Abstract
Risk factors for hepatitis C virus (HCV) infection vary, and there were an estimated 1.75 million new cases worldwide in 2015. The World Health Organization aims for a 90% reduction in new HCV infections by 2030. An HCV vaccine would prevent transmission, regardless of risk factors, and significantly reduce the global burden of HCV-associated disease. Barriers to development include virus diversity, limited models for testing vaccines, and our incomplete understanding of protective immune responses. Although highly effective vaccines could prevent infection altogether, immune responses that increase the rate of HCV clearance and prevent chronic infection may be sufficient to reduce disease burden. Adjuvant envelope or core protein and virus-vectored nonstructural antigen vaccines have been tested in healthy volunteers who are not at risk for HCV infection; viral vectors encoding nonstructural proteins are the only vaccine strategy to be tested in at-risk individuals. Despite development challenges, a prophylactic vaccine is necessary for global control of HCV.
Keywords: HCV, Viral Hepatitis, Vaccines, Prophylactic Vaccination
Abbreviations used in this paper: Ad5, adenovirus serotype 5; bNAb, broadly neutralizing antibodies; CD81bs, CD81 receptor binding site; ChAd, chimpanzee adenovirus; DAA, direct-acting antiviral; HCV, hepatitis C virus; HCVcc, HCV derived from cell culture; HCVpp, HCV pseudoparticles; HVR1, hypervariable region 1; mAbs, monoclonal antibodies; MVA, modified vaccinia Ankara; NAbs, neutralizing antibodies; NS, nonstructural; PD-1, programmed cell death 1; PWID, people who inject drugs; VLP, virus-like particle

Justin R. Bailey

Eleanor Barnes

Andrea L. Cox
The advent of all oral, interferon-sparing direct-acting antivirals (DAAs) that cure hepatitis C virus (HCV) infection has transformed treatment, particularly in high-income countries. Although DAAs have fueled optimism for global control, several limitations of treatment make development of a preventive vaccine necessary to achieve that goal. HCV infections are rarely symptomatic before the onset of advanced liver disease, and HCV screening is rare in most parts of the world, so most persons with HCV infection are not identified.1 In addition, the cost of and practical aspects to delivering therapy result in only a subset of those diagnosed being treated. HCV treatment has been decreasing globally since its peak in 2015, as the HCV-infected people easiest to access have been treated, leaving those more difficult to access without treatment (John McHutchinson and Diana Brainard, Gilead Sciences, personal communication; 2018).
Some treated individuals have developed resistance to DAAs, and transmission of resistant HCV variants was documented in clinical trials before DAAs were even approved.2 With expansion of treatment to patients less able to take medication reliably, antiviral resistance is likely to become more common. Furthermore, liver disease can progress and cancer can develop despite cure of the HCV infection in patients with cirrhosis. So, treatment does not eliminate all of the consequences of HCV infection and prevention of chronic infection offers significant advantages over treatment.
Despite increased cure rates with DAA, HCV elimination continues to be difficult due to reinfection. Immunity after effective treatment has been shown to be insufficient to prevent reinfection with HCV in individuals with ongoing risk of infection, including people who inject drugs (PWID), men having sex with men, and health care workers with frequent exposure to blood and bodily fluids.3, 4, 5, 6 Rates of reinfection in these populations vary, but are high when those most at risk of transmitting infection are treated, in part as a means to interrupt transmission. A recent study in PWID treated while actively injecting showed 6-month and 18-month reinfection rates of 12.6 and 17.1 per 100 person-years, respectively.7 PWID, men who have sex with men, health care workers, infants born to HCV-infected mothers, and those living in the many countries with high HCV incidence would be expected to benefit from a preventive HCV vaccine. The effects of prophylactic vaccines with varying levels of efficacy and delivery strategies have been modeled.8, 9, 10 Based on these models, high vaccination rates of high-risk seronegative PWID, even with a vaccine with only 30% efficacy, would have significant effects on transmission.
Global control will require annual rates of cure that are consistently and significantly higher than new HCV infection rates. Few countries are on target to eliminate HCV as a public health problem by 2030, the goal set by the World Health Organization in 2016, and nearly 60% of surveyed countries had more infections than cures in 2016.11, 12 Consequently, control is unlikely to occur without improved focus on and success in reducing the number of new HCV infections in addition to cure. An effective preventive vaccine would have a significant effects on HCV incidence and would provide a major advance toward global HCV control. However, there are barriers to development, including limitations to HCV culture systems, virus diversity, limited models, and at-risk populations for testing vaccines, and incomplete understanding of protective immune responses.
Feasibility of Traditional Approaches for HCV Vaccine Design
Generation of live-attenuated and inactivated whole virus vaccines has been effective against other viruses, but neither strategy is feasible for generating HCV vaccines. The inability to culture HCV (until recently) and ongoing limitations of HCV culture systems have posed challenges to production of a live-attenuated or inactivated whole HCV vaccine.13 Culture strains of HCV have adaptive mutations that increase replication efficiency in vitro with unknown effects on replication in humans. Live-attenuated vaccines against other viruses have been generated in 2 primary ways: by passage of virus in nonhuman primate cell lines in which natural variants can arise; these have reduced replication in human cells, and by genetic deletion or inactivation of virulence factors. However, HCV does not replicate at high levels in nonhuman primate cell lines and virulence factors for HCV have not been defined. Practical production aspects and the risk of causing HCV infection with live-attenuated vaccines limit their utility.
HCV Genetic Diversity
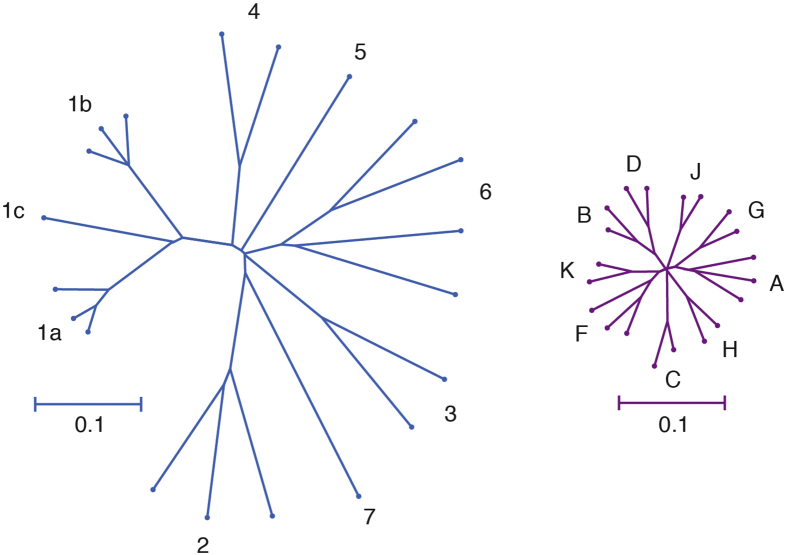
A principal challenge for HCV vaccine development is the extraordinary genetic diversity of the virus. With 7 known genotypes and more than 80 subtypes, the genetic diversity of HCV exceeds that of human immunodeficiency virus-1 (Figure 1). HCV strains from different genotypes differ, on average, at approximately 30% of their amino acids, whereas different subtypes within each genotype differ at an average of approximately 15% of their amino acids.14, 15, 16 In addition to diversity among genotypes and subtypes, immune selection and the error-prone polymerase of the virus generate a diverse quasispecies of related but genetically distinct viral variants within each infected individual, presenting many opportunities for selection of viral variants with resistance to T-cell and antibody responses.17, 18, 19, 20, 21, 22, 23 Several recent studies have demonstrated that antibody resistance can arise from mutations either within or distant from antibody binding epitopes, providing the virus with additional mechanisms of immune escape.24, 25, 26 Given this viral diversity within and between infected individuals, vaccine induction of very broadly reactive immune responses or the generation of immune responses that target genetically conserved regions of the viral genome may be required for protection against HCV infection or persistence.
Figure 1.
