Abstract
Vitiligo is an autoimmune skin disease in which the pigment-producing melanocytes are destroyed by autoreactive CD8+ T cells. As a result, patients develop disfiguring white spots on the skin. This unit discusses the first mouse model of vitiligo that develops epidermal depigmentation, similar to disease in human patients. To achieve epidermal depigmentation, mice are genetically engineered to retain melanocytes in the skin epidermis. Induction of disease occurs by adoptive transfer of melanocyte-specific CD8+ T cells into recipient mice and the subsequent activation of these T cells using a viral vector. Depigmentation of the epidermis occurs within 5–7 weeks in a patchy pattern similar to patients with vitiligo. This unit describes the methods of vitiligo induction, quantification of lesion progression and regression, processing of the skin for detailed analysis, and how to use this model to inform clinical studies.
Keywords: vitiligo, autoimmunity, melanocyte, CD8 T cells, mice
INTRODUCTION
Vitiligo is an under-recognized autoimmune disease that affects the skin. Patients with vitiligo present with white spots on the skin that result from the destruction of epidermal pigment-producing cells, melanocytes. It is widely accepted that melanocyte-specific CD8+ T cells are both necessary and sufficient to mediate vitiligo (Ogg, Dunbar, Romero, Chen, & Cerundolo, 1998; van den Boorn et al., 2009). However, it is also believed that melanocyte stress and activation of the innate immune system may play a role in the initiation of disease (J. E. Harris, 2016; Richmond, Frisoli, & Harris, 2013; van den Boorn, Melief, & Luiten, 2011). A better understanding of the genetic, environmental and immunoregulatory factors that drive vitiligo will provide important insights into treatment modalities.
This unit will focus on a detailed protocol for inducing epidermal depigmentation in mice. Mice that develop epidermal depigmentation exhibit disease characteristics that closely replicate human disease and this unit will discuss the parallels of the vitiligo mouse model to human vitiligo.
Basic Protocol 1 describes detailed methods for inducing epidermal depigmentation in mice. Alternate Protocol 1 describes a method to screen for potential treatment targets and identify those that prevent vitiligo development. Alternate Protocol 2 describes a method to study disease reversal known as repigmentation and to quantitate it using Image J. Support Protocol 1 describes how skin should be processed to facilitate analysis of skin cells by flow cytometry, while Support Protocol 2 includes methods for the detection of effector CD8+ T cells and chemokine production in skin using confocal microscopy.
Basic Protocol 1 - INDUCTION OF PIGMENT LOSS
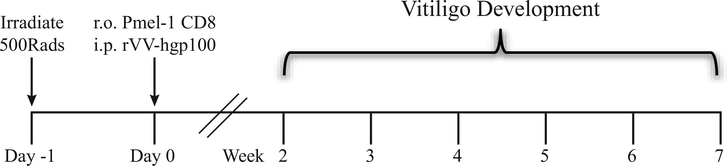
This model of vitiligo is a modification of the adoptive transfer model developed for melanoma immunotherapy (Overwijk et al., 2003). In short, vitiligo is induced by adoptive transfer of melanocyte-specific CD8+ T cells and activation of the transferred CD8+ T cells using a recombinant vaccinia virus that expresses their cognate antigen. The major difference between this model and previous models is the location of depigmentation. C57BL/6 mice have pink skin due to the lack of melanocytes present within the epidermis and most melanocytes reside in the hair follicles (Nishimura et al., 2002). Previous models using C57BL/6 mice displayed only hair depigmentation, which is not representative of human disease, reviewed in Essien & Harris, 2014 and summarized in Table 1. Here, we utilize mice that retain melanocytes in the epidermis due to transgenic expression of a melanocyte growth factor, resulting in black skin and black hair (Kunisada et al., 1998) (Figure 1). Induction of vitiligo in these Stem Cell Factor (SCF) transgenic mice leads to epidermal depigmentation (Figure 2A,B) that spares melanocytes in the hair follicle, similar to human vitiligo. Because of the sparing of these follicular melanocytes, mice in this model are capable of perifollicular repigmentation, similar to how disease is reversed in humans. Induction of vitiligo in mice is dependent on three parameters: sublethal irradiation, transfer of melanocyte-specific CD8+ T cells (Pmel-1), and infection with recombinant vaccinia virus engineered to express their cognate antigen (rVV-hgp100). A graphical representation of the experimental time line of vitiligo induction in mice is shown in Figure 3.
Table 1:
Mouse models of depigmentation
| Model summary | Disease Mechanism | Reference |
|---|---|---|
| Monobenzone induced stress | CD8+ T cells infiltrate the skin after application of monobenzone | Zhu et al |
| Immunization with vaccinia virus expressing human TRP1 | Autoantibody and CD4+ T cell mediated | Overwijk et al |
| Immunization of skin with DNA plasmid encoding human TRP2 | CD8+ T cell and perforin-dependent | Brown et al |
| FH TCR Tg mouse expressing HLA.A2 and tyrosinase specific CD8+ T cells | Dependent on CD8+ T cells | Gregg et al |
| Generated a tyrosinase TCR Tg mouse cloned from CD4+ T cell of melanoma | CD3+ T cell mediated | Mehrotra et al |
| TCR Tg mouse - expresses of HEL under TRP2 promoter | CD4+ T cell and Fas dependent | Lambe et al |
| TRP1 TCR Tg mice | CD4+ T cell mediated | Muranski et al |
| Requires RAG deficient hosts, B16 melanoma cells, Pmel T cells, hgp100 VV, and IL-2 | CD8+ T cells and IFNγ dependent | Antony et al |
| SCF recipient mice, adoptive transfer of Pmel T cells, hgp100 VV | Dependent on CD8+ T cells and IFNγ | Harris et al |
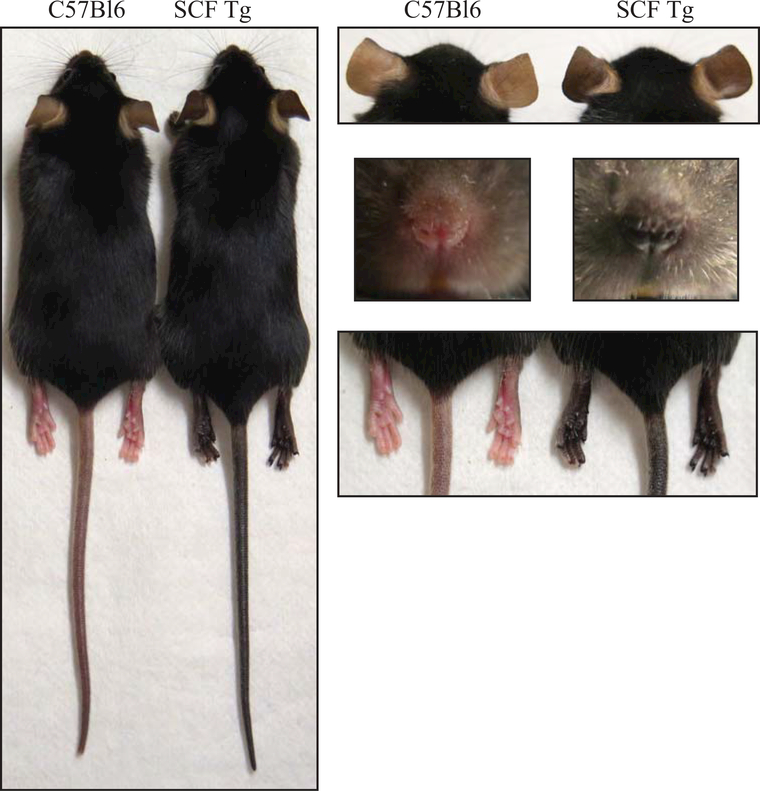
Figure 1:
KRT14-kitl*4XTG2Bjl (SCF) mice have black skin due to melanocyte retention in the epidermis. Images contrast normal (no vitiligo) C57Bl6 and SCF Tg mouse tails, ears, nose, and rear footpads.
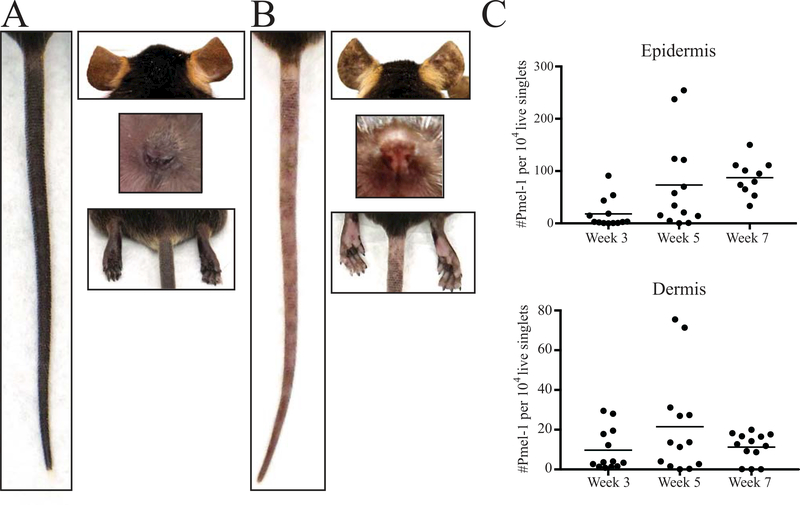
Figure 2:
Vitiligo development in SCF mice. A) Images of the tail, ears, nose and rear footpad of an SCF mouse without vitiligo. B) Images revealing spots of depigmentation on the tail, ears, nose and rear footpads of an SCF mouse 7 weeks post vitiligo induction. C) The number of infiltrating Pmel-1 (normalized to live singlets) in the epidermis and dermis during vitiligo progression.
Figure 3:
Summary of vitiligo induction in mice. Induction of vitiligo in SCF mice includes sublethal irradiation, transfer of Pmel-1 CD8+ T cells, and infection with recombinant vaccinia virus that expresses their cognate antigen gp100. SCF mice develop epidermal depigmentation over the course of 7 weeks.
NOTE: All protocols using live animals must be reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) and must conform to NIH regulations for the care and use of laboratory animals. All solutions, equipment and therapeutics used on living animals must be sterile and aseptic technique should be used.
