Abstract
Neuroinflammation has positive and negative effects. This review focuses on the roles of macrophage in the PNS. Transection of PNS axons leads to degeneration and clearance of the distal nerve and to changes in the region of the axotomized cell bodies. In both locations, resident and infiltrating macrophages are found. Macrophages enter these areas in response to expression of the chemokine CCL2 acting on the macrophage receptor CCR2. In the distal nerve, macrophages and other phagocytes are involved in clearance of axonal debris, which removes molecules that inhibit nerve regeneration. In the cell body region, macrophage trigger the conditioning lesion response, a process in which neurons increase their regeneration after a prior lesion. In mice in which the genes for CCL2 or CCR2 are deleted, neither macrophage infiltration nor the conditioning lesion response occurs in dorsal root ganglia (DRG). Macrophages exist in different phenotypes depending on their environment. These phenotypes have different effects on axonal clearance and neurite outgrowth. The mechanism by which macrophages affect neuronal cell bodies is still under study. Overexpression of CCL2 in DRG in uninjured animals leads to macrophage accumulation in the ganglia and to an increase in the growth potential of DRG neurons. This increased growth requires activation of neuronal STAT3. In contrast, in acute demyelinating neuropathies, macrophages are involved in stripping myelin from peripheral axons. The molecular mechanisms that trigger macrophage action after trauma and in autoimmune disease are receiving increased attention and should lead to avenues to promote regeneration and protect axonal integrity.
Keywords: Axotomy, chemokine, conditioning lesion, dorsal root ganglion, macrophage, neuroinflammation, Wallerian degeneration
1. Introduction: An overview of the molecular and cellular responses of the peripheral nervous system to axonal injury.
Neurons in the peripheral nervous system (PNS) are capable of regeneration after axotomy whereas neurons in the central nervous system (CNS) are generally not (for recent reviews see Curcio and Bradke, 2018; Hilton and Bradke, 2017; Scheib and Hoke, 2013). Because of this distinction, researchers interested in regeneration tend to focus on mechanisms that facilitate regeneration in the PNS or on those that prevent regeneration in the brain and spinal cord. Nevertheless, it should be recognized that, whereas, in the PNS, an injured axon’s regeneration to its original target can be quite precise (Nguyen et al., 2002), it is not always so (Langley, 1897; Lingappa and Zigmond, 2013; Purves and Thompson, 1979). In addition, the number of axons that reach their targets can be limited (Brushart, 2011; Gordon et al., 2009). Therefore, understanding factors that can enhance or that limit PNS regeneration is important for developing therapies for individuals with nerve injury (e.g., Gordon et al., 2003).
Regeneration of axons in vivo is not cell autonomous, rather it is highly influenced by non-neuronal cells, in particular Schwann cells and macrophages. While axonal outgrowth does occur in neurons in dissociated cell culture (e.g., Frey et al., 2015), such cultures often contain appreciable numbers of non-neuronal cells, which may play a role even in vitro. Furthermore, the mechanisms of axon outgrowth in vivo and in vitro may not be identical. In this review article, we first briefly summarize the major changes that occur in the PNS after axonal injury. We then focus largely on the regulation of macrophage accumulation in the PNS examined in rodents, the effects of macrophages in nerve degeneration and regeneration, and the regulation of macrophage phenotype.
We will also review some of the techniques that allow functional studies on macrophage-neuron interactions. The majority of the studies we will review involve studies on the events following axotomy of the sciatic nerve that take place in the distal nerve segment or in the lumbar (L) 4 and/or 5 DRG. In a few places, mention will be made of studies on microglia, the resident macrophages in the CNS.
The inflammatory response in the nervous system in response to injury, referred to as neuroinflammation, has been termed a “double-edged sword”, as it can produce both beneficial and detrimental effects (e.g., Bose and Cho, 2013; Morganti-Kossmann et al., 2002; Stoll et al., 2002). In this review article, we will primarily discuss studies that demonstrate the beneficial effects of macrophages on degeneration and regeneration of neurons after axotomy; however, it is clear that in certain disease states macrophages can be involved in pathological changes (see Section 11).
1.1. Degeneration of the distal axon after axotomy.
When axons are injured by nerve transection or nerve crush, the distal nerve segment fragments and degenerates, and eventually myelin and axonal debris are cleared by a combination of phagocytosis and autophagy (Brosius Lutz et al., 2017; DeFrancesco-Lisowitz et al., 2015; Jessen and Mirsky, 2016a). This process is termed Wallerian degeneration due to its early description by Augustus Waller (1850). The molecular basis for axon degeneration has recently received a great deal of attention (Coleman and Freeman, 2010; Farley et al., 2018; Freeman, 2014; Gerdts et al., 2016; Neukomm et al., 2017) and is assumed to be cell autonomous, but this process is only briefly discussed in this review.
Much of our knowledge of the biochemistry of axon degeneration was initiated from the serendipitous discovery at Oxford University in 1989 of a spontaneous mutant mouse strain unknowingly received from the laboratory’s normal animal supplier that had a very surprising phenotype. Following transection of the sciatic nerve, axonal degeneration and myelin clearance was approximately ten times slower than in wild type mice (Lunn et al., 1989). Given that the accumulation of macrophages in the distal nerve segment of these animals was also slow (Brown et al., 1991; Hall, 1993; Lunn et al., 1989), the question arose as to whether the important locus of this mutation was in the neurons’ axons or in the animal’s macrophages. Through bone marrow transplantation experiments, it was shown that the slow axonal degeneration was an intrinsic property of the axons rather than a mutation in the monocytes (Perry et al., 1990). These mice were initially referred to as C57BL/Ola but are now called C57BL/Wld or just Wlds. A review of studies with these animals was published by Coleman and Freeman (2010).
Following such degeneration, the clearance of myelin and axonal debris is necessary for optimal regeneration because molecules from the degenerating axons are inhibitory to axonal outgrowth (Fournier and Strittmatter, 2001; Qiu et al., 2000; Schwab, 1996). Macrophages and Schwann cells play important roles in the removal of these inhibitory substances (e.g., David et al., 1990; Stoll et al., 1989; also see Section 8.1). In addition, recent studies have established that neutrophils also play a role (Lindborg et al., 2017). In support of the importance of this clearance is the finding that regeneration into peripheral nerve grafts is greatly facilitated if the grafts are predegenerated (Bedi et al., 1992; Hasan et al., 1996; Kerns et al., 1993; Krekoski et al., 2002).
Following transection of the sciatic nerve, initial myelin clearance measured by luxol fast blue staining is detectable by 3 days, and by 7 days the staining is reduced by 80% (Lindborg et al., 2017). The distal nerve segment undergoes additional cellular and molecular changes including the proliferation of Schwann cells, their differentiation into repair cells, and the expression of nerve growth factors (for review see Jessen and Mirsky, 2016b; also see Section 8.2). In the CNS, Wallerian degeneration occurs very slowly and incompletely, and this has been proposed to be one reason for the general failure of regeneration in the CNS (for a review see Vargas and Barres, 2007).
1.2. The cell body response and the expression of regeneration-associated genes.
In addition to changes in the distal nerve segment, axotomy produces profound changes in the cell body of axotomized neurons, changes referred to as “the cell body response” or sometimes “the axon reaction” (Grafstein, 1975; Hanz and Fainzilber, 2006; Hendry, 1992; Lieberman, 1971; Plunet et al., 2002; Zigmond, 2012). Such changes were first described at the histological level as chromatolysis, which involves a breakdown of the layered rough endoplasmic reticulum and the movement of the nucleus from the center of the cell body to an eccentric position (Cragg, 1970; Johnson and Sears, 2013; Matthews and Raisman, 1972). It has been speculated that these changes in the rough endoplasmic reticulum reflect a change from the synthesis of proteins for export (e.g., neuropeptides used as neurotransmitters or neuromodulators) to the synthesis of proteins for intracellular use (i.e., for regeneration) (Matthews and Raisman, 1972).
Early studies established that soon after axotomy there is an overall increase in RNA and protein synthesis (Watson, 1974). More recently, increases and decreases have been documented in the expression of particular mRNAs (Boeshore et al., 2004; Costigan et al., 2002). These changes are thought to be triggered by changes in the expression of specific transcription factors including, for example, cJUN, STAT3, and ATF3 (BenYaakov et al., 2012; Chandran et al., 2016; Smith et al., 2011). The decreases in neuronal gene expression include many proteins involved in synaptic transmission [e.g., in sympathetic neurons the enzyme tyrosine hydroxylase and subunits of ganglionic nicotinic receptors (Cheah and Geffen, 1973; Sun and Zigmond, 1996a; Zhou et al., 1998, 2001)], and the increases include proteins involved in nerve regeneration [e.g., the neuropeptide galanin (Hokfelt et al., 1994; Holmes et al., 2000; Zigmond, 1997, 2001)]. The genes whose expression is increased after axotomy are commonly referred to as “regeneration-associated genes”. Nevertheless, only a small subset of these genes have been proven to play an important role in regeneration (DeFrancesco-Lisowitz et al., 2015; Ma and Willis, 2015; Mahar and Cavalli, 2018).