Genetic diversities of HCV and human immunodeficiency virus 1 (HIV-1). Phylogenetic trees of reference full-genome HCV (left) and HIV-1 (right) nucleotide sequences downloaded from the Los Alamos National Laboratory sequence database. Trees were inferred using the Neighbor-Joining method, with branch lengths drawn to scale. Genotypes or subtypes are labeled. Trees are on the same scale. The HCV tree is based on 8940 positions, and the HIV-1 tree is based on 8193 positions. Distances were computed using the Tamura-Nei method. Analyses were performed in MEGA7.151
Challenges to Testing HCV Vaccines
Another barrier to HCV vaccine development is the lack of in vitro systems and immunocompetent small animal models that facilitate determining whether vaccination induces protective immunity.13 The discovery of an HCV-like virus, the rat Hepacivirus, will generate a new small animal model for vaccine testing. The limitation of this model is that although this virus is structurally analogous to HCV, it has limited sequence homology with HCV.27, 28 Although the success of DAA therapy has resulted in discussions of HCV challenge studies, in which vaccinated subjects would be intentionally infected with HCV, it is not clear how those infections would be achieved. Primary HCV isolates have limited ability to replicate in tissue culture, restricting their production. In addition, primary HCV isolates are not expressed in good manufacturing process-compliant cell lines and do not represent the viral diversity of the quasispecies circulating in natural infection. Direct infusion of HCV-infected human plasma might be considered, but would require careful screening for other pathogens and, even with thoughtful selection of inoculum levels and HCV genotypes, might fail to completely recapitulate natural exposure.
An effective vaccine is therefore difficult to validate unless it is tested in populations with a predictably high risk for HCV infection. Although HCV transmission occurs through blood transfusion and invasive medical procedures, identifying people who could be at risk of transmission via those routes and vaccinating them before exposure is not feasible. PWID are at predictably high risk with the incidence of HCV infection (ranging from 5% to 25% per year), so they could be a suitable test population for HCV vaccines, and there is a continued need for prevention of HCV infection in this population.29 However, few studies have successfully managed the identification, enrollment, and prospective monitoring of PWID before onset of acute HCV infection.30, 31 These cohorts will likely remain of critical importance to vaccine testing and help us increase our understanding of the host responses required for protective immunity. Knowing what immune responses indicate protective immunity would permit testing of candidate vaccines in healthy adults not at risk for infection first. With correlates of protection known, only those vaccines that elicit effective immune responses would advance to testing in at-risk populations for verification of protection, permitting judicious use of these PWID cohorts. Although our incomplete understanding of protective immunity against HCV is a barrier to vaccine development, studies have provided substantial evidence that protective immunity does exist.
Evidence for Protective Immunity
Spontaneous clearance of HCV infection occurs in approximately 25% of acutely infected individuals.32 Chimpanzees and humans who spontaneously control an initial HCV infection can develop recurrent HCV viremia following additional HCV exposure, so spontaneous clearance of primary HCV infection does not generate sterilizing immunity.33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 The discovery that spontaneous immunologic control of HCV does not always result in protective immunity initially decreased confidence that prophylactic vaccination was possible. However, clearance of multiple infections with homologous and heterologous virus has been observed in chimpanzees and humans.35, 40, 41, 42, 44, 45
Furthermore, reinfections are cleared more often than primary infections. Reinfected PWID control secondary HCV infections approximately 80% of the time, essentially the reverse of rates of clearance vs persistence observed in primary infection.40 Although genetic traits and other unmodifiable traits affect overall clearance rates, they would not alter the pace of clearance in reinfection. In contrast, adaptive immune responses would be expected to result in more rapid control of reinfection than occurs in the initial infection. Supporting the hypothesis that adaptive immunity induced in control of a primary infection leads to more effective clearance of subsequent infections, Lanford et al46 reported decreased duration and magnitude of viremia in chimpanzees in reinfection vs primary infection, regardless of whether the chimpanzees were infected with homologous or heterologous viruses.
HCV reinfection in humans is also characterized by a reduced peak and duration of viremia compared with initial infection of the same person.40, 47 Reinfection was associated with broadened cellular immune responses compared with primary infection and the presence of broadly cross-reactive neutralizing antibodies (NAbs).40
The more rapid and effective control of viral replication with subsequent exposures, compared with the initial exposure, indicates that adaptive immunity is induced and, although not sterilizing, protects against chronic infection. Additional research on effective immune control of HCV is needed to develop a vaccine, including studies of people with repeated spontaneous control of diverse HCV infections over time. Decades of research have revealed that HCV-specific CD4+ helper T cells, CD8+ cytotoxic T cells, and antibodies all play a role in protection against persistent HCV infection. Vaccine strategies to induce all 3 adaptive immune responses are in development.
T-Cell–Mediated Protection
Data from human and chimpanzee studies have indicated that HCV-specific CD4+ and CD8+ T cells are crucial in control of primary and secondary HCV infections. There is indirect evidence for T-cell control from genetic studies, which demonstrated an association between HCV clearance and specific class-I and class-II HLAs, which present HCV peptides to CD8+ and CD4+ T cells, respectively.48, 49 A vigorous and multispecific proliferative CD4+ T-cell response against HCV proteins has been correlated with spontaneous control of acute HCV infection.50, 51, 52, 53 Schulze zur Wiesch et al50 demonstrated that broadly directed HCV-specific CD4+ T cells are detectable during early stages of infection, regardless of outcome. However, CD4+ T cells have functional defects during early stages of chronic HCV infection, and rapidly become undetectable with progression, whereas CD4+ T cells are more often maintained with virus clearance. The ineffective CD4+ T cells are thought to impair the response of CD8+ T cells, and are associated with persistent infection, T-cell exhaustion, and selection of HCV variants with escape mutations in class I epitopes.53
Generation of an effective memory response is required for successful vaccination. Reinfection studies provide evidence for the important role of T-cell memory in HCV control. Antibody-mediated depletion of CD4+ T cells before reinfection of 2 immune chimpanzees resulted in persistence of HCV despite functional intrahepatic memory CD8+ T-cell responses.54 Antibody-mediated depletion of CD8+ T cells immediately before the third infection of 2 chimpanzees that had previously cleared infection resulted in prolonged viremia, which was controlled only when CD8+ T cells were again detectable in the liver.38 Similarly, protection against viral persistence in recurrent HCV infection in PWID was associated with broadening of the T-cell response and the expansion of effector memory T cells at the peak of the T-cell response.40, 55 In addition, early-stage high-level expression of a molecule associated with exhaustion, programmed cell death 1 (PD-1) on HCV-specific CD8+ T cells was associated with persistence of reinfection, consistent with findings from studies that associated high expression of PD-1 on T cells during early stages of primary infection with progression to chronic infection.55, 56, 57
Dominant epitope regions of HCV strains isolated from patients with persistent reinfection had sequence variations that were not recognized by preexisting memory T cells. This observation is consistent with escape observed in association with viral persistence following primary infection. Together, these studies demonstrate that memory CD4+ and CD8+ T cells are required for protective immunity on re-exposure to HCV.
Trials of Vaccines Designed to Elicit T-Cell Responses
It is a challenge to translate what we have learned about protective T-cell responses to design of vaccines that elicit these responses. The most effective vaccines might induce sterilizing immunity, but most HCV-associated diseases are caused by chronic infection. Immune responses that prevent persistence of infection could decrease HCV morbidity and mortality without reducing incidence of infection. The feasibility of testing vaccines that reduce rates of chronic HCV infection, rather than reducing incidence of infection, has been studied. Reducing viremia during the early acute phase of infection with a vaccine could reduce HCV transmission by lowering the residual infectious virus titers in injecting equipment.58, 59 So, it might be reasonable to lower the bar for vaccine development, setting goals of decreased persistence or population HCV RNA levels to effect reductions in disease sequelae and transmission.
Another approach is to target the relatively conserved viral proteins within the nonstructural region of the genome to induce a broad T-cell response, with and without including envelope glycoprotein as a target. The nonstructural (NS) proteins (NS3, NS4, and NS5) are more conserved across HCV genotypes than the envelope glycoproteins and are the dominant targets of CD8+ T cells.60 A variety of strategies have been developed to introduce NS protein antigens in an immunogenic way, including DNA-based immunization, DNA priming followed by recombinant virus vector or HCV protein boosting, recombinant adenovirus priming and DNA boosting, combinations of replicating and nonreplicating recombinant viruses for prime and boost, virus-like particles (VLPs), hepatitis B virus surface antigen–HCV recombinants, and pooled synthetic class I peptide epitopes or peptides incorporated in lysosomes.29, 61, 62, 63 An HCV vaccine was recently generated that specifically targets conserved regions of the HCV genome and uses simian adenoviral vectors to generate T cells that target multiple HCV genotypes.64 Most candidate vaccines have elicited humoral and cell-mediated immune responses in rodents, and a small subset of candidate vaccines elicit robust CD8+ T-cell–mediated immunity in macaques.65, 66, 67, 68, 69, 70, 71
Fewer candidate vaccines have been assessed for immunogenicity and ability to protect chimpanzees from HCV. These vaccines include VLP comprising the HCV E1, E2, and core proteins; recombinant nonstructural proteins, formulated with the ISCOMATRIX adjuvant; and genetic vaccines encoding nonstructural proteins.61, 62, 72, 73, 74 The effects of these vaccines in chimpanzees varied among the studies, which included fewer than 6 vaccinated animals per study.61, 62, 72, 74, 75 The vaccines encoding envelope glycoproteins induced antibody as well as T-cell responses, but none provided sterilizing immunity.61, 72 Although not uniformly providing sterilizing immunity, these vaccines reduced primary viremia after challenge with HCV by as much as several orders of magnitude.61, 62, 72, 73, 74, 75 A meta-analysis of all the data from chimpanzee vaccine trials showed that suppression of acute-phase virus replication was associated with recall of vaccine-primed T cells, but the levels of induced T-cell responses did not correlate with vaccine success.75 The analysis also noted that vaccines that contained only structural proteins had clearance rates that were significantly higher than vaccines that contained nonstructural components. However, the vectors and antigens used, the timing of vaccination prime and boost, and the timing and identity of challenge HCV viruses varied among the studies, limiting our ability to pool data, compare results from these studies, or conclude that inclusion of envelope proteins is required for a successful vaccine.