Materials
γ irradiator for small animals
8- to 12-week old SCF Tg host mice (Older mice can be used)
Available from Jackson Laboratory Stock#009687 but the authors can supply breeders upon reader request.
8- to 12-week old Pmel-1 transgenic mice (Jackson Laboratory, Stock#005023)
Red Cell Lysis Buffer (Sigma, R7757–100ML)
MACS CD8+ T cell isolation kit (Miltenyi, 130–104-075)
MACS buffer (see recipe)
quadroMACS separator (Miltenyi, 130–090-976)
MACS MultiStand (Miltenyi, 130–042-203)
LS columns (Miltenyi, 130–042-401)
Scissors (straight or curved)
Curved forceps
1X PBS (Corning cellgro, 21–040-CV))
100-μm Nylon mesh (ELKO)
FALCON Corning Brand tissue culture dish (60 × 15 mm, 353002)
Hemocytometer or automated cell counter
0.5 mL insulin syringes (BD, 329461)
3 or 5 mL syringes (BD,2023–01-31, 2022–02-28)
Ketamine
Xylazine
Frozen rVV-hgp100 stocks (N. Restifo at NCI/NIH, Overwijk et al., 2003)
Day -1 - Irradiation
-
Irradiate SCF recipient mice using a γ irradiator with a sublethal dose (5 Gy) to deplete endogenous lymphocytes. Refer to the correct date/dose chart for your radioactive source to ensure correct dosage time.
In C57Bl/6 mice, 5 Gy is the standard dose. Access to the gamma irradiator at any institution requires irradiation safety training and personnel background checks. To use the y-irradiator follow the institutional and manufactures instructions. It is preferred to use a y- irradiator with a rotating device to ensure equal radiation dose for each mouse. If it is of interest to the investigator, SCF mice can be crossed to different genetic backgrounds to study the role of various factors in vitiligo pathogenesis.
Day 0 - Isolation of PMEL CD8+ T cells
-
2.
Euthanize a same sex donor Pmel-1 transgenic mouse aged 8- to 12- weeks by CO2 asphyxiation followed by cervical dislocation (Donovan & Brown, 2006b).
If a same sex donor is not available, transfer of female Pmel-1 cells into male mice is an option. Induction of vitiligo with older Pmel-1 donors seems to result in more severe disease.
-
3.
Harvest the spleen (J. P. Reeves & Reeves, 2001) of Pmel-1 TCR Tg mice.
One spleen from a Pmel-1 TCR Tg mouse is enough to induce vitiligo in about 10 recipients.
-
4.
Work in the hood using aseptic technique. Crush the spleen into a single cell suspension. Place the spleen into a tissue culture dish containing a cut piece of nylon mesh. Add 1 mL of PBS. Crush the spleen into a cell suspension using the sterile flat end of a 3 or 5 mL syringe. Pipette the cell suspension into a 15 mL conical tube.
-
5.
Spin the spleen cell suspension at 330 × g for 5 minutes. Decant and suspend the spleen in 2 mLs of Red Cell Lysis buffer at RT for 3 minutes. Quench the lysis reaction by adding at least 5 mLs of PBS.
-
6.
Spin the lysed cell suspension at 330 × g for 10 minutes.
-
7.
Follow the commercial instructions for negative selection of CD8+ T cells using MACS CD8+ T cell Isolation Kit (Miltenyi).
More information regarding T cell isolation can be found in Thornton UNIT 3.5A (Thornton, 2003).
-
8.
Count isolated Pmel-1 CD8+ T cells using a hemocytometer or automated cell counter.
-
9.
Bring the final isolated Pmel-1 CD8+ T cells to a concentration of 10×106 cells per 1 ml in sterile PBS. Keep Pmel-1 CD8+ T cells on ice.
On average 10×106 to 20×106 are recovered from one spleen after CD8+ T cell isolation. It is preferred to use isolated Pmel-1 CD8+ T cells same day but they can be frozen at −80°C for up to 3 months. Freeze Pmel-1 CD8+ T cells in DMSO with 10% Fetal Bovine Serum.
Day 0 - Prepare virus
-
10.
Thaw rVV–hgp100 stock in 37°C water bath.
-
11.
Vortex or sonicate virus after thawing to break up clumps.
-
12.
Dilute the virus stock in ice-cold PBS to 5.0×106 pfu/ml. Keep diluted rVV-hgp100 on ice. Virus stocks can be refrozen and thawed several times without a significant reduction in viral titer.
Information regarding the generation of recombinant vaccinia virus can be found in Earl et al., 2001.
Day 0 – Prepare anesthetic
-
13.
Use a sterile injection vial (commercial injection vial). Refer to https://animal.research.uiowa.edu/iacuc-guidelines-anesthesia for guidelines on anesthesia.
-
14.
Dilute the anesthetic to the approved protocol concentration in sterile PBS.
Vitiligo mice are anesthetized with Ketamine/Xylazine cocktail at 100/10 mg/kg.
Day 0 - Induction of vitiligo in mice
-
15.
Anesthetize the mice with Ketamine/Xylazine at 100/10 mg/kg diluted in 100 μl PBS by intraperitoneal (i.p.) injection (Donovan & Brown, 2006c).
Investigators may choose another method of anesthesia such as isofluorane. All protocols must be approved by IACUC and follow institutional guidelines.
-
16.
Inject 1.0×106 (100 μl) of isolated Pmel-1 CD8+ T cells by retro-orbital (r.o.) injection into each SCF recipient mouse.
Retro orbital injection is necessary as the tail vein is not visible in SCF black skinned mice.
-
17.
Infect SCF mice by intraperitoneal injection of 1.0×106 pfu rVV-hgp100 diluted in 200 μl PBS.
rVV-hgp100 is an attenuated strain of vaccinia virus and non-manipulated wild-type SCF mice clear rVV-hgp100 by 10–14 days post infection. All cages containing mice infected with rVV-hgp100 must be kept closed until clearance of virus per IACUC instructions. Because of this, it is important that the mice are put into clean cages prior to infection.
-
18.
Mice are typically scored at 7 weeks post vitiligo induction when the disease has reached a plateau.
No visual or behavioral changes are observed in SCF mice following infection with rVV-hgp100. Wild-type SCF mice begin to develop spots of depigmentation on the footpad and tail first, with spots occurring on the ears and nose in mice with severe disease. Spots of depigmentation begin to appear at week 3 post vitiligo induction as light spots that then become completely white. The number of white spots gradually increase over the 7 week period. After 7 weeks, disease has stabilized and mice do not usually develop additional spots.
Week 7– Quantification of vitiligo disease severity
-
19.
Quantification of epidermal depigmentation in SCF mice is performed using a subjective scoring system based on a point scale (Table 2). A trained investigator who is blinded to the details of the experimental set up should score the mice.
-
20.
Mice can be scored before experimental analyses or after euthanizing. If scoring live mice, bring each experimental cage over to the blinded scorer. The blinded scorer will subjectively score each SCF mouse by estimating the percent depigmentation at each of the 4 skin sites; the nose, ears, rear footpads and tail. Refer to Table 2 for scoring details.
Both the left and right ears and the left and right rear footpads are estimated together and evaluated as a single site. On average about 20% of SCF mice do not develop vitiligo and it is not clear why this occurs.
Table 2:
Each skin site is awarded a score between 0–5; a score of 0 reflects no evidence of depigmentation, and a score of 5 reflects 100% depigmentation at that site. Thus, the highest score each mouse can achieve is 20. For unbiased scoring of vitiligo mice, a blinded but trained investigator who is not aware of the experimental set up details should score the mice.
| Vitiligo Scoring Point System | ||||||
|---|---|---|---|---|---|---|
| SCORE | 0 | 1 | 2 | 3 | 4 | 5 |
| TAIL | 0 | <10% | 10–25% | >25–75% | >75–99% | 100% |
| EAR | 0 | <10% | 10–25% | >25–75% | >75–99% | 100% |
| NOSE | 0 | <10% | 10–25% | >25–75% | >75–99% | 100% |
| FOOTPAD | 0 | <10% | 10–25% | >25–75% | >75–99% | 100% |
| Troubleshooting | ||
|---|---|---|
| Problem | Possible cause | Solution |
| High Pmel-1 CD8+ T cell number | Lymphoma in Pmel-1 donor | Use a different Pmel-1 donor with normal sized spleen. Transfer of Pmel-1 lymphoma cells may cause death of the recipient mouse |
| Poor or no induction of vitiligo | Poor injection technique | Practice retro orbital injections or ask a trained investigator for help with injections |
| Poor or no induction of vitiligo | Pmel-1 cells were killed | Investigate viability of CD8+ T cells after Miltenyi isolation and whole splenocytes after RBC lysis steps |
| Poor or no induction of vitiligo | Poorly mixed cells | Be sure to mix Pmel-1 cells before injection and do not leave cells in syringe for a long time |
| Poor or no induction of vitiligo | Poor viral titer | Re-titer viral stock |
| All the mice die | Mice were lethally irradiated | Be sure to calculate the time of radiation exposure so that mice receive sublethal dose of radiation (500 rads) |
NOTE: For identification of SCF mice, use ear tags or punches (Donovan & Brown, 2006a). When ear punching is performed at the same time as vitiligo induction, depigmentation develops at the site of injury known as Koebner phenomenon. Therefore, ear punch mice at least 3 weeks prior to vitiligo induction.
