Abstract
Introduction:
Neisseria meningitidis serogroup B is the most common form of meningococcal infection in young adults in the U.S. Vaccines have recently become available, but it is not clear that the benefits outweigh the costs. The purpose of this study was to assess cost effectiveness and determine potentially favorable conditions for universal vaccination.
Methods:
Costs and benefits of universal vaccination at college entry versus no universal vaccination with an outbreak response were estimated in 2018 in the context of a mid-sized U.S.-based 4-year college from both a health sector and a societal perspective. Probability, cost, and utility data were obtained from the published literature. Costs (2015 U.S.$) and benefits were discounted at 3%. One-way and multivariable probabilistic sensitivity analyses were performed including variations in the specific vaccine used. Further testing of the model’s parameters at extremes was used to identify favorable conditions for universal vaccination.
Results:
The incremental cost per quality-adjusted life year gained with universal vaccination was $13.9 million under the health sector perspective and $13.8 million under the societal perspective, each perspective was compared with a willingness-to-pay threshold of $150,000 per quality-adjusted life year. Multivariable probabilistic sensitivity analysis showed that universal vaccination was not the preferred strategy for <$15 million per quality-adjusted life year. Under an extremely favorable model, a universal vaccination strategy became cost effective for vaccine series costing <$65.
Conclusions:
This study demonstrates that universal vaccination at college entry is not cost effective. The rarity of Neisseria meningitidis serogroup B contributes to the lack of cost effectiveness for universal vaccination.
INTRODUCTION
Infection with Neisseria meningitidis results in long-term morbidity and high mortality despite improved access to and administration of supportive treatment in critical care settings as well as antibiotic therapies.1,2 Established vaccination programs have reduced the incidence of serogroups A, C, W, and Y. As a result, N. meningitidis serogroup B (MenB) is now the most prevalent form of meningococcal infection in individuals aged 16 to 23 years.3 Two Food and Drug Administration–approved MenB vaccines have overcome the high antigenic variability and the need for unique biotechnologic approaches to production.4 Their introduction has led to mass MenB vaccination for college-aged young adults in outbreak settings given the persistent cases of meningococcal infection in this age group and high incidence relative to other population subgroups.5
Despite the critical contributions of vaccination to public health, existing MenB vaccines may not be able to offer a societal health benefit commensurate with their resource use. MenB vaccines’ immunogenicity quickly wanes over time limiting their ability to provide long-term protection like many other vaccines.6 Moreover, the existing vaccine series for MenB are more expensive than previously approved vaccines for other infectious diseases.7 Advocates for vaccination argue that the devastating consequences of MenB infection limit the ability to perform cost-effectiveness studies.8 The college-aged subpopulation has been specifically identified as a targetable group who would most benefit from vaccine protection given the increased exposure risk and the long life expectancy and large societal contribution of these individuals.9 Prior to widespread use of MenB vaccines in adolescents, further work may need to justify the high cost of the vaccine versus the gains accrued to individuals, payers, and society.
The purpose of this study is to assess the cost effectiveness of universal MenB vaccination of entering college students in the U.S. relative to the current standard of care, where vaccination is an individual decision. This question is addressed from both a health sector perspective that incorporated individual and payer costs and a societal perspective that further accounted for lifetime productivity losses among students experiencing MenB infection.
METHODS
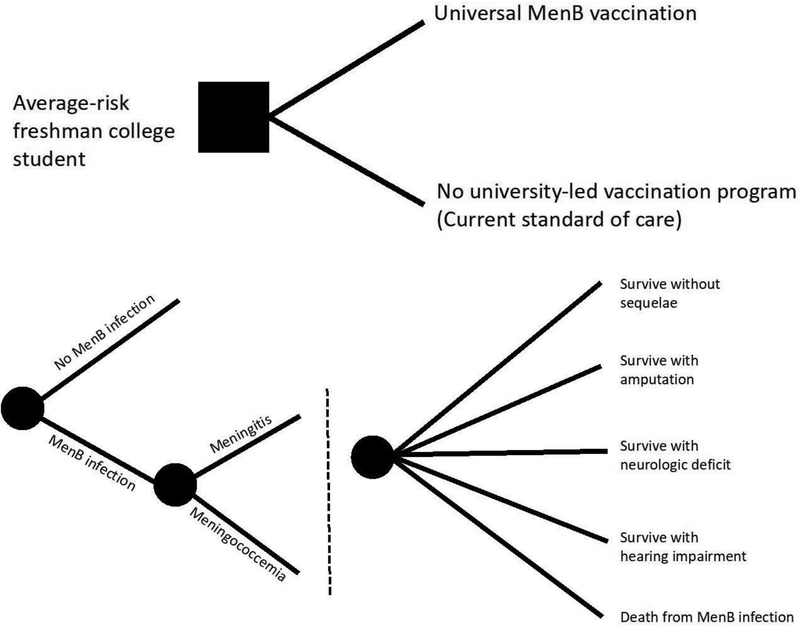
The authors developed a decision tree model using TreeAge Pro 2018, version 18.1 to assess the incremental cost effectiveness and cost per case averted for universal vaccination of incoming students at a mid-sized U.S.-based higher education institution versus no formal vaccination program (current standard of care). This decision point and the resulting risk of MenB infection and associated healthcare events is modeled in Figure 1. All reporting methods described here were adapted from the recommendations of the Second Panel on Cost Effectiveness in Health and Medicine.10,11
Figure 1.