Most chimpanzee vaccine studies have shown reduced HCV persistence rates in vaccinated animals vs controls.75 However, vaccines designed to induce T-cell responses have not uniformly increased HCV control; data indicate that not all vaccine-induced immune responses are beneficial. Vaccination of chimpanzees with recombinant NS3, NS4, and NS5 proteins formulated with the ISCOMATRIX adjuvant resulted in persistent HCV infection on rechallenge in all 5 vaccinated animals, despite the appearance of HCV-specific CD4+ and CD8+ T cells in liver before challenge and infection.76 When naïve chimpanzees were immunized with DNA plasmids expressing the core-E1-E2 and NS3 for priming, and with recombinant modified vaccinia (MVA)-expressing core-E1-E2 and NS3 gene sequences as a boost, they developed HCV-specific antibody and T-cell responses.77 Despite these immune responses, vaccinated animals had a higher rate of virus persistence than control animals.
The observation that vaccines have failed even when they induced T-cell responses indicates that trials should proceed with caution and should include strategies to identify the features of immune responses associated with control. In general, the small number of chimpanzees tested, the diversity of the vaccines tested, and the different methods used to assess induced immune responses make it exceedingly difficult to determine what aspects of the elicited immune responses provided protection. It is also unclear if the results from nonhuman primate studies can be translated to humans. More detailed phenotypic and functional analyses, performed on existing and future trial specimens, are required to identify factors that determine whether a vaccine will reduce the rate of persistent infection in humans.
Two vaccines designed to prevent infection solely by eliciting T-cell–mediated immunity have been tested in phase I (safety and immunogenicity) trials in human volunteers not at risk for HCV infection. A prototype vaccine with the HCV core protein and ISCOMATRIX adjuvant was assessed for its ability to induce T-cell responses in healthy individuals not at risk for HCV infection.78 Although the vaccine was generally well tolerated, CD8+ T-cell responses were detected in only 2 of the 8 participants receiving the highest dose. The second vaccine tested in healthy volunteers and designed to elicit T-cell responses is the foundation of the only candidate vaccine to have advanced to a trial in at-risk subjects. This vaccine is composed of a replication-defective chimpanzee adenovirus (ChAd) vector encoding NS3, NS4, and NS5 proteins.79
Replication-defective adenovirus vectors have been used to introduce antigens from other pathogens and induce a potent immune response. Human adenovirus serotype 5 (Ad5) induces protective immune responses against diverse pathogens and cancer in animal models and elicits robust and sustained cellular immunity in humans. However, most humans have neutralizing antibodies to Ad5, which can reduce immune responses to the antigens carried in Ad5-based vaccines. Replication-defective adenoviral vectors based on serotype 6 (subgroup C) and 24 (subgroup D) have low seroprevalence in humans, reducing immunologic cross-reactivity.66 A segment of DNA coding for NS3–5 of the HCV genotype 1b was delivered by Ad6 followed by Ad24 and finally by electroporated plasmid DNA in chimpanzees.73 Following HCV rechallenge with HCV genotype 1a virus, all vaccinated chimpanzees had a significantly reduced peak of viremia, with the average peak more than 100-fold lower in the HCV vaccination group than in the control group.
These viral kinetics are similar to those observed in PWID who successfully control HCV with repeated exposure.40 All 5 animals that received the vaccine had minimal increases in levels of alanine amino transferase compared with mock-vaccinated control chimpanzees challenged with HCV. Four of 5 vaccinated chimpanzees cleared the virus with a significantly shorter duration of viremia vs the control group, whereas 1 vaccinated chimpanzee maintained low levels of HCV RNA for the duration of the study. A follow-up study revealed that after challenge, vaccinated chimpanzees had early expansion of CD8+ T cells with higher expression of the memory precursor molecule CD127, lower levels of the inhibitory molecule PD1, and increased effector functions compared with primary T cells from the mock-vaccinated controls that developed persistent infections.80 Early expansion of CD127+ HCV-specific T cells with high functionality was previously demonstrated in chimpanzees that spontaneously controlled acute HCV infection.81
Based on these promising results, replication-defective vectors generated from a subset of novel ChAd serotypes were screened to determine whether they were neutralized by human sera and if they were able to grow in human cell lines.66 Of these, ChAd3 expressing the NS region from HCV was found to induce long-lasting T- and B-cell memory responses in mice and macaques. Adenoviral vectors expressing NS proteins from HCV genotype 1b were constructed based on the rare serotypes Ad6 and ChAd3. The Ad6-NS and ChAd3-NS vaccines were tested in an adenovirus prime heterologous adenovirus boost regimens in a safety and immunogenicity phase 1 clinical trial in healthy volunteers not at risk for HCV infection.82 ChAd3-NS was well tolerated and highly immunogenic, with intracellular cytokine staining demonstrating that ChAd3-NSmut primed a large number of polyfunctional CD8+ T cells. Antigen-specific polyfunctional CD4+ T cells were detected at a lower frequency. Memory CD8+ T cells that expressed CD127, but not PD1, were sustained in circulation. Although more robust recognition of HCV genotype 1b peptides (matching the vaccine) was observed, there was recognition of genotype 1a and 3a peptide pools that might provide cross-genotypic protection. Responses were sustained for at least 1 year after boosting with the heterologous adenoviral vector, demonstrated by the persistence of central and effector memory T-cell pools that retained polyfunctionality and proliferative capacity. This response was similar to the sustained T-cell responses (in magnitude and quantity) associated with protective immunity in vaccine trials of other pathogens. However, boosting was not as robust with heterologous adenovirus as it was later found to be with MVA, so the vaccine was advanced to HCV at-risk subjects with ChAd3 prime and MVA boost, both expressing NS3–5.79
The ChAd3-NS prime and MVA-NS boost strategy is being evaluated in a staged phase 1/2 study, under way in Baltimore, San Francisco, and New Mexico (see ClinicalTrials.gov NCT01436357). Vaccine responses were primed with the ChAd3-NS at a dose previously found to be well tolerated and immunogenic in healthy volunteers, followed by boosting 8 weeks later with MVA-NS. The primary endpoint of this study is to prevent HCV persistence in HCV-naïve populations of PWID at high risk for infection. Phase 1 of the trial was completed with safety signal and immunogenicity parameters supporting advancement, and enrollment in phase 2 of the trial began in 2013. Results are expected in 2019. If promising, confirmation that the vaccine reduces the rate of chronic HCV infection in PWID would likely require larger trials; however, this trial will at minimum demonstrate the feasibility of conducting HCV vaccine trials in PWID.
Neutralizing Antibody-mediated Protection
Many licensed prophylactic vaccines against other viral infections induce neutralizing or binding antibody titers that correlate with protection. Although HCV envelope genes are extremely diverse, there is evidence from infected humans and animal models that neutralizing antibodies can be protective. Clearance of HCV infection is associated with the early development of serum antibodies capable of blocking infection by multiple heterologous HCV strains. Antibodies with these characteristics are called broadly neutralizing antibodies (bNAbs).83, 84, 85 The appearance of NAbs later in infection also has been associated with spontaneous clearance of HCV infection.86 Individuals who clear their first infection clear subsequent infections more than 80% of the time. Along with expanded T-cell responses, clearance of reinfection was associated with rapid induction of antibodies capable of neutralizing heterologous HCV strains.40 bNAbs have been isolated from the B cells of individuals who cleared HCV infection without treatment.87, 88 Some of these bNAbs have low levels of somatic hypermutation, similar to levels commonly stimulated by vaccination against other viruses, so vaccine induction of bNAbs against HCV might be feasible.89, 90
There is also evidence of antibody protection against HCV challenge in animal models of infection. Infusion of immunoglobulin isolated from the serum of a chronically infected human before challenge with homologous virus from the same donor prevented infection of most human liver chimeric mice. Similarly, infusion of chronic-phase human immunoglobulin before challenge of a chimpanzee prevented infection with homologous, but not heterologous HCV strains.91, 92, 93 In contrast, it was demonstrated that infusion of bNAbs before challenge with heterologous virus could partially or fully prevent infection in humanized mice94, 95, 96 and chimpanzees,97 and combinations of bNAbs also abrogated established HCV infection in a human liver chimeric mice.98 Together, these studies demonstrate that typical chronic-phase human sera have protective titers of strain-specific NAbs, but bNAbs may be necessary to prevent infection by diverse, heterologous HCV strains.
