Abstract
PURPOSE:
Upper airway exercises for snoring treatment can be effective but difficult to administer and monitor. We hypothesized that a brief, relatively simple daily upper airway exercise regimen, administered by a smartphone application, would reduce snoring and encourage compliance.
METHODS:
Targeted vowel sounds causing tongue base movements were incorporated into a voice-controlled smartphone game application. Participants with habitual snoring, apnea hypopnea index (AHI) ≤ 14 events/hour, and BMI ≤ 32 kg/m2 were randomly assigned to perform 15 minutes of daily gameplay (intervention group) or 5 seconds of daily voice recording (control group) and to audio record their snoring for 2 nights/week for up to 12 weeks. Sounds above 60 dB were extracted from recordings for snore classification with machine learning support vector machine classifiers.
RESULTS:
Sixteen patients (8 in each group) completed the protocol. Groups were similar at baseline in gender distribution (5 males, 3 females), mean BMI (27.5±3.8 vs 27.4±3.8 kg/m2), neck circumference (15.1±1.6 vs 14.7±1.7 inches), Epworth Sleepiness Score (8±3.5 vs 7±4.0) and AHI (9.2±4.0 vs 8.2±3.2 events/hr). At 8 weeks, the absolute change in snoring rate (> 60 dB/hour) was greater for the intervention group than the control group (−49.3±55.3 vs −6.23±23.2; p=0.037), a 22% and 5.6% reduction, respectively. All bed partners of participants in the intervention group reported reduced snoring volume and frequency, whereas no change was reported for the control group.
CONCLUSIONS:
Smartphone application administered upper airway training reduces objective and subjective snoring measures and improves sleep quality.
Keywords: Snoring, Oropharyngeal Exercise, Smartphone Application, Randomized Controlled Trial
Introduction
Snoring is the most common presenting complaint in sleep medicine clinics and is highly pervasive in the general population, affecting up to 44% of men and 28% of women between 30 and 60 years of age[1]. Snoring is caused by the vibration of soft tissue structures during inspiratory airflow through a narrowed upper airway. The primary cause of airway narrowing is sleep-induced hypotony of the upper airway muscles. While snoring may be a predictor of the more serious condition of obstructive sleep apnea (OSA), primary snoring in the absence of clinical diagnosis of OSA is common and may be associated with negative health risk, including increased carotid intima media thickness[2], increased levels of systemic inflammatory markers[3], and greater healthcare utilization[4]. Snoring can also adversely affect the sleep quality of bed partners[5], perhaps the most common reason for self-referral to a sleep health clinic.
Treatment options for snoring include treatment for nasopharyngeal conditions, lifestyle changes (weight loss or avoidance of alcohol), positional therapy, mandibular advancement devices, upper airway surgery, and nasal continuous positive airway pressure (nCPAP). An alternative, less intrusive treatment is myofunctional therapy involving exercises of the oral and facial muscles. An upper airway training approach using the wind instrument didgeridoo was found to reduce the severity of OSA and snoring in comparison to control subjects[6]. In other studies, orofacial and oropharyngeal exercise routines (involving the tongue, soft palate, and lateral pharyngeal wall) - when performed over 3 months for either 8 minutes 3 times a day or 30 minutes daily - were effective in improving sleep quality and snore index (snores >36 dB/h) and in reducing snoring frequency and intensity[7][8]. Although these may be beneficial, oropharyngeal exercises are not well standardized and often cumbersome.
In order to standardize an effective technique that would reduce the complexity of the exercise regimen, optimize adherence and have an objective method for monitoring therapy compliance, we developed a smartphone application to gamify upper airway exercises involving tongue muscles. We hypothesized that a brief daily upper airway exercise regimen administered by smartphone application is effective in the treatment of snoring.
Methods
Exercise Regimen
We used the International Phonetic Association vowel diagram[9] combined with submental ultrasound imaging[10] of the normal airway to identify specific phonemes that produce different forward and backward movements of the tongue base. Key phonemes identified were /i/ (as in key, see, bee), which brings the base of the tongue maximally forward; /a/ (as in saw, law, pawn), which brings it maximally backward; and /u/ (as in do, few, true), an intermediate vowel that places the tongue base between /a/ and /i/. Per ultrasound imaging, these sounds can only be articulated by engaging the tongue base, whereas other vocalizations can be articulated in multiple ways.