Decision tree model of universal vaccination versus no formal vaccination program in college students (A) and schematized sequelae (B). Note: To enhance readability, dashed lines represent downstream sequences of identical chance nodes that stem from each of the associated upstream chance nodes. MenB, Neisseria meningitidis serogroup B.
Study Population
In order to provide estimates for a real-world setting, this study modeled a hypothetical 4-year U.S.-based liberal arts college with 1,000 students per class and 4,000 faculty and staff in the year 2018. Each individual student was assumed to be at equal risk for MenB infection and to enter college at age 18 years. For the decision to not perform universal preemptive vaccination, it was assumed that a suspected outbreak (i.e., one serogroup-confirmed case) led to a belated universal vaccination campaign of all students, faculty, and staff. Such an aggressive mass vaccination following an outbreak was assumed based on an intended conservative bias favoring early vaccination and the likelihood of many on-campus individuals seeking vaccination independently even if not directly administered as part of an outbreak response. In addition, although the Centers for Disease Control and Prevention (CDC) recommends two to three localized cases before initiating an outbreak response, the authors used a single case to reduce unnecessary model complexity and given that many college administrators may be pressured to initiate an outbreak response after even a single high-publicity case.6,12 For the purpose of this model, all students left the institution after 4 years.
Measures
Health sector and societal perspectives were analyzed. The health sector perspective included direct medical costs borne by the individual and third-party payers over a 4-year time horizon. The societal perspective included additional costs of lost productivity attributable to premature death and long-term disability from sequelae of MenB infection over the expected lifetime of an entering freshman college student (80 total years of life).13 The modeled risk of MenB infection spanned 4 years with an assumed zero risk of infection after leaving college to limit model complexity. This assumption is consistent with current epidemiologic trends reported by the CDC demonstrating a tenfold reduction in risk after the college-aged years.3
Parameter estimates of conditional probabilities were derived from previously published sources (Table 1). Death and hospitalization rates for non-meningococcal causes were assumed to be equal in both decision arms and excluded. Harms contributed by vaccine-associated adverse events were excluded due to the exceptionally low reported incidence.14,15
Table 1.
Conditional Probabilities, Costs (US$2015), and Utilities for Cost-Effectiveness Analysis Comparing Universal MenB Vaccination Versus Standard of Care
| Parameter | Estimate | SD | Distribution | Reference |
|---|---|---|---|---|
| Probabilities | ||||
| Risk of meningococcal infection | ||||
| 4-year probability of MenB infection in the absence of | 9.2 × 10−6 | 2.8 × 10−6a | Beta | 3 |
| vaccination | ||||
| Probability of meningococcemia if infected2 | 0.25 | 0.075a | Beta | 1,2 |
| Vaccine efficacy | ||||
| Effectiveness of vaccine | 0.5 | 0.075b | Beta | 6,16–18 |
| Natural history of meningococcal infection | ||||
| Probability of amputation, meningitis/meningococcemia | 0 / 0.0325 | 0 / 0.00975 a | Beta | 1,2,19–23 |
| Probability of death, meningitis/meningococcemia | 0.1 / 0.4 | 0.03 / 0.12 a | Beta | 1,2,19–23 |
| Probability of hearing loss if meningitis only | 0.0547 | 0.01641 a | Beta | 1,2,19–23 |
| Probability of chronic neuro deficit if meningitis only | 0.1245 | 0.03735 a | Beta | 1,2,19–23 |
| Costsd | ||||
| Cost of vaccination | ||||
| Cost of vaccination per person | 323.81 | 48.57b | Gamma | 7,14,15 |
| Costs associated with treating an outbreak | 2,590,480 | 388,572 b | Gamma | 7,14,15,24 |
| Acute hospitalization | ||||