Anti-HCV bNAb Epitopes and Implications for Vaccine Design
The targets of the NAb response against HCV are the viral envelope glycoproteins, E1 and E2. E1 and E2 are membrane-anchored proteins that are believed to form a heterodimer on the surface of viral particles. Although the function of E1 is unclear, E2 interacts with multiple cell-surface receptors, including but not limited to CD81 and scavenger receptor class B member 1, which mediates viral entry.99 Hypervariable region 1 (HVR1), a 27-amino acid region at the N-terminus of E2, is an immunodominant epitope that evolves rapidly under antibody pressure.100, 101 Although most HCV-infected individuals develop strain-specific NAbs against HVR1, viral mutations generally confer resistance to these antibodies.18, 102, 103, 104, 105, 106
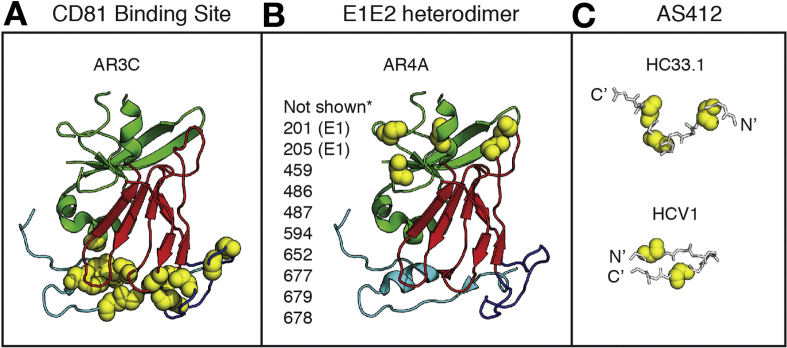
Unlike strain-specific NAbs that target HVR1, bNAbs target relatively conserved epitopes on E2 or on the E1E2 heterodimer.87, 94, 96, 107, 108, 109, 110, 111, 112, 113, 114 Characterization of these bNAbs has helped to identify epitopes that could be incorporated into a vaccine. These studies have also identified challenges that may limit induction of bNAbs, including complex conformational bNAb epitopes and structural flexibility of envelope proteins. Pierce et al115 and Gopal et al116 performed comprehensive alanine scanning mutagenesis across the entire E2 or E1E2 genes followed by binding assays with many bNAbs. These studies identified the binding epitopes of many bNAbs and non-neutralizing antibodies (reviewed in Kinchen et al117 and Fuerst et al118), demonstrating that antibodies that interact with the region of E2 commonly referred to as the back layer are generally weakly neutralizing or non-neutralizing. In contrast, most bNAbs do not interact with the E2 back layer, but instead bind at the CD81 receptor binding site of E2 (CD81bs) or to the E1E2 heterodimer (Figure 2).
Figure 2.
bNAb epitopes are conformationally complex and flexible. (A, B) The crystallized structure of a truncated HCV E2 protein, strain H77, PDB 4MWF, from Kong et al,119 with E2 domains and bNAb binding residues highlighted in Pymol, v1.8.6.2. The E2 front layer is cyan, central beta sheet is red, and the back layer is green. Binding residues of the CD81 binding site-targeting bNAb AR3C (A) or the E1E2 complex-targeting bNAb AR4A (B), identified by alanine scanning mutagenesis and binding assays, are marked with yellow spheres.116 Amino acid side chains are not shown. *Putative AR4A binding residues not present in the E2 crystal structure are listed next to the structure. Binding residues shown or listed are positions at which mutation to alanine had the greatest effect on AR3C or AR4A binding, but minimal effect on control mAbs. Numbering is relative to the H77 polyprotein sequence. (C) Structures of peptide epitopes crystallized in complex with AS412-targeting bNAbs HC33.1 or HCV1. Fab structures are not shown. HC33.1 binds the peptide in an extended conformation (PDB 4XVJ),127 whereas HCV1 binds the peptide in a hairpin conformation (PDB 4DGY).113 Critical binding residues for each bNAb determined by alanine scanning mutagenesis and binding assays are marked with yellow spheres.110, 120 Amino acid side chains are not shown.
The x-ray crystal structure of a truncated strain H77 E2 core, complexed with a CD81bs bNAb designated AR3C, provided the first view of a bNAb in complex with a near full-length, correctly folded envelope protein.119 This structure, along with the previously described binding studies, demonstrated that the AR3C conformational epitope is structurally complex, with extensive AR3C binding residues in multiple E2 domains, including the front layer and the CD81 binding loop (Figure 2A). BNAbs targeting the E1E2 heterodimer, such as the bNAb designated AR4A, recognize epitopes that are still ill-defined, but they also appear to bind to residues spanning multiple envelope protein domains (Figure 2B).96, 98 A third group of bNAbs targets continuous epitopes spanning amino acids 412 to 423, a region commonly referred to as AS412, near the CD81bs of E2 (Figure 2C).110, 120, 121, 122, 123 Crystal structures of peptides spanning amino acids 412 to 423 in complex with monoclonal antibodies (mAbs) HCV-1, HC33.1, AP33, and 3/11 demonstrate that mAbs can bind to this region with different angles of approach.113, 124, 125, 126, 127 In addition, the region assumes an extended conformation in some complexes and a beta hairpin conformation in others, indicating that it is structurally flexible. It is not clear whether any of these epitope conformations predominate in vivo. Overall, these characterizations of bNAbs help to define epitopes that could be incorporated into a vaccine, but they also identify the challenge of discontinuous, conformational epitopes that might be difficult to reproduce for a vaccine, and structural flexibility of some epitopes that could limit bNAb induction.
Trials of Vaccines Designed to Induce a Humoral Response
Strategies developed to induce antibodies against HCV have included protein-based, DNA-based, VLP-based, pox virus–based, and whole virus–based vaccines. A vaccine composed of recombinant full-length E1E2 protein from a single genotype 1a HCV strain with oil-in-water adjuvant has been tested in rodents, nonhuman primates, and humans. This vaccine induced fairly strong heterologous neutralizing activity in guinea pigs,128 and protected chimpanzees against homologous HCV challenge.129 Although the vaccine did not provide sterilizing immunity, it also reduced rates of persistence in chimpanzees after challenge with a neutralization sensitive heterologous virus.76 However, post-vaccination bNAb titers were detectable in only 3 of 16 vaccinees in a phase 1a human trial despite induction of strong CD4+ T-cell proliferation in response to recombinant E1E2. T vaccine antigen or adjuvant might therefore require optimization.130, 131
Studies of other vaccines in chimpanzees, such as DNA vaccines designed to express envelope proteins, DNA priming followed by MVA boost, or expression of envelope proteins on VLPs, have induced relatively disappointing humoral responses (reviewed in Liang132 and Bukh15). Two chimpanzees given a DNA vaccine designed to express cell-surface E2 protein developed low levels of E2-specific antibodies, but it is not clear that the low titers of induced antibodies had a significant role in clearance.133 The DNA priming followed by MVA boost was designed to express core, E1, E2, and NS3 proteins, but failed to induce detectable NAb titers.77 The VLPs (HCV core, E1, and E2 proteins) did not induce an antibody response, or produced barely detectable antibodies against core and E1E2, although the chimpanzees developed robust T-cell responses against these proteins.62 In contrast, this same vaccine induced production of envelope-specific antibodies in baboons, although neutralizing titers were not assessed.67 There are several reasons that chimpanzees could have weak NAb responses; for example, HCV-infected chimpanzees generally mount less vigorous anti-envelope antibody responses than infected humans.134, 135, 136
As the efficiency of production of chimeric replication competent cell culture viruses (HCVcc) has improved, use of whole inactivated virus has also become a more viable vaccine strategy, although there are limitations to this strategy. Vaccination of mice with inactivated J6/JFH-1 HCVcc stimulated NAbs against homologous virus and 2 heterologous HCV strains, and infusion of purified immunoglobulin from vaccinated mice prevented homologous virus infection of mice with humanized livers.137 Other groups have developed multivalent VLP or purified recombinant E1E2 protein vaccines that express envelope genes from multiple genotypes. In early studies, both vaccines induced cross-reactive antibodies in mice, and the multivalent E1E2 protein vaccine induced NAbs against 1 homologous and 1 heterologous HCV strain.63, 138 Further development of vaccines expressing full-length, native E1E2 is supported by a recent study demonstrating that combinations of human NAbs against multiple epitopes can display complementary neutralizing breadth and in some cases neutralizing synergy.139
Truncated protein antigens and rationally designed peptide antigens are also at early stages of development. Studies showing that HVR1 may occlude conserved bNAb epitopes140 led Vietheer et al141 to develop an E2 vaccine antigen with deletion of 3 variable regions. This vaccine induced moderately high titers of bNAbs in guinea pigs.141 However, Law et al142 found that full-length E1E2 or a variant with truncated HVR1 induced equivalent titers of NAbs against heterologous HCV strains in vaccinated guinea pigs, so truncation of HVR1 may not be necessary. Recently, Pierce et al143 developed a molecular scaffold to present the AS412 epitope. Antibody responses in vaccinated mice were modest, so further optimization may be needed to better match the structure of this bNAb epitope. Overall, more work is needed to identify ideal vaccine antigens, as well as optimal vaccine adjuvants. It will be important to perform many trials, evaluating as many vaccine approaches as possible; it is unclear whether anti-HCV antibody responses observed in rodents or nonhuman primates will also occur in humans.