We developed a smartphone application that requires users to articulate these phonemes to achieve voice-controlled on-screen objectives. The exercise routine comprises three different games of 5 minutes each, played consecutively for a total of 15 minutes daily. These games focus on improving endurance, strength, and coordination of upper airway muscles by moving the user’s tongue base forwards and backwards repeatedly. Game 1 prompts the user to repeatedly enunciate the /i/ sound to perform the on-screen objective, with the aim of building endurance by holding the tongue forward. Game 2 is designed to improve strength by inducing pulsing of the tongue in forward and backward motion through vocalization of /i/ and /a/ to control the on-screen object. Game 3 prompts articulation of /i/, /u/, and /a/ to improve coordination by navigating the tongue through different zones.
Participant Selection and Enrollment
We recruited participants from the University of Minnesota Medical Center sleep clinic. Eligible patients were between 20 and 65 years of age, fluent in English, with a BMI ≤ 32 kg/m2. Patients had to be habitual snorers (self-reported or bed partner reported snoring 3 or more nights a week) with a polysomnography (PSG) or home sleep test (HST) within the past year that showed objective snoring with no more than mild obstructive sleep apnea (AHI 0–14). For both PSG and HST, apneas were defined as ≥ 90% drop in peak signal excursion of the apnea sensor for ≥ 10 seconds while hypopneas were defined as ≥ 30% drop from peak signal excursion of the airflow sensor associated with ≥ 4% oxygen desaturation from pre-event baseline. AHI was calculated in accordance with the AASM rules, terminology and technical specifications[11]. We excluded anyone with comorbid sleep disorders (significant insomnia, uncontrolled restless legs syndrome, chronic insufficient sleep intake, or pathological excessive daytime sleepiness, i.e., Epworth Sleepiness Score >11), significant medical comorbidities including decompensated cardiopulmonary disease and chronic rhinitis, self-reported average of 3 or more alcoholic drinks per day, or significant daily opioid use. Those currently using nCPAP were excluded. To minimize variation in OSA risk factors, we excluded anyone with > 5% weight change since their sleep apnea evaluation. Given our data collection procedures, we excluded patients with a less than 10 Mb/s wifi connection where they sleep, inability to sleep in a quiet environment, or a loud snorer as a bed partner.
Study Protocol
The protocol was approved by the University of Minnesota’s Institutional Review Board (IRB#1606S88671). After obtaining informed consent, we randomly assigned participants to either the intervention group, requiring 15 minutes of daily gameplay (3 different games, 5 minutes each), or the control group, requiring a daily “check-in” on a mobile application but without exercises. Videos of activities performed by each group are included as online resource2 and 3. The participants were not blinded to their assignment of the study arm. They were provided microphones to record their sleep environment for an entire night, 2 nights a week (to ensure at least one successful recording per week), for up to 12 weeks. Participants were considered to have completed the protocol if they produced at least 12 recordings of ≥5 hours each (primary end-point). Participants in both groups were also instructed to perform 2 nights of recordings during 1 week preceding initiation of their assigned group activities (run-in period). Participants using mandibular advancement device and nasal dilator strips routinely were restricted from using these on the nights of sleep recording for the study duration.
Data Collection
Our primary outcome was snoring rate, defined as the number of classified snores > 60 dBA per hour of sleep. Snoring analysis comparing amplitude measurements to psychoacoustic assessments have a good correlation[12][13]. A non-contact microphone (Donner DM-1; Micmc Co Ltd, London, England) was positioned 30 cm above the participant’s mouth during sleep to optimize signal quality and patient comfort[14][15]. Microphones were calibrated from a speaker array. The array sound level was set by using a Bruel & Kjaer 2250 Light: Hand-held analyzer and a Bruel & Kjaer Type 4950 microphone. Calibration was confirmed by using a Bruel & Kjaer Type 4188-A-021 microphone to record sound levels the following day. Data were recorded at a sampling rate of 22,050 Hz and 16-bit resolution, then uploaded to a server in real time via the participant’s wifi internet connection. Data were collected and stored in de-identified form, assigned to a unique participant ID. Recordings were checked to ensure successful uploading and audio quality.
In addition to objective snoring rate, we assessed bed-partner reported changes in snoring frequency and intensity, and self-reported changes in sleep quality (continuity), daytime sleepiness, and throat dryness upon awakening via an online questionnaire (likert scale) or a phone interview at the end of the study (Online resource4). Participant demographics, sleep history, and sleep evaluation results were obtained from the electronic medical record.