| Baseline costs of hospitalization for meningitis/meningococcemia | 19,959 / 25,240 | 2,993 / 3,786b | Gamma | 25 |
| Additional costs of hospitalization with amputation | 81,772 | 40,000 | Gamma | 25 |
| Additional costs of hospitalization with hearing loss | 2,928 | 439 a | Gamma | 25 |
| Additional costs of hospitalization with neurologic deficit | 8,775 | 1,316 b | Gamma | 25 |
| Long-term health sector costs | ||||
| Intermediate- and long-term costs of amputation | 427,313 | 213,657 c | Gamma | 26–28 |
| Intermediate- and long-term costs of hearing loss | 129,327 | 64,663c | Gamma | 26–28 |
| Intermediate- and long-term costs of neurologic deficit | 502,173 | 251,087 c | Gamma | 26–28 |
| Productivity (human capital lost) | ||||
| Productivity costs of amputation, neuro deficit, or hearing loss | 706,409 | 658,847 b | Gamma | 27 |
| Productivity costs of early death | 851,775 | 127,766 b | Gamma | 29 |
| Utilitiesd | ||||
| QALYs from decision point | ||||
| Early death from MenB | 1.85 | 0.2775 | Normal | 30 |
| Full life | 25.9 | 3.885 | Normal | 30 |
| Disutilities from surviving MenB infection with sequelae | ||||
| Amputation | 11.93 | 5.965 | Normal | 31–35 |
| Chronic hearing loss | 3.19 | 1.595 | Normal | 31–35 |
| Neurologic disability | 1.51 | 0.755 | Normal | 31–35 |
| MenB hospitalization | 0.02 | 0.01 | Normal | 31–35 |
SD: +/− 30% of estimate. SDs in other literature were not routinely reported and inconsistent amongst sources.
SD: +/− 15% of estimate. SDs in other literature were not reported.
SD: +/− 50% of estimate. SDs in other literature were not routinely reported, inconsistent amongst sources, and projected to long-term with assumed increased variability.
All values discounted to present at 3% per year.
MenB, Neisseria meningitidis serogroup B; QALY, quality adjusted life year.
The MenB risk for this population was calculated based on the annualized incidence of MenB infection in the college-going, aged 18 to 24 years U.S. population for a 4-year cumulative probability. Estimates of MenB infection in this demographic were obtained from government surveillance data.3 Universal vaccination was estimated to provide 50% reduction in the probability of MenB infection given a moderate to strong initial immunogenicity and then marked reduction at 48 months.6,16–18 Herd immunity was not included in this model due to an exploratory sensitivity analysis that demonstrated no change in conclusions with or without community protection. Historical sources reporting the proportion of meningitis (75%) versus meningococcemia (25%) were used.1,2
Four clinically significant sequelae of meningococcal infection were included: death, chronic hearing loss, chronic neurologic disorder including epilepsy and intellectual disability, and limb amputation. Probabilities of these sequelae were derived from the historical incidences.1,2,19–23 MenB infection was assumed to be clinicopathologically similar to other serogroups for modeling although not true in practice. To reduce model complexity, each of the sequelae was treated as a mutually exclusive event. To account for the overlapping sequelae often present in reality, the more severe sequelae were over-weighted by using the high-end estimated incidence in the literature whenever possible.
Table 1 summarizes all cost parameters. Vaccine prices were estimated from publicly reported vaccine price lists with a simple average cost of the two available vaccines for two doses.7,14,15 Vaccine administration costs were estimated to be 10% in both the preventive setting as well as the outbreak response with variation included in the sensitivity analysis described below.24 The cost of an outbreak included vaccination to all students, faculty, and staff, plus rifampin administration to 20 close contacts. Rifampin was used exclusively in the model as a means of model simplification and relative similarity of healthcare resource utilization regardless of the chemoprophylaxis agent used.