ALTERNATE PROTOCOL 1
TESTING TREATMENTS FOR THE PREVENTION OF VITILIGO
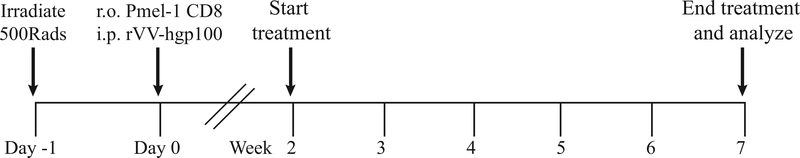
One major goal of treatment for vitiligo patients is to prevent the development of new spots. The prevention protocol described below is used with this purpose in mind to screen for factors that prevent vitiligo development in mice. These experiments will thus identify agents driving vitiligo pathogenesis (Agarwal et al., 2015; J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012b; Rashighi, Agarwal, Richmond, Harris, Dresser, Su, Zhou, Deng, Hunter, Luster, & Harris, 2014a; Richmond, Masterjohn, et al., 2017b). The prevention protocol also serves to “predict” treatments that may be successful in reversing disease, through induction of repigmentation (Alternate Protocol 2). As repigmentation experiments take a minimum of 20 weeks to perform, the 7 week prevention protocol is a quick alternative to narrow down molecular factors that promote vitiligo. Both of these protocols mirror treatment options for vitiligo patients in that treatment can stabilize disease “(prevent new spots)” and/or induce repigmentation, depending on the patient’s disease course. Because vitiligo induction in mice is dependent on infection with rVV-hgp100, it is important to start treatment after virus is cleared in the recipients. Therefore, treatments using this protocol start at 2 weeks post vitiligo induction, at which time wild-type SCF mice have cleared vaccinia virus infection. This schedule prevents any adverse effects of the treatment on vaccinia virus clearance and titer. The protocol uses the same vitiligo induction strategy as described in Basic Protocol 1. Vitiligo is induced and mice are left untreated for 2 weeks until vaccinia virus is cleared. Treatment starts at 2 weeks post vitiligo induction and continues until week 7 for a treatment duration of 5 weeks (Figure 4). Treatment efficacy is determined by quantification of vitiligo disease following 5 weeks of treatment. The anatomical site of treatment and treatment schedule will vary based on the experimental goal and target of interest. Common routes of administration include, but are not limited to, intraperitoneal, intradermal, oral gavage, and topical application.
Figure 4:
Prevention Protocol. For preventative studies in SCF vitiligo mice, induce vitiligo as in Basic Protocol 1. Start treatment of mice at 2 weeks post vitiligo induction when wild-type SCF mice have cleared vaccinia virus. Treat mice for 5 weeks and score mice for disease quantification.
Materials
γ irradiator for small animals
8- to 12-week old SCF Tg host mice (Older mice can be used)
Available from Jackson Laboratory Stock#009687 but the authors can supply breeders upon reader request.
8- to 12-week old Pmel-1 transgenic mice (Jackson Laboratory, Stock#005023)
Red Cell Lysis Buffer (Sigma, R7757–100ML)
MACS CD8+ T cell isolation kit (Miltenyi, 130–104-075)
MACS buffer (see recipe)
quadroMACS separator (Miltenyi, 130–090-976)
MACS MultiStand (Miltenyi, 130–042-203)
LS columns (Miltenyi, 130–042-401)
Scissors (straight or curved)
Curved forceps
1X PBS (Corning cellgro, 21–040-CV))
100-μm Nylon mesh (ELKO)
FALCON Corning Brand tissue culture dish (60 × 15 mm, 353002)
Hemocytometer or automated cell counter
0.5 mL insulin syringes (BD, 329461)
3 or 5 mL syringes (BD,2023–01-31, 2022–02-28)
Ketamine
Xylazine
Frozen rVV-hgp100 stocks
Treatment or drug of interest (e.g. CXCR3 depleting antibody)
Vehicle control treatment (e.g. PBS)
Prevention Protocol
Induce vitiligo in SCF mice (Basic Protocol 1)
-
Start treatment at 2 weeks post vitiligo induction.
Use of the prevention protocol is reported in Richmond et al., 2017 in which mice were treated with a chemokine receptor depleting antibody specific for CXCR3, a skin homing receptor for T cells. Also used in (Rashighi, Agarwal, Richmond, Harris, Dresser, Su, Zhou, Deng, Hunter, Luster, & Harris, 2014b)
-
Treat mice for 5 weeks.
For example, treatment with CXCR3 depleting antibody 3 times weekly at 100 μg per mouse protected mice from developing vitiligo (Richmond et al., 2017).
Determine the clinical disease score at week 7 post vitiligo induction using the scoring system outlined in Basic Protocol 1 and Table 2.
-
Drug efficacy is measured by a reduction in clinical disease score compared to animals treated with vehicle or other appropriate control.
For each experiment, use 5–10 mice per group. For example, one experiment would consist of 5 mice treated with vehicle and 5 mice treated with drug. Drug dosage regimen may vary and optimization may be required.
ALTERNATE PROTOCOL 2
QUANTIFICATION OF REPIGMENTATION IN MICE WITH VITILIGO
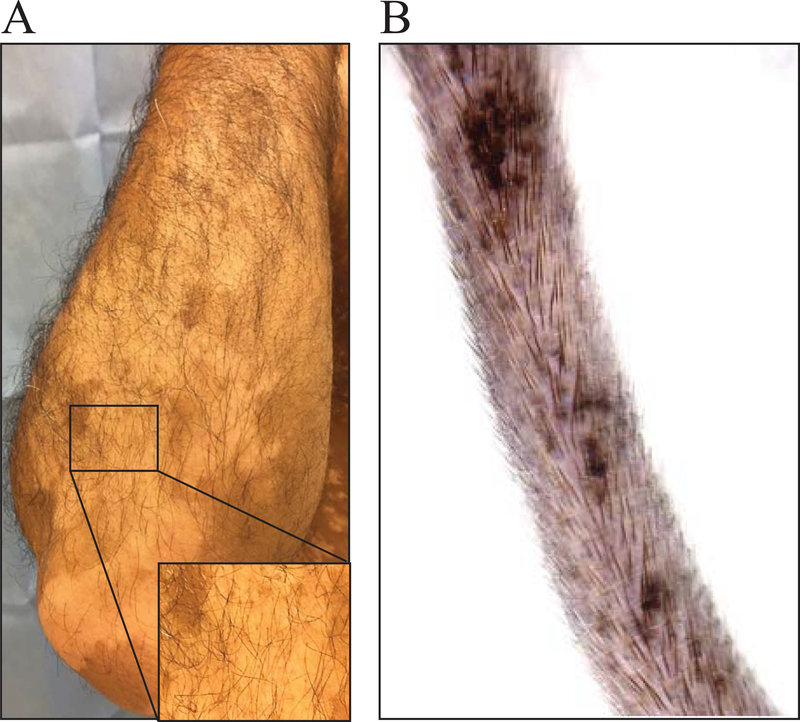
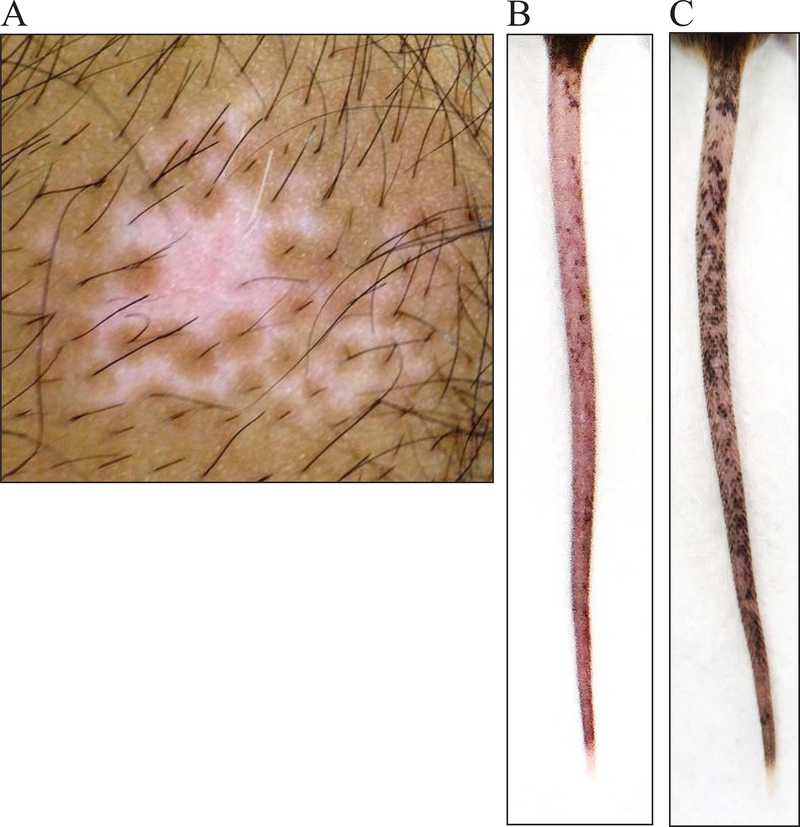
Vitiligo lesions occur when mature epidermal melanocytes are killed by melanocyte-specific CD8+ T cells. In human vitiligo, mature melanocytes and melanocyte precursors that live in the hair follicle are protected from autoimmune attack (Figure 5A)(Falabella, 2009). As a result, melanocytes can be regenerated from the hair follicles in an active process known as perifollicular repigmentation (Birlea, Costin, Roop, & Norris, 2017; Cui, Shen, & Wang, 1991). This occurs when the melanocyte precursors in the hair follicle bulge proliferate, migrate, and differentiate to replenish the lost epidermal melanocytes. The process of repigmentation can be triggered through conventional therapy such as nbUVB and topical immunosuppressants. A vitiligo lesion responding well to therapy will develop dark macules surrounding each hair follicle, visible evidence of perifollicular repigmentation (Figure 6A).
Figure 5:
Hair follicle melanocytes are spared in human vitiligo and in SCF vitiligo mice. A) Human vitiligo lesion with hair follicle pigment maintained. B) SCF vitiligo mouse, 12 weeks post vitiligo induction with pigmented hair follicles within lesions.
Figure 6:
SCF vitiligo mice show perifollicular repigmentation following successful treatment, similar to in human vitiligo. A) Human vitiligo lesion responding to therapy with repigmentation emerging from hair follicles. B) 12 week old SCF vitiligo mouse with severe depigmentation on the tail. C) Tail of SCF vitiligo mouse 8 weeks after CD8 T cell depletion with perifollicular repigmentation.