HCV Panels Used to Quantify Neutralizing Breadth
Accurate measurement of neutralizing breadth of sera is critical to assess antibody responses induced by candidate vaccines. An ongoing challenge that significantly limits comparison of results among preclinical vaccine studies is the use of relatively few HCV strains to assess neutralizing breadth of antibodies, as well as the use of different HCV strains and different neutralization assays in each laboratory.96, 107, 144, 145, 146, 147 Studies have demonstrated that neutralization sensitivity varies widely among HCV strains, within and across HCV genotypes. In a study by Carlsen et al25 50% inhibitory concentrations of the same mAb among different HCVcc strains varied more than 1000-fold, within and among HCV genotypes. Urbanowicz et al148 measured neutralization of more than 70 genotype 1-6 HCV pseudoparticles (HCVpp), which are retroviral particles with HCV envelope proteins on their surface, by 5 mAbs. They also observed wide variation in neutralization sensitivity, within and among subtypes. These findings indicate that the neutralization sensitivity of individual strains from any genotype are not representative of the genotype as a whole. Therefore, it is critical that neutralization panels include as many diverse HCV strains as possible. Moreover, accurate comparison of candidate vaccines would be greatly facilitated by testing of all sera against a more standardized panel of heterologous HCV strains.
Future Directions
There are significant deficits in our tools for development of an HCV vaccine. Elucidating the mechanisms through which antigen-specific immune cell populations mediate long-term protection is an important goal. Vaccine strategies meant to overcome the enormous diversity of HCV must generate a broad immune response, capable of responding to abundant variations. Selecting antigens to maximize the induction of T-cell and antibody responses that elicit successful responses remains an active area of research. At least 4 types of HCV antigens have been proposed to maximize induction of robust and cross-protective responses: specific previously defined T-cell epitopes for inclusion, a single circulating HCV variant quadrivalent genotype 1a/1b/2a/3a VLP, and antigens generated computationally that minimize the degree of sequence dissimilarity between a vaccine strain and contemporary circulating viruses.
One study demonstrated the potential of computer-generated sequences to elicit cross-reactive T-cell responses, compared with previously defined T-cell epitopes or circulating HCV variants. These data support the use of a synthetically generated sequence as vaccine antigen to elicit robust CD8+ T-cell responses.149 However, computational strategies to aid in antigen design have not been used in HCV vaccines. Methods for introducing antigens are also being explored, with novel vectors in development for use against HCV and other viral targets. Studies in small animal models and macaques have shown that the addition of class-II invariant chain to an immunogen encoded in adenoviral and MVA viral vectors may significantly increase specific responses of CD4+ and CD8+ T cells to HCV. Phase 1 studies are under way to assess this strategy in healthy volunteers, with results expected in 2019. EudraCT Number: 2016-000983-41 (Capone et al150).
Going forward, successful control of HCV infection will most likely require a combination of large-scale screening to identify infected individuals, treatment of infected persons, and prevention and harm-reduction strategies for those who are uninfected and at risk. Although pharmaceutical companies have invested more in in drug development, vaccine development requires investment from sources beyond government and charitable foundations. A prophylactic HCV vaccine is an important part of a successful strategy for global control. Although development is not easy, the quest is a worthy challenge.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Justin R. Bailey is funded by National Institutes of Health National Institute of Allergy and Infectious Diseases (NIAID) grants R01AI127469 and U19AI088791. Eleanor Barnes is funded by the Medical Research Council UK as a Senior Clinical Fellow, and is an National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article are those of the author and not necessarily those of the National Health Service, the NIHR, or the Department of Health. Andrea L. Cox is funded by NIAIDU19AI088791.
References
- 1.Gravitz L. Introduction: a smouldering public-health crisis. Nature. 2011;474:S2–S4. doi: 10.1038/474S2a. [DOI] [PubMed] [Google Scholar]
- 2.Franco S., Tural C., Nevot M. Detection of a sexually transmitted hepatitis C virus protease inhibitor-resistance variant in a human immunodeficiency virus-infected homosexual man. Gastroenterology. 2014;147:599–601.e1. doi: 10.1053/j.gastro.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Midgard H., Bjoro B., Maeland A. Hepatitis C reinfection after sustained virological response. J Hepatol. 2016;64:1020–1026. doi: 10.1016/j.jhep.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Martin T.C., Martin N.K., Hickman M. Hepatitis C virus reinfection incidence and treatment outcome among HIV-positive MSM. AIDS. 2013;27:2551–2557. doi: 10.1097/QAD.0b013e32836381cc. [DOI] [PubMed] [Google Scholar]
- 5.Pineda J.A., Nunez-Torres R., Tellez F. Hepatitis C virus reinfection after sustained virological response in HIV-infected patients with chronic hepatitis C. J Infect. 2015;71:571–577. doi: 10.1016/j.jinf.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Martinello M., Grebely J., Petoumenos K. HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat. 2017;24:359–370. doi: 10.1111/jvh.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulkind J., Ahmad F., Stephens B. EASL International Liver Conference, Paris France. 2018. Abstract THU-063 Eradicate hepatitis C: a pilot of treatment as prevention in active drug users. J Hepatol. 2018;68(Suppl 1) [Google Scholar]
- 8.Hahn J.A., Wylie D., Dill J. Potential impact of vaccination on the hepatitis C virus epidemic in injection drug users. Epidemics. 2009;1:47–57. doi: 10.1016/j.epidem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott N., McBryde E., Vickerman P. The role of a hepatitis C virus vaccine: modelling the benefits alongside direct-acting antiviral treatments. BMC Med. 2015;13:198. doi: 10.1186/s12916-015-0440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone J., Martin N.K., Hickman M. The potential impact of a hepatitis C vaccine for people who inject drugs: is a vaccine needed in the age of direct-acting antivirals? PLoS One. 2016;11:e0156213. doi: 10.1371/journal.pone.0156213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill A.M., Nath S., Simmons B. The road to elimination of hepatitis C: analysis of cures versus new infections in 91 countries. J Virus Erad. 2017;3:117–123. doi: 10.1016/S2055-6640(20)30329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization Global Hepatitis Report 2017. http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf?sequence=1 Available at: Accessed May 28, 2017.