All cases of MenB infection were assumed to be treated with an inpatient hospital admission. Index hospital admission costs were calculated with close monitoring in a critical care setting using National Inpatient Sample charges for healthcare services.25 Meningococcal sequelae were assumed to manifest during the index hospital admission and included additional index hospital admission costs appropriate for each sequelae (Appendix 1). Post-hospital and long-term care costs of sequelae were obtained from previously published reports for each condition.26–28
Productivity losses were assessed using a human capital approach, currently recommended over friction cost methods.11 The effect of using a human capital approach versus a friction cost methodology was tested by sensitivity analysis. Deaths from MenB were valued based on lifetime earnings.29 Because of unavailability of specific estimates, the productivity loss previously reported for severe hearing deficit was applied to all nonfatal sequelae (i.e., amputation, neurologic deficit).27
Quality-adjusted life years (QALYs) were estimated based on age and gender (assumed 50/50 male/female population) Health State Valuations adjustments.30 All deaths from meningitis were assumed to occur at the midpoint of college enrollment (2 years). Disutilities were applied for hospital stay, daily life with an amputation, daily life with a severe hearing deficit, and daily life with a neurologic disorder (Appendix 2).31–35 Summative utilities and event-specific disutilities are reported in Table 1. For those with nonfatal sequelae of meningococcal infection, estimated disutility was deducted from the full-life QALY estimate. It was assumed that no reduction in total life expectancy occurred for those with nonfatal sequelae who survived the original infectious episode.
All costs were adjusted to 2015 U.S. dollars based on most common dates of existing literature and applied 3% per year discounting for future costs and future QALYs.
Statistical Analysis
One-way sensitivity analysis was performed of all parameter estimates to identity those most likely to affect overall results. Having identified the college-aged incidence of MenB infection and vaccine price as disproportionately sensitive estimates by one-way sensitivity analysis, an extreme outlier model was constructed with all cases of MenB infection leading to hospitalization and immediate death to determine a conservative threshold of MenB infection incidence and vaccine prices where reconsideration of cost effectiveness may be indicated (Appendix 4, Figure 1). MenB incidence was modeled across a range based on observed real-world MenB infection rates from <10 times to >10 times the current MenB incidence in U.S. college students.3,36 The total cost of a vaccine series from current prices down to zero was also included.
All of the deterministic base parameters described above were incorporated into a multivariable probabilistic sensitivity analysis using probability ranges described in Table 1 including beta distributions for conditional probabilities and gamma distributions for costs. Utilities were assumed to be normally distributed based on summated utilities consistently >0 with adequate sampling to justify a normal distribution.37 The probabilistic distribution of incremental cost-effectiveness ratios was assessed with a 10,000-iteration Monte Carlo simulation plotted with a willingness-to-pay threshold of $150,000 per QALY, as is currently accepted for interventions within the U.S. by both the WHO and expert consensus.38,39 Comparative effectiveness across a wide range of willingness-to-pay thresholds up to $30 million per QALY was ultimately included due to the high cost of vaccination relative to its incidence.
For the assessment of costs in real-world implementation, health sector costs of universal vaccination were compared with outbreak-only vaccination at the college campus level. The probability-weighted expected value of an outbreak response was calculated as the total estimated cost of an outbreak times the probability of an outbreak on an individual college campus. Health sector costs and societal costs were also assessed per case averted.
RESULTS
For both the health sector and societal perspectives, not having a formalized MenB vaccination program was more cost effective than universal vaccination in college-aged individuals with a willingness-to-pay threshold of $150,000 per QALY (Table 2). From the campus-level health sector perspective, vaccinating each entering class of college students per year would cost $323,810 per campus. By contrast, an outbreak for a campus that would only universally vaccinate after an infected case was identified would cost $2,590,480 per campus. However, the probability-weighted expected value of an outbreak would only cost $23.83 per campus. With the low incidence of MenB even among high-risk populations, such as U.S. college students, and published evidence that vaccine efficacy quickly wanes over time,3,6 a universal vaccination program would reduce the number of cases/mid-sized campuses affected in 4 years from 9.2 cases per 1,000 campuses to 4.6 per 1,000 campuses.
Table 2.
Expected Values of Base Case Cost Effectiveness Comparing Universal MenB Vaccination for College Students to Standard of Care
| EV cost (US$, 2015) | Incremental EV cost | EV effectiveness (QALYs) | Incremental EV effectiveness | ICER ($/QALYs) | Cost per case averted | |
|---|---|---|---|---|---|---|
| Health sector perspective | ||||||
| Universal vaccination | 324.25 | 299.53 | 25.89998 | 0.0000215 | 13,899,861 | 65,116,255 |
| Standard of care | 24.72 | 25.89996 | ||||
| Societal perspective | ||||||
| Universal vaccination | 325.55 | 298.24 | 25.89998 | 0.0000215 | 13,839,796 | 64,834,867 |
| Standard of care | 27.31 | 25.89996 |
EV, estimated value; QALYs, quality adjusted life years; ICER, incremental cost-effectiveness ratio; Men B, Neisseria meningitidis serogroup B.
