Abstract
Objective:
The purpose of this study was to test the hypothesis that balanced crystalloids improve quality of recovery more than normal saline (0.9% sodium chloride, NS) in stable ED patients. Secondary outcomes measured differences in health care utilization.
Methods:
A single-site participant- and evaluator-blinded, 2-arm parallel allocation (1:1), comparative effectiveness randomized controlled trial allocated adults receiving IV fluids in the ED before discharge to receive 2 L of Lactated Ringer’s (LR) or NS. The primary outcome was symptom scores measured by the validated Quality of Recovery-40 (QoR-40) instrument (scores 40–200) 24 hours after enrollment. Secondary outcomes included subsequent health care use and medication compliance.
Results:
Participants (N=157) were enrolled and follow-up was analyzed for 94 (follow-up rate of 60%) using intention-to-treat methodology. There was no difference in post-enrollment QoR scores between NS and LR groups (mean difference 2.4; 95% CI −6.8 to 11.6). Although pre-enrollment scores were higher in the LR group (mean difference 10.4; 95% CI 1.9 to 19.0), adjusting for pre-survey imbalances did not change the primary outcome (adjusted difference −4.67; 95% CI −13.48 to 4.14). There were no differences in return to ED (mean difference 7.5%; 95% CI −8.7 to 23.8), prescriptions filled (mean difference 22.2%; 95% CI −3.3 to 47.6), or seeking care from another provider (mean difference −2.0%; 95% CI −19.9 to 15.9) at 7 days.
Conclusions:
NS and LR were associated with similar 24-h recovery scores and 7-day health care utilization in stable ED patients. These results supplement recent trials by informing fluid choice for stable ED patients.
INTRODUCTION
Background
Nearly 25% of emergency department (ED) patients receive intravenous (IV) fluids, making administration of IV fluids the most common ED procedures with many patients receiving IV fluids before ED discharge.1,2 Crystalloid IV fluids are administered to increase effective blood volume and maintain organ perfusion in patients with signs or symptoms of volume depletion and dehydration.3 Since its discovery in the 19th century, normal saline (NS), 0.9% sodium chloride, has been the most frequently administered crystalloid.4,5
High chloride loads associated with NS administration cause hyperchloremic acidosis, and this resulting acidosis has been hypothesized to contribute to poor clinical outcomes and impaired recovery.6–9 NS contributes to acute kidney injury, persistent kidney dysfunction, and mortality among critically ill patients.7 NS is also associated with decreased renal tissue perfusion, as the high chloride concentration increases afferent arteriole resistance.10 Even in healthy adults, NS impairs renal function and causes hyperchloremia, which can lead to myalgias, abdominal discomfort, and persistent kidney dysfunction.10–12 In contrast, balanced crystalloid solutions, like Lactated Ringer’s (LR), have a lower chloride concentration and avoid many of the concerns associated with NS.6,7,9,13–16
Recently, two large pragmatic randomized controlled trials have compared NS and balanced solutions.6,9 In hospitalized, noncritically ill patients, there was no difference in the primary outcome of hospital-free days, however, in the balanced solution group there was lower incidence of the major adverse kidney events (MAKE): a composite outcome of death, new renal-replacement therapy, and persistent renal dysfunction at 30 days (number needed to treat (NNT) = 91).6
In both trials, heterogeneity in the effect of balanced crystalloid solutions was observed by severity of illness, principal hospital diagnosis, and co-morbidities.6,9 Therefore, it is difficult to generalize these recent trial results to a different patient population consisting of mildly ill patients discharged from the ED. Further, the difference in MAKE outcomes suggests there could be differences in subjective recovery based on IV fluids. Given many patients do receive IV fluids before ED discharge, it is important to determine if balanced solutions will have a recovery benefit in this mildly ill population.
Importance
For many patients receiving IV fluids in the ED prior to discharge, the principal indication for fluid administration is to decrease symptoms and improve patients’ perception of well-being. While several studies have recently reported objective effects of fluid selection on clinical outcomes, the lack of patient-reported outcomes significantly impairs clinicians’ ability to fully appreciate the range of effects that fluid selection might have. Furthermore, these patient-reported outcomes may influence ongoing health care resource utilization, even in the absence of differences in mortality or renal failure. Identifying an optimal fluid choice could lead to significant benefits for patients and health care systems.
Goals of This Investigation
The objective of this study was to measure the effect of fluid selection on patient-reported symptoms 24 hours after ED discharge in noncritically ill patients. The primary outcome was patient-reported symptoms after ED discharge using the Quality of Recovery-40 (QoR-40), a validated instrument that quantifies patient’s self-assessment of functional recovery, symptoms, and physical comfort. Secondary outcomes included seven-day health care utilization, defined as return visits to the ED, filled ED prescriptions, and seeking care from another health care provider. We hypothesized that administration of a balanced crystalloid solution would lead to greater symptom improvement and decreased health care utilization compared to NS.
