Abstract
Social behaviors largely constitute mutual exchanges of social cues and the responses to them. The adaptive response also requires proper interpretation of the current context. In fear behaviors, social signals have bidirectional effects—some cues elicit or enhance fear whereas other suppress or buffer it. Studies on the social facilitation and social buffering of fear provide evidence of competition between social cues of opposing meanings. Co-expression of opposing cues by the same animal may explain the contradicting outcomes from the interaction between naive and frightened conspecifics, which reflect the fine balance between fear facilitation and buffering. The neuronal mechanisms that determine that balance provide an exciting target for future studies to probe the brain circuits underlying social modulation of emotional behaviors.
Keywords: alarm pheromone, appeasing pheromone, avoidance, observational fear, social buffering
1 |. INTRODUCTION
When rodents encounter a threat, they respond with flight, vocalization, defecation and urination, or freezing, which act as signals of danger and help conspecifics’ survival by initiating defensive behaviors. These signals recruit multiple sensory systems—visual, auditory, olfactory and sometimes mechanosensory. Paradoxically, even during threatening situations, animals generate appeasing social cues that serve as a safety signal.1–3 Furthermore, other factors—social status, familiarity and prior experience of stress—influence how the animal interprets and responds to social cues.4,5 Such process may need the higher brain circuits that integrate the multimodal and conflicting information, and that have not been identified yet. Meanwhile, extensive data is accumulating about distinct effects of social signals on fear learning. At the same time, significant progress has been made in identifying the pheromones and the olfactory circuits that signal threat and safety. Here, we review the distinct effects of social signals on defensive behaviors in rodents, the underlying olfactory pathways and possible mechanisms of integrating complex information to produce the proper behavioral response.
We will describe the effects of social signals that are emitted by naive animals or by demonstrators undergoing fear learning or fear expression. Such signals promote (Section 2) and buffer (Section 4) defensive responses in the “receiver” subject. First, they can reinforce aversive learning acting as the unconditioned stimulus (Section 2.1) in the avoidance (Section 2.1.1) and fear conditioning paradigms (Section 2.1.2). Notably, the demonstrators’ unconditioned and condi tioned defensive responses both can reinforce fear learning. Second, social signals can enhance the learning (Section 2.2). Third, the repeated vicarious social defeat produces non-associative defensive behaviors—the anxiety and depression-like traits—as in the regular social defeat paradigm (Section 2.3). Importantly, the other factors— social status, familiarity and prior experience of stress—modulate these effects (Section 3).
Social signals emitted by naive conspecifics, when presented during fear training, memory consolidation and expression, usually buffer defensive responses (Section 4). Remarkably, even when the animal is distressed and frightened, it generates fear-buffering cues (Section 5 and Section 6). Among the multimodal social signals, studies on the olfactory pathways responsible for these phenomena made bigger progress—the putative alarm and appeasing pheromones, their receptors, and the downstream targets have been identified (Section 7). Finally, we discuss potential brain nodes which process the multi-modal social signals and interact with the fear and reward circuits in distinct social contexts, and the contribution of the higher brain structures to integrating social signals of the opposing meanings (Section 8).
2 |. SOCIAL FACILITATION OF DEFENSIVE BEHAVIORS
2.1 |. Social signals work as unconditioned stimulus during aversive learning
2.1.1 |. Reinforcement of avoidance
The very first evidence that rodents can learn avoidance by observing conspecific, came from experiments by Lore et al, in which observer rats watched demonstrator rats learning to avoid candles with fire. After the exposure, the observer rats learned the avoidance faster than controls.6 Such observational avoidance learning was not always successful, for example, rats did not learn the step-through inhibitory avoidance from watching demonstrator rats, which crossed in the dark chamber and received the resulting footshock.3 So far, there is no evidence that mice can learn such task either.
Another example of successful avoidance in mice was self-burying to escape from natural micropredators, stable flies Stomoxys calcitrans, developed by Kavaliers et al. In this ethologically relevant paradigm, first, naive laboratory mice did not avoid the flies that were altered not to bite. Next, the demonstrator mice learned to avoid bites from intact flies, and the subject mice watched how the trained demonstrators, attacked by the flies, buried under the bedding. Finally, 1–3 days later, the subjects buried themselves under the bedding upon encounter with the non-biting flies. Here, the observers learned the task without ever experiencing biting or expressing defensive responses during training.7–9
2.1.2 |. Reinforcement of conditioned fear
The term “observational conditioning” was originally introduced to describe experiments in monkeys, in which the subject acquired fear of snakes vicariously, by observing the fearful behavior of a conspecific exposed to a snake.10,11 Several studies indicate that social signals can also act, in rodents, as an aversive unconditioned stimulus, though less effective than electrical footshocks.
There are two rodent paradigms for vicarious fear learning. In the observational fear conditioning, an observer subject watches a naive demonstrator undergoing fear conditioning training. Upon exposure to the conditioned stimulus (CS) and unconditioned stimulus (US), the demonstrator expresses the unconditioned response, which includes vocalizations, jumps and freezing. In the fear conditioning by proxy, the observer subject watches the trained demonstrator expressing the conditioned response, freezing to the CS.
Observational fear conditioning
Jeon et al have shown that mice remember the context in which they observed another mouse been shocked.12 The subject observer mice were trained by exposure, via a transparent partition, to the demonstrator mouse receiving repeated electrical footshocks. When returned to the training box 24 hours later, the observers exhibited freezing. Replacing the transparent partition with an opaque one attenuated fear learning but did not abolish it, which indicated that, in addition to vision, multiple sensory modalities conveyed the information about fear in the demonstrator.
