFS 10.6 and 12.3 months, respectively). Elderly compared to younger patients experienced more grade 5 AE’s (8% vs 3%, p<0.01), more grade ≥ 3 dyspnea (11% vs 7%, p=0.03), but less grade ≥ 3 esophagitis/dysphagia (14% vs 19%, p=0.04) and grade ≥ 3 vomiting (11% vs 17%, p=0.01). Elderly patients completed treatment less often, and discontinued treatment due to AE’s, patient refusal, and died during treatment more frequently.
Conclusions:
Elderly patients with LS-SCLC had a worse PFS and OS, and more difficulty tolerating therapy. Future trials should incorporate assessments of elderly patients, novel monitoring of AE’s, and more tolerable radiation and systemic therapies.
Keywords: adverse events related to age, clinical trial, small cell lung cancer, chemotherapy, thoracic radiation therapy
Condensed abstract:
This retrospective analysis compared outcomes of patients age ≥ 70 and < 70 years using individual patient data from National Clinical Trials Network of clinical trials for limited-stage small cell lung cancer. Elderly patients had a worse progression-free and overall survival, more grade 5 adverse events, and completed treatment less frequently.
Lung cancer is the leading cause of cancer-related mortality in the United States, and approximately 15% of patients have the small cell lung cancer (SCLC) subtype.1, 2 For patients with limited stage SCLC (LS-SCLC) concurrent chemoradiotherapy followed by prophylactic cranial irradiation (PCI) with curative intent is the standard therapy.3 The median overall survival (OS) observed with concurrent chemoradiotherapy on recent phase 3 trials was 25–30 months and the 5-year OS rate was 25–35%.4, 5 However, the trial eligibility criteria limited enrollment to a carefully selected patient population, and elderly patients are underrepresented in lung cancer clinical trials.6 Clinicians have concerns about the ability of elderly patients to tolerate concurrent chemoradiotherapy, and the frequency of severe adverse events. Clinicians rely on subset analyses of elderly patients from clinical trials to estimate the efficacy and frequency of adverse events, or extrapolate the benefit of the study population to elderly and frailer patients.
Most patients with SCLC have a history of tobacco use, are elderly, and have significant co-morbidities.7 Previous retrospective and subset studies have investigated the outcomes of elderly patients, defined as age ≥ 70 years, with LS-SCLC treated with chemoradiotherapy on clinical trials.8–11 However, only 13–21% of the patients enrolled in these trials were age ≥ 70 years and these subsets were small (n=33 to 88). The small sample size limits the interpretation of the previous analyses. The United States National Cancer Institute (NCI) National Clinical Trials Network (NCTN) performed clinical trials investigating concurrent chemoradiotherapy for LS-SCLC. These trials were widely available, and enrolled patients at both community and academic centers. We investigated the outcomes as assessed by OS, progression-free survival (PFS) and the adverse events of patients enrolled in NCTN trials of concurrent chemoradiotherapy for LS-SCLC.
Patients and Methods
Data sharing agreements with the relevant cooperative groups were developed, and individual patient data (IPD) were obtained for patients with non-small cell lung cancer and SCLC treated on NCTN trials from 1990–2012. A centralized database was developed, and for this analysis, IPD was restricted to trials of concurrent chemoradiotherapy for LS-SCLC. We reviewed the study protocols and final publications for inclusion, and only trials that included concurrent chemoradiotherapy were included. We defined patients age < 70 years as younger and patients age ≥ 70 years as elderly since this age cut-off was used in previous retrospective analyses of chemoradiotherapy for patients with LS-SCLC.9–11 The primary end-point was OS, and secondary end-points were PFS, the rate of severe adverse events, and off-treatment reasons. OS was defined as the time between randomization/registration to death from any cause. PFS was defined as the time between randomization/registration to disease progression or death (whichever comes first). Severe adverse events were defined as grade ≥ 3 using the NCI Common Terminology Criteria for Adverse Events. We compared Individual grade ≥ 3 adverse events and all grade adverse events in elderly or younger patients (excluding leukopenia/lymphopenia). The adverse events were for the entire study treatment, and included the chemoradiotherapy and chemotherapy alone portions of the treatment. The study team assessed the off treatment reasons which were reported as part of the protocol. Off treatment reasons reported included treatment completed, adverse events, disease progression, patient refused further treatment, died during treatment, treatment never started, developed other disease, and no response to treatment. Off treatment reasons not included above were categorized as other. Grade 5 adverse events include all fatal events whether related to treatment, co-morbidity or intercurrent illness. The classification of death related treatment was based on the study team attributing the cause of death was due to the treatment, which was collected as a component of the study protocol.