METHODS
Study Design and Setting
This study was a single-center, participant- and evaluator-blinded, 2-arm parallel allocation (1:1), comparative effectiveness randomized controlled trial. The study compared the quality of recovery of a single 2-liter (L) IV bolus of LR or NS. Study enrollment was conducted in a Midwestern academic ED with an annual census of 60,000 patients. The study was approved by the local institutional review board and registered on ClinicalTrials.gov (NCT03133767). All participants provided written informed consent for study participation, and this trial is reported in accordance with the CONsolidated Standards Of Reporting Trials (CONSORT) guidelines.17
Selection of Participants
Participants were enrolled from May 2017 to October 2017 from a convenience sample of adult (18 – 100 years old) ED patients presenting with one of the following complaints: nausea, vomiting or emesis, diarrhea, abdominal pain, dizziness, weakness, heat stroke or heat exhaustion, dehydration, fatigue, or volume depletion. Participants were included if they (1) were being administered IV fluids by their ED treatment team, (2) could tolerate 2 L of IV fluids (according to the treating clinician), and (3) were expected to be discharged from the ED without hospital admission. Between 0800 and 2300, trained research assistants identified potential study participants based on chief complaint and discussed eligibility criteria with the ED treatment team. Patients were excluded if they were pregnant, prisoners, did not speak English, were undergoing current chemotherapy, had signs of jaundice, had already received greater than 250 mL of IV fluids, or were unable to provide informed consent. No financial incentive was given for study participation.
Intervention
Participants were randomized to receive 2 L of either LR (treatment) or NS solution (control) using 1:1 allocation in randomized, permuted blocks by computerized random-generated sequence (block sizes 2 to 6). Allocation was concealed in opaque, sealed numbered envelopes. Participants received fluids in a peripheral IV line in the upper extremity placed for clinical care, and fluids were delivered directly from a standard preparation (Baxter Healthcare Corporation, Deerfield, IL). IV fluid bags were prepared outside the room and covered with an opaque bag to maintain participant blinding before being brought into the clinical care room by the treating nurse and administered. Participants continued to receive standard clinical care while in the ED, and the remainder of care was not dictated by the study protocol.
Methods of Measurement
Primary Outcome.
The primary outcome was the QoR-40 score, measured at 24 hours after the ED visit. The QoR-40 is a validated survey tool previously used in anesthesia and surgical populations that measures patient-reported recovery across five independent domains: Comfort, Emotion, Physical Independence, Patient Support, and Pain (Table S1). The QoR-40 was selected for use in this study since it measures quality of life during short-term recovery. Survey scores range from 40 to 200 with 40 indicating a poor quality of recovery and 200 indicating an excellent quality of recovery.18,19
Trained research assistants (RAs) administered the QoR-40 to participants immediately following study enrollment and consent, but prior to randomization and fluid administration (to confirm the adequacy of randomization). The same RA enrolled the participant, administered the survey, randomized the participant, and oversaw fluid administration. Twenty-four to 48 hours following ED discharge, a different RA blinded to treatment allocation administered the same QoR-40 by telephone (primary outcome). Participants that did not respond to three phone calls were considered lost to follow-up, but were still eligible for seven-day follow-up of secondary outcomes. Survey data were recorded first on paper and then transcribed into an electronic database (REDCap, version 8.1.1, Nashville, TN). A randomly selected sample (10%) of paper records was validated by an independent reviewer (to ensure accuracy of transcription between paper and electronic data) and achieved perfect concordance.
Secondary Outcomes.
Seven days after discharge from the ED, the same blinded RA (who had collected primary outcome) contacted participants via text message to evaluate their health care utilization. Participants were asked three questions: 1) “Have you returned to the emergency department for the same problem?”, 2) “Have you filled any prescriptions from the emergency department?”, and 3) “Have you seen another medical provider for the same complaint?”. Responses were provided by text message or telephone as dichotomous responses, and participants who did not respond to three queries were considered lost to follow-up.
Definitions.
Covariates were abstracted from the participant’s electronic medical record by a trained research assistant. Co-morbidities, including psychiatric disease, chronic kidney disease, and chronic gastrointestinal disease, were defined as the presence of a previous diagnosis in the medical record. For example, chronic gastrointestinal disorders was defined as a past medical history of chronic disorders of the gastrointestinal tract, including Crohn’s disease, Celiac disease, chronic pancreatitis/pancreatic insufficiency, lactose intolerance, diverticulosis, chronic diarrhea, gastroparesis, tubular adenoma, IBS, and diverticulitis.
Sample Size.