Mice and rats can learn, not only the contextual fear, but also the auditory fear conditioning by observing tone-footshock pairing on a demonstrator. In mice, such learning was stronger in the gregarious C57BL/6J strain than in the less social BALB/cJ mice.13 In rats, social isolation impaired such conditioning.14
Fear conditioning by proxy
In the paradigm established by Bruchey et al, fear learning was reinforced by the cues from a previously conditioned demonstrator expressing the conditioned response.15 At first, the demonstrator rats were trained in a classical Pavlovian paradigm, in which a tone, paired with an electrical footshock, was repeated three times. Twenty-four hours later, the observer subjects were exposed to the demonstrators while the tone was played and the demonstrators expressed fear. When tested 24 hours later in the same chamber and with the same tone, the demonstrator-exposed subject exhibited more freezing than the control rats. However, the freezing was very modest, and the half of the animals did not freeze at all. Females also learned the fear conditioning by proxy and showed higher freezing than males.15,16
Interestingly, the social reinforcing stimuli during the fear conditioning by proxy appears different from those during the observational fear conditioning—conditional responses to CS in the proxy learning, and unconditional responses to US in the observational fear, respectively. Despite the demonstrators that express the unconditional and conditional fear, exhibit very different patterns of movements (jumps vs immobility) and vocalizations (robust vs rare17), the observers learned fear in both paradigms. It suggests that other sensory modalities beyond visual and auditory ones can reinforce fear learning. Moreover, the fear leaning by proxy did not correlate to the amount of freezing in demonstrators. Those dissociations of the putative reinforcing signals from behaviors suggest that the demonstrators expressed fear without freezing and emitted social cues that act as a reinforcement contingency for the observer. Accordingly, the fear learning did correlate with the social interaction between the two animals during the fear by proxy training, suggesting the physical proximity determined the effectiveness of observational learning.
2.2 |. Social signals as enhancers of aversive learning
Besides acting as the US, as discussed in Section 2.1, social signals can enhance the aversive learning that is reinforced by electrical footshock as US. The stress-enhanced fear learning is well documented for various stressors18–23 and examples below show such short-term and long-term enhancement by social cues.
Knapska et al documented the short-term effects on learning in the two-way avoidance and contextual fear conditioning in rats.24 The authors distressed the demonstrator rat by training in the contextual fear conditioning paradigm and then immediately returned it to the home cage where the demonstrator interacted with the subject rat for 10 minutes. The control subjects interacted with non-shocked demonstrators. Immediately afterwards, the observers were trained in a shuttle box to avoid electrical footshocks, signaled by an auditory stimulus, or underwent contextual fear conditioning. The avoidance learning was higher in the observers that interacted with the fear conditioned demonstrators than with controls. Similarly, such observers showed stronger contextual fear memories.
Our laboratory has found that a 4 minutes exposure to distressed conspecific has a lasting effect on subsequent inhibitory avoidance learning in mice.25 Subject mice were exposed to the cagemate demonstrators that were receiving repeated electrical footshocks, as during the observational fear conditioning described in the Section “Observational Fear Conditioning”.12 A transparent perforated Plexiglas partition separated the two animals, which allowed transmission of visual, olfactory and auditory signals. Control mice watched the demonstrators that were not receiving footshocks. On the next day, the observers underwent inhibitory avoidance training. When tested 24 hours later, the subjects exposed to the shocked demonstrators showed higher retention of the inhibitory avoidance memory than the controls. Parallel ex vivo electrophysiology experiments revealed that the prefrontal-amygdala pathway formed new silent synapses25 and had more plasticity, measured as LTP in the amygdala slices (data not shown), which may explain the enhanced inhibitory avoidance learning.
2.3 |. Social induction of non-associative defensive responses
Social signals not only reinforce and modulate fear memories but also change emotional states, defined by the behavioral traits relevant to anxiety and depression. The vicarious social defeat is a robust model of such modulation,26 which is a non-associative phenomenon. The observer C57BL/6J mouse watches, 10 minutes per day for 10 days, how an aggressive CD-1 mouse attacks another C57BL/6 J mouse. Then, the observer and the aggressor are housed in the same cage, but separated by a transparent perforated partition. After this treatment, the observers decreased sociability in the three chamber task, acquired the anxiety-like trait of spending less time in the open arms of the elevated plus maze, and acquired the depression-like traits of decreased sucrose preference and increased immobility in the forced swim test. These effects were similar to the effects of the physical, non-vicarious social defeat and were reproduced in females.27 Given the observer received two kinds of cues, the distress cues from the defeated demonstrator and the threatening cues from the CD-1 aggressor, the role of each type of cues in eliciting specific behavioral traits remains to be determined.