Analysis
Because snoring can be inconsistent even in habitual snorers, we analyzed snoring metrics longitudinally in each participant across all recording nights to provide a more accurate assessment of the intervention’s efficacy. We used the middle four hours of each recording night for analyses to control for room noise while participants were falling asleep and waking up, increasing the likelihood they were sleeping throughout the recording, and increasing the likelihood of capturing a full sleep cycle. Recordings were segmented into potential snores by extracting any sound above 60 dB. Sounds were then classified into snores or not snores by using a trained support vector machine using pyAudioAnalysis[16]. To train the classification models, 1000 sleep sounds were randomly selected from each participant and adjudicated as snore/not snore by two blinded members of the research team. Discrepancies that could not be resolved were removed from analysis; these tended to be short (< 100 ms) or ambiguous breathing noises.
We used R (R Foundation for Statistical Computing, Vienna, Austria) for statistical analyses. Two-tailed unpaired t-tests were performed to compare baseline characteristics of the intervention and control groups. Change in snoring rate over time was measured by the change in starting and ending values of the linear regression of snoring rate versus days for 56 days (8 weeks). Paired t-tests were performed to evaluate within-group changes over the study period. An unpaired t-test, assuming unequal variances, was used to evaluate group differences in the absolute change in snoring rate and the percent change in snoring rate. Results are reported as mean±SD. A p-value < 0.05 was considered significant.
Results
We identified 729 patients from clinic database who met the inclusion criteria. Potential participants were contacted by phone for additional screening (wifi connectivity, loud bed partners, etc.) until our recruitment goal of 32 was met. Despite instructions to record their sleep environment sounds twice weekly, participants’ recording frequency varied. Over the study period, 16 participants (8 intervention group, 8 control group) met the requirement of recording 12 nights of sleep for 5 hours or longer. Data from these 16 participants were analyzed (Figure 1). Their baseline characteristics are presented in Table 1. Participants were predominantly overweight men. The intervention and control groups did not differ significantly in their demographics or baseline sleep measures.
Figure 1:
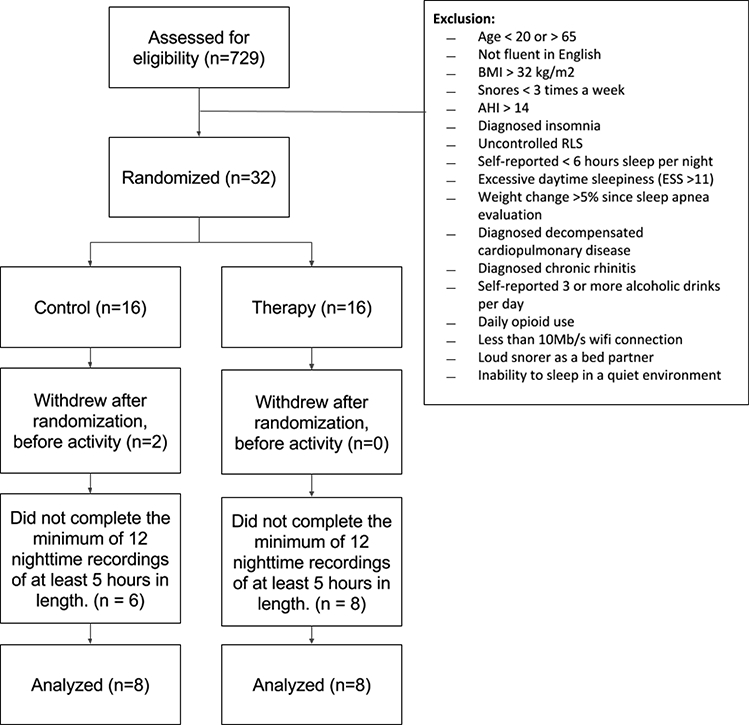
Flow of patients through the study.