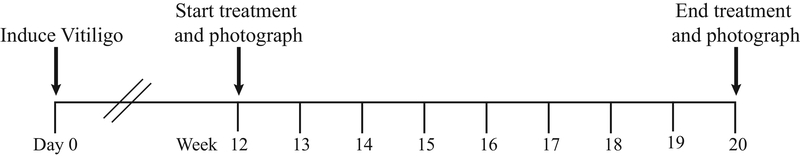
Vitiligo initiated in mice using the protocol outlined in Basic Protocol 1 induces epidermal depigmentation with hair follicle melanocytes remaining intact, similar to disease in human vitiligo (Figure 5B). Because of this, vitiligo mice can be used to test potential treatments that activate repigmentation. By 10 to 12 weeks after vitiligo induction, one fourth of the mice develop between 75% and 100% depigmentation on the tail and disease stabilizes. Figure 6B depicts the tail of an SCF mouse 12 weeks post vitiligo induction with almost no pigment remaining. Similar to disease reversal in human patients, induction of repigmentation by treatment using this protocol results in perifollicular repigmentation of the mouse tail (Figure 6C). For pre-clinical studies and to induce repigmentation, mice with at least 50% tail depigmentation are selected. Treatment is started at week 12 post vitiligo induction when disease progression has stabilized. Wild-type SCF mice can undergo some spontaneous repigmentation over time after vitiligo induction. Starting treatment at 12 weeks minimizes spontaneous repigmentation and helps in the interpretation of results. On average, mice are treated for 8 weeks using this protocol, although treatment duration can be varied (Figure 7). Efficacy of treatment is quantified objectively by comparing the tail photographs before and after treatment using ImageJ analysis (Support Protocol 2).
Figure 7:
Repigmentation Protocol. For pre-clinical studies in mice, induce vitiligo as described in Basic Protocol 1. Allow SCF vitiligo mice to develop vitiligo until disease is stabilized, 10–12 weeks post induction. Start treatment by 12 weeks post vitiligo induction and treat for 8 weeks. After treatment is stopped, quantify the percent repigmentation using ImageJ.
Materials
γ irradiator for small animals
8- to 12-week old SCF Tg host mice (Older mice can be used)
Available from Jackson Laboratory Stock#009687 but the authors can supply breeders upon reader request.
8- to 12-week old Pmel-1 transgenic mice (Jackson Laboratory, Stock#005023)
Red Cell Lysis Buffer (Sigma, R7757–100ML)
MACS CD8+ T cell isolation kit (Miltenyi, 130–104-075)
MACS buffer (see recipe)
quadroMACS separator (Miltenyi, 130–090-976)
MACS MultiStand (Miltenyi, 130–042-203)
LS columns (Miltenyi, 130–042-401)
Scissors (straight or curved)
Curved forceps
1X PBS (Corning cellgro, 21–040-CV))
100-μm Nylon mesh (ELKO)
FALCON Corning Brand tissue culture dish (60 × 15 mm, 353002)
Hemocytometer or automated cell counter
0.5 mL insulin syringes (BD, 329461)
3 or 5 mL syringes (BD,2023–01-31, 2022–02-28)
Ketamine
Xylazine
Frozen rVV-hgp100 stocks
Treatment or test drug (e.g. CD8 depleting antibody)
Vehicle/isotype or other control treatment (e.g. PBS)
Camera (Canon PowerShot G16 58 mm)
ImageJ software (NIH) https://imagej.nih.gov/ij/download.html
Solid white background
Tail photographs
Repigmentation Protocol
-
Induce vitiligo in SCF mice using Basic Protocol 1.
Repigmentation experiments require the tail to have developed at least 50% depigmentation after 10–12 weeks. Only half of vitiligo mice will develop at least 50% depigmentation on the tail. Therefore, induce vitiligo in approximately two times the number of SCF animals required for repigmentation experiments. For example, to ensure 10 useable animals for the study, it is recommended to induce vitiligo in 20 mice.
Wait 10–12 weeks after vitiligo induction.
Select mice for repigmentation experiment by choosing those with at least 50% tail depigmentation.
Take ventral and dorsal tail photographs of all mice before starting treatment to determine baseline depigmentation (Figure 8A). Take photographs without flash or if flash is used, use with a polarized lens to minimize shadows and glare.
To photograph tails, first anesthetize mice with ketamine/xylazine at 100/10 mg/kg by i.p. injection.
Place the anesthetized mice on a white background and carefully photograph both the dorsal and ventral sides of the tail.
Treat mice (e.g. CXCL10 neutralizing antibody, Rashighi et al., 2014 (Richmond, Masterjohn, et al., 2017b) as necessary based on treatment dose and schedule. Stimulation of repigmentation by treatment occurs gradually over time but following 8 weeks of treatment, repigmentation efficacy is easily detectable and quantifiable using ImageJ.
-
Take photographs of mouse tails again at end of treatment schedule. This is often performed on the same day of cellular analysis such as flow cytometry so that you can correlate percent repigmentation with cellular phenotypes (Support protocol 1). Mice can be identified by ear tagging or punching.
Perform ventral and dorsal tail photographs before and after treatment course to quantify percent repigmentation. To quantify repigmentation over time, take photographs during the course of treatment, for example every 2 weeks.
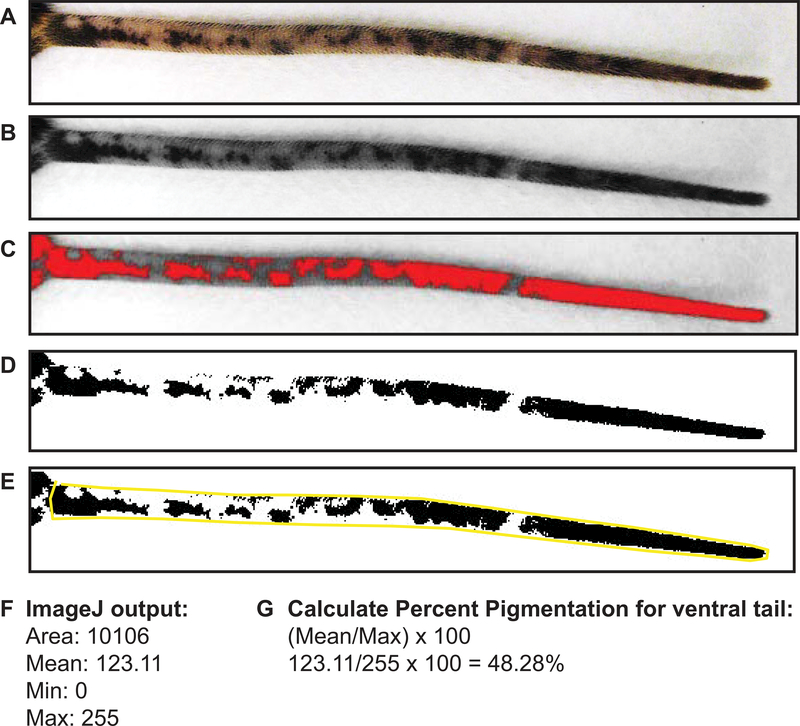
Figure 8:
Quantification of repigmentation using ImageJ analysis. A) Imported ventral tail image. B) Ventral tail image converted to 8-bit black and white. C) Ventral tail image with adjusted brightness threshold to detect areas of pigmentation (red) and depigmentation (grey). D) End result of adjusted ventral tail. E) Ventral tail outlined using the polygon tool. ImageJ will analyze the area outlined to determine the mean area that is pigmented. F) The output from ImageJ after measuring mean pigmentation. G) Formula to calculate percent pigmentation.
Repigmentation analysis using ImageJ
-
9.
Import tail photograph into ImageJ (Figure 8A).
-
10.
Convert photograph to 8-bit black and white (Figure 8B).
From the tool bar go to Image and then select Type and 8-bit.
-
11.
Adjust the brightness threshold so all pigmented areas are black and all depigmented areas are white (Figure 8C, D).
From the tool bar, go to Image, and then select Adjust and Threshold.
-
12.
Outline the entire tail using the freehand/polygon tool (Figure 8E).
-
13.
Calculate the mean area over threshold. This area represents the fraction of pigmented tail skin (Figure 8F). Steps 9–13 are performed for both the ventral and dorsal side of each tail and added together to get the total tail pigment per mouse.
From the tool bar, go to Analyze and then Measure.
-
14.
Divide the mean threshold area calculated above by the maximum (255) and then by one hundred to convert the fraction to percent pigmentation of the tail (Figure 8G).
Take the mean threshold divided by the maximum (255) × 100
-
15.
To calculate the percent change over baseline, take the percent pigmentation after treatment minus the percent pigmentation before treatment.
A positive number indicates repigmentation while a negative number indicates further depigmentation.
SUPPORT PROTOCOL 1
PROCESSING OF SKIN TISSUE FOR FLOW CYTOMETRY ANALYSIS
This section describes the procedure for isolating, separating, and processing of the skin from experimental mice. For analysis of vitiligo mouse skin, the epidermal and dermal tissue is separated using enzymes that release immune cells from the tissue. It is helpful to analyze each skin compartment (epidermis and dermis) separately to determine the location and phenotype of Pmel-1 within each location. Dispase II is used for separation of the epidermis from the dermis. Dispase II is a nonspecific metalloprotease that works by cleaving at the basement membrane of the epidermis. For release of immune cells from the dermal tissue, collagenase is used. Collagenase breaks down the tissue by dismantling collagen, a major structural protein found in the dermis. Note that Dispase II and collagenase can cleave and therefore impact the detectability of some immune cell surface markers, affecting phenotypic analyses (Autengruber, Gereke, Hansen, Hennig, & Bruder, 2012). However, minimal marker deterioration has been found using the protocol below.
Materials
PBS (CORNING cellgro)
RPMI 1640-Medium (Sigma)
Deoxyribonuclease I from bovine pancreas (Sigma, DN25–5G)
Dispase II - neutral protease grade II (Roche Diagnostics, 04942078001)
Collagenase from Clostridium histolyticum (Sigma, C6885–1G)
Falcon tissue culture dish (60 × 14 mm)
100-μm Nylon mesh (Fisher Scientific)
37° incubator or hot room
24 well plate (Thermo Scientific)
5 ml Corning Falcon Round-Bottom polystyrene tubes (Fisher Scientific)
Fetal Bovine Serum (FBS)- heat inactivated (Sigma)
FACS Staining Buffer (see recipe)
FACS staining buffer with Deoxyribonuclease I (1mg/ml)
Harvesting skin tissues from SCF mice
Sacrifice SCF mice according to IACUC regulations.