- 13.Thomas E., Liang T.J. Experimental models of hepatitis B and C—new insights and progress. Nat Rev Gastroenterol Hepatol. 2016;13:362–374. doi: 10.1038/nrgastro.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith D.B., Bukh J., Kuiken C. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukh J. The history of hepatitis C virus (HCV): basic research reveals unique features in phylogeny, evolution and the viral life cycle with new perspectives for epidemic control. J Hepatol. 2016;65:S2–S21. doi: 10.1016/j.jhep.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Smith D.B., Meyers G., Bukh J. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J Gen Virol. 2017;98:2106–2112. doi: 10.1099/jgv.0.000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martell M., Esteban J.I., Quer J. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J Virol. 1992;66:3225–3229. doi: 10.1128/jvi.66.5.3225-3229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farci P., Bukh J., Purcell R.H. The quasispecies of hepatitis C virus and the host immune response. Springer Semin Immunopathol. 1997;19:5–26. doi: 10.1007/BF00945022. [DOI] [PubMed] [Google Scholar]
- 19.Forns X., Purcell R.H., Bukh J. Quasispecies in viral persistence and pathogenesis of hepatitis C virus. Trends Microbiol. 1999;7:402–410. doi: 10.1016/s0966-842x(99)01590-5. [DOI] [PubMed] [Google Scholar]
- 20.Cox A.L., Mosbruger T., Mao Q. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson A.L., Houghton M., Choo Q.L. Hepatitis C virus-specific CTL responses in the liver of chimpanzees with acute and chronic hepatitis C. J Immunol. 1993;151:4189–4199. [PubMed] [Google Scholar]
- 22.Liu L., Fisher B.E., Dowd K.A. Acceleration of hepatitis C virus envelope evolution in humans is consistent with progressive humoral immune selection during the transition from acute to chronic infection. J Virol. 2010;84:5067–5077. doi: 10.1128/JVI.02265-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timm J., Lauer G.M., Kavanagh D.G. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593–1604. doi: 10.1084/jem.20041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey J.R., Wasilewski L.N., Snider A.E. Naturally selected hepatitis C virus polymorphisms confer broad neutralizing antibody resistance. J Clin Invest. 2015;125:437–447. doi: 10.1172/JCI78794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsen T.H., Pedersen J., Prentoe J.C. Breadth of neutralization and synergy of clinically relevant human monoclonal antibodies against HCV genotypes 1a, 1b, 2a, 2b, 2c, and 3a. Hepatology. 2014;60:1551–1562. doi: 10.1002/hep.27298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Diwany R., Cohen V.J., Mankowski M.C. Extra-epitopic hepatitis C virus polymorphisms confer resistance to broadly neutralizing antibodies by modulating binding to scavenger receptor B1. PLoS Pathog. 2017;13:e1006235. doi: 10.1371/journal.ppat.1006235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billerbeck E., Wolfisberg R., Fahnoe U. Mouse models of acute and chronic hepacivirus infection. Science. 2017;357:204–208. doi: 10.1126/science.aal1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trivedi S., Murthy S., Sharma H. Viral persistence, liver disease and host response in Hepatitis C-like virus rat model. Hepatology. 2017;68:435–448. doi: 10.1002/hep.29494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox A.L., Thomas D.L. Hepatitis C virus vaccines among people who inject drugs. Clin Infect Dis. 2013;57(Suppl 2):S46–S50. doi: 10.1093/cid/cit329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox A.L., Page K., Bruneau J. Rare birds in North America: acute hepatitis C cohorts. Gastroenterology. 2009;136:26–31. doi: 10.1053/j.gastro.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edlin B.R., Shu M.A., Winkelstein E. More rare birds, and the occasional swan. Gastroenterology. 2009;136:2412–2414. doi: 10.1053/j.gastro.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micallef J.M., Kaldor J.M., Dore G.J. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 33.Farci P., Alter H.J., Govindarajan S. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258:135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 34.Prince A.M. Immunity in hepatitis C virus infection. Vox Sang. 1994;67(Suppl 3):227–228. doi: 10.1111/j.1423-0410.1994.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 35.Bassett S.E., Guerra B., Brasky K. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 36.Major M.E., Mihalik K., Puig M. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586–6595. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nascimbeni M., Mizukoshi E., Bosmann M. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781–4793. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoukry N.H., Grakoui A., Houghton M. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta S.H., Cox A., Hoover D.R. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 40.Osburn W.O., Fisher B.E., Dowd K.A. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prince A.M., Brotman B., Lee D.H. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis. 2005;192:1701–1709. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- 42.Micallef J.M., Macdonald V., Jauncey M. High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. J Viral Hepat. 2007;14:413–418. doi: 10.1111/j.1365-2893.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 43.van de Laar T.J., Molenkamp R., van den Berg C. Frequent HCV reinfection and superinfection in a cohort of injecting drug users in Amsterdam. J Hepatol. 2009;51:667–674. doi: 10.1016/j.jhep.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Page K., Hahn J.A., Evans J. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–1226. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grebely J., Prins M., Hellard M. Hepatitis C virus clearance, reinfection, and persistence, with insights from studies of injecting drug users: towards a vaccine. Lancet Infect Dis. 2012;12:408–414. doi: 10.1016/S1473-3099(12)70010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanford R.E., Guerra B., Chavez D. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575–1581. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacks-Davis R., Grebely J., Dore G.J. Hepatitis C virus reinfection and spontaneous clearance of reinfection—the InC3 Study. J Infect Dis. 2015;212:1407–1419. doi: 10.1093/infdis/jiv220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuniholm M.H., Kovacs A., Gao X. Specific human leukocyte antigen class I and II alleles associated with hepatitis C virus viremia. Hepatology. 2010;51:1514–1522. doi: 10.1002/hep.23515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duggal P., Thio C.L., Wojcik G.L. Genome-wide association study of spontaneous resolution of hepatitis C virus infection: data from multiple cohorts. Ann Intern Med. 2013;158:235–245. doi: 10.7326/0003-4819-158-4-201302190-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulze zur Wiesch J., Ciuffreda D., Lewis-Ximenez L. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J Exp Med. 2012;209:61–75. doi: 10.1084/jem.20100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diepolder H.M., Zachoval R., Hoffmann R.M. The role of hepatitis C virus specific CD4+ T lymphocytes in acute and chronic hepatitis C. J Mol Med (Berl) 1996;74:583–588. doi: 10.1007/s001090050062. [DOI] [PubMed] [Google Scholar]
- 52.Chang K.M., Thimme R., Melpolder J.J. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 53.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grakoui A., Shoukry N.H., Woollard D.J. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 55.Abdel-Hakeem M.S., Bedard N., Murphy D. Signatures of protective memory immune responses during hepatitis C virus reinfection. Gastroenterology. 2014;147:870–881.e8. doi: 10.1053/j.gastro.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rutebemberwa A., Ray S.C., Astemborski J. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215–8225. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMahan R.H., Golden-Mason L., Nishimura M.I. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White B., Madden A., Prins M. Assessing the feasibility of hepatitis C virus vaccine trials: results from the Hepatitis C Incidence and Transmission Study-community (HITS-c) vaccine preparedness study. Vaccine. 2014;32:5460–5467. doi: 10.1016/j.vaccine.2014.07.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Major M., Gutfraind A., Shekhtman L. Modeling of patient virus titers suggests that availability of a vaccine could reduce hepatitis C virus transmission among injecting drug users. Sci Transl Med. 2018;10:eaao4496. doi: 10.1126/scitranslmed.aao4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward S., Lauer G., Isba R. Cellular immune responses against hepatitis C virus: the evidence base 2002. Clin Exp Immunol. 2002;128:195–203. doi: 10.1046/j.1365-2249.2002.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Youn J.W., Hu Y.W., Tricoche N. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J Virol. 2008;82:10896–10905. doi: 10.1128/JVI.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elmowalid G.A., Qiao M., Jeong S.H. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci U S A. 2007;104:8427–8432. doi: 10.1073/pnas.0702162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christiansen D., Earnest-Silveira L., Chua B. Immunological responses following administration of a genotype 1a/1b/2/3a quadrivalent HCV VLP vaccine. Sci Rep. 2018;8:6483. doi: 10.1038/s41598-018-24762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.von Delft A., Donnison T.A., Lourenco J. The generation of a simian adenoviral vectored HCV vaccine encoding genetically conserved gene segments to target multiple HCV genotypes. Vaccine. 2018;36:313–321. doi: 10.1016/j.vaccine.2017.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garrone P., Fluckiger A.C., Mangeot P.E. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med. 2011;3:94ra71. doi: 10.1126/scitranslmed.3002330. [DOI] [PubMed] [Google Scholar]