A power calculation was performed to select the necessary sample size prior to enrollment, and this calculation assumed that a minimum difference of 10-points on the QoR-40 survey score was clinically meaningful. This 10-point difference is equivalent to a 15% clinical recovery which has been widely used in previous research, established from a distribution-based method of inferring a clinically relevant difference.18–21 Expected loss to follow-up was estimated from a previous ED pilot study with similar telephone follow-up methods.20 The estimated necessary sample size (α = 0.05, β = 0.20, 40% expected loss to follow-up, difference of 10 points) was 156 total participants.
Analysis
Baseline characteristics were analyzed using a t-test for continuous variables and Pearson chi-square test for categorical variables and reported with basic descriptive statistics and 95% confidence intervals. The primary outcome, the 24-hour QoR-40 score, was analyzed using a Wilcoxon-Mann-Whitney test in an intention-to-treat (ITT) analysis, as QoR-40 scores were not expected to follow a normal distribution.18 An a priori-defined sensitivity analysis was planned using per-protocol analysis, to measure the sensitivity of results to treatment crossover. Individual analyses of the five survey domains (Pain, Patient Support, Emotions, Comfort, and Physical Independence) were conducted to identify specific areas of recovery. The seven-day survey responses were analyzed using a chi-square test to evaluate health care utilization following discharge.
Covariate Adjustment.
An a priori plan was followed to develop a multivariable linear regression model if there were imbalances in the baseline demographic variables or pre-treatment QOR-40 scores. Variables to be included in the regression model included any of the collected covariates with imbalances between the two treatment groups. Linear regression was planned, as the error terms followed a normal distribution.
Interim Safety Analysis.
A blinded, monthly safety analysis was performed by treatment received during the enrollment period by an independent safety monitor. No interim efficacy analysis was included (QoR-40 scores were not reported at interim analyses). The trial continued to the estimated sample size. There were no adverse drug events during the study.
Missing Data.
After data collection, several pre-treatment QoR-40 surveys were found to have missing values on individual questions. These values were imputed using multiple imputation to preserve variance in the variable with missing data. The missing-at-random assumption was supported by visualization of missing data patterns and assessment of correlation of other variables with a missing pre-survey score (threshold of r>0.4). One hundred imputed data sets were generated based on the distribution of the non-missing pre-survey scores. Then, regression parameters for each of the 100 data sets were pooled in a final model. Results are reported with complete case analysis and multiple imputation. No values were missing in the primary outcome QoR-40 at 24 hours, and no imputation was performed for participants lost to follow-up.
A post hoc sensitivity analysis was conducted to test the robustness of the primary outcome to participants lost to follow-up by estimating the necessary difference is the participants lost to follow-up to change the conclusion of the study. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Characteristics of study subjects
Of 777 patients screened, 217 were eligible for study participation, and the accrual rate was 71.7% (n=157, Figure 1). One participant was withdrawn from the study after randomization due to an unrecognized current pregnancy. The primary outcome was available for analysis in 60.3% of participants, and follow-up was higher in the NS group (68.8%) compared to the LR group (51.9%) (difference 17.0%, 95%CI: 1.8 to 32.1%). Participants were primarily female (62%) and Caucasian (84%) with a median age of 33.5 (IQR 23.0 to 44.0) years. The study arms were similar in most demographics, including ED and prehospital medications, ED chief complaint, and laboratory values. However, the NS group was more likely to have diabetes, chronic gastrointestinal disease, and psychiatric disease (Table 1). Approximately 5% (n=8) of participants had a missing value for a question in the pre-survey, resulting in a missing pre-survey score, and these scores were imputed.
Figure 1.
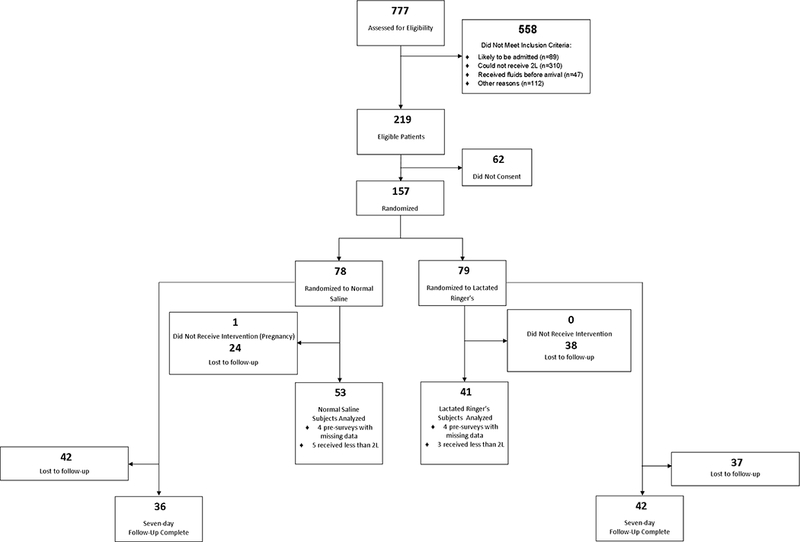
Flowchart of study participants.