TAIL
Use a razor blade to cut the tail off at the base.
EAR
Use scissors to cut the ear off at their base.
FOOTPAD
Use a razor blade to cut slits on each site of the footpad. Use forceps to pull the skin away from the foot.
Prepare Dispase II solution for separation of epidermis and dermis
-
For tail skin digestion, dilute dispase II to 5 mg/ml in PBS. Keep on ice.
Tail samples require 1 ml dispase solution per skin sample. For example, for 10 SCF mouse tails make 10 mls of a 5 mg/ml dispase solution.
-
For ear and footpad skin, dilute dispase II to 50 mg/ml in PBS. Keep on ice.
Ear and footpad samples require 500μl dispase solution per skin sample.
Prepare Collagenase solution for digestion of dermis
Prepare a 1 mg/ml collagenase and 2 mg/ml Deoxyribonuclease I solution in RPMI. Make enough for 1 ml per dermis sample. Keep on ice.
Enzymatic digestion to separate the epidermis and dermis
To remove the tail skin, use a razor blade to cut a small slit at the base of the tail. Separate the tail skin from the tail bone by using forceps to hold the tail skin down on the benchtop while peeling the tail bone away from the skin. Once the skin is free from the tail bone, cut it into 6–10 pieces. Place the tail skin pieces into the same well of a 24-well plate.
For ear, split the anterior and posterior halves using a pair of forceps. Place ear skin in one well of a 24- well plate.
Footpad skin is ready for digestion once harvested from the mice. Place footpad skin into one well of a 24- well plate.
For tail skin, add 1 ml of prepared 5 mg/ml dispase solution to each well/sample. Use forceps to make sure all tail skin pieces are completely submerged in the dispase solution. Incubate for 1 hr at 37°C.
For ear and footpad skin, incubate in 500μl prepared 50 mg/ml dispase solution for 1 hr at 37°C.
After incubation at 37°C, place each skin sample into a labeled tissue culture dish.
Use curved forceps to separate the epidermis from the dermis. Leave the isolated epidermis in the tissue culture dish and place the isolated dermis into a labeled 24- well plate.
Add 1 ml of prepared Collagenase solution to each dermis sample and make sure the tissue is completely submerged in the solution. Incubate in collagenase for 45 minutes at 37°C.
To crush epidermis tissue, place a cut piece of nylon mesh into the culture dish. Add 1 ml of staining buffer with Deoxyribonuclease I. Use the back of the plunger of a 3- or 5-ml syringe to crush the tissue into a single cell suspension.
Filter the single cell suspension into a 5 ml FALCON tube using nylon mesh. Wash the nylon mesh with 1 ml of staining buffer to collect any remaining cells from the mesh.
-
After all epidermis tissue is crushed, spin cells at 330 × g for 10 minutes.
Sometimes the skin tissue does not pellet during centrifugation because there is a lot of dead cells and debris, so be sure to check before decanting. The addition of FACS DNase helps the tissue pellet because it breaks down expelled DNA from dead cells, which prevents clumping.
-
Pour off the supernatant and suspend the epidermis cell pellet in 400 μl FACS staining buffer.
Typically, one quarter to one half of the collected skin tissue is stained for flow cytometry analysis.
For dermis, use the back of the plunger of a 3 ml syringe to crush the tissue in the 24-well plate. The solution will become cloudy and this indicates release of dermal cells.
Pipette out the cell suspension and filter into a 5 ml Falcon tube using nylon mesh. Wash nylon mesh with 1 ml of staining buffer with Deoxyribonuclease I.
-
Spin the cell suspension at 330 × g for 10 minutes. Decant the supernatant and suspend the dermis cell pellet in 400 μl FACS buffer.
Again, one quarter to one half of the dermis tissue is stained for flow cytometry.
-
For staining of skin samples, pipette 200 μl of tissue into a 96-well plate or in 5 ml FACS tubes depending on the number of samples. Spin by centrifugation at 330 × g for 10 minutes. Decant the supernatant and suspend the samples in desired antibody staining cocktail.
For detailed protocol on flow cytometry staining of tissue single cell suspensions see Holmes et al, 2001 (Holmes, Lantz, Fowlkes, Schmid, & Giorgi, 2001).
SUPPORT PROTOCOL 2
DETECTION OF PMEL-1 AND CHEMOKINE EXPRESSION IN SKIN USING CONFOCAL MICROSCOPY
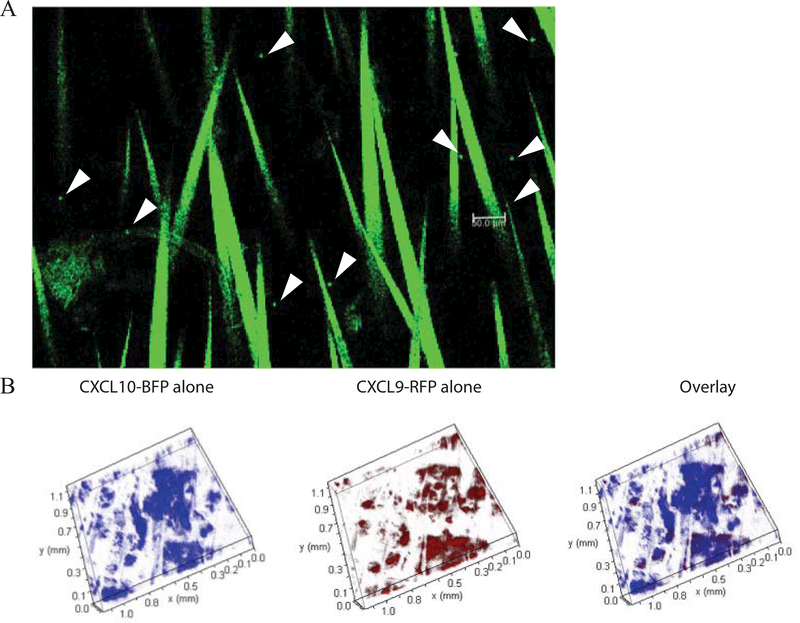
Confocal microscopy of vitiligo mouse skin can be used to identify the position of Pmel-1 and chemokines within the epidermal and dermal tissue. To visualize Pmel-1 in the skin, Pmel-1 TCR transgenic mice were crossed to DPEGFP mice (Manjunath et al., 1999). Induction of vitiligo using GFP+ Pmel-1 CD8+ T cells from these mice allows for the visualization of Pmel-1 by confocal imaging (Figure 9A). Hair follicles are naturally autofluorescent and are visible as linear structures in the image. Additionally, confocal microscopy is used to detect expression of the chemokines CXCL9 and CXCL10 that play a critical role in vitiligo progression. A transgenic mouse was generated that reports RNA expression of CXCL9 and CXCL10 using distinct fluorescent reporter proteins (Groom et al., 2012). In reporting the expression of CXCR3 ligands (REX3) transgenic mice, CXCL9 reports through red fluorescent protein (RFP) and CXCL10 through blue fluorescent protein (BFP). REX3 mice were crossed to SCF mice and vitiligo can be induced in these recipients to visualize chemokine expression as vitiligo develops (Figure 9B). RFP (CXCL9) and BFP (CXCL10) fluorescence can be detected using the confocal microscope, which will elucidate the location of chemokine expression during disease and the skin cell types responsible for producing these chemokines (Richmond, Bangari, et al., 2017a).
Figure 9:
Visualization of Pmel-1 and chemokine expression in the skin by confocal imaging. A) Sample image from a vitiligo mouse at week 7 depicting GFP+ Pmel-1 CD8+ T cells in the tail skin. Pmel-1 are rounded punctate cells (arrows). Bar = 50 μm. Hairs are visible due to autofluorescence. B) Tail skin of REX3 SCF vitiligo mouse showing clouds of CXCL10-BFP and CXCL9-RFP chemokine expression (10× z-stack).
Materials
Vitiligo mouse tail or ear skin tissue (SCF REX3 OR PMEL-1-GFP) –REX3 mice are available from A. Luster at MGH, Harvard, Groom et al., 2012 and DPE-GFP mice are available from Ulrich H.von Andiran at the Ragon Institute.
SCF control mouse tail or ear skin tissue
1X PBS
Glass Slides
Coverslips
Leica SP8 Confocal Microscope
Leica LAS-AF software version 3.3.0 (Leica Biosystems)
Whole mounting of tissue for confocal microscopy
-
Dissect a small piece of tail from a euthanized SCF REX3 mouse.
For ear tissue split the anterior and posterior halves.
Whole-mount the full thickness tail or ear skin on glass slides in PBS.
Keep sample slides on ice until ready to image.
Set laser voltages using the control SCF non-REX3 expressing mouse. This process can take up to 30 minutes. Each slide takes an average of 10–15 minutes to image. A minimum of 3 separate fields of view are taken per mouse ear or tail. 10× magnification is used to see REX3 expression in the skin and 40× magnification is used for imaging GFP positive Pmel-1 in the skin tissue.