3 |. NON-THREAT SOCIAL INFORMATION MODULATES THE PERCEPTION OF THREAT SIGNALS
In humans, knowledge about the source of information biases the interpretation. Likewise, observer animals do not treat the same signals of threat equally when they come from different demonstrators. There is evidence that the social cues that do not signal threat but inform about familiarity or dominance of demonstrator, powerfully modulate threat response. In the study described in the previous section, on the vicarious learning to avoid biting flies, subordinate mice learned better from dominant demonstrators than the dominant learned from the subordinates. Mice also learned better from littermates than from non-kin demonstrators, even after single housing for over 120 days before the experiment.5 The fear conditioning by proxy showed a similar effect of dominance: the fear learning was more robust from dominant to subordinate rat, but not in the opposite direction.4
The familiarity impacts the observational fear conditioning too: the learning of contextual fear was stronger in siblings than in strangers. Interestingly, when subject male mice were co-housed with female mating partners, who were later used as demonstrators, the learning was stronger with the co-housed females than with strangers, but only if the animals lived together for more than 10 weeks.12 The role of dominance in the observational fear conditioning needs additional studies.
These findings suggest that social ranking (dominance) and social identity (familiarity) modulate the perception of the social signals of threat as either reliable or not. The perception may be influenced by the level of arousal to the demonstrator and the attraction to the particular odor or behavioral pattern. The brain regions that process signals of familiarity, dominance or something else, must interact with the circuits that process social signals of threat to enhance or devalue the threat information.
4 |. SOCIAL BUFFERING OF FEAR
“Better recovery from stress” is a broad definition of social buffering.28,29 The types of social buffering and their dependency on social organization, sex, developmental stage, and social status within groups are thoroughly reviewed elsewhere.28,30 Here, we focus on the social buffering in several fear learning paradigms in adult rodents—the buffering takes effects not only at fear learning and expression, but also before the learning and during the consolidation of memory.
Davitz and Mason were the first to report social buffering of learned fear in rats, and described the buffering that takes place during the retrieval of fear memories.31 They conditioned rats by repeated exposures to a 2 seconds blinking light followed a 3 seconds footshock, and then tested for fear expression to the light, either with or without a companion rat. The companion rats attenuated fear in the subjects, measured as the behavioral inactivity. The authors did not use the term, buffering, and attributed the fear attenuation to distracting subjects from CS by triggering exploratory drive.
A series of recent studies expanded the effective time point of social buffering, showing the effect not only during retrieval, but also before and during fear learning. Guzman et al demonstrated that pre-exposure to social cues buffered contextual fear in mice.32 They exposed subjects to training context together with another mouse for 3 minutes per day during 2 consecutive days. On the third day, the subjects were trained by 3 min exposure to the same context followed by a single 2 s 0.7 mA footshock. When tested 24 hours later, the group, pre-exposed with another mouse, showed less contextual freezing than the controls. The buffering effect was long-lasting, and remained for 10 days after the pre-exposure. The effect was also context-dependent, which indicates that the underlying mechanism was an associative learning of safety of the specific context, rather than a non-specific reduction of fear.
Social buffering during fear memory consolidation and expression was reported by Kiyokawa et al, using both cued and contextual fear training. They used two behavioral protocols to distinguish the differential effects of buffering. In the “pair-housing” protocol, the subject is housed with conspecific after the training. In the “pair-exposure” protocol, the conspecific is present during the retrieval of fear memories.2,30 Both protocols attenuated fear expression, but via different mechanisms. The pair-housing suppressed hypothermia—an autonomic response, but did not affect freezing. Conversely, the pair-exposure eliminated freezing but did not affect the hypothermia. When combined, by co-housing with the partner after the training (pair-housing) and by presenting the partner during the retrieval, the autonomic and behavioral responses were both suppressed. Moreover, the selective elimination of freezing by “pair-exposure” only happened in the cued fear conditioning paradigm. In the contextual fear conditioning, “pair-exposure” attenuated both responses.1 The fact that “pair-exposure” buffers the expression of the hippocampus-dependent contextual fear33 more strongly than the hippocampus-independent cued fear may indicate that hippocampus plays an important role in the social buffering of fear, possibly by processing the social signals of safety and modifying the perception of the feared context. Notably, in these experiments, the buffering was obtained using unfamiliar rats.
5 |. MIXED SOCIAL SIGNALS OF BUFFERING AND FACILITATION OF FEAR FROM THE SAME ANIMAL
The evidence of competition between social cues that facilitate fear and buffer fear, come from the comparisons of the effects from the frightened vs non-frightened demonstrator. In most experiments described below, the demonstrator, even when it expressed fear, was able to buffer fear in the subject animal. However, the buffering effects were weaker than from non-frightened demonstrators.
The first evidence of such competition during the retrieval of fear memory is documented in the pioneering study of Davitz and Mason.31 In this study, they tested how fear expression was modulated by the companion demonstrators that were either naïve or trained as the subjects. Remarkably, not only the naïve companions, but the fearful ones also showed a tendency to attenuate fear expression in the subjects, though the effect did not reach statistical significance as with the naïve ones.
A similar finding was reported by Kiyokawa et al,1 assessing the fear-buffering effects from demonstrators, either fearful or not. The fearful ones still decreased fear in the subjects, but the effect was smaller than from the non-fearful demonstrators. Interestingly, fear can be buffered by the training context paired in advance with safety cues emitted by naïve mice—when subject mice were pre-exposed to training contexts with a naïve demonstrator, it buffered subsequent fear conditioning in the same context. However, pre-exposure with freezing demonstrator had no effect,32 suggesting that the fear-buffering signals canceled the effects of fear facilitating signals.