Statistical methods
Baseline patient characteristics’ distributions between the two age groups were tested by chi-square test for categorical variables and Kruskal-Wallis test for continuous variables.12, 13 OS and PFS analysis of two age groups were evaluated by Kaplan-Meier curves and differences were tested by log-rank test.14, 15 Univariate and multivariable frailty Cox models were then fitted to calculate hazard ratios (HR) and 95% confidence intervals (CI) for OS and PFS, where the heterogeneity between trials was accounted as frailty.16, 17 For multivariable frailty Cox models, candidate covariates were age group, gender, race, weight loss, performance status, body mass index (BMI), prior chemotherapy or not, prior surgery or not, number of chemotherapy agents, and recent trial or not (first patient enrolled in trial before January 1, 1996). We selected the date of January 1, 1996 for this exploratory analysis to divide the patients into approximately equal cohorts. Since age effect was of primary interest, it was included in all multivariable models after stepwise selection. For OS, covariates selected into the final model were age group, gender, race, weight loss, performance status, BMI, prior surgery or not, number of chemotherapy agents, and recent trial or not. For PFS, covariates selected into the final model were age group, gender, race, weight loss, performance status, and BMI.
Adverse events were categorized into seven categories based on grade (≥3, ≥4, and 5), and type (hematologic and non-hematologic). Adverse events grade ≥ 3 with a frequency ≥ 2 % were compared between age groups by chi-square test. Reasons for treatment discontinuation in the two groups were summarized in categories and for each category compared by Fisher’s Exact test or chi-square test.12 All p-values were two-sided and statistical significance was defined as p<0.05. Sensitivity analysis for the age cut-off 65 years was performed using same methodologies. We performed an exploratory analysis dividing the patients into three age cohorts (age <50, ≥ 50 to <70, and ≥ 70 years)
Duke University Institutional Review Board approved this study. Statistical Software used is SAS (version 9.4; SAS Institute, Cary, NC).
Results
IPD from 1,303 patients enrolled in 11 trials were included in the analysis (Supplemental Figure 1).18–28 The trials enrolled patients between 1993 to 2006. All the trials used concurrent chemotherapy with cisplatin or carboplatin with concurrent thoracic radiation, most investigated the integration of novel agent (e.g. tamoxifen, topotecan, paclitaxel, irinotecan, tirapazamine), and all permitted PCI at the discretion of the investigator. Of the 11 trials, two were phase 3 trials and nine were phase 2 trials, and the trials ranged in size from 58 to 324 patients (Supplemental Table 1). Nine of the 11 trials included a cisplatin-based therapy.
Of the 1,303 patients, 1,049 patients were < 70 years (81%) and 254 were ≥ 70 years (19%). The majority of the patients were male, white, and did not have any weight loss (Supplemental Table 2). There was an imbalance in the performance status (p=0.02). Among patients < 70 years, 49% of patients had a performance status of 0 and 45% of patients had a performance status of 1. Among patients age ≥ 70 years, 39% of patients had performance status of 0, and 53%, had a performance status of 1. Among patients age < 70 years 94% of patients had a performance status of 0 or 1; among patients age ≥ 70 years 92% of patients had a performance status of 0 or 1.