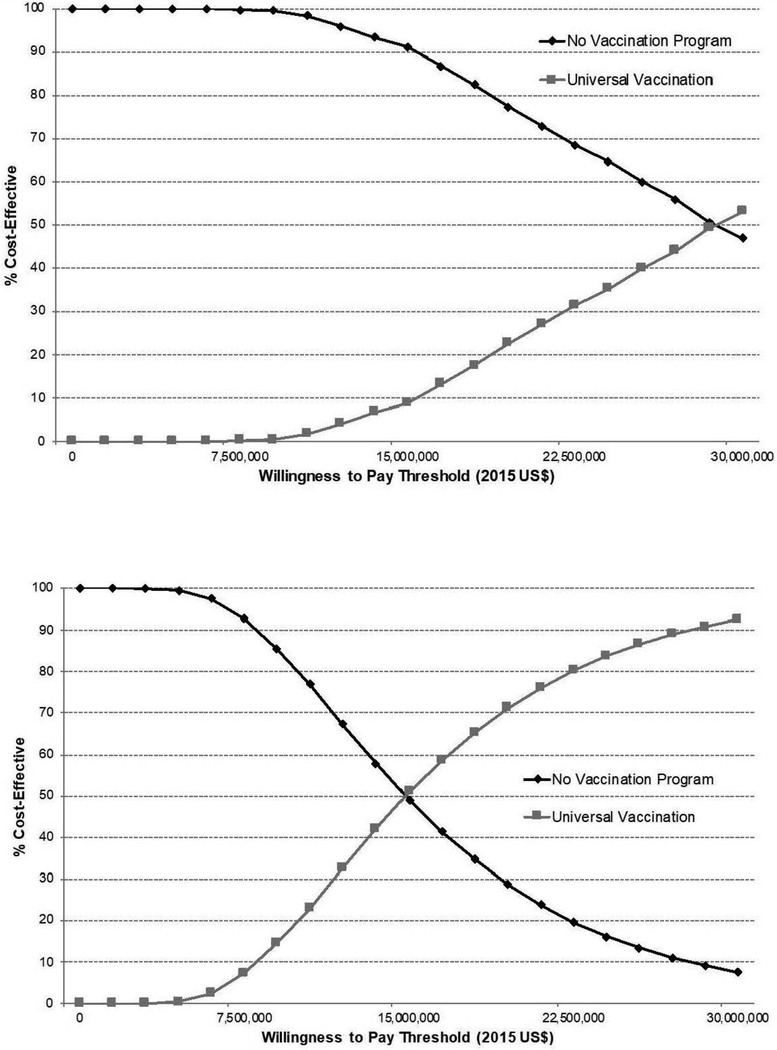
The incremental cost per QALY gained with universal vaccination was $13.9 million under the health sector perspective and $13.8 million under the societal perspective each compared with a willingness-to-pay threshold of $150,000 per QALY. Multivariable probabilistic sensitivity analysis demonstrated that universal vaccination was not the probability-weighted preferred strategy up to a willingness-to-pay threshold of $28.2 million per QALY and $15.0 million per QALY under the health sector and societal perspectives, respectively (Figure 2). Sensitivity analysis of these findings using a friction cost approach demonstrated a <1% difference in resulting estimates (Appendix 3).
Figure 2.
Cost-effectiveness acceptability curve comparing universal vaccination with no formal vaccination program for college students using a health sector perspective (A) and a societal perspective (B).
Potential extremes of real-world variation in MenB with a simplified model (Appendix 4, Figure 1) and one-way sensitivity analysis of MenB incidence demonstrated a universal vaccination strategy becoming cost effective at a 4-year cumulative risk of 4.6 cases per 100,000 individuals (Appendix 4, Figure 2). Under this same extreme model, a universal vaccination strategy became cost effective for vaccine series pricing <$65 (Appendix 4, Figure 3). In the more conservative model used throughout the rest of this manuscript, the breakeven price for a vaccine series was $28 or a 4-year cumulative risk rising to 10.3 cases per 100,000 individuals.
DISCUSSION
The purpose of this study was to assess the cost effectiveness of universal MenB vaccination amongst those entering college in the U.S. These results demonstrate that universal MenB vaccination at entry to college is not cost effective at any accepted willingness-to-pay threshold. Even under a series of conservative assumptions including the high cost of pan-campus vaccination in response to an outbreak, a decision maker’s willingness-to-pay would need to be more than ten times greater than standard assumptions for MenB vaccination to be a cost-effective decision for the payer/payee or for society at large. In the context of a resource-constrained health system, these results reject current advocacy for broader MenB vaccination of college-bound adolescents.