Masuda and Aou have shown a similar competition in the inhibitory avoidance paradigm.3 First, they individually trained rats to avoid the dark compartment, where rats were shocked, and then tested fear expression with or without a companion—the companions were either trained or naïve. Either type of companion attenuated avoidance memory in the subjects, but the attenuation was greater with the naïve ones. The research also describes an interesting vicarious reinstatement—watching a partner crossing into the dark compartment and then being shocked, indeed reinstated the avoidance memory that was extinguished by habituating the subject to the dark compartment. Yet, naïve subjects did not learn avoidance from such observation of the partner.
Together, these experiments suggest that a frightened conspecific emits simultaneously the two types of social signals—the fear-buffering and fear-promoting ones. The contradicting information must compete to determine the directionality and magnitude of the effect, depending on the behavioral task and prior experience of the subject.
6 |. FACTORS THAT DETERMINE THE BALANCE BETWEEN FEAR BUFFERING AND FEAR FACILITATION
It is fair to state that non-distressed conspecific suppresses fear, but the effect from frightened animals often varies. Here, we summarize the factors that influence fear buffering and facilitation by frightened conspecifics.
In fear conditioning with footshock as US, the presence of a frightened partner, during both training or testing, typically buffered fear memories.1,2,31 Such buffering may result from (1) distracting subjects from the CS or (2) pairing the CS and/or context with safety cues emitted by demonstrators. Presence of a footshocked partner in the home cage after training, also buffered fear.2 Presumably, the non-single housing condition ameliorates posttraining stress and attenuates memory consolidation.
As mentioned earlier, exposure to a naïve conspecific in the training context before training, buffered the fear, but exposure to a frightened demonstrator had no effect.32 Interestingly, a similar pre-exposure, but in a non-training context, shifted the effects towards fear promotion—a naïve conspecific had no effects, but a frightened one facilitated subsequent fear learning.24,25 The shift can be explained by the lack of association between safety cues and the training context and by lasting effects on subjects from fear promoting cues emitted by a frightened demonstrator. The fear cues may sensitize animals or cause lasting changes in fear circuitry, resulting in stronger subsequent learning in a new context.
In naturalistic social situations, it is worthwhile to consider the bidirectional flow of social information between two interacting animals. After the initial exchange of social signals elicits bilateral responses, the responding signals come back to the original sources to elicit new responses. Through such iterations of the signal-response exchanges, the animals can either exaggerate or devalue the meaning of the signals they received in the beginning. Such bidirectional social process is apparent when a fear conditioned animal and a naïve animal, together, are exposed to CS. The naïve animal emits safety cues and buffers conditioned response to the CS in the trained partner.34 On the other hand, the trained animal freezes and acts as unconditioned stimulus for the naïve partner, which learns fear by proxy.4,15,16
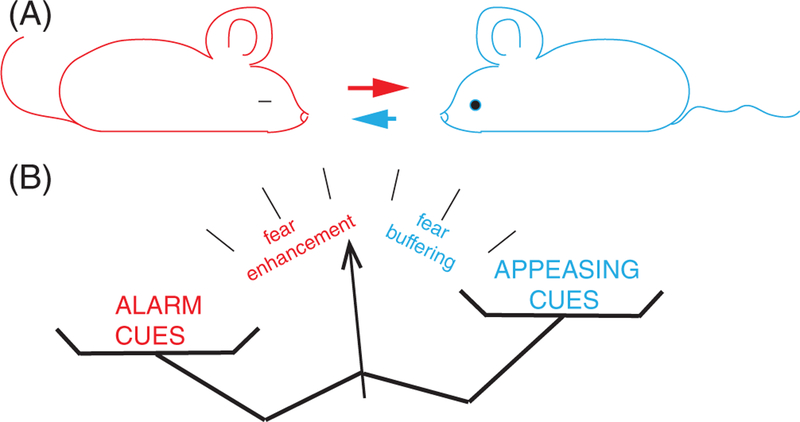
Obviously, there are somewhat contradicting findings in these studies. Kiyokawa reported that naïve rats completely abolished CS-induced freezing in the conditioned rats,34 whereas Bruchey reported that the conditioned rats still froze.15 In regards to the fear learning by proxy, Monfils’s group reported low efficiency and high variance among pairs of rats.4,15,16 Interaction of the two, the fear buffering and the fear learning by proxy, may explain the fluctuating behavioral outcome from the social exchanges—fear buffering attenuates the fear promoting signal from trained animals; conversely, fear learning by proxy attenuates the safety cues from naïve animals. The neuronal circuits integrating social signals of the opposite meaning could be a determinant for the behavioral outcome. The discrepancy between the studies could be attributed to some technical factors, like the strains of rats. Notably, in contrast to the findings in rats,34 Nowak et al found no effect from unstressed familiar conspecific on the cued fear in mice,35 which suggests the directionality of social modulation may also differ between species. Despite the conflicting results, these studies draw attention to an idea that the effectiveness of fear buffering/promoting is determined by the reciprocal interaction in which the animals exchange the buffering and alarm cues (Figure 1), and such exchange shapes the emotional state among several animals living together as a social group.
FIGURE 1.
Bidirectional flow of social information. A, The alarm (red arrow) cues from the frightened animal (left) elicit fear in the naive animal (right) and attenuate release of the appeasing cues (blue arrow). Conversely, the appeasing cues from the naive animal buffer fear in the frightened one and attenuate the release of the alarm cues. B, The competition between the effects of opposing cues determines the emotional state of the social group
Which factors account for the differences among social groups of rodents living together, is an intriguing question. Recent findings that observational fear learning can be enhanced by a single genetic mutation,36 and varies strongly among mouse strains,37 encourage such search.