- 66.Colloca S., Barnes E., Folgori A. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4:115ra2. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeong S.H., Qiao M., Nascimbeni M. Immunization with hepatitis C virus-like particles induces humoral and cellular immune responses in nonhuman primates. J Virol. 2004;78:6995–7003. doi: 10.1128/JVI.78.13.6995-7003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fattori E., Zampaglione I., Arcuri M. Efficient immunization of rhesus macaques with an HCV candidate vaccine by heterologous priming-boosting with novel adenoviral vectors based on different serotypes. Gene Ther. 2006;13:1088–1096. doi: 10.1038/sj.gt.3302754. [DOI] [PubMed] [Google Scholar]
- 69.Lang Kuhs K.A., Ginsberg A.A., Yan J. Hepatitis C virus NS3/NS4A DNA vaccine induces multiepitope T cell responses in rhesus macaques mimicking human immune responses [corrected] Mol Ther. 2012;20:669–678. doi: 10.1038/mt.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polakos N.K., Drane D., Cox J. Characterization of hepatitis C virus core-specific immune responses primed in rhesus macaques by a nonclassical ISCOM vaccine. J Immunol. 2001;166:3589–3598. doi: 10.4049/jimmunol.166.5.3589. [DOI] [PubMed] [Google Scholar]
- 71.Rollier C., Verschoor E.J., Paranhos-Baccala G. Modulation of vaccine-induced immune responses to hepatitis C virus in rhesus macaques by altering priming before adenovirus boosting. J Infect Dis. 2005;192:920–929. doi: 10.1086/432517. [DOI] [PubMed] [Google Scholar]
- 72.Rollier C., Depla E., Drexhage J.A. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J Virol. 2004;78:187–196. doi: 10.1128/JVI.78.1.187-196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Folgori A., Capone S., Ruggeri L. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 74.Zubkova I., Duan H., Wells F. Hepatitis C virus clearance correlates with HLA-DR expression on proliferating CD8+ T cells in immune-primed chimpanzees. Hepatology. 2014;59:803–813. doi: 10.1002/hep.26747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dahari H., Feinstone S.M., Major M.E. Meta-analysis of hepatitis C virus vaccine efficacy in chimpanzees indicates an importance for structural proteins. Gastroenterology. 2010;139:965–974. doi: 10.1053/j.gastro.2010.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houghton M. Prospects for prophylactic and therapeutic vaccines against the hepatitis C viruses. Immunol Rev. 2011;239:99–108. doi: 10.1111/j.1600-065X.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 77.Rollier C.S., Paranhos-Baccala G., Verschoor E.J. Vaccine-induced early control of hepatitis C virus infection in chimpanzees fails to impact on hepatic PD-1 and chronicity. Hepatology. 2007;45:602–613. doi: 10.1002/hep.21573. [DOI] [PubMed] [Google Scholar]
- 78.Drane D., Maraskovsky E., Gibson R. Priming of CD4+ and CD8+ T cell responses using a HCV core ISCOMATRIX vaccine: a phase I study in healthy volunteers. Hum Vaccin. 2009;5:151–157. doi: 10.4161/hv.5.3.6614. [DOI] [PubMed] [Google Scholar]
- 79.Swadling L., Capone S., Antrobus R.D. A human vaccine strategy based on chimpanzee adenoviral and MVA vectors that primes, boosts, and sustains functional HCV-specific T cell memory. Sci Transl Med. 2014;6:261ra153. doi: 10.1126/scitranslmed.3009185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park S.H., Shin E.C., Capone S. Successful vaccination induces multifunctional memory T-cell precursors associated with early control of hepatitis C virus. Gastroenterology. 2012;143:1048–1060.e4. doi: 10.1053/j.gastro.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin E.C., Park S.H., Nascimbeni M. The frequency of CD127(+) hepatitis C virus (HCV)-specific T cells but not the expression of exhaustion markers predicts the outcome of acute HCV infection. J Virol. 2013;87:4772–4777. doi: 10.1128/JVI.03122-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barnes E., Folgori A., Capone S. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Osburn W.O., Snider A.E., Wells B.L. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology. 2014;59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pestka J.M., Zeisel M.B., Blaser E. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A. 2007;104:6025–6030. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Logvinoff C., Major M.E., Oldach D. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A. 2004;101:10149–10154. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raghuraman S., Park H., Osburn W.O. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis. 2012;205:763–771. doi: 10.1093/infdis/jir835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bailey J.R., Flyak A.I., Cohen V.J. Broadly neutralizing antibodies with few somatic mutations and hepatitis C virus clearance. JCI Insight. 2017;2:92872. doi: 10.1172/jci.insight.92872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merat S.J., Molenkamp R., Wagner K. Hepatitis C virus broadly neutralizing monoclonal antibodies isolated 25 years after spontaneous clearance. PLoS One. 2016;11:e0165047. doi: 10.1371/journal.pone.0165047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scherer E.M., Smith R.A., Simonich C.A. Characteristics of memory B cells elicited by a highly efficacious HPV vaccine in subjects with no pre-existing immunity. PLoS Pathog. 2014;10:e1004461. doi: 10.1371/journal.ppat.1004461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C., Liu Y., Cavanagh M.M. B-cell repertoire responses to varicella-zoster vaccination in human identical twins. Proc Natl Acad Sci U S A. 2015;112:500–505. doi: 10.1073/pnas.1415875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meuleman P., Bukh J., Verhoye L. In vivo evaluation of the cross-genotype neutralizing activity of polyclonal antibodies against hepatitis C virus. Hepatology. 2011;53:755–762. doi: 10.1002/hep.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vanwolleghem T., Bukh J., Meuleman P. Polyclonal immunoglobulins from a chronic hepatitis C virus patient protect human liver-chimeric mice from infection with a homologous hepatitis C virus strain. Hepatology. 2008;47:1846–1855. doi: 10.1002/hep.22244. [DOI] [PubMed] [Google Scholar]
- 93.Bukh J., Engle R.E., Faulk K. Immunoglobulin with high-titer in vitro cross-neutralizing hepatitis C virus antibodies passively protects chimpanzees from homologous, but not heterologous, challenge. J Virol. 2015;89:9128–9132. doi: 10.1128/JVI.01194-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Law M., Maruyama T., Lewis J. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 95.Keck Z.Y., Wang Y., Lau P. Affinity maturation of a broadly neutralizing human monoclonal antibody that prevents acute HCV infection. Hepatology. 2016;64:1922–1933. doi: 10.1002/hep.28850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giang E., Dorner M., Prentoe J.C. Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci U S A. 2012;109:6205–6210. doi: 10.1073/pnas.1114927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morin T.J., Broering T.J., Leav B.A. Human monoclonal antibody HCV1 effectively prevents and treats HCV infection in chimpanzees. PLoS Pathog. 2012;8:e1002895. doi: 10.1371/journal.ppat.1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.de Jong Y.P., Dorner M., Mommersteeg M.C. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colpitts C.C., Baumert T.F. Hepatitis C virus cell entry: a target for novel antiviral strategies to address limitations of direct acting antivirals. Hepatol Int. 2016;10:741–748. doi: 10.1007/s12072-016-9724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ray S.C., Wang Y.M., Laeyendecker O. Acute hepatitis C virus structural gene sequences as predictors of persistent viremia: hypervariable region 1 as a decoy. J Virol. 1999;73:2938–2946. doi: 10.1128/jvi.73.4.2938-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimizu Y.K., Igarashi H., Kiyohara T. A hyperimmune serum against a synthetic peptide corresponding to the hypervariable region 1 of hepatitis C virus can prevent viral infection in cell cultures. Virology. 1996;223:409–412. doi: 10.1006/viro.1996.0497. [DOI] [PubMed] [Google Scholar]
- 102.Dowd K.A., Netski D.M., Wang X.H. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377–2386. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farci P., Alter H.J., Wong D.C. Prevention of hepatitis C virus infection in chimpanzees after antibody-mediated in vitro neutralization. Proc Natl Acad Sci U S A. 1994;91:7792–7796. doi: 10.1073/pnas.91.16.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimizu Y.K., Hijikata M., Iwamoto A. Neutralizing antibodies against hepatitis C virus and the emergence of neutralization escape mutant viruses. J Virol. 1994;68:1494–1500. doi: 10.1128/jvi.68.3.1494-1500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.von Hahn T., Yoon J.C., Alter H. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 106.Gal-Tanamy M., Keck Z.Y., Yi M. In vitro selection of a neutralization-resistant hepatitis C virus escape mutant. Proc Natl Acad Sci U S A. 2008;105:19450–19455. doi: 10.1073/pnas.0809879105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keck Z.Y., Xia J., Wang Y. Human monoclonal antibodies to a novel cluster of conformational epitopes on HCV e2 with resistance to neutralization escape in a genotype 2a isolate. PLoS Pathog. 2012;8:e1002653. doi: 10.1371/journal.ppat.1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hadlock K.G., Lanford R.E., Perkins S. Human monoclonal antibodies that inhibit binding of hepatitis C virus E2 protein to CD81 and recognize conserved conformational epitopes. J Virol. 