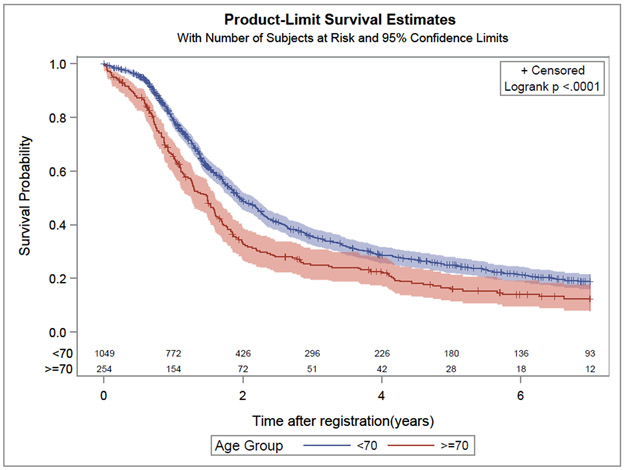
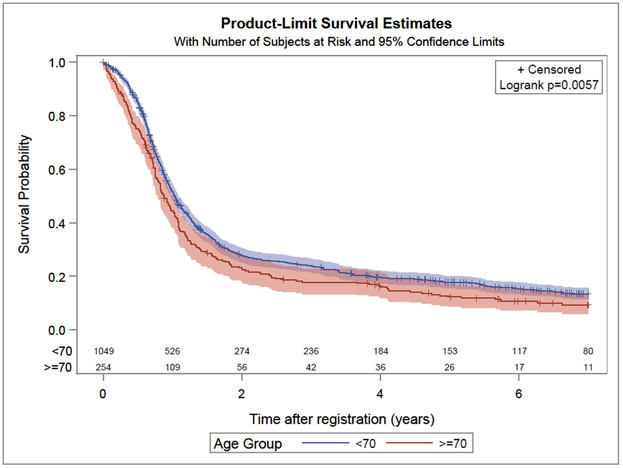
In univariate and multivariable Cox frailty models elderly compared to younger patients experienced a worse OS (HR 1.40, 95% CI: 1.20–1.65; p<0.01 and HR 1.38, 95% CI: 1.18–1.63; p<0.01, respectively). The median OS observed in the elderly and younger patients were 17.8 and 23.5 months, respectively (Figure 1). In univariate and multivariable Cox frailty models elderly compared to younger patients experienced a worse PFS (HR 1.23, 95% CI: 1.06 −1.43; p< 0.01 and HR 1.19, 95% CI: 1.03–1.39; p= 0.02, respectively). The median PFS for elderly and younger patients were 10.6 and 12.3 months, respectively (Figure 2).
Figure 1:
Kaplan Meier curve for overall survival for elderly (age ≥ 70 years) and younger patients (age < 70 years)
Figure 2:
Kaplan Meier curve for progression-free for elderly (age ≥ 70 years) and younger patients (age < 70 years)
Elderly patients compared to younger patients experienced a similar frequency of adverse events grade ≥ 3 (p=0.47), hematologic adverse events grade ≥ 3 (p=0.64), and non-hematologic adverse events ≥ 3 (p=0.75) (Table 1). Elderly patients compared to younger patients experienced more adverse events grade ≥4 (p<0.01) and hematologic adverse event grade ≥ 4 (p<0.01), but a similar frequency of non-hematologic adverse events grade ≥ 4 (p=0.26). Elderly patients experienced more grade 5 adverse events (deaths regardless of cause) (8% vs 3%, p<0.01). When the specific adverse events observed in ≥2% of patients were analyzed, elderly compared to younger patients experienced more grade ≥ 3 dyspnea (11% vs 7%, p=0.03), and less esophagitis/dysphagia (14% vs 19%, p=0.04) and vomiting (11% vs 17%, p=0.01) (Table 2). When all grade adverse events were examined elderly patients experienced less nausea (69% vs 77%, p<0.01) and esophagitis (50% vs 63%, p<0.01) (Supplemental Table 3).