Rates of meningococcal disease in the U.S. have decreased by more than 75% in the last decade, and population-based protection afforded tetravalent vaccines have been an important contributor to this trend.40,41 Currently, serogroup B meningococcal infections represent more than half of all meningococcal cases that occur each year in the U.S among adolescents and young adults.3,40 The unique antigenic variability of serogroup B N. meningitidis has historically inhibited vaccine development, but two approved MenB vaccines offer a new opportunity for enhanced meningococcal protection. Unfortunately, the short-term immunogenicity of these vaccines and low disease incidence limits the benefit from these technologic advances.6 Currently, the CDC’s Advisory Committee on Immunization Practices maintains a Category B recommendation for MenB vaccination in adolescents and young adults, supporting its use in the context of individualized decision making. This recommendation effectively represents an intermediate state in between a Category A recommendation for the widespread use of a vaccine and a recommendation to not use a vaccine.
These results demonstrate that despite the poor prognosis of MenB infection and reasonable nominal cost of MenB vaccination, the extreme rarity of MenB infection even amongst its second modal peak in college-age individuals make vaccination not cost effective. Vaccinating 100,000 college students would prevent less than five cases of MenB. Such a small population-wide benefit makes support of vaccination economically untenable even when accounting for individual costs, payer costs, community outbreak costs, and productivity lost by society.
Given the lack of cost effectiveness that was observed for MenB vaccination under current epidemiologic trends, it was also tested under what conditions universal MenB vaccination may warrant further consideration. The one-way sensitivity analysis highlighted that the incidence of MenB may be a particularly critical assumption to the analysis described above. For the last part of this study, extremes of MenB disease incidence were tested for the effect on conclusions. First, all other constraints were relaxed that may limit the favorability of MenB vaccination to create a conservative model for predicting a reconsideration threshold (Appendix 4, Figure 1). For example, for this extreme case, it was assumed that any case of MenB infection would result in hospitalization and immediate death with a lifetime of associated productivity loss and QALYs. It was also assumed that current MenB vaccines were perfectly effective leading to zero cases of MenB in the targeted population. Even under such favorable conditions, a rate of MenB cumulative risk during college would have to be 4.6 cases per 100,000 students for universal vaccination to be cost effective under a willingness-to-pay threshold of $150,000 per QALY. This incidence rate is five times higher than the historically stable rate observed in the U.S. and 50% greater than the infection rate observed in Ireland, the highest reported in industrialized countries.36 Recognizing this threshold is important for the public health community and highlights the importance of ongoing long-term surveillance.
Extremes of vaccine price were also considered as part of the sensitivity analysis. Using the same simplified model as for MenB incidence sensitivity analysis, MenB vaccination should be further considered when vaccine series pricing is less than $65. In the more conservative model, the price of the vaccine series would have to drop to less than $28. This threshold is substantially below the $324 average current price of meningococcal vaccine series and does not materially impact the conclusions in this study but may be an important consideration in the future if the cost of MenB vaccines dramatically decreased.
Limitations
These findings have important limitations when considering MenB vaccination in other contexts. The CDC recommends certain high-risk groups for vaccination that include those exposed to an MenB outbreak as well as those with underlying immune system dysfunction.5 The model population in this analysis is composed of exclusively average-risk college-aged individuals. Higher-risk individuals that would likely disproportionately benefit from vaccination were excluded. In the related case of outbreak response, inadequate data currently exists to assess the utility of immediate vaccination when an outbreak occurs. Current outbreak vaccination practices were incorporated in the standard of care arm to model a real-world response. However, there is also limited data on how current outbreak responses are implemented in terms of which vaccine is used, compliance with intermediate-term follow-up vaccination, and antibiotic prophylaxis selected. These unknown factors have been simplified in the model reported here by assuming similar usage as in the universal vaccination case. Finally, and most importantly, these findings cannot address one’s personal decision making when considering vaccination. For any individual, the decision to vaccinate is whether to spend a few hundred dollars today for short-term protection from a disease that they will not likely contract with devastating consequences if they are. It is well known that individual decision makers are excessively loss averse,42 and it may be consistent with an individual’s risk preferences to risk a relatively small amount of income for even imperfect peace of mind.
CONCLUSION
Despite the safety and short-term efficacy of MenB vaccination, the extreme low incidence of MenB and high cost of vaccination prevent universal vaccination of college-aged individuals from being a cost-effective strategy. Based on economic modeling, the standard of care should remain: no formal vaccination program with an outbreak response as needed. However, ongoing epidemiologic surveillance is necessary and universal vaccination should be reconsidered if rates of MenB infection markedly increase in the college-age population.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Gregory de Lissovoy, PhD for study design recommendations and comments on the final manuscript. I.L.L. received salary support during the conduct of this study from an NIH National Cancer Institute institutional training grant (5T32CA126607). V.N. and P.S. received salary support during the conduct of this study from the Republic of India.