7 |. NEURONAL PATHWAYS FOR FEAR BUFFERING AND FACILITATION
The rich information from the behavioral studies on fear buffering and facilitation subserved identification of their molecular and cellular substrates in the olfactory pathway—pheromones, their receptors.
7.1 |. Alarm pheromones and their neuronal targets
In a series of studies, Kiyokawa et al have identified the sources and types of alarm pheromones (APs) in rodents. Initially, they found that footshocks in male rats cause the release of alarm pheromones that elicit behavioral and autonomic responses. The behavioral responses included sniffing, rearing and locomotor activity. The autonomic response was hyperthermia. The behavioral responses were testosterone-dependent whereas the autonomic—were not, suggesting that two different types of APs were produced.38 Kiyokawa identified two separate sources for these pheromones by electrically stimulating different areas of the body of anesthetized rats. The whisker pads secreted the pheromone that elicited the behavioral responses, whereas the perianal region secreted the pheromone that elicited hypothermia.39
Subsequent studies revealed three neuronal targets for the APs: the Gruenberg ganglion cells, vomeronasal epithelium and the main olfactory epithelium. Brechbuhl et al identified Gruenberg ganglion (GG) cells as the AP target in mice. Authors have demonstrated that APs activated the GG cells and that the GG cells were necessary for the alarm responses.40 At that time, the chemical nature of AP remained unknown, but its secretion was induced by euthanasia of mice with CO2. Because this method was different from the electrical stimulation method used by Kiyokawa, it remains unclear whether the CO2-induced pheromone originates from the whisker pads or the perianal region.
The GG cells are located at the tip of the nose and express a VNO receptor V2R83, guanylyl cyclase-D and heterotrimeric GTP-binding40 proteins Gαo and Gαi2.40 They connect to the glomeruli in the Neck-less Complex of the Olfactory Bulb (NCOB),41 which belongs to the non-canonical olfactory subsystem. The neckless glomeruli have functional connectivity to other neckless glomeruli and also to canonical glomeruli.42 Unlike the canonical glomeruli, which receive axons from the olfactory neurons that contain a single G-protein-coupled receptor (GPCR), the neckless glomeruli receive inputs from the olfactory neurons that do not express GPCR. Instead, they express multiple types of MS4A receptors, which are evolutionarily older than GPCRs and are activated by 2,6-dimethylpyrazine, a putative mouse aversive pheromone. The MS4A expressing olfactory neurons are also activated by exposure to CO2, which cause avoidance, and by carbon disulfide (CS2), and peptides of guanylin and uroguanylin, which are implicated in the social transmission of food preference. They express the single-pass transmembrane protein guanylyl cyclase-D (GC-D), which is required for responding to the gases and peptides and interacts with the MS4A receptors.43
In a follow-up study, Brechbuhl and colleagues identified 2-sec-butyl-4,5-dihydrothiazole (SBT) as the alarm pheromone that activated GG cells,44 elicited avoidance, and raised blood pressure. The last two effects required transmembrane guanylyl cyclase G.45 The components of predator odors, TMT from fox feces, and 2-PT from the stoat anal gland, act similarly with the APs by activating GG.44 Furthermore, TMP is structurally similar to SBT. Thus, the sensory pathways that transmit the alarm signals from conspecific and the threat signals from predators appear to be the same or highly overlapping.
Inagaki et al have identified additional pheromones, 4-methylpentanal and hexanal, which target the vomeronasal and main olfactory epithelium, respectively. They were found by electrically stimulating the perianal region of the anesthetized rat. When acting together, these molecules induced defensive and risk-assessment behaviors and activated BNST neurons.46 Another alarm pheromone, 2-heptanone, was found in the urine of electrically shocked rats. It elicited despair in naïve rats.47 However, it did not activate the mouse GG cells.40,44
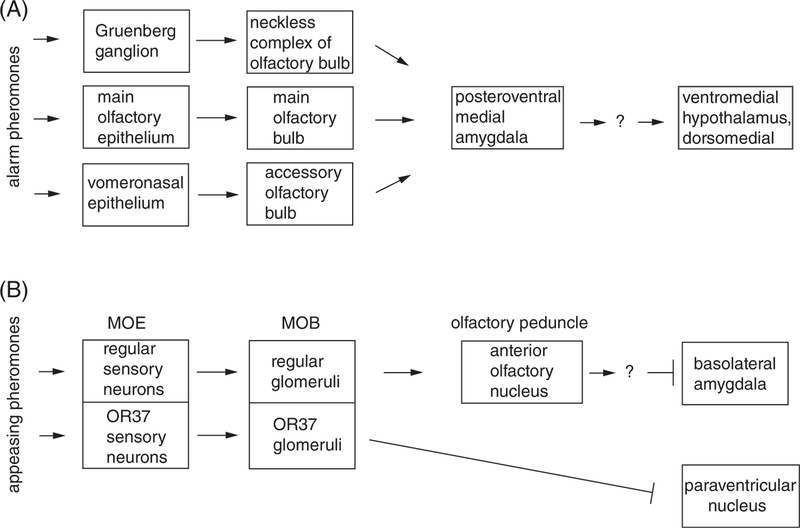
Further downstream targets of the alarm pathway and the relevant synaptic connections are not well defined, but the c-Fos expression induced by APs48 and by components of predator smell49 revealed activation of the brain regions typically involved in the aversive responses. APs from the perianal region (60 minutes exposure to a box in which perianal region of the anesthetized donor was electrically stimulated) caused the strongest neuronal activation (around two folds c-Fos) in the BNST anterior lateral division, BLA, ventrolateral PAG and laterodorsal tegmental nucleus. Smaller but significant activation was found in the medial BNST, PVN, dorsomedial hypothalamus, MeA and locus coeruleus.48 Exposure to the components of predator smells in mice revealed neuronal activation in the posteroventral division of the medial amygdala and the dorsomedial subdivision of the ventromedial hypothalamus49 (Figure 2A).