1.3. Changes in satellite cells, afferent synapses, nerve activity, and cell survival.
In addition to changes in axotomized neurons themselves, changes also occur in their surrounding satellite cells, such as an increase in their proliferation and their expression of glial fibrillary acidic protein (GFAP) (Gehrmann et al., 1991; Hanani, 2005, 2010; Woodham et al., 1989), and in the accumulation of macrophages (Lu and Richardson, 1993; Schreiber et al., 1995). In a study on the guinea pig superior cervical ganglia (SCG), satellite cells were estimated to outnumber neurons by 6 to 1 in control ganglia and to increase by 30% 1–3 weeks after axotomy (Purves, 1975). Satellite cells are thought to be the source of the gp130 cytokine leukemia inhibitory factor (LIF) found in ganglia after injury (Banner and Patterson, 1994; Sun et al., 1994), and, as discussed below, LIF is involved in injury-induced changes in neuronal gene expression.
After axotomy, dramatic changes occur in the synapses onto axotomized autonomic and motor neurons, a process termed “synaptic stripping” (Blinzinger and Kreutzberg, 1968; Matthews and Nelson, 1975; Pilar and Landmesser, 1972; Purves, 1975). The functional significance of these changes is not clear; however, one can speculate that changes produced by afferent nerve activity might oppose changes that occur after axotomy. An example of this is the enzyme tyrosine hydroxylase, which increases in sympathetic neurons following preganglionic nerve stimulation (Biguet et al., 1989; Zigmond and Ben-Ari, 1977) but decreases in these neurons after axotomy (Cheah and Geffen, 1973; Sun and Zigmond, 1996a). The mechanism behind these changes in neuron-neuron contact is also unclear. Blinzinger and Kreutzberg (1968) proposed that microglia are involved in the displacement of synaptic boutons onto axotomized facial motor neurons but that this process did not involve phagocytosis of presynaptic terminals. In the SCG, Matthews and Nelson (1975) proposed that satellite cells processes are responsible for the disruption of the afferent synapses. A third view of this phenomenon was presented more recently by Perry and O’Connor (2010), who argued that non-neuronal cells were not involved but rather that synaptic stripping was a neuron autonomous event.
Although there are no synapses in DRG, nerve activity has also been proposed to be involved in nerve regeneration in sensory neurons; however, there is a discrepancy in the conclusions drawn about this area. Electrical stimulation of adult sensory neurons in culture inhibited neurite outgrowth, an effect based on the L-type calcium channel (Enes et al., 2010). Transection of the sciatic nerve leads to decreased firing of sensory neurons and a downregulation of the pore forming subunit of the L-type calcium channel (Enes et al., 2010). These authors proposed that “the cessation of electrical activity after peripheral lesion contributes to the regenerative response observed upon conditioning”. In apparent conflict with this conclusion are the findings of Gordon and colleagues that brief (1 hour) low-frequency electrical stimulation of peripheral nerve in vivo enhances regeneration of sensory and motor axons (Al-Majed et al., 2000; Gordon, 2016). With regard to sensory neurons, but not motor neurons, when stimulation was continued for longer than 1 hour no enhancement of regeneration occurred (Geremia et al., 2007). Thus, the role of nerve activity on regeneration seems to depend on the exact parameters of stimulation that are used.
Axotomy can eventually lead to neuronal cell death, though this can be quite delayed (Purves, 1975). In the rat, according to Hendry (1992), there is little cell death in the axotomized SCG, although there is one report of apoptosis in a small number of neurons after axotomy very close to the ganglion (Hou et al., 1998). Purves (1975) reported in the guinea pig SCG that there was no neuronal loss during the first week after axotomy but that a decrease of approximately half of the neurons occurred between 1–3 months. One month after transection of the mouse sciatic nerve, there was about a 50% loss of neurons in the mouse L5 DRG (Lyu et al., 2017). It would be interesting to know whether those neurons that die are neurons that do not successfully reinnervate their target tissues.
Survival of facial motor neurons after axotomy is dependent on CD4+ T-cells (Serpe et al., 2003). The number of these motor neurons decreased after axotomy in knockout mice for either CD4 or recombinase activating gene-2, which causes the loss of all B and T-cells. Replenishment of either mouse strain with CD4+ T-cells restored survival to wild type levels (Serpe et al., 2003). A small number of T-cells was found in the DRG and sympathetic ganglia after sciatic nerve transection (Hu and McLachlan, 2002, 2004); however, whether these T-cells promote survival of sensory and/or sympathetic neurons is not known.
1.4. Molecular signals triggering the changes in ganglia following axotomy.
In certain instances, the molecular and cellular changes reviewed above have been shown to result from either negative (downregulated) or positive (upregulated) biochemical signals (Ambron and Walters, 1996; Cragg, 1970; Purves and Nja, 1976; Zigmond, 2012). An example of a negative signal is nerve growth factor (NGF), a factor that is reduced in axotomized sympathetic neurons as a result of interruption of its retrograde transport from target tissues to neuronal cell bodies following axotomy (Hyatt Sachs et al., 2007; Korsching and Thoenen, 1985; Nagata et al., 1987; Nja and Purves, 1978). An example of a positive signal is LIF, which is barely detectable in the intact nervous system but is expressed following axotomy both within ganglia, perhaps by satellite cells (Banner and Patterson, 1994; Sun et al., 1994), and in Schwann cells in the distal nerve segment (Curtis et al., 1994). The study by Curtis et al. (1994) demonstrated that LIF can be transported retrogradely by sensory and motor axons where it might be involved in neuronal gene expression.
Both NGF and LIF affect the expression of galanin in sympathetic and sensory neurons. Regulation of galanin expression is of particular interest because galanin is an example of a regeneration-associated gene that has been shown to play a role in sensory neuron regeneration after sciatic nerve injury (Holmes et al., 2000; Zigmond, 2001). Injections of rats with antiserum against NGF led to an increase in galanin expression in both SCG and DRG, thus mimicking the effects of axotomy (Shadiack et al., 2001). Axotomy of these ganglia in Lif knockout mice, on the other hand, reduced the increase in ganglionic galanin expression compared to that seen in WT mice after axotomy (Corness et al., 1996; Rao et al., 1993; Sun and Zigmond, 1996a, b). Further, it was established that there is an interaction between the effects of NGF and those of LIF. Somewhat surprisingly, when a pellet of LIF was placed adjacent to the SCG, no change in galanin expression occurred. However, if these animals were injected with anti-NGF, the increase in galanin that occurred was significantly greater than if the animals only received the antibody (Shadiack et al., 1998). These results suggest that LIF only induces galanin in neurons that no longer receive NGF from their target tissues, which is an indication that the axons have been injured (Shadiack et al., 1998). A similar interplay between LIF and NGF in the regulation of galanin was found in primary cultures of embryonic DRG neurons (Corness et al., 1998).
2. The two general classes of macrophages: Resident and infiltrating.
Macrophages play multiple roles in the PNS after axonal injury. In most tissues, including in the nervous system, two classes of macrophages are distinguishable: resident macrophages and infiltrating macrophages (Griffin et al., 1993). The former are present under homeostatic conditions, the latter infiltrate into tissues in response to injury or infection. In many tissues, resident macrophages originate from the yolk sac and fetal liver, enter tissues during embryonic development, and maintain their numbers through their longevity and local proliferation rather than through replacement by cells from the bone marrow (for reviews see Ginhoux and Guilliams, 2016; Hashimoto et al., 2013; Varol et al., 2015). Nevertheless, there are exceptions to this rule concerning cellular origin. For example, the resident macrophages in the adult intestine, which derive from the bone marrow (Bain et al., 2014).
The embryonic origin of microglia, the resident macrophages in the CNS, has recently received a great deal of attention (e.g., Li and Barres, 2017), whereas, resident macrophages in the PNS have been less studied recently. Using lineage-tracing techniques, microglia have been shown to originate from the yolk sac (Schulz et al., 2012). Whereas it might seem natural to assume that resident macrophages in the PNS have the same origin (e.g., see Ton et al., 2013), this is not the case. Vass et al. (1993) and Muller et al. (2010) asked whether macrophages derived from the transplanted cells contributed to the population of resident macrophages in the sciatic nerve and dorsal root ganglia (DRG) by using bone marrow transplantation from mice carrying a traceable cellular marker [(i.e., a histocompatibility antigen or green fluorescent protein (GFP)]. They found that over a few months 50 – 60% of the resident macrophages were replaced by circulating monocytes. The developmental origin of the remaining host macrophages has not been determined. Interestingly, when Mueller et al. (2003) compared GFP+ macrophages with host GFP- macrophages, no differences were found in morphology, in staining for the macrophage antigens F4/80, CD68, Iba-1, or CD11b, or in phagocytosis of myelin basic protein.
Resident macrophages are found both in peripheral nerves and in ganglia (Gehrmann et al., 1991). In the adult rat sciatic nerve, endoneurial resident macrophages were estimated to account for 2–6% of the total endoneurial cells (Mueller et al., 2003; Oldfors, 1980). In postmortem examination of human sensory and autonomic ganglia, it was reported that 5–20% of the cells present were resident macrophages (Esiri and Reading, 1989).
Infiltrating macrophages originate from bone marrow-derived monocytes that enter tissues in response to injury or infection (Mildner et al., 2016). The ratio of infiltrating macrophages to resident macrophages in the sciatic nerve seven days after transection is about 3 to 1 (Mueller et al., 2003). The most well characterized chemoattractive peptide (or chemokine) that brings monocytes into tissues is chemokine C-C motif ligand 2 [CCL2, formerly referred to as monocyte chemoattractive protein (MCP-1)]. This chemokine acts primarily through the receptor CCR2 on monocytes. In addition, there is a second population of monocytes in the circulation that do not express CCR2 and are referred to as “patrolling monocytes” (Auffray et al., 2007).