Footnotes
No other financial disclosures were reported by the authors. No conflicts of interest were reported by the authors.
Initial findings from this study were presented at the International Society of Pharmacoeconomics and Outcomes Research in Baltimore, Maryland on May 21, 2018.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344(18):1378–1388. 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 2.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–2210. 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Enhanced Meningococcal Disease Surveillance Report. www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf. Published 2016. Accessed April 23, 2018.
- 4.Soeters HM, Dinitz-Sklar J, Kulkarni PA, et al. Serogroup B meningococcal disease vaccine recommendations at a university, New Jersey, USA, 2016. Emerg Infect Dis. 2017;23(5):867–869. 10.3201/eid2305.161870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Advisory Committee on Immunization Practices, CDC. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for Use of Serogroup B Meningococcal (MenB) Vaccines in Persons at Increased Risk for Serogroup B Meningococcal Disease. www.cdc.gov/vaccines/acip/recs/grade/mening-serogroup-b.html. Published 2015. Accessed April 24, 2018.
- 6.Patton ME, Stephens D, Moore K, MacNeil JR. Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine—Advisory Committee on Immunization Practices, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(19):509–513. 10.15585/mmwr.mm6619a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. Vaccines for Children Program. www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html. Published 2018. Accessed March 15, 2018.
- 8.National Meningitis Association. Beyond the Science: Putting a Face on Meningococcal Disease. Fort Meyers, FL; National Meningitis Association; www.nmaus.org/wp-content/uploads/2015/02/nma-beyond-the-science-report.pdf. Published 2015. Accessed September 27, 2018. [Google Scholar]
- 9.Herzog K Separate vaccine can protect against meningitis B strain. USA Today http://college.usatoday.com/2016/08/17/separate-vaccine-can-protect-against-meningitis-b-strain/. Published August 17, 2016. Accessed September 27, 2018. [Google Scholar]
- 10.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses. JAMA. 2016;316(10):1093–1103. 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 11.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. 2nd ed. New York, NY: Oxford University Press; 2017. [Google Scholar]
- 12.CDC. Guidance for the Evaluation and Public Health Management of Suspected Outbreak of Meningococal Disease. Atlanta, GA: CDC; www.cdc.gov/meningococcal/downloads/meningococcal-outbreak-guidance.pdf. Published 2017. Accessed September 27, 2018. [Google Scholar]
- 13.Social Security Administration. Actuarial Life Table. www.ssa.gov/oact/STATS/table4c6.html. Published 2014. Accessed April 6, 2018.
- 14.Pfizer. STN 125549–360. PI Approved Final Draft. (Trumemba package insert). www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM421139.pdf. Published 2014. Accessed April 23, 2018.
- 15.GlaxoSmithKline. 125546–189. PI Approved Final Draft. (Bexsero package insert). www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM431447.pdf. Published 2015. Accessed April 23, 2018.
- 16.Parikh SR, Andrews NJ, Beebeejaun K, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet. 2016;388(10061):2775–2782. 10.1016/S0140-6736(16)31921-3. [DOI] [PubMed] [Google Scholar]
- 17.Parikh SR, Newbold L, Slater S, et al. Meningococcal serogroup B strain coverage of the multicomponent 4CMenB vaccine with corresponding regional distribution and clinical characteristics in England, Wales, and Northern Ireland, 2007–08 and 2014–15: a qualitative and quantitative assessment. Lancet Infect Dis. 2017;17(7):754–762. 10.1016/S1473-3099(17)30170-6. [DOI] [PubMed] [Google Scholar]
- 18.Granoff DM. Commentary: European Medicines Agency recommends approval of a broadly protective vaccine against serogroup B meningococcal disease. Pediatr Infect Dis J. 2013;32(4):372–373. 10.1097/INF.0b013e318282942f. [DOI] [PubMed] [Google Scholar]
- 19.Stein-Zamir C, Shoob H, Sokolov I, Kunbar A, Abramson N, Zimmerman D. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dis J. 2014;33(7):777–779. 10.1097/INF.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 20.Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(suppl 2):B3–B9. 10.1016/j.vaccine.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Haji Ali Afzali H, Marshall H. The inpatient costs and hospital service use associated with invasive meningococcal disease in South Australian children. Vaccine. 2014;32(37):4791–4798. 10.1016/j.vaccine.2014.05.069. [DOI] [PubMed] [Google Scholar]