Table 1.
Characteristics of Study Participants
| Normal Saline (N=53) | Lactated Ringer’s (N=41) | |||
|---|---|---|---|---|
| Age, median (IQR) | 39 (25, 45) | 29 (22, 41) | ||
| IV Fluid Volume Administered, mean (SD) | 1921 (252) | 1954 (237) | ||
| Calculated GFR, median (IQR) | 84 (72, 91) | 90 (81, 91) | ||
| N | % | N | % | |
| Female | 34 | 64.2 | 24 | 58.4 |
| Race | ||||
| African American | 7 | 13.2 | 2 | 4.9 |
| Asian | 0 | 0.0 | 3 | 7.3 |
| Caucasian | 45 | 84.9 | 34 | 82.9 |
| Other | 1 | 1.9 | 2 | 4.9 |
| ED Chief Complaint | ||||
| Abdominal Pain | 28 | 52.8 | 15 | 36.6 |
| Diarrhea | 1 | 1.9 | 2 | 4.9 |
| Dizziness | 3 | 5.7 | 4 | 9.8 |
| Fever | 2 | 3.8 | 2 | 4.9 |
| Flank Pain | 6 | 11.3 | 4 | 9.8 |
| General Illness | 1 | 1.9 | 0 | 0.0 |
| Headache | 3 | 5.7 | 5 | 12.2 |
| Vomiting or Emesis | 7 | 13.2 | 7 | 17.1 |
| Other | 2 | 3.8 | 2 | 4.9 |
| Prehospital Medications | 5 | 9.4 | 6 | 14.6 |
| ED Medications before Pre-Survey | ||||
| Opiates | 10 | 18.9 | 12 | 29.3 |
| NSAIDs | 6 | 11.3 | 4 | 9.8 |
| Anti-emetics | 14 | 26.4 | 11 | 26.8 |
| Benzodiazepines | 0 | 0.0 | 1 | 2.4 |
| None | 35 | 66.0 | 25 | 61.0 |
| ED Medications following Pre-Survey | ||||
| Opiates | 19 | 35.9 | 16 | 39.0 |
| NSAIDs | 12 | 22.6 | 11 | 26.8 |
| Anti-emetics | 26 | 49.1 | 17 | 41.4 |
| Benzodiazepines | 0 | 0.0 | 1 | 2.4 |
| None | 17 | 32.1 | 13 | 31.7 |
| ED Disposition | ||||
| Discharged from ED | 44 | 83.0 | 37 | 90.2 |
| Admitted to Inpatient Floor | 5 | 9.4 | 2 | 4.9 |
| Admitted for Observation | 4 | 7.6 | 1 | 2.4 |
| Other | 0 | 0.0 | 1 | 2.4 |
| Co-morbidities | ||||
| Chronic Kidney Disease | 0 | 0.0 | 1 | 2.4 |
| Congestive Heart Failure | 0 | 0.0 | 0 | 0.0 |
| Diabetes | 7 | 13.2 | 0 | 0.0 |
| Chronic Gastrointestinal Disease | 11 | 20.8 | 3 | 7.3 |
| Migraine History | 7 | 13.2 | 6 | 46.2 |
| Psychiatric Disorder | 24 | 45.3 | 8 | 19.5 |
| Urine Specific Gravity | ||||
| Not Measured | 21 | 55.3 | 19 | 46.3 |
| < 1.000 | 2 | 3.8 | 1 | 2.4 |
| 1.000 – 1.030 | 26 | 49.1 | 19 | 46.3 |
| >1.030 | 4 | 7.8 | 2 | 4.9 |
| BUN/Cr Ratio | ||||
| < 10 | 17 | 32.1 | 17 | 41.5 |
| 10 – 20 | 31 | 58.5 | 21 | 51.2 |
| >20 | 5 | 9.4 | 3 | 7.3 |
| ED Discharge Diagnoses | ||||
| Abdominal Pain/Disease | 13 | 31.7 | 15 | 28.3 |
| Fever | 3 | 5.7 | 1 | 2.4 |
| Flank Pain/Disease | 10 | 18.9 | 4 | 9.8 |
| Headache or Migraine | 4 | 7.6 | 5 | 12.2 |
| Nausea, Vomiting, or Diarrhea | 7 | 13.2 | 6 | 14.6 |
| Syncope | 0 | 0.0 | 3 | 7.3 |
| Urinary Tract Infection | 1 | 1.9 | 1 | 2.4 |
| Viral Syndrome | 2 | 3.8 | 1 | 2.4 |
| Other | 11 | 20.8 | 7 | 17.1 |
| Any GI (Abdominal Pain or Nausea, Vomiting, or Diarrhea) | 19 | 46.3 | 22 | 53.7 |
SD= Standard Deviation; BUN/Cr = Blood Urea Nitrogen/Creatinine Ratio
The analysis population on the primary outcome (n=94) was mostly similar to the population lost to follow-up (Table S2). However, they were less likely to receive anti-emetic medication in the ED (45.2 vs. 26.6%, difference 18.6%, 95%CI: 3.3 to 33.8%) and less likely to receive non-steroidal anti-inflammatory medications after survey administration in the ED (24.5% vs. 9.7%, difference 14.8%, 95%CI: 3.4 to 26.2%) when compared to participants lost to follow-up (n=62). Pre-analysis scores between participants who responded to the follow-up survey and those who did not were not statistically different (difference= −1.23; 95% CI −10.3 to 7.8). Study participants admitted to the hospital (18.0%) were included in the analysis.