An open question in the field is whether resident and infiltrating macrophages have distinct functions after nerve injury or infection. One challenge in approaching this issue is to find markers by which these cells can be distinguished. Using bone marrow transplantation, Mueller et al. (2001) observed that resident macrophages began phagocytosing myelin within two days after sciatic nerve crush, which is before the influx of infiltrating macrophages. They also observed proliferation of the resident macrophages at this early time point. These data suggest that resident macrophages along with neutrophils (Lindborg et al., 2017) and Schwann cells (Stoll et al., 1989) are first responders after nerve injury. In the CNS, efforts have been made recently to distinguish between microglia and monocyte-derived macrophages (e.g., Bennett et al., 2016; Butovsky et al., 2014; Greter et al., 2015), and evidence exists that these two cell types do perform different functions (see for example Kim and Cho, 2016; London et al., 2013; Yamasaki et al., 2014).
It is worth noting that resident macrophages in nerves are sensitive to irradiation. This feature is important because it means that during irradiation for bone marrow transplantation, both bone marrow precursor cells and resident macrophages would be reduced (Monaco et al., 1992).
3. Some issues regarding the detection of macrophages in tissues.
Macrophages are generally detected in neural tissue by either immunohistochemistry or flow cytometry. A variety of antibodies is used in such studies making it sometimes difficult to compare results from different groups. The most commonly used antibodies are those against the antigens CD68 (ED1), F4/80, ionized calcium binding adaptor molecule 1 (Iba1), and CD11b (Mac-1). As in any study utilizing antibodies, an important question is how specific the antibody is to a particular cell type. A very extreme example of this problem was demonstrated in a study in which these four antibodies were examined by flow cytometry of fibrotic tissue from animals whose fibroblasts had been labelled with a reporter gene (GFP). All of the antibodies with the exception of F4/80 were found to cross react with GFP-labeled fibroblasts (Inoue et al., 2005).
Another example is that antibodies to CD11b, which are commonly used to label macrophages, also label neutrophils (Barrette et al., 2008). Nevertheless, macrophages and neutrophils can be distinguished in at least two ways. Neutrophils can also be labelled with antibodies to Ly6G (lymphocyte antigen 6 complex locus G6D), whereas macrophages are not (e.g., Lindborg et al., 2017). Also relevant is the time after injury at which the antibody to CD11b is used, as the time course of infiltration of neutrophils is much quicker and of shorter duration than that of macrophages (Lindborg et al., 2017).
Another important question is whether different antibodies label the same population of macrophages or whether there is heterogeneity among macrophages with respect to binding these probes. In an IHC study on the sciatic nerve, CD68 and Iba-1 co-localized to the same cells (Mueller et al., 2003). On the other hand, in flow cytometry studies on the sciatic nerve and dorsal root ganglia (DRG), cells characterized as CD11b+ and Ly6G− were significantly more numerous than cells that were both CD11b+ and F4/80+ (Lindborg et al., 2017). The latter result raises questions as to how best quantitate the total number of macrophages in a tissue and whether different macrophage subsets defined by particular antibodies subserve different physiological functions. Interestingly, in our study on the rat superior cervical sympathetic ganglion, we found that antibodies to CD163 (ED2) stained macrophages in intact ganglia (i.e., resident macrophages), while antibodies to CD68 (ED1) only stained macrophages after axotomy (i.e., infiltrating macrophages) (Schreiber et al., 1995).
4.1. Sites of macrophages accumulation within the PNS after an injury: The distal nerve segment.
Until recently, studies on macrophages in the PNS after axotomy focused almost entirely on those that accumulated in the distal segment of peripheral nerves after axotomy (Fig. 1). Ramon Y Cajal, in his classic book Degeneration and Regeneration of the Nervous System, described the arrival of blood cells into the distal nerve segment and their participation in the phagocytosis of axonal debris (Ramon y Cajal, 1928). Cajal’s interpretation of what he observed turned out to be extremely accurate as will be discussed later in this review.
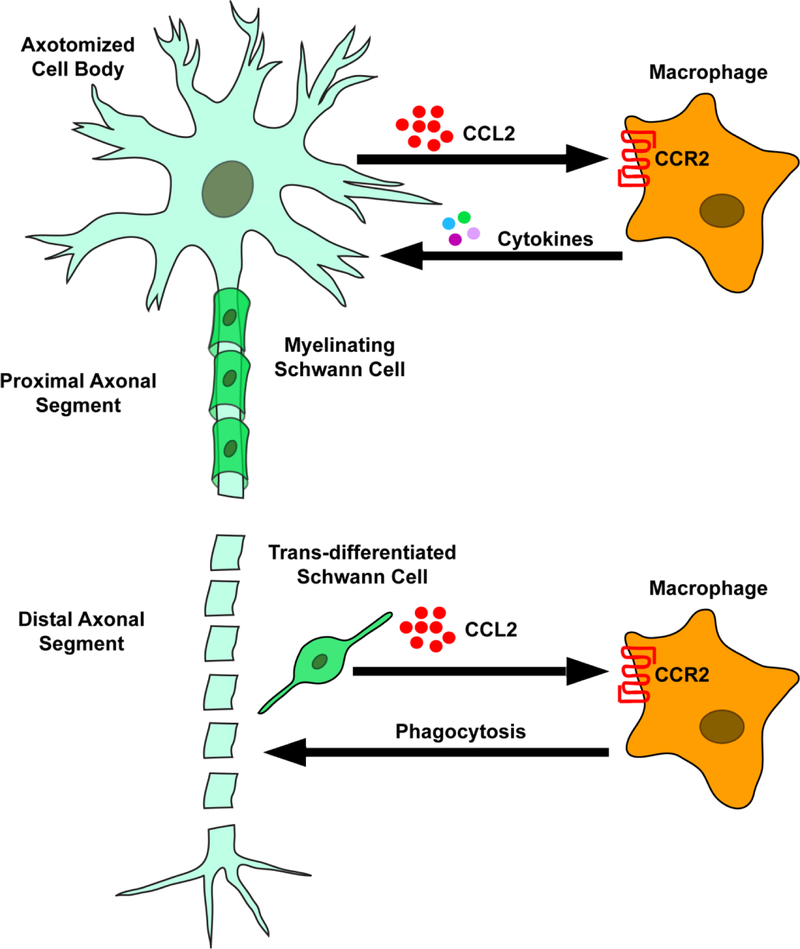
Fig. 1.
A diagram of a hypothetical peripheral neuron following axonal transection or crush showing the two sites of macrophage accumulation.. The lower part of the figure shows the degeneration of the distal axonal segment, the trans-differentiation of Schwann cells, the secretion by the Schwann cells of the chemokine CCL2, and the attraction of macrophages to the distal degenerating segment. This is the classical view of macrophage accumulation after axotomy. The upper part of the figure shows that macrophages also accumulate around the axotomized neuronal cell body and these neurons themselves secrete the chemokine CCL2 following axotomy.
“…we might conjecture that the decomposition of the axon and myelin liberate positive chemotactic substances capable of attracting the wandering cells. Perhaps these enticing substances are produced by the rejuvenated cells of Schwann. At any rate this attractive action reaches its maximum from the fifth to the eighth day, at the critical period for the breaking up of the nerve fibre” (page 97 of Ramon y Cajal, 1928).
A summary of other early studies in this field can be found in Griffin and George (1993) and Perry’s monograph Macrophages and the Nervous System (Perry, 1994). The former give credit for the identification of resident macrophages in peripheral nerve to Arvidson (1977) who injected animals with horseradish peroxidase and found it in the mouse sciatic nerve concentrated in cells with the ultrastructural features of macrophages.
Accumulation of macrophages in the distal segment of the transected sciatic nerve can be seen at 3 days, peaks at about 14 days, and is low but still detectable at 56 days. At 3 days, macrophages are more concentrated in the epineurium than in the endoneurium but by 14 days the opposite is true (Taskinen and Roytta, 1997). Although macrophage accumulation is greatest in the distal segment of the sciatic nerve after an injury, a minor accumulation also occurs proximal to the lesion (Taskinen and Roytta, 1997).
Very little is known about the eventual exit of macrophage from the PNS. However, in one study, elimination from the injured sciatic nerve was found to occur via a combination of local apoptosis and lymphatic elimination to lymph nodes and spleen (Kuhlmann et al., 2001).
4.2 . Sites of macrophages accumulation within the PNS after an injury: Peripheral ganglia.
A second, more recently recognized site of macrophage accumulation after axotomy is the peripheral ganglion (Fig. 1). After transection of the sciatic nerve, macrophages were shown to accumulate in lumbar DRG (Lu and Richardson, 1993) and after transection of the internal and external carotid nerves, in the SCG (Schreiber et al., 1995). Accumulation of macrophages in the DRG is seen 4 days after axotomy and is still detectable at 32 days (Lu and Richardson, 1993). In the SCG, macrophage accumulation is seen by 2 days after axotomy, reaches a peak at 8 d, and is sustained until 14 days, which was the latest time examined (Schreiber et al., 1995). Although macrophage accumulation in the lumbar DRG after unilateral sciatic nerve transection is primarily ipsilateral, a much smaller increase is also seen in the contralateral DRG. No change is seen in cervical DRG, making it unlikely that macrophage accumulation results from systemic inflammation (Lu and Richardson, 1993).
In one study using the spared nerve procedure (in which the peroneal and tibial branches of the sciatic are transected but the sural nerve is left intact), macrophages in the L4 and L5 DRG were localized with respect to the sizes of nearby sensory neurons (Vega-Avelaira et al., 2009). Following surgery, the retrograde tracer fluorogold was injected into the cut nerves to identify neurons in the DRG that had been axotomized. Macrophages were found to form “ring-like” structures preferentially around large diameter axotomized neurons, with many fewer rings formed around non-axotomized neurons and around small diameter axotomized neurons.