Table 1:
Rate of grade ≥ 3 adverse events among older (age < 70 years) and younger (age ≥ 70 years) patients
| Adverse event category | Age ≥ 70 years (n=254) |
Age < 70 years (n=1,049) |
P-value a |
|---|---|---|---|
| All AE’s grade ≥3 | 83% (n=212) | 82% (n=855) | 0.47 |
| Hematologic AE’s grade ≥3 | 62% (n=158) | 61% (n=636) | 0.64 |
| Non-hematologic AE’s ≥3 | 54% (n=136) | 52% (n=550) | 0.75 |
| All AE’s grade ≥ 4 | 62% (n=157) | 50% (n=527) | < 0.01 |
| Hematologic AE’s ≥ 4 | 50% (n=128) | 41% (n=428) | < 0.01 |
| Non-hematologic AE’s ≥ 4 | 24% (n=61) | 21% (n=218) | 0.26 |
| Grade 5 AE’s | 8% (n=20) | 3% (n=32) | <0.01 |
| Treatment related deaths b | 6% (n=9) | 4% (n=23) | 0.22 |
Chi-square test for adverse events comparison
Treatment related death data were available in only 7 trials (n=729)
Table 2:
The rate of non-hematologic and hematologic grade ≥ 3 adverse events with a frequency in ≥ 2% in older (age < 70 years) and younger (age ≥ 70 years) patients.a
| Adverse event | Age ≥ 70 years (n=254) |
Age < 70 years (n=1049) |
P-value |
|---|---|---|---|
| Anemia | 19% (n=49) | 20% (n=211) | 0.77 |
| Anorexia | 9% (n=22) | 9% (n=97) | 0.77 |
| Constipation | 2% (n=4) | 1% (n=12) | 0.58 |
| Dehydration | 7% (n=19) | 7% (n=69) | 0.61 |
| Diarrhea | 6% (n=15) | 4% (n=46) | 0.30 |
| Dyspnea | 11% (n=27) | 7% (n=70) | 0.03 |
| Esophagitis/dysphagia | 14% (n=35) | 19% (n=203) | 0.04 |
| Fatigue | 13% (n=34) | 10% (n=104) | 0.11 |
| Hyponatremia | 3% (n=4) | 3% (n=34) | 0.16 |
| Hypotension | 3% (n=8) | 3% (n=33) | 1.00 |
| Nausea | 18% (n=48) | 22% (n=229) | 0.15 |
| Neutropenia | 56% (n=142) | 52% (n=549) | 0.31 |
| Pain | 7% (n=17) | 8% (n=79) | 0.65 |
| Pneumonitis/Pulmonary infiltrates | 2% (n=4) | 1% (n=6) | 0.10 |
| Vomiting | 11% (n=28) | 17% (n=183) | 0.01 |
Chi-square test for adverse events comparison
Elderly compared to younger patients completed treatment less often (p=0.02) and discontinued treatment due to an adverse events (p=0.02), refused further treatment (p<0.01) and died during treatment more frequently (p<0.01) (Table 3). Elderly patients compared to younger patients stopped therapy due to disease progression significantly less often (p=0.04). Data were available on 729 patients on the study teams attribution of cause death, and deaths attributed to treatment occurred in elderly patients and younger patients in 6% and 4% of patients, respectively (p=0.22).
Table 3:
End of treatment reasons with a frequency of ≥1% in older (age < 70 years) and younger (age ≥ 70 years) patients a
| End of treatment reason | Age ≥ 70 years (n=250) |
Age < 70 years (n=1,016) |
P-value b |
|---|---|---|---|
| Treatment completed | 46% (n=115) | 54% (n=551) | 0.02 |
| Adverse event | 14% (n=36) | 9% (n=96) | 0.02 |
| Disease progression | 13% (n=32) | 18% (n=186) | 0.04 |
| Patient refused further treatment | 10% (n=26) | 5% (n=52) | < 0.01 |
| Died during treatment | 11% (n=28) | 4% (n=40) | <0.01 |
| Other | 5% (n=13) | 8% (n=81) | 0.13 |
Data missing for 37 patients
Chi square test for p-value except for no response to treatment, developed other disease and treatment never started which used Fisher’s exact test
In the exploratory analysis using the age cut-off of 65, patients ≥ 65 (n=540) compared to < 65 years (n=763) had a worse OS in multivariable analysis (p<0.01), and a worse PFS in multivariable analysis (p=0.01). The median OS for patients ≥ 65 and < 65 years was 24.6 and 19.3 months, respectively (supplemental figure 2). In an exploratory analysis to investigate the potential contribution of younger patients (defined as age < 50 years) we investigated the OS patients age < 50 (n=146), age ≥ 50 to < 70 (n=903), and age ≥ 70 years (n=254). Patients younger than age 50 compared to patients age ≥ 50 to <70, had a better OS (p< 0.01), and patients age ≥ 70 years compared to patients age ≥50 to <70 years had a worse OS (p<0.01). The median OS observed in patients age <50, ≥ 50 to <70 years, and ≥ 70 years were 27.2, 23.0 and 17.8 months respectively (supplemental figure 3). In an exploratory analysis of OS in patients enrolled in recent versus later trials, patients enrolled in trials with the first patient enrolled before January 1 1996 (n=602) were compared patients enrolled in trials with the first patient enrolled after January 1, 1996 (n=701). A difference in OS between recent and later trials was not observed (p=0.13).