- 22.Sadarangani M, Scheifele DW, Halperin SA, et al. Outcomes of invasive meningococcal disease in adults and children in Canada between 2002 and 2011: a prospective cohort study. Clin Infect Dis. 2015;60(8):e27–e35. 10.1093/cid/civ028. [DOI] [PubMed] [Google Scholar]
- 23.Kirsch EA, Barton RP, Kitchen L, Giroir BP. Pathophysiology, treatment and outcome of meningococcemia: a review and recent experience. Pediatr Infect Dis J. 1996;15(11):967–978. 10.1097/00006454-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Trotter CL. Modelling cost effectiveness of meningococcal serogroup C conjugate vaccination campaign in England and Wales. BMJ. 2002;324(7341):809–809. 10.1136/bmj.324.7341.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project Databases. www.hcup-us.ahrq.gov/nisoverview.jsp. Published 2016. Accessed April 23, 2018. [PubMed]
- 26.Wright C, Wordsworth R, Glennie L. Counting the cost of meningococcal disease. Pediatr Drugs. 2013;15(1):49–58. 10.1007/s40272-012-0006-0. [DOI] [PubMed] [Google Scholar]
- 27.Mohr PE, Feldman JJ, Dunbar JL, et al. The societal costs of severe to profound hearing loss in the United States. Int J Technol Assess Health Care. 2000;16(4):1120–1135. 10.1017/S0266462300103162. [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie EJ, Jones AS, Bosse MJ, et al. Health-care costs associated with amputation or reconstruction of a limb-threatening injury. J Bone Joint Surg Am. 2007;89(8):1685–1692. 10.2106/JBJS.F.01350. [DOI] [PubMed] [Google Scholar]
- 29.Liu G Measuring the stock of human capital for international and intertemporal comparisons In: Jorgenson DW, Landefeld S, Schreyer P, eds. Measuring Economic Sustainability and Progress. Chicago, IL: University of Chicago; 2014:493–544. 10.7208/chicago/9780226121475.003.0015. [DOI] [Google Scholar]
- 30.Mathers CD, Murray CJL, Lopez AD, et al. Estimates of Health Life Expectancy for 191 Countries in the Year 2000: Methods and Results. Geneva, Switzerland: WHO; www.who.int/choice/publications/d_2000_gpe38.pdf?ua=1. Published 2000. Accessed September 27, 2018. [Google Scholar]
- 31.Vega-Hernandez G, Wojcik R, Schlueter M. Cost-effectiveness of liraglutide versus dapagliflozin for the treatment of patients with type 2 diabetes mellitus in the UK. Diabetes Ther. 2017;8(3):513–530. 10.1007/s13300-017-0250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng X, Zhao Y, Zhang X, Jin H, Min J. Health economic evaluation of immunization strategies of hepatitis E vaccine for elderly population. Hum Vaccin Immunother. 2017;13(8):1873–1878. 10.1080/21645515.2017.1316913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiragami M, Mizukami A, Leeuwenkamp O, et al. Cost-effectiveness evaluation of the 10-valent pneumococcal non-typeable haemophilus influenzae protein d conjugate vaccine and 13-valent pneumococcal vaccine in Japanese children. Infect Dis Ther. 2014;4(1):93–112. 10.1007/s40121-014-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martí SG, Colantonio L, Bardach A, et al. A cost-effectiveness analysis of a 10-valent pneumococcal conjugate vaccine in children in six Latin American countries. Cost Eff Resour Alloc. 2013;11(1):21 10.1186/1478-7547-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pomeroy SL, Holmes SJ, Dodge PR, Feigin RD. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med. 1990;323(24):1651–1657. 10.1056/NEJM199012133232402. [DOI] [PubMed] [Google Scholar]
- 36.European Center for Disease Prevention and Control. Annual Epidemiological Report on Communicable Diseases in Europe. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/1111_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf. Published 2011. Accessed April 24, 2018.
- 37.Briggs A, Sculpher M, Claxton K. Decision modeling for health economic evaluation. Oxford, England: Oxford University Press; 2006. [Google Scholar]
- 38.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163(14):1637–1641. 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 39.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93(2):118–124. 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.CDC. Meningococcal disease. www.cdc.gov/meningococcal/surveillance/index.html. Published 2018. Accessed April 24, 2018.
- 41.Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clin Infect Dis. 2010;50(suppl 2):S37–S44. 10.1086/648963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeil BJ, Pauker SG, Sox HC, Tversky A. On the elicitation of preferences for alternative therapies. N Engl J Med. 1982;306(21):1259–1262. 10.1056/NEJM198205273062103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.