FIGURE 2.
Olfactory pathways for social signals of fear and safety. Structures activated by putative alarm pheromone (A) and appeasing pheromone (B) are shown. MOE, main olfactory epithelium; MOB, main olfactory bulb. Arrows indicate activation, blunted lines—suppression
7.2 |. Pathways for social buffering of fear
Kiyokawa et al provided the first evidence that volatile compounds mediate social buffering of conditioned fear in rats. The buffering occurred even when the subject was separated from another rat by a 5 cm double wire mesh partition.50,51 The follow-up experiments identified olfaction as the main buffering pathway. (1) An appeasing pheromone that decreases the heart rate in rats was found to be secreted by the neck region in the male rats.52 (2) The inactivation of olfactory epithelium with ZnSO4 abolished social buffering of fear in rats51; and (3) the disconnection of the olfactory peduncle and the basolateral amygdala also impaired social buffering.53 Furthermore, c-Fos analysis showed that the appeasing olfactory signal activated neurons in the posteromedial region of the olfactory peduncle, but suppressed neurons in the lateral and basal amygdala nuclei.54 Consistently, electrophysiological recordings from basolateral amygdala showed that the buffering of cued fear conditioning coincided with a reduction in the CS-induced local field potentials, gamma oscillations (25–75 Hz) and high-frequency oscillations (100–300 Hz).55 Together, these studies suggest that the main olfactory epithelium detects the appeasing signal and transmits it to the main olfactory bulb and then to the BLA via the posterior complex of the anterior olfactory nucleus within the posteromedial region of the olfactory peduncle30 (Figure 2B).
While the molecular nature of appeasing signal and its receptor remains unknown, a recent study suggests that the OR37 family of olfactory receptors, which are activated by the long-chain aliphatic aldehydes pentadecanal, hexadecanal and heptadecanal mediate fear buffering.56 When mice were exposed to a clean test box, it caused stress response, detected as activation of neurons in the paraventricular nucleus. When the box contained bodily secretions from conspecific, there was a robust activation of the OR37 glomeruli, but fewer activated PVN neurons. The OR37 glomeruli, which receive inputs from olfactory sensory neurons with OR37 receptors, are different from other glomeruli. They are not wired to the olfactory cortex but make direct projections to the paraventricular nucleus of the hypothalamus,57 which provides a potential direct pathway for suppressing stress response (Figure 2B).
8 |. FUTURE DIRECTIONS: SEARCH FOR NEURONAL MECHANISMS TO INTEGRATE SOCIAL SIGNALS OF FEAR AND SAFETY
Where and how does the brain integrate information about social signals, aversive and appeasing, and past experiences to elicit adaptive responses? A simple model is that pathways transmitting different types of information converge on the central “integrator-neurons.” However, the multimodal nature of incoming information is more likely to require that the distributed groups of brain nodes, responsible for each modality, operate the integrations through the hierarchical connections and undergo opposing adaptations in response to fear buffering and fear enhancing cues. As one of the nodes, Sterley et al have found such properties in the hypothalamic corticotropin-releasing hormone neurons, which acquired short-term plasticity in mice that were footshocked or interacted with a distressed conspecific, but lost the plasticity after the animal interacted with the naïve conspecific that buffered fear.58 Thus, the CRH neurons participate in integrating the competing effects from aversive and appeasing social signals.
The medial amygdala (MeA), which projects to the hypothalamus, is another potential site for such integration, because the olfactory pathways for various social signals converge on MeA. Furthermore, oxytocin, whose activity in MeA is required for social recognition59 and female preference in male mice,60 was found to enhance both the social buffering of fear28,30 and observational fear learning.61 Apparently, oxytocin by itself neither promotes nor suppresses fear, but it increases the salience of social signals,62 regardless whether they indicate threat or safety. It is likely that oxytocin receptors in MeA, which processes social signals, mediate some of these effects. Finally, the MeA also processed the signals of familiarity and dominance,59,63 which do not inform about the threat of safety directly but strongly influence the perception of the alarm signals.4,5,12
While the pathways activated by the alarm and appeasing pheromones can be tracked down to the MeA and hypothalamus, olfaction is not the only sensory modality for perceiving threat and safety. For example, visual signals contribute to observational fear learning12 and non-noxious tactile stimulation mediates some of social buffering29 and activates hypothalamic neurons that produce oxytocin.64 Thus, social signals of threat and safety are multimodal and require the brain regions capable of multimodal sensory processing. In addition, the integration of the past experience may involve recall of lasting contextual memories, which require the anterior cingulate cortex.65
Given the complexity of integration of multimodal social signals, the responsible distributed brain nodes likely involve the frontal cortex, which is required for the observational fear learning,12 and the basolateral amygdala, whose activity is suppressed by social buffering of fear conditioning.30 Then, the interaction between neuronal ensembles that encode the appeasing and threatening signals within these structures may determine the outcome of the competition between fear buffering and fear facilitation. Studying how these ensembles interact at the synaptic level may help explain individual differences in the susceptibility to social signals of distress.