Main Results
Primary Outcome.
Participants in both treatment groups (NS and LR) showed an improvement in their QoR-40 score 24 hours after receiving IV fluids in the ED (NS 22.7, 95%CI 14.6 to 30.4; and LR 14.7, 95%CI 7.2 to 20.0). There was no difference in the primary outcome between the LR and NS group (166.8 LR vs. 164.4 NS, mean difference 2.4; 95%CI −6.8 to 11.6; Table 2). There was a difference between pre-enrollment QoR-40 scores (152.1 LR vs. 141.7 NS, mean difference 10.5; 95%CI 1.9 to 19.0). As a post-hoc exploratory analysis, 62.3% (n=33) of the NS group experienced a significant improvement (defined as a change in 10 points) in QoR-40 compared to 51.2% (n=21) of the LR group (difference 11.0%; 95%CI −9.1 to 31.2%).
Table 2.
Primary Outcome by Treatment Allocation: Quality of Recovery-40 scores.
| Survey Domain (possible range of scores) | Pre-survey | Post-survey | ||||
|---|---|---|---|---|---|---|
| Normal Saline (n=70) | Lactated Ringer’s (n=67) | Mean Difference (95% CI) | Normal Saline (n=53) | Lactated Ringer’s (n=41) | Mean Difference (95% CI) | |
| QoR-40* | 141.7 | 152.1 | 10.5 (1.9, 19.0) | 164.4 | 166.8 | 2.4 (−6.8, 11.6) |
| Emotional State (9–45) | 30.7 | 32.2 | 1.6 (−0.9, 4.0) | 36.2 | 37.4 | 1.3 (−1.4, 3.9) |
| Comfort (12–60) | 37.6 | 40.4 | 2.8 (−0.3, 5.9) | 48.6 | 49.0 | 0.4 (−3.1, 3.8) |
| Patient Support (7–35) | 31.0 | 32.1 | 1.1 (−0.5, 2.7) | 31.8 | 32.7 | 0.8 (−1.1, 2.8) |
| Physical Independence (5–25) | 20.1 | 21.4 | 1.3 (−0.3, 2.8) | 22.8 | 22.4 | −0.4 (−1.7, 0.9) |
| Pain (7–35) | 23.7 | 25.1 | 1.4 (−0.4, 3.2) | 28.6 | 28.8 | 0.1 (−2.0, 2.2) |
The QoR-40 ranges from 40 to 200 with higher scores indicating a higher quality of recovery.
Covariate Adjustment.
A multivariable linear regression analysis was conducted adjusting for the pre-enrollment score differences and unbalanced covariates (Table 3), and this continued to show no relationship between group allocation and post-survey scores (adjusted difference −3.85; 95%CI −12.94 to 5.24).
Table 3.
Results of Multivariable Regression to Predict Post-Survey Scores
| Mean Difference | 95%CI | Adjusted Mean Difference | 95%CI | |
|---|---|---|---|---|
| Complete Case Analysis a | ||||
| Treatment Group | −2.81 | (−11.31, 5.69) | −4.91 | (−14.44, 4.63) |
| Pre-Treatment QoR-40 Score | - | - | 0.44 | (0.27, 0.61) |
| Diabetes | - | - | −5.44 | (−26.57, 15.69) |
| Psychiatric Disease | - | - | −4.87 | (−14.28, 4.53) |
| Chronic Gastrointestinal Disease | - | - | −2.14 | (−15.48, 11.21) |
| Imputed Pre-Survey Score Analysis b | ||||
| Treatment Group | −1.70 | (−10.09, 6.68) | −3.85 | (−12.94, 5.24) |
| Pre-Treatment QoR-40 Score | - | - | 0.40 | (0.23, 0.57) |
| Diabetes | - | - | −4.98 | (−22.96, 13.00) |
| Psychiatric Disease | - | - | −4.72 | (−13.65, 4.22) |
| Chronic Gastrointestinal Disease | - | - | −2.58 | (−15.64, 10.47) |
Complete case analysis includes participants that had no missing responses for the pre-treatment QoR-40 survey.