REAGENTS AND SOLUTIONS
MACS Buffer
1X PBS (500 mL)
grams or 2.5% Bovine Serum Albumin (BSA)
Add 2 mLs of 0.5M EDTA (Fisher scientific, #PR V4231) for a final concentration of 2mM EDTA
Sterile filter (ThermoFisher Scientific #166–0045)
Store protected from light at 2–8°C for up to 3 months
FACS Staining Buffer
1X PBS (500 mL)
Add 1% or 5 mL Fetal Bovine Serum
Store protected from light at 2–8°C for up to 3 months
COMMENTARY
Background Information
Vitiligo is a common autoimmune disease of the skin in which the pigment producing cells of the epidermis, melanocytes, are targeted for destruction. Death of melanocytes leads to the appearance of patchy depigmentation of the skin. Vitiligo affects roughly 1% of the population, is a severely disfiguring disease, and patients’ quality of life and self-esteem are significantly impacted (Alikhan, Felsten, Daly, & Petronic-Rosic, 2011; Ezzedine et al., 2015; J. E. Harris, 2017; Picardo et al., 2015; Taïeb & Picardo, 2009). Patients may also develop numerous adverse effects from vitiligo and its systemic associations. There are multiple other autoimmune diseases associated with vitiligo including Addison’s disease, type I diabetes, and thyroiditis (Alkhateeb, Fain, Thody, Bennett, & Spritz, 2003; Cunliffe, Hall, Newell, & Stevenson, 1968; Gill et al., 2016; Spritz, 2013). The standard treatment for vitiligo patients is narrow band ultraviolet B (nbUVB) therapy in combination with topical corticosteroids and calcineurin inhibitors. This therapy is effective for many patients with up to 100% repigmentation possible, depending on the anatomical site that is affected (Rodrigues et al., 2017). However, vitiligo is a chronic disease that requires lifelong therapy and currently there are no FDA approved treatments for vitiligo. Because of the few options for treatment, limited access to nbUVB, their slow and sometimes poor efficacy, and only short-term benefit, there is a significant need to develop targeted, durable treatments for disease that are safe and have high efficacy (Frisoli & Harris, 2017).
It has been demonstrated that melanocyte-specific CD8+ T cells are both necessary and sufficient to mediate melanocyte destruction in vitiligo. Melanocyte specific CD8+ T cells isolated from HLA A2+ vitiligo patients were able to lyse A2 melanoma cells in vitro (Ogg et al., 1998). Additionally, perilesional CD8+ T cells isolated from vitiligo patient skin could cause melanocyte apoptosis in normal pigmented skin isolated from the same patient when cultured together ex vivo (van den Boorn et al., 2009). These studies highlight the importance of CD8+ T cells in causing melanocyte death, but how autoreactive CD8+ T cells find their melanocyte target in vivo and how CD8+ T cells cooperate to kill melanocytes was largely unknown. While early clinical and translational studies of vitiligo were important for defining vitiligo as an autoimmune disease, animal models have played an important role in the discovery of factors that promote disease progression (Essien & Harris, 2014).
Multiple mouse models have been developed that induce depigmentation, but all of these models of vitiligo measured hair follicle melanocyte death and depigmentation rather than epidermal depigmentation that is seen in human vitiligo (Table 1)(Essien & Harris, 2014). In normal skin, melanocytes reside in two major compartments. Mature melanocytes reside in the epidermis, the uppermost layer, and provide pigment to the skin. Additionally, mature melanocytes reside in the hair follicles to which they provide pigment to the hair shaft. The hair follicle also serves as a stem cell reservoir and houses epidermal precursor cells including melanocyte stem cells (Cichorek, Wachulska, Stasiewicz, & Tymińska, 2013). In human vitiligo, epidermal melanocytes are specifically targeted for destruction while the melanocytes residing in the hair follicle are often spared from autoimmune attack, presumably due to immune privilege of the hair follicle (Falabella, 2009). It is from the melanocyte precursors within the hair follicle that mature melanocytes can be replenished in the epidermis. Patients responding well to therapy will develop macules of pigment surrounding the hair follicles, which will then spread to the interfollicular epidermis, a pattern called perifollicular repigmentation (Figure 6A) (Birlea et al., 2017; Cui et al., 1991). Patients with vitiligo lesions in areas without hair (such as the knuckles, elbows, and ventral wrists) or where the hair has lost pigment, typically have poor treatment responses and do not regain their pigment. The difference in targeting of the epidermal melanocytes and hair follicle melanocytes in vitiligo remains unclear but is likely due to “immune privilege” of hair follicles, which protects them from inflammation (Falabella, 2009).
This unit describes the first mouse model of vitiligo that develops epidermal depigmentation and spares the hair follicles, similar to disease in humans. This distinction is important as the location of T cell trafficking into the epidermis is different from trafficking into the hair follicles. Trafficking to these different locations is likely mediated by distinct cytokines and requires overcoming different barriers. Involvement of the hair follicle in human vitiligo is thought to be a more severe form of the disease. Most importantly, reversal of disease with treatment depends on the return of melanocyte stem cells from the hair follicle, visible as perifollicular repigmentation. Thus, if hair follicles are involved, reversal of disease is not possible. Therefore, a preclinical model to test treatments that reverse disease requires maintenance of hair pigmentation. For comparisons of human and mouse vitiligo skin see Figure 5.
Previous research using the mouse model of vitiligo described in this unit identified multiple pathways involved in the pathogenesis of disease that parallel findings in vitiligo patients (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012a; Rashighi, Agarwal, Richmond, Harris, Dresser, Su, Zhou, Deng, Hunter, Luster, & Harris, 2014a; Strassner, Rashighi, Ahmed Refat, Richmond, & Harris, 2017a). The studies found that Interferon-(IFN)γ and the interferon-induced chemokines C-X-C Motif Ligand-(CXCL)9 and CXCL10 are critical for disease progression (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012a; Rashighi, Agarwal, Richmond, Harris, Dresser, Su, Zhou, Deng, Hunter, Luster, & Harris, 2014a). Blocking IFNγ, or using mice lacking CXCL10, prevents vitiligo development in mice. Interestingly, CXCL9-deficiency does not protect mice from developing vitiligo, but it was found to play a non-redundant role in the recruitment of CD8+ T cells to the skin. Additional studies identified an IFNγ-specific gene signature in the lesional skin of both vitiligo mice and human vitiligo patients. The pathways identified using vitiligo mice discussed in this unit have closely paralleled those in human patients. A recent report sought to identify biomarkers of disease activity in vitiligo patients comparing skin samples of healthy controls to active and stable vitiligo patients. These samples were obtained using a blistering technique that was modified to obtain immune cells and interstitial skin fluid from the lesions and control skin. Results revealed that CD8+ T cell number and CXCL9 were excellent biomarkers of vitiligo disease activity (Strassner, Rashighi, Ahmed Refat, Richmond, & Harris, 2017b). It is clear that the pathways and mechanisms identified using this mouse model of vitiligo have been crucial to the understanding of human vitiligo and will help to improve patient care and to develop targeted therapies.
Critical Parameters
Recipient mice for epidermal depigmentation
A critical element to generating a mouse model of epidermal depigmentation was to use mice containing melanocytes within the epidermis. Melanocytes depend on the expression of Kit protein, a receptor tyrosine kinase, for their development, migration, and survival. The ligand for kit, also known as stem cell factor (SCF), is produced locally in human skin by multiple cell types including keratinocytes, fibroblasts, and endothelial cells (Grichnik, Burch, Burchette, & Shea, 1998; Wehrle-Haller, 2003). It is the expression of SCF by epidermal cells that directs and positions melanocytes within the interfollicular epidermis in human skin. In contrast to human skin, the epidermis of adult C57Bl6 mice does not express SCF and instead expression is limited to the dermis and hair follicles. As a result, melanocytes in C57Bl6 mice are restricted to the hair follicles with only a small number of melanocytes found in the tail and footpad epidermis (Nishimura et al., 2002; Nishimura, Yoshida, Kunisada, & Nishikawa, 1999).
SCF has two forms, soluble or transmembrane, depending on alternative splicing of the protein. Previous research on SCF identified two proteolytic domains required for the generation of soluble SCF, and found that mutation of the proteolytic domains restricted the protein to the membrane while remaining biologically active (Majumdar, Feng, Medlock, Toksoz, & Williams, 1994). Transgenic mice were generated to express the membrane bound form of SCF in keratinocytes, which are the majority of cells that make up the epidermis. To accomplish this, engineered mice express SCF driven from the human keratin 14 promoter, KRT14-kitl*4XTG2Bjl (Kunisada et al., 1998). In contrast to wild-type C57Bl6 mice that lack SCF expression in the epidermis, KRT14-kitl*4XTG2Bjl mice express SCF by epidermal keratinocytes and have black skin owing to the retention of melanocytes in the interfollicular epidermal basal layer and follicular epidermis (Figure 1). In this article, recipient mice for vitiligo induction are referred to as SCF mice.
Melanocyte-specific CD8+ T cells
Studies of melanoma pathogenesis and immunotherapy led to the identification of multiple melanocyte specific-proteins targeted during the cytotoxic T lymphocyte response. Not surprisingly, these proteins are also vitiligo autoantigens. One such protein is premelanosome protein (Pmel-1) which is highly enriched in the melanin-producing organelles of melanocytes, called melanosomes. Pmel-1 T cell receptor (TCR) transgenic mice were generated by the Restifo lab (NIH) and Pmel-1 T cells recognize the melanocyte differentiation antigen gp10025–33 presented on H-2Kb (Overwijk et al., 2003; 1998). For induction of vitiligo, Pmel-1 TCR transgenic mice are used as the source of effector CD8+ T cells and are adoptively transferred into SCF recipients. Purification of naïve CD8+ T cells from Pmel-1 mice is performed using a magnetic- isolation technique. The spleen from a Pmel-1 TCR transgenic donor is harvested, crushed, and the splenic cell suspension is run over a magnetic cell separation (MACS) CD8+ T cell negative selection isolation column to avoid premature activation or blocking of CD8 surface receptor that might occur from a positive selection column. Purity of CD8+ T cells after column isolation is usually 80%−90%. Recovery of CD8+ T cells per Pmel-1 donor spleen is about 10 million cells and therefore one Pmel-1 donor provides cells for about 10 experimental SCF mice. Total time for harvest of spleen to isolation of CD8+ T cells is about 90 to 120 minutes. Pmel-1 CD8+ T cells are transferred into SCF recipients by retro-orbital (r.o.) injection. For r.o. injections, SCF mice are anesthetized using a combination of Ketamine/Xylazine. Pmel-1 CD8+ T cells are tracked in the SCF recipient by expression of the T lymphocyte Thy1a congenic marker or by fluorescent protein expression such as green fluorescent protein (GFP). Pmel-1 TCR transgenic mice are available for purchase at the Jackson Laboratory.