Discussion
Previous studies comparing elderly to younger patients have revealed contradictory OS results. The larger sample size of our study provided a more definitive analysis of OS and a better estimate of the median OS in each of the age subgroups which will assist future analyses. A retrospective study of two randomized trials performed by the National Cancer Institute of Canada revealed similar OS in patients age < 70 years and age ≥ 70 years (n=608).11 In a retrospective study of patients enrolled in a trial of once or twice daily radiotherapy starting with the third cycle, elderly and younger patients experienced similar OS.10 A retrospective analysis of a phase 3 trial of cisplatin/etoposide with once or twice daily radiation used the age cut-off of 70 years (n=381), and revealed a statistically significant worse 5-year OS rate.9 A previous retrospective study of three trials investigating high dose thoracic radiation revealed younger age (< 60 years) was independently associated with improved OS on multivariable analysis (n=200).8 Heterogeneity in the patient population and the treatments investigated as well as the smaller sample size may have contributed to the discrepant results from previous studies. Elderly patients experienced a worse progression-free survival suggesting that disease progression contributed to the worse OS. The difficulty tolerating and completing chemoradiotherapy probably contributed to the worse PFS. We do not have data on the rate of subsequent therapies and this may have differed between the elderly and younger patients and been a contributing factor to the worse OS as well.
One challenge in performing analyses based on age is selecting the optimal age cut-off. We used the age cut-off of ≥ 70 years in this analysis to ease comparison to previous studies in SCLC and non-small cell lung cancer.9–11, 29–31 However, the use of this age is arbitrary and not based on physiological data or clinical evidence. Our exploratory analyses revealed similar results for PFS and OS with the age cut-off of 65 years, and differences in OS when patients were divided age < 50 years, ≥ 50 to < 70 years, and ≥ 70 years. Younger patients may have tolerated therapy better, had less co-morbidities, or received subsequent therapies at a higher rate which may contribute to the differences in OS. A previous retrospective analysis of LS-SCLC revealed age > 70 years was not associated with a higher frequency of radiation therapy interruption.32 However, patients age ≤ 50 compared to > 50 years were less likely to have treatment interruptions, but an association with radiation treatment interruptions and OS was not observed. Additional studies will assist in elucidating the treatment compliance and tolerability of therapy is young and elderly patients with LS-SCLC.
Nine of the 11 trials used cisplatin-based chemotherapy, which contributed to the adverse events observed. Importantly, half of the patients received platinum-based therapy in combination with a novel agent, which may have contributed to the rate of adverse events observed. Elderly patients may have been more susceptible to the adverse events associated with the investigational agent due to differences drug clearance or drug-drug interactions due to more concurrent medications.
There have been improvements in anti-emetics used with cisplatin and carboplatin based therapies since these trials were performed which would reduce the rate of nausea or vomiting in both groups.
We report the frequency of specific adverse events but we do not have data on the number of cycles of chemotherapy or dose adjustments and we cannot calculate the rate of adverse events per chemotherapy cycle or chemotherapy delivery. For example, the lower rate of grade ≥ 3 vomiting observed in elderly patients may be due to a lower frequency of vomiting, more frequent dose reductions, or fewer cycles of chemotherapy in the elderly patients. Elderly patients also experienced a lower frequency of grade ≥ 3 esophagitis/dysphagia, and we do not have data available to determine if this was due to more radiation treatment interruptions or patients not completing the intended radiation therapy. Elderly patients reported a higher rate of grade ≥ 3 dyspnea (defined as dyspnea with activities of daily living), but a similar rate of radiation pneumonitis and anemia, which can cause dyspnea. It is possible that elderly patients had less pulmonary reserve or more underlying cardiovascular disease and were more likely to develop symptomatic dyspnea with lower grade anemia or radiation pneumonitis. The assessment of adverse events is complex since a combination of underlying co-morbidities, treatment related adverse events, disease status, and investigator interpretation contribute to attribution and grade.