Imputed pre-survey score analysis uses multiple imputation to estimate the pre-treatment QoR-40 score for subjects with missing pre-treatment survey data (n=8) prior to developing the adjusted model.
QoR-40 Survey Sub-Domain Analysis.
There was no difference between the groups in emotional state, comfort, patient support, physical independence, or pain twenty-four hours after receiving IV fluids in the ED (Table 2). Overall, comfort scores had the largest increase from pre- to post-survey scores, while pain and emotional state increased modestly. None of the differences were statistically significant.
Secondary Outcomes.
Table 4 shows the results of the 7-day health care utilization survey. Response rates for the secondary outcomes were 47.4% (n=36) in the NS group and 53.3% (n=42) in the LR group (difference −5.9%, 95%CI: −21.6 to 9.8%). The most common way patients utilized more health care was filling a prescription (42.3%). There were no differences in health care utilization between the treatment groups. Overall, 15.4% of participants returned to the ED for the same complaint within 7 days of the study visit.
Table 4.
Secondary Outcomes by Treatment Group
| Normal Saline (N=36) | Lactated Ringer’s (N=42) | Mean Difference | |||
|---|---|---|---|---|---|
| n | % | n | % | 95% CI | |
| Returned to the ED | 7 | 19.4 | 5 | 11.9 | 7.5 (−8.7, 23.8) |
| Sought care from another provider | 7 | 19.4 | 9 | 21.4 | −2.0 (−19.9, 15.9) |
| Filled prescription* | 19 | 76.0 | 14 | 53.9 | 22.2 (−3.3, 47.6) |
Patients without ED prescriptions (n=27; 11 NS, 16 LR) were not included in the analysis of prescriptions filled.
Per-Protocol Analysis.
There were no treatment crossovers during the study. A per-protocol analysis was completed with subjects that received the full 2L of intervention (90.6%, n=48 of NS group, 92.7%, n=38 of LR group). The primary outcome, post-treatment QoR-40 scores remained similar between the two treatment groups (167.3 LR vs. 164.4 NS, mean difference 2.9; 95% CI −5.0 to 23.2).
Sensitivity Analysis.
Due to differential loss to follow-up between the two treatment groups, a sensitivity analysis estimated the magnitude of a hypothetical difference between the two groups’ post-survey scores needed to change the interpretation of the primary outcome. The control group (NS) was assumed to have the mean value, while the treatment group’s value was varied to identify the minimum increase or decrease in QoR-40 score that would change the conclusion of the study results. If all participants had responded to the 24-h survey, a six-point reduction or an eight-point increase in the post QoR-40 scores of participants lost to follow-up would be needed to change the study conclusion of no difference between the two treatment groups.
LIMITATIONS
Our study has several important limitations. First, this was a single-center study. Second, only 11% of participants had an abnormal BUN-to-creatinine ratio (Table 1), suggesting that many participants may not have been objectively volume-depleted. This finding could reflect broad inclusion criteria, but because this clinical judgment reflected current ED practice, we think that our findings apply broadly to the population receiving IV fluids prior to discharge. Considering recent findings of differences in hospitalized patients,6 the inclusion of admitted patients in our study population may have biased the reported results toward finding a significant difference. However, our results still showed no difference between the treatment groups. An exploratory analysis was conducted for hospitalized patients, which showed that the QoR-40 scores improved by 17.8 (SD 24.0) points in the hospitalized participants compared with 18.8 (SD 24.8) in the discharged participants. It is also possible that some patients received IV fluids for alternate reasons (e.g. hydration prior to intravenous contrast agents).
Third, our study design included a heterogeneous ED patient population. Participants might have been expected to have different recovery patterns based on the varying indications for care, which could dilute our observed effect. Similar to other studies on this topic,6 though, this participant heterogeneity reflects current ED practice, and no imbalances on indication for fluids were observed in the randomization. Additionally, the primary outcome measure, QoR-40, has been validated in anesthesia and surgery populations, but has not been used in emergency medicine populations. While some questions directly relate to general anesthesia, the majority of response items capture relevant dimensions of recovery for the study population.