One limitation of inducing vitiligo as in Basic Protocol 1 is that a clonal population of Pmel-1 CD8+ T cells is used to mediate disease. While Pmel-1 is a relevant target antigen in human vitiligo, human disease appears to be driven by polyclonal CD8+ T cells and it is likely that each patient varies in the specificity of their auto-reactive CD8+ T cell populations.
Infection with vaccinia virus
As stated above, Pmel-1 CD8+ T cells recognize the peptide gp10025–33. Recombinant vaccinia viruses (rVV) encoding mouse gp100 and human gp100 were generated and tested in immunization experiments to target melanoma (Overwijk et al., 1998). Studies showed that only immunization with rVV encoding human gp100 (rVV-hgp100) induced immune reactivity to both mouse and human gp100 and that rVV-hgp100 induced a robust CD8+ T response in mice. Recombinant VV-hgp100 was a gift from the Restifo lab (NIH) and recipient SCF mice are infected by intraperitoneal (i.p.) injection with rVV-hgp100 to induce activation of transferred Pmel-1 CD8+ T cells in vivo. The infection induces the proliferation, expansion, and activation of Pmel-1 CD8+ T cells (Overwijk et al, 2003). For more information regarding the generation of recombinant vaccinia virus see (Earl, Moss, Wyatt, & Carroll, 2001).
NOTE: rVV-hugp100 is a highly attenuated strain of vaccinia virus for infection in mice. However, vaccinia virus can replicate in human cells and should be treated with caution. Those working with vaccinia virus are able to request a vaccination with vaccinia (the smallpox vaccine) should they choose.
Summary of Mouse Model
Induction of vitiligo in SCF mice is dependent on sublethal irradiation, Pmel-1 CD8+ T cell transfer, and infection with rVV-hgp100 to activate Pmel-1 CD8+ T cells in vivo (Figure 3). The day before Pmel-1 CD8+ T cell transfer, SCF recipient mice are sublethally irradiated (500 rad). This step is critical for the synchronization of vitiligo and may work by causing lymphocyte cell death to make space for the transferred Pmel-1 cells, although the exact role of irradiation is still not clear. The following day, one million purified Pmel-1 CD8+ T cells are transferred to SCF recipient mice by retro-orbital injection. Retro-orbital injections are used because the tail vein of SCF mice is not visible, owing to their black skin. On the same day, SCF recipients are infected with 106 pfu rVV-hgp100 i.p., which causes the activation and expansion of Pmel-1 CD8+ T cells in vivo (Overwijk et al., 2003). Induction of vitiligo in SCF mice results in epidermal white spots of depigmentation on the tail, ears, footpad, and nose (Figure 2A, B). The number of infiltrating Pmel-1 in most mice peaks at 5 weeks post vitiligo induction (Figure 2C). Infiltration of Pmel-1 to the epidermis correlates with depigmentation and melanocyte death. Melanocyte transcripts slowly decrease over time and significantly drop by 5 weeks post vitiligo induction (J. E. Harris, Harris, Weninger, Wherry, Hunter, & Turka, 2012b). Spots of depigmentation appear as early as 3–4 weeks and continue to develop in severity over time. SCF mice reach a plateau of vitiligo around 7–8 weeks, after which disease stabilizes and the majority of Pmel-1 adopt a resident memory phenotype defined as CD103+CD69+CD44+CD62L−(Richmond et al., 2018).
Because vitiligo is intentionally initiated through transfer and activation of naïve Pmel-1 CD8+ T cells in vivo, this model is not positioned to study mechanisms of disease initiation, such as the role of melanocyte stress and its downstream induced pathways. It is not fitted for studying environmental factors of disease initiation and SCF mice are not genetically predisposed to spontaneously develop vitiligo. Vitiligo mice can be used to study innate immune pathways that assist Pmel-1 CD8+ T cells in mediating disease but is not necessarily appropriate for studying innate pathways involved in the initiation of vitiligo.
The most important aspect to successful induction of vitiligo in mice is proper and consistent injection technique. Practicing the injection of Pmel-1 and rVV-hgp100 are critical for reproducible results and for efficient completion of the procedure. The number of Pmel-1 cells injected and the correct viral titer of rVV-hgp100 can affect vitiligo development. Thus, careful attention to Pmel-1 isolation and count is important. The full activation of Pmel-1 by rVV-hgp100 also proves critical. Virus stocks can clump during the freezing process so mix virus stocks vigorously by sonication or vortexing when thawing to break up clumps. Previous studies revealed that injecting Pmel-1 alone resulted in inconsistent vitiligo development in terms of disease severity and kinetics. Proper injection of Pmel-1 and rVV-hgp100 will result in measurable vitiligo at synchronized and expected time intervals. Vitiligo induction results in variable disease severity among wild-type mice. Because of variability in vitiligo disease scores it is important to set up at least 5 to 10 mice per group in order to measure changes in disease score. Scoring of vitiligo is subjective and is highly susceptible to observer bias, and therefore should be done by a blinded investigator.
Troubleshooting
For troubleshooting instructions please see table.
Statistical Analyses
A variety of parametric statistical tests can be used depending on the number of comparisons. For one comparison between two groups, the student’s T test is used. For two or more comparisons, ANOVA with subsequent multiplicity adjusted post T test (Tukey’s) is used.
Understanding Results
Mice treated as in Basic Protocol 1 clear vaccinia virus infection between 10–14 days. Early observations of epidermal depigmentation include patchy light spots that then become completely white. Epidermal depigmentation starts to develop at 3 weeks post induction although melanocyte transcripts begin to decrease earlier at week 2. The requirement for keratinocyte turnover to see the spots is likely the reason for the delay in observing depigmentation. Mice will typically develop white spots on the tail and rear footpads first, with spots developing on the ears and nose in mice with severe vitiligo. Mice with more severe tail depigmentation typically go on to develop depigmentation at multiple sites. Pmel-1 CD8+ T cells are recruited into the skin as early as one week after vitiligo induction (unpublished observation) but infiltration peaks at 5–7 weeks post vitiligo induction. We have found that Pmel-1 CD8+ T cells remain in the skin until the end of natural life as resident memory CD8+ T cells(Richmond et al., 2018). Between 10–12 weeks post vitiligo induction some wild-type SCF mice spontaneously repigment and the reasons behind this are unknown. Other mice remain depigmented. Vitiligo mice do not succumb to disease unless lymphoma cells are unknowingly transferred into recipients (see troubleshooting). The mice do not develop additional sequela related to vaccinia virus or Pmel-1 CD8+ T cell transfer. All observations are general and may differ when genetically modified SCF recipients or Pmel-1 CD8+ T cells are used in experiments.
Time Considerations
The induction of vitiligo in mice requires significant planning, as the time between experimental set up and analysis takes up to two months or more, depending on the variables to be tested. Vitiligo induction requires availability of SCF and Pmel-1 mice of appropriate age. If comparing genetically altered SCF mice to wild-type SCF mice, be sure to have appropriate littermate controls. Vitiligo induction requires hands on work for two consecutive days. The first day, SCF mice are irradiated. Animal Biosafety Level 2 (ABSL2) handling and containment is required for in vivo infection with rVV-hgp100. Thus, before infection with vaccinia virus, mice must be moved to an ABSL2 facility. The second day requires multiple hours of uninterrupted work isolating Pmel-1 CD8+ T cells and performing in vivo transfer and infection with rVV-hgp100. To minimize the time Pmel-1 cells remain on ice, it is critical to work efficiently. A typical vitiligo experiment takes about 7 weeks. For repigmentation experiments, planning ahead is even more important. Vitiligo disease in mice must be stabilized before treatment, which takes about 10– 12 weeks. Then mice are treated with target molecules for 8 weeks before disease quantification (18–20 weeks total time).
Significance Statement.
Vitiligo is a severely disfiguring autoimmune skin disease that affects about 1% of the population. Vitiligo patients develop patchy white spots on the skin that result from the loss of pigment-producing cells in the epidermis known as melanocytes. Mouse models of vitiligo have been critical to the understanding of vitiligo pathogenesis and research using these models has revealed key immune pathways responsible for human vitiligo pathogenesis. More broadly, there is also considerable overlap between the pathways responsible for vitiligo and those of other organ-specific autoimmune diseases. Therefore, vitiligo mice are not only an excellent tool for the investigation of vitiligo disease pathogenesis, but also reveal basic scientific principles that lead to the identification of new targets for therapeutic use in autoimmunity.
ACKNOWLEDGEMENT
We thank B.J Longley for KRT14-Kitl* mice, U. von Andrian for DPEDPE mice, A. Luster for REX3 mice, and N. Restifo for recombinant vaccinia virus. We thank James Strassner for his insightful comments on the article. This work was supported by the National Institutes of Health through grants AR09114 and AR07302 to JEH, American Skin Association and Dermatology Foundation grants to JMR and training grant AI007349 to RLR.