Prior to these findings in the PNS, studies in the brainstem demonstrated that microglia accumulate around the cell bodies of axotomized neurons that undergo regeneration. Following transection of two cranial motor nerves (i.e., the facial and hypoglossal nerves), microglia were activated near the cell bodies of the affected neurons (Graeber et al., 1988; Schiefer et al., 1999; Svensson et al., 1994). Although facial and hypoglossal neuronal cell bodies are located within the CNS, they are considered peripheral neurons based on their projection into the periphery and their ability to regenerate after axotomy. When two intrinsic CNS pathways that do not normally regenerate, the thalamic reticular nucleus and the red nucleus, little or no microglial activation was seen (Shokouhi et al., 2010). Interestingly, if these neurons were induced to regenerate by providing them with a peripheral nerve graft, microglial activation was seen around the axotomized cell bodies (Shokouhi et al., 2010).
1. Axotomy of the central process of DRG neurons does not mimic the effects of axotomy of their peripheral process.
Unlike sciatic nerve transection, transection of dorsal roots does not lead to macrophage accumulation in the DRG (Kwon et al., 2013). In an influential paper published in 1970 entitled “What is the signal for chromatolysis” Cragg speculated on what signals triggered the cell body response (Cragg, 1970). In this paper, he discussed the intriguing fact that, whereas a DRG neuron responds with chromatolysis to injury to its peripheral process, this does not occur in response to injury to its central process (for references on this phenomenon see Cragg, 1970; Lieberman, 1971). This distinction was found not only for DRG neurons but also for visceral sensory neurons in the nodose ganglion (Lieberman, 1969). Subsequently, Oblinger and Lasek (1984) reported that whereas crushing of the peripheral process of DRG neurons leads to regeneration of these peripherally projecting axons, crushing of the dorsal roots does not lead to regeneration of the centrally projecting axons. Since then, it has been shown that some of the molecular changes that occur after lesioning the peripheral process do not occur after lesioning the central process, including increased expression of growth associated protein 43 (GAP43) (Chong et al., 1994; Schreyer and Skene, 1993) and cJun (Broude et al., 1997). Another example of a gene that does not increase after transecting a dorsal root is CCL2 (Kwon et al., 2015). For a more complete profile of the gene changes that occur after cutting the dorsal root and how they compare to those seen after sciatic nerve axotomy see Stam et al. (2007).
2. The chemoattractive molecules that bring macrophages into the PNS.
Chemokines (or chemotactic cytokines) are small peptides that attract leukocytes into injured or infected tissue (Ransohoff, 2009; Ransohoff et al., 2007; Rollins, 1997). A number of cell types can secrete these molecules. Chemokines act on G-protein-coupled receptors found on leukocytes. Whereas the primary study of chemokines has concerned their chemotactic properties, they can produce other effects, an example of which will be noted later in this review.
CCL2 is the major chemokine for monocytes, and it binds with highest affinity to the receptor CCR2 (Charo and Ransohoff, 2006; Deshmane et al., 2009). CCR2 is expressed by monocytes and macrophages (Abbadie et al., 2003; Mack et al., 2001); however, as will be discussed later, it is also expressed after injury by sensory neurons (Jung et al., 2008; White et al., 2005) and satellite glial cells (Takeda et al., 2017). In Ccl2 −/− or Ccr2 −/− animals, decreased macrophage accumulation occurs in the distal sciatic nerve and in the DRG after nerve injury (Lindborg et al., 2017; Niemi et al., 2013; Siebert et al., 2000). Neutralizing antibodies to CCL2 suppressed macrophage accumulation in the distal sciatic nerve and inhibited clearance of myelin (Perrin et al., 2005). Similarly, antibodies to CCR2 (i.e., MC-21) decrease monocyte/macrophage accumulation in the blood and in the sciatic nerve (Lindborg et al., 2017).
After axotomy, Ccl2 mRNA increases in the sciatic nerve. At first it is detectable at the injury site; however, over time it is seen throughout the distal nerve segment (Carroll and Frohnert, 1998). In the proximal nerve segment, the message is only expressed adjacent to the injury site. In the distal sciatic nerve, Ccl2 mRNA is expressed within 12 hours after nerve transection or crush and reaches peak values between 1–3 days (Carroll and Frohnert, 1998; Cheepudomwit et al., 2008; Toews et al., 1998). In the nerve, CCL2 is mainly expressed in Schwann cells (e.g., Subang and Richardson, 2001; Taskinen and Roytta, 2000). Ccl2 mRNA levels also increased in the sciatic nerve when unmyelinated nerve fibers were lesioned selectively by injecting neonatal animals with the neurotoxin capsaicin, though the duration of this effect was shorter lived than after axotomy, which injures both myelinated and unmyelinated axons (Cheepudomwit et al., 2008).
In addition to accumulating in the distal nerve segment, macrophages accumulated in sensory and sympathetic ganglia after axotomy. Strikingly, although Ccl2 mRNA in the distal nerve is expressed by glial cells, in axotomized sensory and sympathetic ganglia and in the axotomized facial motor nucleus, Ccl2 is expressed by axotomized neurons (Flugel et al., 2001; Schreiber et al., 2001; Tanaka et al., 2004). These increases in ganglia can be detected within 6 hours after axotomy (Flugel et al., 2001; Niemi et al., 2013; Schreiber et al., 2001). In the DRG, CCL2 protein is found specifically in neurons and not in satellite cells after axotomy or after administration of chemotherapeutic agents like paclitaxel (Liu et al., 2016; Tanaka et al., 2004; Zhang et al., 2016). When facial skin inflammation was produced by injection of Freund’s adjuvant, CCL2 immunoreactivity was localized in the trigeminal ganglion in both small and medium diameter sensory neurons (Takeda et al., 2017). Following chronic constriction injury of the sciatic nerve, a common model of neuropathic pain, CCL2 was most commonly expressed in P2X3- positive non-peptidergic C-fibers, with a lower percentage found in calcitonin gene-related peptide (CGRP)-positive and NF200-positive neurons (Thacker et al., 2009). The level of Ccl2 mRNA was reduced by 40% in DRG from capsaicin-treated animals, corroborating the fact that a portion of the message is contained in nociceptive neurons (Van Steenwinckel et al., 2011). A small increase in Ccl2 was seen in the contralateral DRG after unilateral injury to the sciatic nerve (Tanaka et al., 2004). In most studies, very low amounts of Ccl2 mRNA or protein were detected in sham-operated rat or mouse DRG (e.g., Jung et al., 2008; Subang and Richardson, 2001; Tanaka et al., 2004); however, Dansereau et al. (2008) reported that both were constitutively expressed in small and medium sized DRG neurons from male Sprague-Dawley rats. Many of these neurons also expressed CGRP or substance P. It is unclear how to reconcile this discrepancy.
An interesting question with regard to the differential expression of CCL2 in subtypes of DRG neurons is how locally or how diffusely does the chemokine act. Specifically, are there more macrophages surrounding the neurons with the highest expression of CCL2, i.e., the small diameter P2X3-positive neurons? This could have implications for which neurons are affected by cytokines released by infiltrating macrophages. Although this question has not be addressed in detail, the study by Vega-Avelaira et al. (2009) suggests somewhat surprisingly that macrophages are preferential located surrounding large diameter DRG neurons.
In addition to its role in attracting macrophages into DRG, CCL2 from sensory neurons acts in the dorsal horn of the spinal cord. The chemokine is expressed in large granules in DRG cell bodies and is transported into neurites in culture and into the dorsal horn in vivo. Depolarization or nerve stimulation leads to the release of CCL2 in vitro and in vivo in the dorsal horn (Jung et al., 2008; Thacker et al., 2009; Van Steenwinckel et al., 2011). Data from several studies indicate that this release of CCL2 in the spinal cord plays an important role in neuropathic pain.
The signal(s) by which axotomy triggers the increase in CCL2 expression in Schwann cells or sensory neurons is not known. Incubation of rat Schwann cells in culture with TNF-α (Subang and Richardson, 2001) or the gp130 cytokines LIF or IL-6 (Tofaris et al., 2002) leads to an increase in expression of Ccl2 mRNA. Macrophage accumulation in the sciatic nerve after axotomy is reduced in Lif−/− mice; however, this may not be due to a direct effect of LIF on Ccl2 mRNA levels as LIF can have a chemotactic effect on its own on isolated macrophages (Sugiura et al., 2000).
In a recent paper, Wang et al. (2018) examined the role of Sarm1 in Ccl2 induction. Sarm1 was originally identified as a key molecule in axonal degeneration and nerve transection (Osterloh et al., 2012) and has since been implicated in the neuropathies produced by administration of the chemotherapeutic agent paclitaxel and by feeding mice with a high fat diet (Turkiew et al., 2017). Wang et al. (2018) reported that Sarm1 acting via JUN kinase and phospho-JUN is also involved in triggering the expression of several chemokines in DRG neurons including CCL2. At this point, what is not known is what activates this signaling cascade following axotomy.