The study dose of 2 L may be a larger dose than generally administered for noncritical patients, but dosing was selected to ensure that patients received a sufficient chloride load to observe effects if they existed. Two liters has shown to induce physiologic changes,7 and a recent study of noncritical, admitted ED patients showed differences in renal function with only 1 L.15 As participants in our study received a higher dose of chloride, our conclusion of no observed effect of fluid choice on subjective outcomes is strengthened.
Loss to follow-up is a significant limitation of this study, with nearly a 40% loss to follow-up and more participants in the NS group completing follow-up surveys compared to the LR group. While we powered the study for a high loss to follow-up, the high loss to follow-up remains a major limitation. The differential loss to follow-up between treatment groups creates an unequal group distribution that was not predicted in the sample size calculation. As pre-survey score was a strong predictor of post-survey score, it is likely that differences in the lost-to-follow-up population compared to the analysis population are minimal. A sensitivity analysis showed that the study results were robust to changes in the responses of the participants loss to follow-up, as a −6 or +8 difference was required to change the study conclusion. Since pre-survey scores and ED diagnoses did not differ based on follow-up status, the loss-to-follow-up population is unlikely to be substantially different from the analysis population.
DISCUSSION
While prior studies evaluated the effects of fluid choice in critical patients and patients admitted to the general ward, our study evaluated fluid choice in ED patients who were anticipated to be discharged home. It also evaluated the patient’s subjective perception of recovery. Patient-reported recovery is an important outcome for patients in whom fluid administration is being considered, and differences in patient-reported symptom scores would support selection of fluids better targeting improved symptoms.
In noncritically ill patients, crystalloid choice may not affect recovery time or health care utilization. The SALT-ED study reported no difference in hospital-free days between admitted patients receiving balanced solutions compared to NS. Our study supports these findings, as fluid choice had no effect on subjective recovery of participants, even when administered at higher doses.
The studies by Semler et al. and Self et al. suggest that fluid choice has a larger effect on outcomes in critically ill patients when compared to noncritically ill patients.6,9 In critically ill patients, balanced solutions resulted in a benefit in MAKE outcomes.9 Similar decreases in MAKE outcomes were observed in noncritically ill patients, but the lower incidence in MAKE outcomes was driven by differences in persistent renal dysfunction (defined as final serum creatinine concentration, ≥200% of the baseline value).6 The increased creatinine could be reflective of sentinel microvascular changes and interstitial edema in encapsulated renal tissue due to NS’s chloride load.21 The participants we enrolled may not have been ill enough for the acidosis associated with NS administration to lead to symptoms, and the ability to buffer that chloride load may have preserved their rapid recovery.
Our study population included patients who received IV fluids based on clinician judgment and as part of standard clinical care. However, only 10% of our participants had an elevated BUN-to-creatinine ratio out of those with a ratio measured. This suggests IV fluid administration may not be necessary in this population, and that liberal use of IV fluids may not prevent repeat health care visits. Our data are not sufficient to recommend against IV fluid use, but little data supports liberal crystalloid fluid administration in patients with normal laboratory studies discharged to home despite its ubiquitous practice. IV fluid administration in our study population may not be necessary or therapeutic. During recent IV fluid shortages, some hospitals have reserved IV fluid treatment for critically ill patients, while utilizing oral rehydration protocols for ED patients who would have previously received IV fluids.22,23 Future studies could evaluate the effect of different rehydration protocols, such as a comparison of oral rehydration and IV fluid treatments, on quality of recovery in these ED patients. Moreover, our secondary outcomes, while underpowered, demonstrated a notable ED re-visit rate during the first seven days following discharge. This suggests a potential need to identify predictors of increased health care utilization among noncritically ill patients discharged from the ED.
In summary, LR and NS were associated with similar patient-reported recovery in patients anticipated to be discharged home from the ED. LR does not lead to improved quality of recovery when compared to NS. Our findings supplement the conclusions of recent large trials that either LR or NS may be reasonable choices for fluid resuscitation in patients who are not critically ill.
Supplementary Material
Acknowledgements:
The authors specifically acknowledge the statistical support and expertise of Patrick Ten Eyck, PhD, for his help with multiple imputation, the University of Iowa Department of Emergency Medicine, and the University of Iowa Emergency Department – Research Enrollers Program.
GRANTS: Research reported in this publication was supported by the University of Iowa Department of Emergency Medicine and the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number U54TR001356 and NIH training grant number 5 T35 HL 7485-38. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
MEETINGS (WHERE PRESENTED W/ LOCATION & DATE): These findings were presented at the Society for Academic Emergency Medicine (SAEM) Great Plains Regional Meeting on September 7, 2017 in Columbia, MO and at the SAEM Annual Meeting in Indianapolis, IN on May 17, 2018.
CONFLICTS OF INTEREST: The authors report no conflicts of interest.