The higher rate of grade 5 adverse events is concerning. Grade 5 adverse events includes deaths from all causes. Elderly patients may have had a higher prevalence of co-morbidities and these may have been responsible or contributed to their death while receiving study therapy. In order to better assess the cause of the higher rate of grade 5 adverse events in elderly patients we investigated the attribution according to the study team’s assessment, and data were available from seven studies on 729 patients. Deaths were attributed to study therapy in elderly and younger patients in 6% and 4% of patients (p=0.22). The smaller sample size limits interpretation and attributing causality is difficult, and this analysis should be interpreted cautiously.
One hazard of using clinical trial data is the patient population enrolled in clinical trials may differ substantially from patients seen in routine clinical practice. The eligibility criteria, especially organ function, co-morbidity, and history of prior malignancy restrict the elderly patients who can enroll in clinical trials.33 The median age for patients with SCLC in recent cohort study was 69 years, but the median age on a recent trial of chemoradiotherapy for LS-SCLC was approximately 62 years.4, 7 The use of cisplatin and physician practice patterns may have limited enrollment of elderly patients to the “fit” elderly or the patients perceived as the “fit” elderly. However, physicians may have been more lenient in enrolling younger patients with comorbidities and poor performance status, which was observed in advanced stage non-small cell lung cancer.34 Without a more in depth prospective assessment of patients, such as a Comprehensive Geriatric Assessment (CGA), it is difficult to detect the variation in co-morbidities in younger and older patients enrolled in studies. 35
This analysis has weaknesses. The retrospective design of this pooled analysis may have introduced uncontrolled biases. PCI was included in the treatment plan at the discretion of the treating physician in all of the trials. However, we were unable to determine if individual patients or patients on specific trial received PCI, and if the rate differed between the elderly and younger patients. Thus, we are unable to determine if PCI contributed to the difference in PFS or OS or perform subset analyses. The adverse events were recorded over the entire treatment so we cannot determine if the chemotherapy alone or the concurrent chemoradiotherapy portion of the treatment contributed to the differences in the severe adverse events. Patients enrolled in the trials over an extended period, and the characteristics of the elderly patients studied in this analysis may differ from the current elderly patient population with LS-SCLC. Another issue is that we did not identify a specific adverse event, such as febrile neutropenia, that is easy to address to reduce fatal adverse events. The staging work-up has changed, and there is greater use of positron emission tomography/computed tomography staging. These studies also lack central confirmation of the diagnosis of SCLC.
Despite the difficulty tolerating the therapy the median OS among elderly patients was 17.8 months and a significant percentage experienced long term survival. Appropriate elderly patients should be offered chemoradiotherapy, but these indicate the need for better assessment of elderly patients. The decision to pursue chemoradiotherapy should include patient preferences and a discussion about the benefits and risks associated the therapy. CGA is a method of assessing vulnerabilities and identifying patients who are more likely to experience chemotherapy adverse events, which may facilitate a conversation about the risks and benefits of therapy.35–37 Elderly patients frequently have co-morbidities that necessitated concurrent medications and these can exacerbated adverse events (e.g. anti-hypertensive medication may exacerbated dehydration or contribute to episodes of hypotension). We recommend a careful review of concurrent medications prior to starting therapy.
For patients who do not be meet the common eligibility criteria for chemoradiotherapy due to organ dysfunction, performance status, or co-morbidities the use of sequential chemotherapy and radiation can be considered. Radiotherapy techniques associated with lower exposure of critical normal tissues (heart, lungs, esophagus, and immune system) would be desirable in the elderly patients.38,39 The substitution of carboplatin for cisplatin is an option for patients who have contraindications to cisplatin
Future LS-SCLC trials should prospectively assess the vulnerability of elderly patients for adverse events, incorporate novel methods of monitoring adverse events through patient reported outcomes, and investigate novel radiation treatments, or systemic therapies.36, 40 A phase 3 trial of carboplatin and etoposide alone or with atezolizumab in extensive stage SCLC revealed an improvement in OS.41 Recently the United States Food and Drug Administration approved single agent nivolumab for patients extensive stage SCLC with disease progression after platinum-based therapy and at least one other therapy.42 Immunotherapy alone and in combination with chemotherapy has the potential to improve efficacy, and may have fewer severe adverse events than the chemotherapy combinations investigated in these trials. Future trials could prospectively investigate reduced dose chemotherapy with immunotherapy in elderly patients to improve the tolerability.