CCL2 may not be the only chemokine that brings monocytes into nervous tissue. Injections of CCL3 (macrophage inflammatory peptide-1α) and CX3CL1 (fracktalkine) directly into the fifth lumbar DRG leads to macrophage accumulation in the ganglion comparable to that which occurred after sciatic nerve lesion or after injection of CCL2 (Kwon et al., 2015). It is noteworthy that no increase in Cx3cl1 mRNA was found in the distal sciatic nerve after axotomy (Lindborg et al., 2017). Currently there is no evidence as to whether these chemokines, or any chemokine other than CCL2, is involved in bringing monocytes into the DRG in vivo. In the distal sciatic nerve, however, neutralizing antibodies to both CCL2 and CCL3 diminished macrophage accumulation (Perrin et al., 2005). In addition, Cobos et al. (2018), in an RNAseq study, reported increases in the DRG of a number of chemokines after injury including CCL4, CCL7, CCL9, and CCL12; however, the effects of these molecules on immune cell infiltration in vivo remains to be tested.
Another question to consider is whether CCR2 is the only receptor through which CCL2 acts to bring monocytes into nervous tissue? When macrophage accumulation in the sciatic nerve was measured in Ccr2 knockout mice by flow cytometry, there was still an increase in macrophages 7 days after axotomy, albeit a substantially smaller increase than seen in wild type mice (Lindborg et al., 2017). This increase could result from an involvement of a second receptor that can bind CCL2, perhaps CCR4 (Kwon et al., 2015; Power et al., 1995), or from proliferation of resident macrophages (e.g., Leonhard et al., 2002; Schreiber et al., 2002).
3. Methods employed to deplete macrophages.
An important approach to elucidating the function of macrophages in the nervous system is the examination of the effect of blocking or reducing monocytes and macrophages in nervous tissue (e.g., Lund et al., 2017). Several methods have been employed in such studies, and these are described below. In addition, an example is given for each method where effects on nerve degeneration and/or regeneration were studied.
Clodronate liposomes.
A method was introduced by Van Rooijen to deplete circulating monocytes and therefore reduce macrophage accumulation in tissues by injecting animals intravenously with liposomes encapsulating the compound clodronate (dichloromethylene diphophonate) (van Rooijen, 1989; Van Rooijen and Sanders, 1994). The liposomes are taken up by monocytes/macrophages via phagocytosis, the liposomal membrane is broken down, and the clodronate causes the death of the cells. Clodronate inhibits mitochondrial oxygen consumption via inhibition of the ADP/ATP translocase (Lehenkari et al., 2002). It is reported that there is no change in circulating neutrophils (Ferenbach et al., 2012; Van Rooijen and Sanders, 1994), though they are also phagocytic cells, but the molecular basis for this cellular specificity has not been determined. In some but not all tissues, resident macrophages are also targets of the liposomes (e.g., Yue et al., 2017). In the sciatic nerve, Bruck et al. (1996) claim that the resident macrophages are not depleted by clodronate and that this is because the liposomes do not cross the blood-nerve barrier; however, no evidence is provided for this mechanism. In one recent experiment, the liposomes were injected directly into the trigeminal ganglion, and a local depletion of macrophages occurred (Batbold et al., 2017).
In a study on the mouse spleen, depletion of macrophages could be seen within 24 hours after a single injection and recovery to normal levels took about 4 weeks (van Rooijen and van Nieuwmegen, 1984). Differences in the time course of macrophage repopulation have been reported in different tissues and between mouse and rat (Van Rooijen et al., 1990). Our laboratory and some colleagues have found depletion of macrophages in the PNS by clodronate liposomes to be quite variable, perhaps due to uncertainty as to the best dose to use and the time course of the onset and recovery for maximum macrophage depletion. In a recent publication, Brosius Lutz et al. (2017) reported that they were unable to reduce macrophages by more than 50% using clodronate. Nevertheless, it was also recently reported that a single injection led to a marked depletion of monocytes in the blood and macrophages in the axotomized DRG, and this depletion was still observable 8 days after the injection (Cobos et al., 2018). In terms of their physiological consequences, clodronate liposomes have been shown to reduce significantly myelin clearance after sciatic nerve injury (Bruck et al., 1996).
Transduction with a viral thymidine kinase transgene followed by ganciclovir.
Barrette et al. (2008) expressed a mutated herpes simplex virus thymidine kinase— known as a “suicide gene”--under the CD11b promoter. When these animals are injected with ganciclovir, a nontoxic nucleoside, the nucleoside is phosphorylated by thymidine kinase, and the resulting compound inhibits DNA polymerase leading to cell death in dividing cells. In their transgenic animals, Barrette et al. (2008) found decreases in myelin clearance, nerve regeneration, and functional recovery after sciatic nerve injury. A limitation of this approach is that CD11b expression is not limited to macrophages but also occurs in neutrophils (Barrette et al., 2008). In fact, thus far, no truly macrophage specific promoter has been identified (Abram et al., 2014; Hume, 2011).
Chemokine knockout animals.
Monocytes and macrophages have been reduced by using mice in which the genes for the chemokine Ccl2 (Lu et al., 1998) or its primary receptor Ccr2 (Boring et al., 1997) have been knocked out. These mutants have been used to decrease macrophage accumulation in the sciatic nerve and in sensory and sympathetic ganglia (Kwon et al., 2015; Lindborg et al., 2017; Niemi et al., 2013; Niemi et al., 2017; Siebert et al., 2000). In Ccr2 knockout mice, the conditioning lesion response following sciatic nerve transection was abolished (Niemi et al., 2013; see Section 8.2). These animals, however, showed normal Wallerian degeneration (Lindborg et al., 2017; Niemi et al., 2013)
Depletion of complement with cobra venom factor.
Complement can be depleted by intravenous administration of cobra venom factor. When the sciatic nerve was crushed a day later, a reduction of CD68+ macrophage accumulation was seen in the distal nerve segment (Dailey et al., 1998; Vriesendorp et al., 1998; Vriesendorp et al., 1995, 1997). Complement-depleted animals showed decreased myelin clearance and decreased sciatic nerve regeneration (Dailey et al., 1998).
Use of neutralizing antibodies.
MC-21 is a monoclonal antibody raised against CCR2, whereas MC-67 is an isotype control antibody (Mack et al., 2001; Maus et al., 2002). A single injection of MC-21 depleted CCR2-positive monocytes and macrophages in the blood and spleen respectively by 8–24 hours. Monocytes were almost back to normal by 72 hours (Bruhl et al., 2007). After four daily injections of MC-21, monocyte depletion was sustained for 5 days; however, by 8 days monocyte recovery had occurred probably by a humoral immune response against the antibody. Monocytes were decreased in the blood and macrophages in the distal sciatic nerve compared to animals given MC-67 (Lindborg et al., 2017). Injections of MC-21 (like the use of Ccr2 knockout animals) did not reduce Wallerian degeneration (Lindborg et al., 2017).
Neutralizing antibodies against the chemokine CCL2 administered directly into the lesioned sciatic nerve substantially decreased the accumulation of macrophages (Perrin et al., 2005). Similarly, intrathecal injection of antibodies to CCL2 inhibited macrophage accumulation in the DRG following injection of the cancer chemotherapeutic drugs bortezomib and paclitaxel (Liu et al., 2016; Zhang et al., 2016).
Pharmacological agents.
Minocycline has been used because of its actions on monocytes and microglia. Kwon et al. (2013) found that minocycline inhibits the conditioning lesion effect on DRG neurons and hypothesized that this action was due to its inhibition of macrophages accumulation in the DRG. However, minocycline produces other effects in the nervous system which could affect nerve regeneration, including actions on Schwann cells and on revascularization, not all of which are necessarily secondary to its effects on macrophages and microglia (Keilhoff et al., 2007; Keilhoff et al., 2008; Stirling et al., 2005).
Colony-stimulating factor-1 acting on the colony-stimulating factor-1 receptor plays an important role in the differentiation, proliferation, and survival of macrophages (Hume and MacDonald, 2012; Jenkins and Hume, 2014; Naito et al., 1997). In peripheral nerve, the main source of colony-stimulating factor-1 is endoneurial fibroblasts (Groh et al., 2012). PLX5622, a colony-stimulating factor-1 receptor inhibitor, can be given either by gavage or in laboratory chow. When the drug was given 2 days before partial sciatic nerve ligation, macrophage accumulation in the nerve was decreased 3 days after the lesion (Lee et al., 2018). However, this decrease was only about 50% and was restricted to M1like macrophages (i.e., CD86+CD206-, see Section 9). Prolonged treatment with the inhibitor for 3 or 9 months led to an approximately 70% decrease in macrophages in the femoral quadriceps nerve in wild type mice and in a mutant mouse model of Charcot-Marie-Tooth disease and to a reduction in the clinical symptoms of the disease (Klein et al., 2015).
8.1. Function of macrophages in the PNS after injury: Are infiltrating macrophages necessary for the clearance of myelin and axonal debris?