2000;74:10407–10416. doi: 10.1128/jvi.74.22.10407-10416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keck Z.Y., Olson O., Gal-Tanamy M. A point mutation leading to hepatitis C virus escape from neutralization by a monoclonal antibody to a conserved conformational epitope. J Virol. 2008;82:6067–6072. doi: 10.1128/JVI.00252-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keck Z., Wang W., Wang Y. Cooperativity in virus neutralization by human monoclonal antibodies to two adjacent regions located at the amino terminus of hepatitis C virus E2 glycoprotein. J Virol. 2013;87:37–51. doi: 10.1128/JVI.01941-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krey T., Meola A., Keck Z.Y. Structural basis of HCV neutralization by human monoclonal antibodies resistant to viral neutralization escape. PLoS Pathog. 2013;9:e1003364. doi: 10.1371/journal.ppat.1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johansson D.X., Voisset C., Tarr A.W. Human combinatorial libraries yield rare antibodies that broadly neutralize hepatitis C virus. Proc Natl Acad Sci U S A. 2007;104:16269–16274. doi: 10.1073/pnas.0705522104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kong L., Giang E., Robbins J.B. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc Natl Acad Sci U S A. 2012;109:9499–9504. doi: 10.1073/pnas.1202924109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Owsianka A., Tarr A.W., Juttla V.S. Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol. 2005;79:11095–11104. doi: 10.1128/JVI.79.17.11095-11104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pierce B.G., Keck Z.Y., Lau P. Global mapping of antibody recognition of the hepatitis C virus E2 glycoprotein: implications for vaccine design. Proc Natl Acad Sci U S A. 2016;113:E6946–E6954. doi: 10.1073/pnas.1614942113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gopal R., Jackson K., Tzarum N. Probing the antigenicity of hepatitis C virus envelope glycoprotein complex by high-throughput mutagenesis. PLoS Pathog. 2017;13:e1006735. doi: 10.1371/journal.ppat.1006735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kinchen V.J., Cox A.L., Bailey J.R. Can broadly neutralizing monoclonal antibodies lead to a hepatitis C virus vaccine? Trends Microbiol. 2018;26:854–864. doi: 10.1016/j.tim.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fuerst T.R., Pierce B.G., Keck Z.Y. Designing a B cell-based vaccine against a highly variable hepatitis C virus. Front Microbiol. 2017;8:2692. doi: 10.3389/fmicb.2017.02692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kong L., Giang E., Nieusma T. Hepatitis C virus E2 envelope glycoprotein core structure. Science. 2013;342:1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Broering T.J., Garrity K.A., Boatright N.K. Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J Virol. 2009;83:12473–12482. doi: 10.1128/JVI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sabo M.C., Luca V.C., Prentoe J. Neutralizing monoclonal antibodies against hepatitis C virus E2 protein bind discontinuous epitopes and inhibit infection at a postattachment step. J Virol. 2011;85:7005–7019. doi: 10.1128/JVI.00586-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tarr A.W., Owsianka A.M., Timms J.M. Characterization of the hepatitis C virus E2 epitope defined by the broadly neutralizing monoclonal antibody AP33. Hepatology. 2006;43:592–601. doi: 10.1002/hep.21088. [DOI] [PubMed] [Google Scholar]
- 123.Flint M., Thomas J.M., Maidens C.M. Functional analysis of cell surface-expressed hepatitis C virus E2 glycoprotein. J Virol. 1999;73:6782–6790. doi: 10.1128/jvi.73.8.6782-6790.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Potter J.A., Owsianka A.M., Jeffery N. Toward a hepatitis C virus vaccine: the structural basis of hepatitis C virus neutralization by AP33, a broadly neutralizing antibody. J Virol. 2012;86:12923–12932. doi: 10.1128/JVI.02052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kong L., Giang E., Nieusma T. Structure of hepatitis C virus envelope glycoprotein E2 antigenic site 412 to 423 in complex with antibody AP33. J Virol. 2012;86:13085–13088. doi: 10.1128/JVI.01939-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meola A., Tarr A.W., England P. Structural flexibility of a conserved antigenic region in hepatitis C virus glycoprotein E2 recognized by broadly neutralizing antibodies. J Virol. 2015;89:2170–2181. doi: 10.1128/JVI.02190-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y., Pierce B.G., Wang Q. Structural basis for penetration of the glycan shield of hepatitis C virus E2 glycoprotein by a broadly neutralizing human antibody. J Biol Chem. 2015;290:10117–10125. doi: 10.1074/jbc.M115.643528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stamataki Z., Coates S., Evans M.J. Hepatitis C virus envelope glycoprotein immunization of rodents elicits cross-reactive neutralizing antibodies. Vaccine. 2007;25:7773–7784. doi: 10.1016/j.vaccine.2007.08.053. [DOI] [PubMed] [Google Scholar]
- 129.Choo Q.L., Kuo G., Ralston R. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci U S A. 1994;91:1294–1298. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Law J.L., Chen C., Wong J. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS One. 2013;8:e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frey S.E., Houghton M., Coates S. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28:6367–6373. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang T.J. Current progress in development of hepatitis C virus vaccines. Nat Med. 2013;19:869–878. doi: 10.1038/nm.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Forns X., Payette P.J., Ma X. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618–625. doi: 10.1053/jhep.2000.9877. [DOI] [PubMed] [Google Scholar]
- 134.Bassett S.E., Brasky K.M., Lanford R.E. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bassett S.E., Thomas D.L., Brasky K.M. Viral persistence, antibody to E1 and E2 and HVR-1 sequence stability in hepatitis C virus-inoculated chimpanzees. J Virol. 1998;73:1118–1126. doi: 10.1128/jvi.73.2.1118-1126.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lanford R.E., Bigger C., Bassett S. The chimpanzee model of hepatitis C virus infections. ILAR J. 2001;42:117–126. doi: 10.1093/ilar.42.2.117. [DOI] [PubMed] [Google Scholar]
- 137.Akazawa D., Moriyama M., Yokokawa H. Neutralizing antibodies induced by cell culture-derived hepatitis C virus protect against infection in mice. Gastroenterology. 2013;145:447–455.e1–4. doi: 10.1053/j.gastro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 138.Krapchev V.B., Rychlowska M., Chmielewska A. Recombinant Flag-tagged E1E2 glycoproteins from three hepatitis C virus genotypes are biologically functional and elicit cross-reactive neutralizing antibodies in mice. Virology. 2018;519:33–41. doi: 10.1016/j.virol.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mankowski M.C., Kinchen V.J., Wasilewski L.N. Synergistic anti-HCV broadly neutralizing human monoclonal antibodies with independent mechanisms. Proc Natl Acad Sci U S A. 2018;115:E82–E91. doi: 10.1073/pnas.1718441115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Prentoe J., Jensen T.B., Meuleman P. Hypervariable region 1 differentially impacts viability of hepatitis C virus strains of genotypes 1 to 6 and impairs virus neutralization. J Virol. 2011;85:2224–2234. doi: 10.1128/JVI.01594-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vietheer P.T., Boo I., Gu J. The core domain of hepatitis C virus glycoprotein E2 generates potent cross-neutralizing antibodies in guinea pigs. Hepatology. 2017;65:1117–1131. doi: 10.1002/hep.28989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Law J.L.M., Logan M., Wong J. Role of the E2 hypervariable region (HVR1) in the immunogenicity of a recombinant hepatitis C virus vaccine. J Virol. 2018;92 doi: 10.1128/JVI.02141-17. e02141–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pierce B.G., Boucher E.N., Piepenbrink K.H. Structure-based design of hepatitis C virus vaccines that elicit neutralizing antibody responses to a conserved epitope. J Virol. 2017;91 doi: 10.1128/JVI.01032-17. e01032–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bartosch B., Dubuisson J., Cosset F.L. Infectious hepatitis C virus pseudo-particles containing functional E1- E2 envelope protein complexes. J Exp Med. 2003;197:633–642. doi: 10.1084/jem.20021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gottwein J.M., Scheel T.K., Jensen T.B. Development and characterization of hepatitis C virus genotype 1–7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology. 2009;49:364–377. doi: 10.1002/hep.22673. [DOI] [PubMed] [Google Scholar]
- 146.Tarr A.W., Urbanowicz R.A., Hamed M.R. Hepatitis C patient-derived glycoproteins exhibit marked differences in susceptibility to serum neutralizing antibodies: genetic subtype defines antigenic but not neutralization serotype. J Virol. 2011;85:4246–4257. doi: 10.1128/JVI.01332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bartosch B., Bukh J., Meunier J.C. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci U S A. 2003;100:14199–14204. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Urbanowicz R.A., McClure C.P., Brown R.J. A diverse panel of hepatitis C virus glycoproteins for use in vaccine research reveals extremes of monoclonal antibody neutralization resistance. J Virol. 2016;90:3288–3301. doi: 10.1128/JVI.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burke K.P., Munshaw S., Osburn W.O. Immunogenicity and cross-reactivity of a representative ancestral sequence in HCV infection. J Immunol. 2012;188:5177–5188. doi: 10.4049/jimmunol.1103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Capone S., Naddeo M., D'Alise A.M. Fusion of HCV nonstructural antigen to MHC class II-associated invariant chain enhances T-cell responses induced by vectored vaccines in nonhuman primates. Mol Ther. 2014;22:1039–1047. doi: 10.1038/mt.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]