ACKNOWLEDGMENTS
This work was funded in part by NIH grant R21MH107970.
Funding information
National Institute of Mental Health, Grant/Award Number: R21MH107970
Footnotes
Conflict of interest
The authors declare no potential conflict of interests.
REFERENCES
- 1.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behav Neurosci 2004c;118: 798–804. [DOI] [PubMed] [Google Scholar]
- 2.Kiyokawa Y, Takeuchi Y, Mori Y. Two types of social buffering differentially mitigate conditioned fear responses. Eur J Neurosci 2007;26: 3606–3613. [DOI] [PubMed] [Google Scholar]
- 3.Masuda A, Aou S. Social transmission of avoidance behavior under situational change in learned and unlearned rats. PLoS One 2009;4: e6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones CE, Monfils MH. Dominance status predicts social fear transmission in laboratory rats. Anim Cogn 2016;19:1051–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavaliers M, Colwell DD, Choleris E. Kinship, familiarity and social status modulate social learning about “micropredators” (biting flies) in deer mice. Behav Ecol Sociobiol 2005;58:60–71. [Google Scholar]
- 6.Lore R, Blanc A, Suedfeld P. Empathic learning of a passive-avoidance response in domesticated Rattus-norvegicus. Anim Behav 1971; 19:112. [Google Scholar]
- 7.Kavaliers M, Colwell DD, Choleris E. NMDA-mediated social learning of fear-induced conditioned analgesia to biting flies. Neuroreport 2001;12:663–667. [DOI] [PubMed] [Google Scholar]
- 8.Kavaliers M, Colwell DD, Choleris E. Learning to fear and cope with a natural stressor: individually and socially acquired corticosterone and avoidance responses to biting flies. Horm Behav 2003;43:99–107. [DOI] [PubMed] [Google Scholar]
- 9.Kavaliers M, Colwell DD, Choleris E, Ossenkopp KP. Learning to cope with biting flies: rapid NMDA-mediated acquisition of conditioned analgesia. Behav Neurosci 1999;113:126–135. [PubMed] [Google Scholar]
- 10.Cook M, Mineka S, Wolkenstein B, Laitsch K. Observational conditioning of snake fear in unrelated rhesus monkeys. J Abnorm Psychol 1985;94:591–610. [DOI] [PubMed] [Google Scholar]
- 11.Mineka S, Davidson M, Cook M, Keir R. Observational conditioning of snake fear in rhesus-monkeys. J Abnorm Psychol 1984;93:355–372. [DOI] [PubMed] [Google Scholar]
- 12.Jeon D, Kim S, Chetana M, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 2010;13:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One 2009;4:e4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yusufishaq S, Rosenkranz JA. Post-weaning social isolation impairs observational fear conditioning. Behav Brain Res 2013;242:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruchey AK, Jones CE, Monfils MH. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav Brain Res 2010; 214:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones CE, Riha PD, Gore AC, Monfils MH. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim Cogn 2014;17:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohr M, Borta A, Schwarting RK. Overt behavior and ultrasonic vocalization in a fear conditioning paradigm: a dose-response study in the rat. Neurobiol Learn Mem 2005;84:228–240. [DOI] [PubMed] [Google Scholar]
- 18.Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav 2003;44: 338–345. [DOI] [PubMed] [Google Scholar]
- 19.Perusini JN, Meyer EM, Long VA, et al. Induction and expression of fear sensitization caused by acute traumatic stress. Neuropsychopharmacology 2016;41:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev 2005;29:1207–1223. [DOI] [PubMed] [Google Scholar]
- 21.Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem 2001;75:10–29. [DOI] [PubMed] [Google Scholar]
- 22.Shors TJ, Weiss C, Thompson RF. Stress-induced facilitation of classical conditioning. Science 1992;257:537–539. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Rosenkranz JA. Repeated restraint stress enhances cue-elicited conditioned freezing and impairs acquisition of extinction in an age-dependent manner. Behav Brain Res 2013;248:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learn Mem 2010;17:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito W, Erisir A, Morozov A. Observation of distressed conspecific as a model of emotional trauma generates silent synapses in the prefrontal-amygdala pathway and enhances fear learning, but ketamine abolishes those effects. Neuropsychopharmacology 2015;40: 2536–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren BL, Vialou VF, Iniguez SD, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry 2013; 73:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iniguez SD, Flores-Ramirez FJ, Riggs LM, et al. Vicarious social defeat stress induces depression-related outcomes in female mice. Biol Psychiatry 2018;83:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol 2009;30:470–482. [DOI] [PubMed] [Google Scholar]
- 29.Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond Ser B Biol Sci 2006;361:2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiyokawa Y Social odors: alarm pheromones and social buffering. Curr Top Behav Neurosci 2017;30:47–65. [DOI] [PubMed] [Google Scholar]
- 31.Davitz JR, Mason DJ. Socially facilitated reduction of a fear response in rats. J Comp Physiol Psychol 1955;48:149–151. [DOI] [PubMed] [Google Scholar]
- 32.Guzman YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res 2009;201:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. Eur J Pharmacol 2003;463:217–223. [DOI] [PubMed] [Google Scholar]
- 34.Kiyokawa Y, Takeuchi Y. Social buffering ameliorates conditioned fear responses in the presence of an auditory conditioned stimulus. Physiol Behav 2017;168:34–40. [DOI] [PubMed] [Google Scholar]