Table 1:
Baseline characteristics for intervention and control group participants.
| Characteristic | Intervention Group (n=8) |
Control Group (n=8) |
P-value |
|---|---|---|---|
| Age | 51±11 | 51±10 | 1.00 |
| Sex No. (% Male) | 5 (63) | 5 (63) | 1.00 |
| Neck circumference (inches) |
15.1±1.6 | 14.7±1.7 | 0.65 |
| Weight (kg) | 84.2±17.0 | 78.2±13.8 | 0.44 |
| BMI | 27.5±3.4 | 27.4±3.8 | 0.97 |
| Epworth Sleepiness Scale | 8±3.5 | 7±4.0 | 0.60 |
| AHI | 9.2±4.0 | 8.2±3.2 | 0.59 |
| HST for diagnosis No. (%) | 2 (25) | 2 (25) | 1.00 |
Data presented as mean±SD unless otherwise noted
BMI, body mass index; AHI, apnea hypopnea index; HST, Home sleep test
P-values are for comparison of the 2 groups using 2 tail unpaired t test
The participants in the intervention group completed the therapy on 77%±22% of days during the study period with 1712±331 vocalizations per 15 minutes gameplay session. Participants achieved the primary end-point of 12 nightly recordings in 8 weeks. As shown in Figure 2, after 8 weeks we observed a significant decrease in mean snoring rate for the intervention group (272.5±169.5 vs 223.3±164.9 snores/hr, p=0.019), but no change in the control group (245.7±174.8 vs 239.5±158.8 snores/hr, p=0.23). The absolute change in mean snoring rate was significantly greater for the intervention group (−49.3±55.3 snores/hr) than the control group (−6.23±23 snores/hr; p= 0.037). Because snoring rates between individuals varied widely, we also calculated the mean percent change in snoring rate. For this metric (Figure 3), the intervention group demonstrated greater declines (−22%±22%) than the control group (−5.7%±29%; p-value = 0.028).
Figure 2:
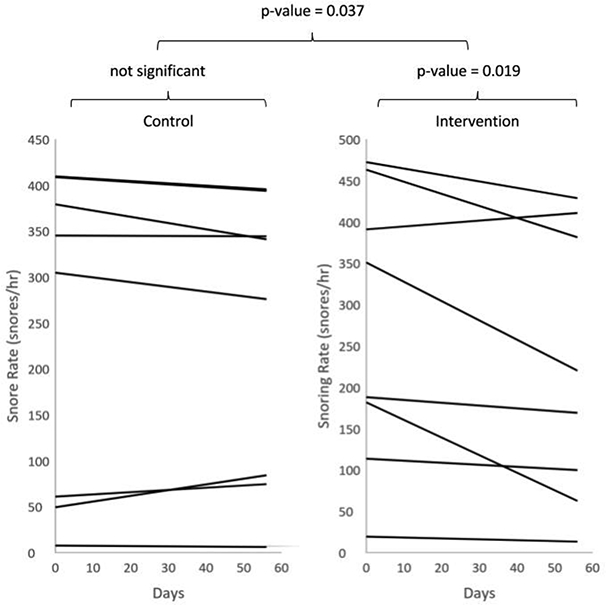
Individual trend lines for snoring rate over time, calculated by using the linear regression of snore rate vs time for each participant. The control group showed no significant change over time while the intervention group did (p-value = 0.019). There was also a significant difference between the absolute change in snoring rate between the control group and experimental group (p-value = 0.037).
Figure 3:
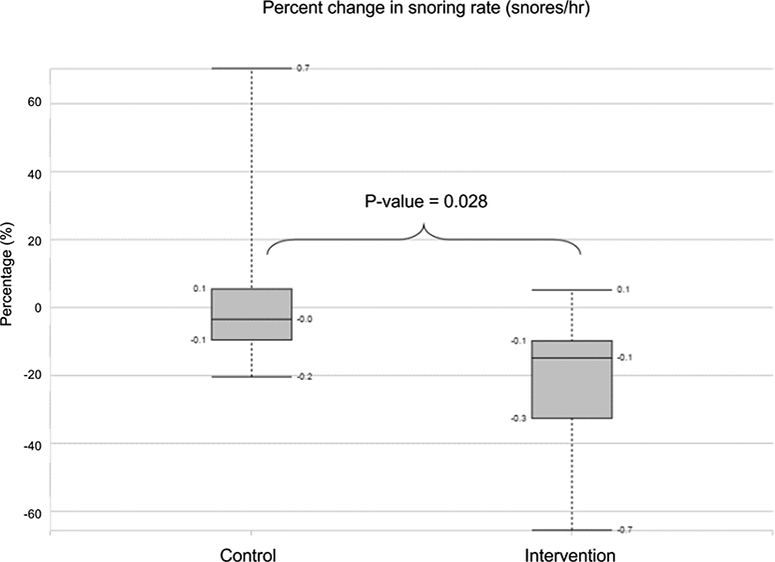
Percent change in snoring rate (snores/hr) between the control group and the intervention group.