Supplementary Material
Acknowledgments
Funding support: This work was supported by NIH grant: R21-AG042894 (AG, HP, TS, EV, and XW) and 5P30CA014236–44 (WF and XW)
Footnotes
Conflict of interest:
The following authors do not report any conflicts of interest: Stinchcombe, Cohen, Pang, Le, Fan, Bogart, Vokes, Horn, Edelman, Komaki, Schild, Bogart, Thomas, and Wang
Dr. Ganti reports grants and personal fees from Pfizer, grants from New Link Genetics, personal fees from Ariad Pharmaceuticals, grants from Amgen, grants from AstraZeneca, grants from Merck, grants from Janssen, grants from Bristol-Myers Squibb, personal fees from Biodesix, outside the submitted work.
References
- 1.National Cancer Institute: SEER stat fact sheets: Lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html-accesse 3/19/2018.
- 2.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24: 4539–4544. [DOI] [PubMed] [Google Scholar]
- 3.Fruh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6: vi99–105. [DOI] [PubMed] [Google Scholar]
- 4.Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol 2017;18: 1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun JM, Ahn YC, Choi EK, et al. Phase III trial of concurrent thoracic radiotherapy with either first- or third-cycle chemotherapy for limited-disease small-cell lung cancer (correction). Annals of Oncology. 2014;25: 1672. [DOI] [PubMed] [Google Scholar]
- 6.Pang HH, Wang X, Stinchcombe TE, et al. Enrollment Trends and Disparity Among Patients With Lung Cancer in National Clinical Trials, 1990 to 2012. J Clin Oncol 2016;34: 3992–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khakwani A, Rich AL, Tata LJ, et al. Small-cell lung cancer in England: trends in survival and chemotherapy using the National Lung Cancer Audit. PLoS One. 2014;9: e89426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama JK, Hodgson L, Pang H, et al. A pooled analysis of limited-stage small-cell lung cancer patients treated with induction chemotherapy followed by concurrent platinum-based chemotherapy and 70 Gy daily radiotherapy: CALGB 30904. J Thorac Oncol 2013;8: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuen AR, Zou G, Turrisi AT, et al. Similar outcome of elderly patients in intergroup trial 0096: Cisplatin, etoposide, and thoracic radiotherapy administered once or twice daily in limited stage small cell lung carcinoma. Cancer. 2000;89: 1953–1960. [DOI] [PubMed] [Google Scholar]
- 10.Schild SE, Stella PJ, Brooks BJ, et al. Results of combined-modality therapy for limited-stage small cell lung carcinoma in the elderly. Cancer. 2005;103: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 11.Quon H, Shepherd FA, Payne DG, et al. The influence of age on the delivery, tolerance, and efficacy of thoracic irradiation in the combined modality treatment of limited stage small cell lung cancer. Int J Radiat Oncol Biol Phys. 1999;43: 39–45. [DOI] [PubMed] [Google Scholar]
- 12.Agresti A An introduction to categorical data analysis. Second edition ed. New York: John Wiley and Sons, 1996. [Google Scholar]
- 13.Kruskal WH, Wallis WA. Use of Ranks in One-Criterion Variance Analysis. Journal of the American Statistical Association. 1952;47: 583–621. [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53: 457–481. [Google Scholar]
- 15.Harrington D Linear Rank Tests in Survival Analysis. 2005. [Google Scholar]
- 16.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological). 1972;34: 187–220. [Google Scholar]
- 17.Hougaard P Frailty models for survival data. Lifetime Data Anal 1995;1: 255–273. [DOI] [PubMed] [Google Scholar]
- 18.McClay EF, Bogart J, Herndon JE 2nd, et al. A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B Study (9235). Am J Clin Oncol 2005;28: 81–90. [DOI] [PubMed] [Google Scholar]
- 19.Bogart JA, Herndon JE 2nd, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys 2004;59: 460–468. [DOI] [PubMed] [Google Scholar]