- 35.Nowak A, Werka T, Knapska E. Social modulation in extinction of aversive memories. Behav Brain Res 2013;238:200–205. [DOI] [PubMed] [Google Scholar]
- 36.Keum S, Kim A, Shin JJ, Kim JH, Park J, Shin HS. A missense variant at the Nrxn3 locus enhances empathy fear in the mouse. Neuron 2018; 98:588–601.e5. [DOI] [PubMed] [Google Scholar]
- 37.Keum S, Park J, Kim A, et al. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav 2016;15:231–242. [DOI] [PubMed] [Google Scholar]
- 38.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Modulatory role of testosterone in alarm pheromone release by male rats. Horm Behav 2004; 45:122–127. [DOI] [PubMed] [Google Scholar]
- 39.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Alarm pheromones with different functions are released from different regions of the body surface of male rats. Chem Senses 2004;29:35–40. [DOI] [PubMed] [Google Scholar]
- 40.Brechbuhl J, Klaey M, Broillet MC. Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science 2008;321:1092–1095. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo T, Rossier DA, Kan C, Rodriguez I. The wiring of Grueneberg ganglion axons is dependent on neuropilin 1. Development 2012;139: 2783–2791. [DOI] [PubMed] [Google Scholar]
- 42.Uytingco CR, Puche AC, Munger SD. Interglomerular connectivity within the canonical and GC-D/necklace olfactory subsystems. PLoS One 2016;11:e0165343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greer PL, Bear DM, Lassance JM, et al. A family of non-GPCR chemosensors defines an alternative logic for mammalian olfaction. Cell 2016;165:1734–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brechbuhl J, Moine F, Klaey M, et al. Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci USA 2013;110:4762–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao YC, Fleischer J, Yang RB. Guanylyl cyclase-G is an alarm pheromone receptor in mice. EMBO J 2017;37:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inagaki H, Kiyokawa Y, Tamogami S, Watanabe H, Takeuchi Y, Mori Y. Identification of a pheromone that increases anxiety in rats. Proc Natl Acad Sci USA 2014;111:18751–18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutierrez-Garcia AG, Contreras CM, Mendoza-Lopez MR, Garcia-Barradas O, Cruz-Sanchez JS. Urine from stressed rats increases immobility in receptor rats forced to swim: role of 2-heptanone. Physiol Behav 2007;91:166–172. [DOI] [PubMed] [Google Scholar]
- 48.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Mapping the neural circuit activated by alarm pheromone perception by c-Fos immunohistochemistry. Brain Res 2005;1043:145–154. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Gomez A, Bleymehl K, Stein B, et al. Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr Biol 2015;25:1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiyokawa Y, Hiroshima S, Takeuchi Y, Mori Y. Social buffering reduces male rats’ behavioral and corticosterone responses to a conditioned stimulus. Horm Behav 2014;65:114–118. [DOI] [PubMed] [Google Scholar]
- 51.Kiyokawa Y, Takeuchi Y, Nishihara M, Mori Y. Main olfactory system mediates social buffering of conditioned fear responses in male rats. Eur J Neurosci 2009;29:777–785. [DOI] [PubMed] [Google Scholar]
- 52.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Chem Senses 2005;30:513–519. [DOI] [PubMed] [Google Scholar]
- 53.Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y. The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci 2012;36:3429–3437. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res 2013;240:46–51. [DOI] [PubMed] [Google Scholar]
- 55.Fuzzo F, Matsumoto J, Kiyokawa Y, Takeuchi Y, Ono T, Nishijo H. Social buffering suppresses fear-associated activation of the lateral amygdala in male rats: behavioral and neurophysiological evidence. Front Neurosci 2015;9:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein B, Bautze V, Maier AM, Deussing J, Breer H, Strotmann J. Activation of the mouse odorant receptor 37 subsystem coincides with a reduction of novel environment-induced activity within the paraventricular nucleus of the hypothalamus. Eur J Neurosci 2015;41:793–801. [DOI] [PubMed] [Google Scholar]
- 57.Bader A, Klein B, Breer H, Strotmann J. Connectivity from OR37 expressing olfactory sensory neurons to distinct cell types in the hypothalamus. Front Neural Circuits 2012;6:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sterley TL, Baimoukhametova D, Fuzesi T, et al. Social transmission and buffering of synaptic changes after stress. Nat Neurosci 2018;21: 393–403. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci 2001;21:8278–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao S, Bergan J, Lanjuin A, Dulac C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife 2017;6: e31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC. Oxytocin enhances observational fear in mice. Nat Commun 2017;8:2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carter CS. The oxytocin-vasopressin pathway in the context of love and fear. Front Endocrinol 2017;8:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Timmer M, Cordero MI, Sevelinges Y, Sandi C. Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology 2011;36:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okabe S, Yoshida M, Takayanagi Y, Onaka T. Activation of hypothalamic oxytocin neurons following tactile stimuli in rats. Neurosci Lett 2015;600:22–27. [DOI] [PubMed] [Google Scholar]
- 65.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron 2004;44: 101–108. [DOI] [PubMed] [Google Scholar]