After 8 weeks, bed partner-reported snoring volume and frequency were either greatly or slightly reduced for all participants in the intervention group, but remained the same for the control group (Table 2). At least half of participants in the intervention group reported improved daytime sleepiness and more continuous sleep, while 37.5% reported reductions in morning throat dryness (Table 3).
Table 2:
Bed partner reported changes in snoring volume and snoring frequency (number of nights snoring) at study completion. (Intervention n = 8, control n = 8). Units: number (%)
| Snoring volume | Snoring Frequency | |||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| Greatly reduced | 4 (50) | 0 (0) | 3 (37.5) | 0 (0) |
| Slightly reduced | 4 (50) | 0 (0) | 5 (62.5) | 0 (0) |
| Stayed the same | 0 (0) | 8 (100) | 0 (0) | 8 (100) |
| Slightly increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Greatly increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Table 3:
Self-reported changes in sleep continuity, daytime sleepiness, and morning dry mouth at study completion. (Intervention n = 8, control n = 8). Units: number (%)
| My sleep is more continuous |
I am less sleepy throughout the daytime |
My throat is less dry/painful in the morning |
||||
|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |
| Strongly disagree |
0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Slightly disagree |
0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) |
| Neutral | 4 (50) | 7 (87.5) | 3 (37.5) | 8 (100) | 4 (50) | 8 (100) |
| Slightly agree | 3 (37.5) | 1 (12.5) | 4 (50) | 0 (0) | 2 (25) | 0 (0) |
| Strongly agree | 1 (12.5) | 0 (0) | 1 (12.5) | 0 (0) | 1 (12.5) | 0 (0) |
Discussion
In this small randomized controlled study of primary snorers and snorers with mild OSA, a 15-minute upper airway exercise regimen performed daily through smartphone gameplay for 8 weeks decreased mean snoring rate (measured acoustically) by 22% and reduced snoring frequency and volume (reported by bed partners). Moreover, at least half of snorers had self-reported improvements in sleep continuity and daytime sleepiness. The majority of participants in the intervention group reported improved daytime sleepiness, half reported more continuous sleep, and a third reported reductions in morning throat dryness. The findings demonstrate the efficacy of a small subset of oropharyngeal exercises using targeted vowel sounds aimed at promoting strength, endurance, and neuromuscular control of upper airway muscles.
Other studies have shown similar degree of reductions in snoring and OSA through myofunctional therapy that incorporates elaborate sets of oropharyngeal exercises[7], singing[17] or didgeridoo playing[6]. Our approach is unique in its reliance on a very limited set of vowel sounds to produce repeated forward and backward movements of the tongue base, and its delivery through gameplay on a smartphone. In addition to increasing user engagement, smartphone gameplay permits tracking of participants’ compliance.
Our therapy focuses on rapid movements of the tongue base for improving neuromuscular control and overall strength. The current study demonstrated efficacy of this technique in treatment of snoring, but it remains completely unknown whether or not it may also be used for treatment of OSA. Given the documented deficiencies in neuromuscular control of the genioglossus muscle[18] in patients with OSA, evidence exists that vocal exercises aimed at preserving or improving this control are effective in correcting these deficiencies[19]. Patients with severe OSA (AHI >50) also experience a significant reduction in tongue movement[20]. Electromyogram changes in genioglossus muscle activity in OSA patients persist during wakefulness, suggesting that these changes are neuropathic, rather than muscular[21]. Abnormal cortical sensory processing to respiratory stimuli suggests that a repetitive task may have more functional relevance to OSA than a sustained task[22], which is the approach that our games were developed with.
Despite adequate compliance, some participants did not show improvement in objective measures of snoring, whereas the greatest individual reduction in snoring rate was over 65%. A similarly high degree of variance in treatment efficacy was reported in previous studies of myofunctional therapy for snoring[7][8]. The source of snoring sounds within the upper airway could influence our regimen’s effectiveness. Mechanisms of snoring may include nasal and palatal causes that may not respond to genioglossus muscle training. Therefore, we would expect people with primarily tongue-based snoring to obtain the greatest benefit from this therapy, while those with palatal-based snoring might see little improvement. Additionally, our youngest participant had the greatest snoring rate reduction, raising the possibility that airway musculature may be more responsive to exercise in younger snorers. We did not notice any similar trends among participants based on their gender. Our study’s small sample size limits identification of sub groups of participants that will benefit most from this therapy. More prospective studies are needed to identify which habitual snorers will benefit most from oropharyngeal exercise therapies.