Clinical Trials Registry Number: NCT03133767
Contributor Information
Andrew Friederich, Department of Emergency Medicine, University of Iowa Carver College of Medicine, Iowa City, IA.
Natalie Martin, Department of Emergency Medicine, University of Iowa Carver College of Medicine, Iowa City, IA.
Morgan Bobb Swanson, Department of Emergency Medicine, University of Iowa Carver College of Medicine, Iowa City, IA.
Brett A. Faine, Department of Emergency Medicine, Department of Pharmaceutical Care, University of Iowa Carver College of Medicine, Iowa City, IA.
Nicholas M. Mohr, Department of Emergency Medicine, Department of Anesthesia, University of Iowa Carver College of Medicine, Iowa City, IA.
REFERENCES
- 1.Rui P, Kang K. National Hospital Ambulatory Medical Care Survey: 2014 Emergency Department Summary Tables.: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 2.Moore B, Stocks C, Owens P. Trends in Emergency Department Visits, 2006–2014. In: AHRQ, ed. Vol HCUP Statistical Brief #227. Rockville, MD: 2017. [Google Scholar]
- 3.Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100(4):1093–1106. [DOI] [PubMed] [Google Scholar]
- 4.Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(13):1243–1251. [DOI] [PubMed] [Google Scholar]
- 5.Awad S, Allison SP, Lobo DN. The history of 0.9% saline. Clin Nutr. 2008;27(2):179–188. [DOI] [PubMed] [Google Scholar]
- 6.Self WH, Semler MW, Wanderer JP, et al. Balanced Crystalloids versus Saline in Noncritically Ill Adults. N Engl J Med. 2018;378(9):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg. 2015;102(1):24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yunos NM, Bellomo R, Glassford N, Sutcliffe H, Lam Q, Bailey M. Chloride-liberal vs. chloride-restrictive intravenous fluid administration and acute kidney injury: an extended analysis. Intensive Care Med. 2015;41(2):257–264. [DOI] [PubMed] [Google Scholar]
- 9.Semler MW, Self WH, Wanderer JP, et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018;378(9):829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(1):18–24. [DOI] [PubMed] [Google Scholar]
- 11.Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88(5):999–1003. [DOI] [PubMed] [Google Scholar]
- 12.Yunos NM, Bellomo R, Hegarty C, Story D, Ho L, Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. Jama. 2012;308(15):1566–1572. [DOI] [PubMed] [Google Scholar]
- 13.Martini WZ, Cortez DS, Dubick MA. Comparisons of normal saline and lactated Ringer’s resuscitation on hemodynamics, metabolic responses, and coagulation in pigs after severe hemorrhagic shock. Scand J Trauma Resusc Emerg Med. 2013;21:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker ME. 0.9% saline induced hyperchloremic acidosis. J Trauma Nurs. 2015;22(2):111–116. [DOI] [PubMed] [Google Scholar]
- 15.Shaw AD, Raghunathan K, Peyerl FW, Munson SH, Paluszkiewicz SM, Schermer CR. Association between intravenous chloride load during resuscitation and in-hospital mortality among patients with SIRS. Intensive Care Med. 2014;40(12):1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellum JA, Song M, Li J. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care. 2004;8(5):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rennie D CONSORT revised--improving the reporting of randomized trials. Jama. 2001;285(15):2006–2007. [DOI] [PubMed] [Google Scholar]
- 18.Myles PS, Hunt JO, Nightingale CE, et al. Development and psychometric testing of a quality of recovery score after general anesthesia and surgery in adults. Anesth Analg. 1999;88(1):83–90. [DOI] [PubMed] [Google Scholar]
- 19.Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth. 2013;111(2):161–169. [DOI] [PubMed] [Google Scholar]
- 20.Faine B, Denning G, Bell G. A pilot comparison of the efficacy of a 3-day course of nitrofurantoin versus 3-day ciprofloxacin in females with uncomplicated bacterial cystitis in the Emergency Department. Paper presented at: American College of Emergency Physicians Scientific Assembly2012; Denver CO Poster Presentation. [Google Scholar]
- 21.Ding X, Cheng Z, Qian Q. Intravenous Fluids and Acute Kidney Injury. Blood Purif. 2017;43(1–3):163–172. [DOI] [PubMed] [Google Scholar]
- 22.Mazer-Amirshahi M, Fox ER. Saline Shortages — Many Causes, No Simple Solution. New England Journal of Medicine. 2018;378(16):1472–1474. [DOI] [PubMed] [Google Scholar]
- 23.Patiño AM, Marsh RH, Nilles EJ, Baugh CW, Rouhani SA, Kayden S. Facing the Shortage of IV Fluids — A Hospital-Based Oral Rehydration Strategy. New England Journal of Medicine. 2018;378(16):1475–1477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