- 20.Miller AA, Wang XF, Bogart JA, et al. Phase II trial of paclitaxel-topotecan-etoposide followed by consolidation chemoradiotherapy for limited-stage small cell lung cancer: CALGB 30002. J Thorac Oncol 2007;2: 645–651. [DOI] [PubMed] [Google Scholar]
- 21.Kelley MJ, Bogart JA, Hodgson LD, et al. Phase II study of induction cisplatin and irinotecan followed by concurrent carboplatin, etoposide, and thoracic radiotherapy for limited-stage small-cell lung cancer, CALGB 30206. J Thorac Oncol 2013;8: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komaki R, Paulus R, Ettinger DS, et al. Phase II study of accelerated high-dose radiotherapy with concurrent chemotherapy for patients with limited small-cell lung cancer: Radiation Therapy Oncology Group protocol 0239. Int J Radiat Oncol Biol Phys 2012;83: e531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59: 943–951. [DOI] [PubMed] [Google Scholar]
- 24.Schild SE, Bonner JA, Hillman S, et al. Results of a phase II study of high-dose thoracic radiation therapy with concurrent cisplatin and etoposide in limited-stage small-cell lung cancer (NCCTG 95–20-53). J Clin Oncol 2007;25: 3124–3129. [DOI] [PubMed] [Google Scholar]
- 25.Thomas CR Jr., Giroux DJ, Stelzer KJ, et al. Concurrent cisplatin, prolonged oral etoposide, and vincristine plus chest and brain irradiation for limited small cell lung cancer: a phase II study of the Southwest Oncology Group (SWOG-9229). Int J Radiat Oncol Biol Phys 1998;40: 1039–1047. [DOI] [PubMed] [Google Scholar]
- 26.Le QT, Moon J, Redman M, et al. Phase II study of tirapazamine, cisplatin, and etoposide and concurrent thoracic radiotherapy for limited-stage small-cell lung cancer: SWOG 0222. J Clin Oncol 2009;27: 3014–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edelman MJ, Chansky K, Gaspar LE, et al. Phase II trial of cisplatin/etoposide and concurrent radiotherapy followed by paclitaxel/carboplatin consolidation for limited small-cell lung cancer: Southwest Oncology Group 9713. J Clin Oncol 2004;22: 127–132. [DOI] [PubMed] [Google Scholar]
- 28.Horn L, Bernardo P, Sandler A, et al. A phase II study of paclitaxel + etoposide + cisplatin + concurrent radiation therapy for previously untreated limited stage small cell lung cancer (E2596): a trial of the Eastern Cooperative Oncology Group. J Thorac Oncol 2009;4: 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Socinski MA, Zhang C, Herndon JE 2nd, et al. Combined modality trials of the Cancer and Leukemia Group B in stage III non-small-cell lung cancer: analysis of factors influencing survival and toxicity. Ann Oncol 2004;15: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 30.Ademuyiwa FO, Johnson CS, White AS, et al. Prognostic factors in stage III non-small-cell lung cancer. Clin Lung Cancer. 2007;8: 478–482. [DOI] [PubMed] [Google Scholar]
- 31.Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol 2012;13: 671–678. [DOI] [PubMed] [Google Scholar]
- 32.Bogart JA, Watson D, McClay EF, et al. Interruptions of once-daily thoracic radiotherapy do not correlate with outcomes in limited stage small cell lung cancer: analysis of CALGB phase III trial 9235. Lung Cancer. 2008;62: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerber DE, Laccetti AL, Xuan L, Halm EA, Pruitt SL. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol 2010;28: 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol 2015;33: 3826–3833. [DOI] [PubMed] [Google Scholar]
- 36.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 2016;34: 2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf-accessed5.1.2018.
- 38.Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117: 3004–3013. [DOI] [PubMed] [Google Scholar]
- 40.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318: 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. https://www.roche.com/media/releases/med-cor-2018-06-25.htm.
- 42. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm617370.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.