We utilized a brief 1-week run-in period that required only 2 nights of sleep recording before initiating exercise routine. Because most habitual snorers do not have the same intensity and frequency of snoring each night, we identified significant intraparticipant variation in snoring rate. Future longitudinal studies of oropharyngeal exercise therapies might be improved by incorporating multiple week run-in period, allowing participants to serve as their own controls. To explore this, we asked one control participant after 8 weeks to crossover to the intervention group for an additional 6 weeks. The result was a reduction from 355±115 snores/hr to 266±121 snores/hr (p-value = 0.25) or an average reduction of 25%.
Our study had several limitations. First, although we designed the therapy to improve strength, endurance, and coordination of the upper airway musculature (particularly the tongue base), we did not include specific tests (e.g., EMG) to evaluate changes in muscle electrophysiology that would have identified the precise mechanism of snoring reduction. Although feasible, such measures would have significantly added to the participant burden. Second, all participants in the control and the intervention group may not be similar in most measures despite the use of randomization technique. In particular, we used the results of either the HST or the PSG to identify our participants. As HST can frequently underestimate the AHI, it is possible that some participants had more than reported burden of OSA. Similarly, our study design did not account for changes in snoring that occur with body position or any changes in participants’ weight between the start and the end of the study. A repeat assessment of sleep disordered breathing with positional assessment and demographic variables could potentially address these important limitations. Third, although, participants were compliant with the exercise regimen, obtaining sufficient sleep recordings twice a week proved challenging. Nearly half of the initially enrolled participants had to be excluded because they did not meet nightly recording requirements, mostly because they either slept <5 hours on a nightly basis or had difficulties with real time data transmission for analysis. It is unclear if this had any impact on the final results of the study.
Conclusion
This study shows the efficacy of smartphone based gamification application in reducing snoring through delivery of a limited subset of targeted oropharyngeal exercises, resulting in repetitive forward and backward movements of tongue base. This technique addresses several important limitations of existing myofunctional therapies for treatment of snoring by reducing the complexity of exercise routines, reducing the training requirement for mastering exercises (e.g. playing an app-based game versus playing a didgeridoo), and enabling the patient and/or physician to track compliance with the therapy.
Supplementary Material
Acknowledgements:
The authors would like to acknowledge Dr. Peter Watson for his guidance in ultrasound imaging studies; Andrew Byrne for helping with microphone calibration; Dr. Steven Reinitz for contribution to study design, Max Anderson for contribution to participant recruitment and Dr. Anne Webber-Main for feedback on manuscript writing.
Funding: This study was funded by NIH Research Evaluation and Commercialization Hub (MN-REACH) Grant # 5U01HL127479–03.
Footnotes
Compliance with ethical standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Conflict of interest: Umesh Goswami: Holds equity in the entity aimed at commercialization of the technology described in this manuscript; Adam Black: Holds the provisional patent of the technology described in this manuscript and holds equity in the entity aimed at commercialization of the technology; Brian Krohn: Holds the provisional patent of the technology described in this manuscript and holds equity in the entity aimed at commercialization of the technology; Wendy Meyers: None; Conrad Iber: None
TRIAL REGISTRY: ClinicalTrials.gov; No.: NCT03264963; URL:www.clinicaltrials.gov
References
- 1.Young T, Palta M, Dempsey J, et al. (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235 [DOI] [PubMed] [Google Scholar]
- 2.Deeb R, Judge P, Peterson E, et al. (2014) Snoring and carotid artery intima-media thickness. Laryngoscope 124:1486–1491. doi: 10.1002/lary.24527 [DOI] [PubMed] [Google Scholar]