LITERATURE CITED
- Agarwal P, Rashighi M, Essien KI, Richmond JM, Randall L, Pazoki-Toroudi H, et al. (2015). Simvastatin Prevents and Reverses Depigmentation in a Mouse Model of Vitiligo. Journal of Investigative Dermatology, 135(4), 1080–1088. 10.1038/jid.2014.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhan A, Felsten LM, Daly M, & Petronic-Rosic V (2011). Vitiligo: A comprehensive overview. Journal of American Dermatology, 65(3), 473–491. 10.1016/j.jaad.2010.11.061 [DOI] [PubMed] [Google Scholar]
- Alkhateeb A, Fain PR, Thody A, Bennett DC, & Spritz RA (2003). Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Research, 16(3), 208–214. [DOI] [PubMed] [Google Scholar]
- Autengruber A, Gereke M, Hansen G, Hennig C, & Bruder D (2012). Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function. European Journal of Microbiology & Immunology, 2(2), 112–120. 10.1556/EuJMI.2.2012.2.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birlea SA, Costin G-E, Roop DR, & Norris DA (2017). Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization. Medicinal Research Reviews, 37(4), 907–935. 10.1002/med.21426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichorek M, Wachulska M, Stasiewicz A, & Tymińska A (2013). Skin melanocytes: biology and development. Postepy Dermatologii I Alergologii, 30(1), 30–41. 10.5114/pdia.2013.33376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shen LY, & Wang GC (1991). Role of hair follicles in the repigmentation of vitiligo. Journal of Investigative Dermatology, 97(3), 410–416. [DOI] [PubMed] [Google Scholar]
- Cunliffe WJ, Hall R, Newell DJ, & Stevenson CJ (1968). Vitiligo, thyroid disease and autoimmunity. British Journal of Dermatology, 80(3), 135–139. [DOI] [PubMed] [Google Scholar]
- Donovan J, & Brown P (2006a). Animal identification. Current Protocols in Immunology, Chapter 1(1), Unit 1.5–1.5.4. 10.1002/0471142735.im0105s73 [DOI] [PubMed] [Google Scholar]
- Donovan J, & Brown P (2006b). Euthanasia. Current Protocols in Immunology, Chapter 1(1), Unit 1.8–1.8.4. 10.1002/0471142735.im0108s73 [DOI] [PubMed] [Google Scholar]
- Donovan J, & Brown P (2006c). Parenteral injections. Current Protocols in Immunology, Chapter 1(1), Unit 1.6–1.6.10. 10.1002/0471142735.im0106s73 [DOI] [PubMed] [Google Scholar]
- Earl PL, Moss B, Wyatt LS, & Carroll MW (2001). Generation of recombinant vaccinia viruses. Current Protocols in Molecular Biology, Chapter 16(1), Unit16.17–16.17.19. 10.1002/0471142727.mb1617s43 [DOI] [PubMed] [Google Scholar]
- Essien KI, & Harris JE (2014). Animal models of vitiligo: Matching the model to the question. Dermatologica Sinica, 32(4), 240–247. 10.1016/j.dsi.2014.09.008 [DOI] [Google Scholar]
- Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, et al. (2015). Vitiligo is not a cosmetic disease. Journal of the American Academy of Dermatology, 73(5), 883–885. 10.1016/j.jaad.2015.07.039 [DOI] [PubMed] [Google Scholar]
- Falabella R (2009). Vitiligo and the melanocyte reservoir. Indian Journal of Dermatology, 54(4), 313–318. 10.4103/0019-5154.57604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisoli ML, & Harris JE (2017). Vitiligo: Mechanistic insights lead to novel treatments. The Journal of Allergy and Clinical Immunology, 140(3), 654–662. 10.1016/j.jaci.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, & Hamzavi I (2016). Comorbid autoimmune diseases in patients with vitiligo: A cross-sectional study. Journal of the American Academy of Dermatology, 74(2), 295–302. 10.1016/j.jaad.2015.08.063 [DOI] [PubMed] [Google Scholar]
- Grichnik JM, Burch JA, Burchette J, & Shea CR (1998). The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. Journal of Investigative Dermatology, 111(2), 233–238. 10.1046/j.1523-1747.1998.00272.x [DOI] [PubMed] [Google Scholar]
- Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, et al. (2012). CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4+ T Helper 1 Cell Differentiation. Immunity, 37(6), 1091–1103. 10.1016/j.immuni.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE (2016). Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunological Reviews, 269(1), 11–25. 10.1111/imr.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE (2017). Optimizing Vitiligo Management: Past, Present, and Future. Dermatologic Clinics, 35(2), xi 10.1016/j.det.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, & Turka LA (2012a). A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFNγ for Autoreactive CD8+ T-Cell Accumulation in the Skin, 132(7), 1869–1876. 10.1038/jid.2011.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, & Turka LA (2012b). A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-γ for Autoreactive CD8+ T-Cell Accumulation in the Skin. Journal of Investigative Dermatology, 132(7), 1869–1876. 10.1038/jid.2011.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K, Lantz LM, Fowlkes BJ, Schmid I, & Giorgi JV (2001). Preparation of cells and reagents for flow cytometry. Current Protocols in Immunology, Chapter 5(1), Unit 5.3–5.3.24. 10.1002/0471142735.im0503s44 [DOI] [PubMed] [Google Scholar]
- Kunisada T, Lu S-Z, Yoshida H, Nishiawa S, Nishikawa S-I, Mizoguchi M, et al. (1998). Murine Cutaneous Mastocytosis and Epidermal Melanocytosis Induced by Keratinocyte Expression of Transgenic Stem Cell Factor. Journal of Experimental Medicine, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar MK, Feng L, Medlock E, Toksoz D, & Williams DA (1994). Identification and mutation of primary and secondary proteolytic cleavage sites in murine stem cell factor cDNA yields biologically active, cell-associated protein. Journal of Biological Chemistry, 269(2), 1237–1242. [PubMed] [Google Scholar]
- Manjunath N, Shankar P, Stockton B, Dubey PD, Lieberman J, & Andrian, von UH (1999). A transgenic mouse model to analyze CD8(+) effector T cell differentiation in vivo. Proceedings of the National Academy of Sciences, 96(24), 13932–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, et al. (2002). Dominant role of the niche in melanocyte stem-cell fate determination. Nature, 416(6883), 854–860. 10.1038/416854a [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Yoshida H, Kunisada T, & Nishikawa SI (1999). Regulation of E- and P-cadherin expression correlated with melanocyte migration and diversification. Developmental Biology, 215(2), 155–166. 10.1006/dbio.1999.9478 [DOI] [PubMed] [Google Scholar]
- Ogg GS, Dunbar PR, Romero P, Chen J-L, & Cerundolo V (1998). High Frequency of Skin-homing Melanocyte-specific Cytotoxic T Lymphocytes in Autoimmune Vitiligo. Journal of Experimental Medicine, 188, 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. (2003). Tumor Regression and Autoimmunity after Reversal of a Functionally Tolerant State of Self-reactive CD8 +T Cells. Journal of Experimental Medicine, 198(4), 569–580. 10.1084/jem.20030590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. (1998). gp100/pmel 17 Is a Murine Tumor Rejection Antigen: Induction of “Self-”reactive, Tumoricidal T cells Using High-affinity, Altered Peptide Ligand. Journal of Experimental Medicine, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardo M, Dell’Anna ML, Ezzedine K, Hamzavi I, Harris JE, Parsad D, & Taïeb A (2015). Vitiligo. Nature Reviews. Disease Primers, 1, 15011 10.1038/nrdp.2015.11 [DOI] [PubMed] [Google Scholar]
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su MW, Zhou Y, Deng A, Hunter CA, Luster AD, & Harris JE (2014a). CXCL10 Is Critical for the Progression and Maintenance of Depigmentation in a Mouse Model of Vitiligo. Science Translational Medicine, 6(223), 223ra23–223ra23. 10.1126/scitranslmed.3007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su M-W, Zhou Y, Deng A, Hunter CA, Luster AD, & Harris JE (2014b). CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Science Translational Medicine, 6(223), 223ra23–223ra23. 10.1126/scitranslmed.3007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves JP, & Reeves PA (2001). Removal of lymphoid organs. Current Protocols in Immunology, Chapter 1(1), Unit 1.9–1.9.3. 10.1002/0471142735.im0109s01 [DOI] [PubMed] [Google Scholar]
- Richmond JM, Bangari DS, Essien KI, Currimbhoy SD, Groom JR, Pandya AG, et al. (2017a). Keratinocyte-Derived Chemokines Orchestrate T-Cell Positioning in the Epidermis during Vitiligo and May Serve as Biomarkers of Disease. Journal of Investigative Dermatology, 137(2), 350–358. 10.1016/j.jid.2016.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JM, Frisoli ML, & Harris JE (2013). Innate immune mechanisms in vitiligo: danger from within. Current Opinion in Immunology, 25(6), 667–669. 10.1016/j.coi.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, & Harris JE (2017b). CXCR3 Depleting Antibodies Prevent and Reverse Vitiligo in Mice. The Journal of Investigative Dermatology, 137(4), 982–985. 10.1016/j.jid.2016.10.048 [DOI] [PubMed] [Google Scholar]
- Richmond JM, Strassner JP, Zapata L, Garg M, Riding RL, Refat MA, et al. (2018). Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Science Translational Medicine, 10(450), eaam7710 10.1126/scitranslmed.aam7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. (2017). Current and emerging treatments for vitiligo. Journal of the American Academy of Dermatology, 77(1), 17–29. 10.1016/j.jaad.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Spritz RA (2013). Modern vitiligo genetics sheds new light on an ancient disease. The Journal of Dermatology, 40(5), 310–318. 10.1111/1346-8138.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner JP, Rashighi M, Ahmed Refat M, Richmond JM, & Harris JE (2017a). Suction blistering the lesional skin of vitiligo patients reveals useful biomarkers of disease activity. Journal of American Dermatology, 76(5), 847–855.e5. 10.1016/j.jaad.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner JP, Rashighi M, Ahmed Refat M, Richmond JM, & Harris JE (2017b). Suction blistering the lesional skin of vitiligo patients reveals useful biomarkers of disease activity. Journal of American Dermatology, 76(5), 847–855.e5. 10.1016/j.jaad.2016.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïeb A, & Picardo M (2009). Vitiligo Clinical practice. New England Journal of Medicine, 160–169. [DOI] [PubMed] [Google Scholar]
- Thornton AM (2003). Fractionation of T and B cells using magnetic beads. Current Protocols in Immunology, Chapter 3(1), Unit 3.5A–3.5A.11. 10.1002/0471142735.im0305as57 [DOI] [PubMed] [Google Scholar]
- van den Boorn JG, Konijnenberg D, Dellemijn TAM, van der Veen JPW, Bos JD, Melief CJM, et al. (2009). Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. The Journal of Investigative Dermatology, 129(9), 2220–2232. 10.1038/jid.2009.32 [DOI] [PubMed] [Google Scholar]
- van den Boorn JG, Melief CJ, & Luiten RM (2011). Monobenzone-induced depigmentation: from enzymatic blockade to autoimmunity. Pigment Cell Melanoma Res, 24(4), 673–679. 10.1111/j.1755-148X.2011.00878.x [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B (2003). The Role of Kit-Ligand in Melanocyte Development and Epidermal Homeostasis. Pigment Cell Research, 287–296. [DOI] [PubMed] [Google Scholar]