It is commonly stated that monocyte-derived macrophages are essential for clearance of myelin and axonal debris. For example, in their review article, Gaudet et al. (2011) stated that “hematogenous macrophages are essential for effective myelin phagocytosis”. While there is no doubt that macrophages phagocytose myelin after axotomy (e.g., Bruck, 1997; Monaco et al., 1992; Perry et al., 1987; Scheidt and Friede, 1987), infiltrating macrophages do not appear to be necessary for clearance of myelin or axonal debris. Thus, in Ccr2 knockout mice, there is efficient clearance of myelin and of the neurofilament light protein in the distal sciatic nerve after axotomy even though there is a dramatic reduction in the infiltration of macrophages into the nerve in these animals (Fig. 2 and Niemi et al., 2013). Subsequent studies established a role for neutrophils in PNS Wallerian degeneration and indicate that the implications from two key papers in the field need to be reconsidered. Barrette et al. (2008) reported a large decrease in myelin clearance in mice expressing thymidine kinase on the CD11b promoter after the animals were given ganciclovir. The authors observed reduced myelin clearance after axotomy and concluded that “overall, these findings suggest that CD11b macrophages are primarily, although perhaps not entirely, responsible for the phagocytosis and removal of myelin debris and its inhibitory effects” (Barrette et al., 2008). However, as already noted, the CD11b promoter is active in both monocytes/macrophages and granulocytes. While the authors showed that their procedure decreased macrophages in the distal nerve, they found also a striking reduction in Gr-1+ granulocytes (a population that includes neutrophils). In contrast, the Ccr2 knockout mice we have studied, which display normal myelin clearance, are selective for monocyte/macrophage depletion (Auffray et al., 2007; Nahrendorf et al., 2007). A second relevant study is that of Perry et al. (1995), who irradiated mice to deplete blood monocytes, and observed normal clearance of myelin basic protein from the distal sciatic nerve 5 days after injury, though clearance was slower at later time points (Perry et al., 1995). However, irradiation would be expected to deplete both monocytes and neutrophils (Heylmann et al., 2014). Since the authors did not examine the levels of specific bone-marrow derived cells in the blood, it is unclear what the relative impact their procedure had on monocytes and neutrophils and how complete their depletion of either was. Nonetheless, Perry et al. (1995) interpreted their findings as resulting solely from macrophage depletion.
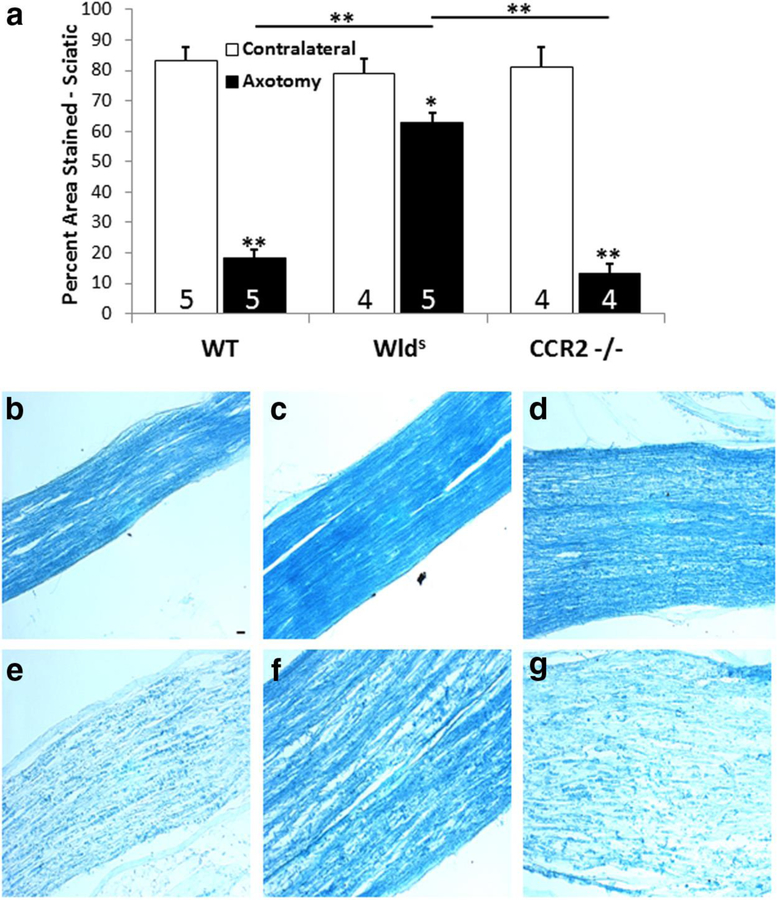
Fig. 2.
Seven days after unilateral transection of the sciatic nerve, myelin in the distal nerve segment was stained with luxol fast blue. (a) Ipsilateral nerves from wild type and Ccr2 knockout mice retained about 20% of the myelin reactivity seen in contralateral control nerves. On the other hand, the ipsilateral distal nerves from Wlds mice retained about 80% of the myelin seen in contralateral controls. Micrographs from sections of the ipsilateral (e-g) and contralateral (b-d) from wild type (WT) (b,e), Wlds (c,f) and Ccr2 knockout (d,g). *p<0.05, **p<0.001. Scale bar, 20 µm. From Niemi et al., 2013.
8.2. Function of macrophages in the PNS after injury: Promotion of regeneration.
Lu and Richardson (1991) reported that causing inflammation in the DRG by injecting the ganglion with the bacterium Corynebacterium parvum increased regeneration in the dorsal root after it had been crushed. A milder increase in regeneration was seen if macrophages were injected directly into the DRG. Interestingly, the bacterial injection did not lead to regeneration of axons in the sciatic nerve after the sciatic nerve was crushed.
Our studies on the relationship of macrophages to nerve regeneration began with studies on the Wlds mouse. In addition to delayed Wallerian degeneration, these mice have impaired regeneration (Bisby and Chen, 1990; Brown et al., 1991; Chen and Bisby, 1993). It has generally been assumed that the decrease in regeneration is the direct consequence of the delayed Wallerian degeneration and the delayed infiltration of macrophages into the distal nerve segment. However, in addition, these mice do not show an increase in macrophage infiltration into the DRG when examined 7 days after sciatic nerve axotomy (Niemi et al., 2013). The decrease in macrophage accumulation was accompanied by a decrease in the axotomy-induced expression of CCL2. We also examined macrophage accumulation under these conditions in mice in which the chemokine receptor CCR2 was knocked out. We found that there was no axotomy- induced macrophage accumulation in the DRG (Niemi et al., 2013). To examine whether there was any relationship between macrophage infiltration in sensory ganglia and nerve regeneration, we turned to the conditioning lesion response.
The conditioning lesion response involves an increase in the rate of axonal regeneration after a nerve lesion as a result of a prior lesion to that nerve. McQuarrie and Grafstein (1973) discovered that regeneration in the sciatic nerve after a nerve crush (referred to as a test lesion) is enhanced if the axons received a prior lesion (a conditioning lesion) distal to the site of the ensuing test lesion. This conditioning lesion effect was shown to occur in both sensory and motor axons (McQuarrie, 1978; McQuarrie et al., 1977). McQuarrie et al. (1978) reported that, in contrast, the rate of regeneration actually decreases in sympathetic axons in the sciatic; however, a later study on sympathetic-cholinergic axons that innervate the sweat gland established that these neurons do show acceleration of regeneration after a conditioning lesion (Navarro and Kennedy, 1990).
Though conditioning lesion experiments were initially performed entirely in vivo, the effect of an in vivo conditioning lesion can subsequently be studied in either explant or dissociated cell culture. Both sensory and sympathetic neurons were shown to increase their neurite outgrowth in these cultures (Fig. 3; Edstrom et al., 1996; Hu-Tsai et al., 1994; Niemi et al., 2013; Shoemaker et al., 2005). In our study in Wlds and Ccr2 knockout mice (Niemi et al., 2013), we examined the conditioning lesion effect in explant DRG to minimize any impact of delayed Wallerian degeneration. In both mutant mouse strains, no conditioning lesion response was seen (see Fig. 3 for Ccr2 data).
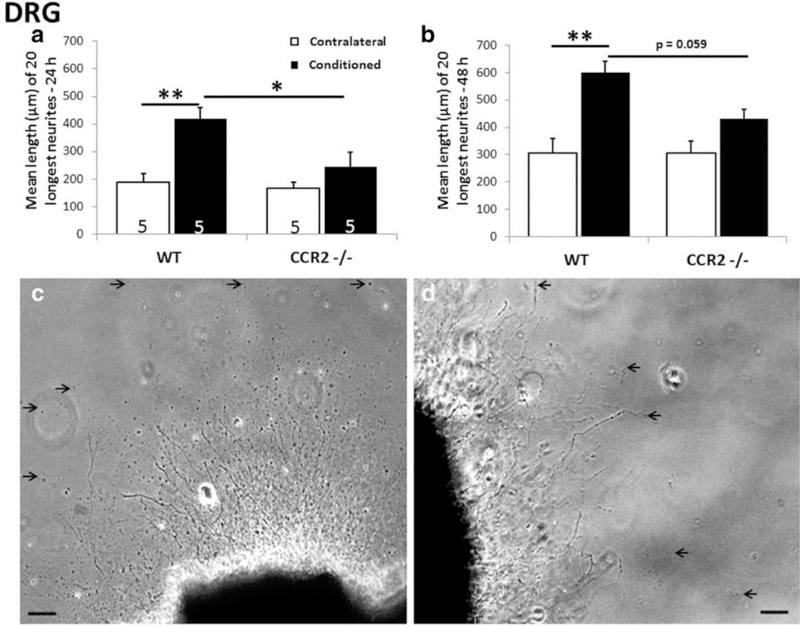
Fig. 3.
Seven days after sciatic nerve transection, a conditioning lesion effect was seen in explanted DRGs from wild type but not from Ccr2 mice measured at 24 (a) and 48 (b) hours. Phase micrographs are shown of DRG explants from wild type (WT) (c) and Ccr2 (d) ater 48 hours in culture. Arrows point to endings of individual neurites. *p<0.05, **p<0.001, Scale bars, 100 µm. From Niemi et al., 2013.