- 3.Jahn C, Gouveris H, Matthias C (2016) Systemic inflammation in patients with compromised upper airway anatomy and primary snoring or mild obstructive sleep apnea. Eur Arch Oto-Rhino-Laryngology 273:3429–3433. doi: 10.1007/s00405-016-4103-5 [DOI] [PubMed] [Google Scholar]
- 4.Dunai A, Keszei AP, Kopp MS, et al. (2008) Cardiovascular disease and health-care utilization in snorers: a population survey. Sleep 31:411–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beninati W, Harris CD, Herold DL, Shepard JW (1999) The Effect of Snoring and Obstructive Sleep Apnea on the Sleep Quality of Bed Partners. Mayo Clin Proc 74:955–958. doi: 10.4065/74.10.955 [DOI] [PubMed] [Google Scholar]
- 6.Puhan MA, Suarez A, Lo Cascio C, et al. (2006) Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ 332:266–70. doi: 10.1136/bmj.38705.470590.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guimarães KC, Drager LF, Genta PR, et al. (2009) Effects of Oropharyngeal Exercises on Patients with Moderate Obstructive Sleep Apnea Syndrome. Am J Respir Crit Care Med 179:962–966. doi: 10.1164/rccm.200806-981OC [DOI] [PubMed] [Google Scholar]
- 8.Ieto V, Kayamori F, Montes MI, et al. (2015) Effects of Oropharyngeal Exercises on Snoring. Chest 148:683–691. doi: 10.1378/chest.14-2953 [DOI] [PubMed] [Google Scholar]
- 9.Lawson E, Stuart-Smith J, Scobbie JM, Nakai S, Beavan D, Edmonds F, Edmonds I, Turk A, Timmins C, Beck J, Esling J, Leplatre G, Cowen S, Barras W, Durham M (2015) Seeing Speech: an articulatory web resource for the study of Phonetics. University of Glasgow. In: http://www.seeingspeech.ac.uk/. http://www.seeingspeech.ac.uk/ipachart/display.php?chart=4&datatype=1&speaker=l Accessed 18 Apr 2018
- 10.Bilici S, Engin A, Ozgur Y, et al. (2017) Submental Ultrasonographic Parameters among Patients with Obstructive Sleep Apnea. Otolaryngol Neck Surg 156:559–566. doi: 10.1177/0194599816684109 [DOI] [PubMed] [Google Scholar]
- 11.Berry RB, Brooks R, Gamaldo CE, et al. The AASM Manual for the Scoring of Sleep and Associated Events
- 12.Ng AK, Koh TS (2008) Using psychoacoustics of snoring sounds to screen for obstructive sleep apnea. In: 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE, pp 1647–1650 [DOI] [PubMed] [Google Scholar]
- 13.Rohrmeier C, Herzog M, Haubner F, Kuehnel TS (2012) The annoyance of snoring and psychoacoustic parameters: a step towards an objective measurement. Eur Arch Oto-Rhino-Laryngology 269:1537–1543. doi: 10.1007/s00405-011-1878-2 [DOI] [PubMed] [Google Scholar]
- 14.Rohrmeier C, Fischer R, Merz A-K, et al. (2015) Are subjective assessments of snoring sounds reliable? Eur Arch Oto-Rhino-Laryngology 272:233–240. doi: 10.1007/s00405-014-3211-3 [DOI] [PubMed] [Google Scholar]
- 15.Herzog M, Kühnel T, Bremert T, et al. (2009) The impact of the microphone position on the frequency analysis of snoring sounds. Eur Arch Oto-Rhino-Laryngology 266:1315–1322. doi: 10.1007/s00405-008-0858-7 [DOI] [PubMed] [Google Scholar]
- 16.Giannakopoulos T (2015) py Audio Analysis: An Open-Source Python Library for Audio Signal Analysis. PLoS One 10:e0144610 . doi: 10.1371/journal.pone.0144610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai I, Lo S, Wolf D, Kajieker A (2008) The effect of singing on snoring and daytime somnolence. Sleep Breath 12:265–268. doi: 10.1007/s11325-007-0159-1 [DOI] [PubMed] [Google Scholar]
- 18.Pierce R, White D, Malhotra A, et al. (2007) Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J 30:345–353. doi: 10.1183/09031936.00063406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGinley BM, Schwartz AR, Schneider H, et al. (2008) Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105:197–205. doi: 10.1152/japplphysiol.01214.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown EC, Cheng S, McKenzie DK, et al. (2013) Respiratory Movement of Upper Airway Tissue in Obstructive Sleep Apnea. Sleep 36:1069–1076. doi: 10.5665/sleep.2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilston LE, Gandevia SC (2014) Biomechanical properties of the human upper airway and their effect on its behavior during breathing and in obstructive sleep apnea. J Appl Physiol 116:314–324. doi: 10.1152/japplphysiol.00539.2013 [DOI] [PubMed] [Google Scholar]
- 22.Eckert DJ, Lo YL, Saboisky JP, et al. (2011) Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol 111: 1644–1653. doi: 10.1152/japplphysiol.00653.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