Having established a correlation between macrophage accumulation in DRG and neurite outgrowth, we next overexpressed CCL2 in DRG of intact mice in an attempt to cause macrophage infiltration in the absence of nerve injury. Intrathecal injection of AAV5-CCL2 or AAV5-yellow fluorescent protein (YFP) were made between lumbar segments 5 and 6. YFP labeling was found in sensory neurons in all of the lumbar DRG. Ccl2 mRNA expression increased in the L5 DRG after a week and plateaued at a maximal level at 3 and 4 weeks (Niemi et al., 2016). Even though axotomy was not performed in these animals, macrophage accumulation was seen in this ganglion between 2 and 4 weeks. To determine the effect of this accumulation on the growth capacity of the neurons, outgrowth was examined in explant and dissociated cell cultures. Neurite outgrowth from AAV5-CCL2 administered mice was enhanced in both culture systems compared to neurons from AAV5-YFP mice (Fig. 4). Administration of AAV5-CCL2 to Ccr2 knockout mice also led to an increased expression of CCL2 but no accumulation of macrophages in the DRG and no increase in neurite outgrowth. Incubation of wild type neurons with recombinant CCL2 had no effect on neurite outgrowth in either culture system, indicating that the chemokine did not stimulate outgrowth by acting directly on DRG neurons (Fig. 4).
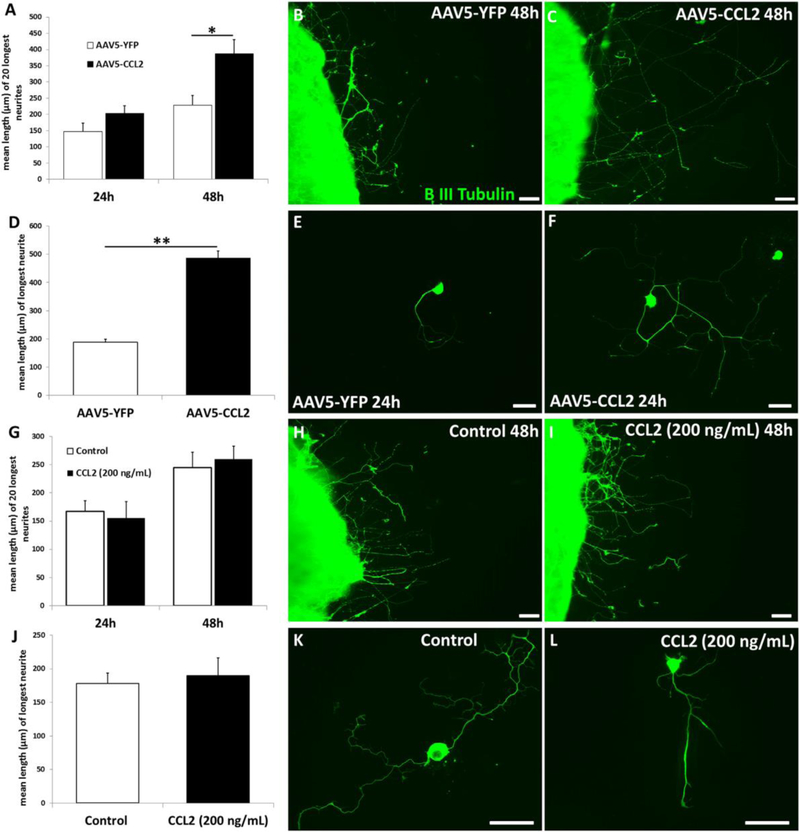
Fig. 4.
CCL2 overexpression causes a conditioning lesion-like increase in neurite outgrowth by DRG neurons from mice that received 3 weeks earlier an injection of AAV5-CCL2. Neurite outgrowth was measured in explant (A-C) and in cell (D-F) cultures. In contrast, if CCL2 (200 ng/ml) was added directly to such cultures, no effect on neurite outgrowth was seen (G-L). *p<0.05, **p<0.001. Scale bar, 100 µm. From Niemi et al., 2016.
We next looked at the expression of eight regeneration-associated genes in the DRG: LIF, IL-6, GAP-43, c-Jun, ATF3, galanin SMAD1 and Sox11. Strikingly, the only one of these that increased in the CCL2 overexpressing animals was LIF. Since LIF acts through gp130 to activate JAK2, which phosphorylates STAT3, we looked at STAT3 phosphorylation in DRG neurons and found that it was increased. To see if this activation of STAT3 was important in producing the change in neurite outgrowth in cell culture, we blocked this step using either of two inhibitors: AG490 and STATTIC. AG490 reduced the increase in neurite length seen in CCL2 overexpressed neurons by about 50%, and STATTIC abolished the increase (Niemi et al., 2016).
A different approach to studying the relationship of macrophages to the conditioning lesion was taken by Kwon et al. (2013). These researchers administered minocycline intrathecally through an osmotic minipump, which decreased macrophage accumulation in the DRG and, in addition, decreased the conditioning lesion effect measured 7 days later in dissociated cell culture. As mentioned earlier in this review, minocycline does have actions beyond its effects on macrophages. Interestingly, Kwon et al. (2013) reported that minocycline decreased the axotomy-induced expression of IL-6, a cytokine that has been shown to increase in sensory neurons after axotomy (Murphy et al., 1995). While it is possible that macrophages trigger IL-6 expression by sensory neurons, it is noteworthy that IL-6 was not induced in ganglia when macrophages accumulated in the DRG after CCL2 overexpression (Niemi et al., 2016).
In a subsequent paper, Kwon et al. (2015) examined CCL2 knockout mice and showed that there was no accumulation of macrophages in the DRG and that no conditioning lesion effect was seen in cell cultures. They also injected directly into the DRG in intact wild type mice each of three chemokines: CCL2, CCL3 and fractalkine. Each of these chemokines had an identical ability to lead to macrophage accumulation; however, only CCL2 led to a conditioning lesion effect. When the macrophages that accumulated in response to these chemokines were examined for M1 and M2 markers (see Section 9), the CCL2 group expressed some M2 markers but no M1 markers. CCL3 and fractalkine, on the other hand, expressed some M1 markers and no M2 markers. Finally, CCL2 seems to have an effect on macrophages in addition to its role as a chemoattractant. When neurons were cocultured with peritoneal macrophages, addition of a neutralizing antibody to CCL2 largely abolished the increase in neurite length normally seen in these co-cultures. Whether this effect represents an effect on macrophage polarization is unknown.
9. Macrophage polarization.
As with macrophages in other tissues, the macrophage population in the peripheral ganglia and their respective nerves after injury is heterogeneous. Upon exposure to a number of stimuli, resident and recruited macrophages alter their genomic/proteomic signature to produce a spectrum of phenotypes and functions. Despite its inherent complexity, this spectrum has been historically separated into two “polarizing” phenotypes- M1 and M2. Traditionally, M1 macrophages are associated with pro-inflammatory functions and neurodegenerative outcomes, while M2 macrophages are broadly seen as anti-inflammatory and promoting repair. In this section, we discuss how M1 and M2 macrophages are characterized as well as reviewing studies that describe their distribution in the PNS after injury and their roles in Wallerian degeneration and subsequent regeneration.
9.1. Background on macrophage polarization.
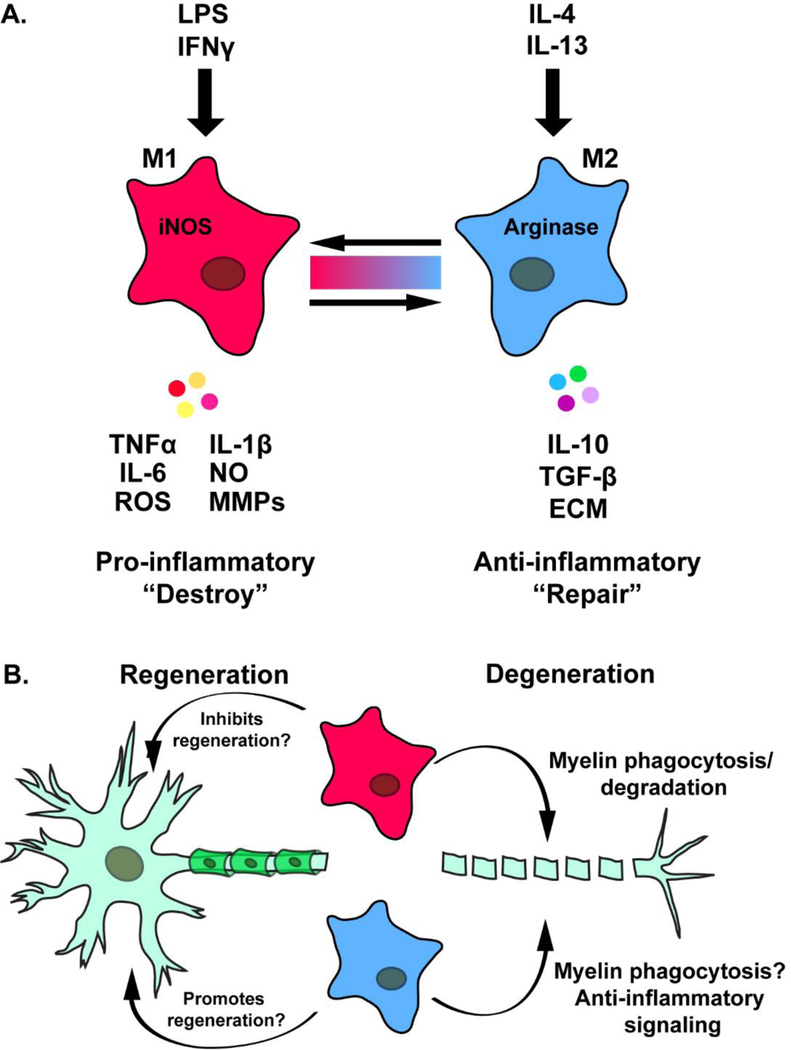
Prior to the use of the M1/M2 nomenclature, macrophage phenotype/function was commonly described as classically- and alternatively-activated, respectively. This classification was based on activity from distinct helper T-cell populations (i.e., Th1/Th2), which are thought to influence macrophage polarization based on the cytokines they release following an immune response (Martinez and Gordon, 2014). For example, Th1 cells secrete IFNγ, a cytokine which leads to classical activation of macrophages. Classically activated macrophages are typically seen as pro-inflammatory, due to their expression of pro-inflammatory cytokines [IL-1β, IL-6, tumor necrosis factor (TNFα)] and noxious agents (nitric oxide, reactive oxygen species, and matrix metalloproteinases) (Fig. 5A). On the other hand, Th2 dependent release of IL-4 and IL-13 promotes alternative activation of macrophages, a state characterized by release of anti-inflammatory cytokines (i.e., IL-10, TGF-β), and the upregulation expression of extracellular matrix proteins, growth factors and arginase (Fig. 5; Martinez and Gordon, 2014; Wynn and Vannella, 2016).
Fig. 5.
A. Diagram showing the polarization of macrophages in vitro by LPS and IFNγ to an M1-like phenotype and by IL-4 and IL-13 to an M2-like phenotype. The color spectrum between the two subtypes indicates the spectrum of macrophage phenotypes that are thought to exist in vivo. M1 macrophages express nitric oxide synthase (iNOS) and secrete TNFα, IL-6, reactive oxygen species (ROS), IL-1β, NO (nitric oxide), and matrix metalloproteinases (MMPs). M2 macrophages express arginase and secrete IL10, TGF-β, and extracellular matrix molecules (ECM). B. Questions remain as to the exact function of M1 (pink)- and M2 (blue)-like macrophages on the axotomized neuronal cell bodies and the distal nerve segment.
The M1/M2 nomenclature was first introduced by Mills et al. (2000). However, the author’s designation of M1 and M2 revolved not on how Th1/Th2 cytokines influenced different macrophage phenotypes, but on an intrinsic property of macrophages to adopt specific phenotypes in response to these cytokines. Specifically, the authors utilized mouse strains with different Th1/Th2 profiles and determined how macrophages processed arginine in response to IFNγ stimulation. For example, in response to IFNγ, cultured peritoneal macrophages from Th1 dominant mouse strains (C57Bl/6 and B10D2) produced the inflammatory marker nitric oxide, a toxic byproduct of arginine and nitric oxide synthase. Conversely, macrophages from the Th2 dominant mouse strain, BALB/C, produced ornithine, a byproduct of arginine and arginase commonly associated with tissue repair. Additionally, the authors also reported an increase in anti-inflammatory marker TGFβ in BALB/C, but not in C57BL/6 or B10D2 mice following stimulation with IFNγ. While the study from Mills et al. (2000) suggests that polarization is not a T-cell dependent process, others have suggested this theory requires more investigation (Murray et al., 2014).
Recently, it has been demonstrated that other proteins/molecules outside of the Th1/Th2 paradigm provoke similar M1/M2 phenotypes in macrophages. For example, the gram-negative bacterial endotoxin and Toll-like receptor-4 (TLR4) agonist lipopolysaccharide (LPS) is capable of inducing an M1 phenotype. Additionally, several ligands produce similar, yet distinct M2-like phenotypes (i.e., M2a, M2b, M2c, and M2d) that are each defined by stimulus, genetic/proteomic signature, and function. For comprehensive reviews on M2 subtypes, refer to the following articles (Martinez and Gordon, 2014; Pinhal-Enfield et al., 2003; Rőszer, 2015).
Currently, it is generally agreed that too much emphasis has been put on macrophages strictly adopting an M1 or M2 phenotype when activated (Martinez and Gordon, 2014; Murray, 2017). Indeed, macrophages are continuously influenced by a blend of M1 and M2 associated signals in their microenvironment, suggesting macrophage phenotype is not strictly bipolar or fixed but, in fact, highly fluid and can exist on a spectrum. In vitro studies demonstrate that macrophage populations are capable of transitioning between M1 and M2 phenotypes (Davis et al., 2013; Khallou-Laschet et al., 2010; Van den Bossche et al., 2016), albeit the transition from M1 to M2 is more difficult than the reverse (Van den Bossche et al., 2016). Additionally, macrophages can express both M1/M2 markers in response to pathological conditions (Bazzan et al., 2017; Lee et al., 2018; Vogel et al., 2013). This added complexity not only makes it difficult to define subpopulations of M1 and M2 macrophages in vivo, but also makes it challenging to infer accurate comparisons between in vivo and in vitro experiments. However, the simplicity of this over-generalized nomenclature and lack of acceptable agreed-upon alternatives (particularly for in vivo studies), leads to its continued use. Therefore, for the purpose of this review, we will continue to use the M1 and M2 nomenclature.
9.2. Presence of M1 and M2 macrophages in the PNS after injury.
Earlier in the review, we described how macrophage accumulation following a peripheral nerve injury occurs at both the distal portion of the injured nerve as well as the respective ganglia (Lu and Richardson, 1993; Schreiber et al., 1995; Siebert et al., 2000). The phenotype of these macrophages can inform whether M1 and M2 macrophages are relevant at these locations and their potential influence on Wallerian degeneration and subsequent regeneration (Fig. 5B). Historically, phenotype is determined based on genomic and/or proteomic signatures. A number of such markers, which consists of cell surface proteins, cytokines, chemokines and enzymes have been reviewed by others (Martinez and Gordon, 2014; Murray, 2017; Murray et al., 2014).
At the onset of macrophage infiltration, Ydens et al. (2012) reported upregulation of certain M1 (Il-6, Il-1β) and M2 (Il-4ra, Il-13ra1) genes in the distal stump. At the protein level, the authors show that while M1 marker iNOS is not upregulated, M2 marker arginase is upregulated at all time points (3, 7, 14 days) after axotomy. Other studies using similar surgical and technical paradigms, also report increases in Tnfα (M1) and Il10 (M2) in the distal stump at these time points (Nadeau et al., 2011; Siqueira Mietto et al., 2015). Recently, Tomlinson et al. (2018) expanded on these results by looking at temporal gene expression from macrophages isolated from distal sciatic nerves at various time points. In terms of specific genes, the authors report that M1 marker Nos2 is upregulated at day 3, yet is significantly decreased by day 14, while M2 marker Retnla (Fizz1) is upregulated at day 14 compared to day 5. Interestingly, both results conflict with that seen from Ydens et al. (2012), which showed no change in these specific genes. While the different techniques used to quantify gene expression between the two studies could explain the different results, it is also likely that the use of whole sciatic nerve tissue by Ydens et al. (2012) diluted cell specific changes.
Phenotypic assessment of each individual macrophage to specifically quantify M1 and M2 macrophages from the injured sciatic nerve at various time points after sciatic nerve transection yielded similar results. Lee et al. (2018) found that at all time points measured after injury (3, 14, 28 days), four populations of macrophages existed based on the cell surface expression of CD86 (M1) or CD206 (M2). At day 3, the majority of macrophages presented with an M1 phenotype (CD86+/CD206-). By day 14 however, the majority of macrophages were not specific to M1 or M2 (CD86-/CD206- or CD86+/CD206+). Similarly, Nadeau et al. (2011) reported an early increase in M1 macrophages 1–2 days after injury. At day 3 however, M1 macrophages were replaced by M2 macrophages with this phenotype maintaining dominance from days 3–7. However, it should be noted that Nadeau et al. (2011) did not include any macrophages that were of mixed phenotype in their analysis. Overall, while these data suggest that macrophage phenotype in the distal stump may shift from M1 to non-M1 or M2 within the first 2 weeks after injury, it also suggests that the majority of macrophages that accumulate within the first 2 weeks after injury may be of a mixed phenotype. This begs the question of whether a specific phenotype is responsible for Wallerian degeneration. Studies that seek to answer this question will be discussed in Section 9.4.
In terms of the macrophages that accumulate in the DRG following sciatic nerve injury, Kwon et al. (2015) measured M1 and M2 associated gene expression in macrophages that had been separated from axotomized DRG and found increases in Cd206 and Arg-1, M2 associated genes, with no changes in the M1 associated genes Cd36, iNos, and Il2. Niemi et al. (2016) found significant elevation of three M2 associated genes (Cd206, Arg-1, Ym1) in whole DRG after axotomy compared to upregulation of just one M1 associated gene (Cd86). In a study using IHC, the vast majority of CD68+ macrophages in the DRG 3 and 7 days after axotomy were positive for the M2 marker CD206 (Lindborg et al., 2018). This result was similar to a study by Komori et al. (2011), which showed a significant elevation of M2 positive macrophages (CD206+, Arg-1+, or CD163+) in the DRG 3 days after partial sciatic nerve ligation compared to that of the uninjured contralateral DRG. The number of macrophages positive for M1 markers iNOS or CD86 was unchanged. Each of these studies suggests a predominant M2 macrophage phenotype in the DRG following sciatic nerve injury.
Role of polarized macrophages in the injured PNS: In vitro studies.
Studies in vitro have also provided understanding on how macrophage phenotype affects myelin phagocytosis and DRG regeneration. In the majority of in vitro studies, bone marrow-derived macrophages are polarized over 24–48 hours using LPS/IFNγ (M1) and IL-4 (M2). For experiments using M1/M2 bone marrow-derived macrophages, it is possible to distinguish them by the signal used to polarize them [e.g., M(LPS-IFNγ) and M(IL-4)] instead of M1 and M2 respectively, as multiple signals can be used to obtain an “M2” phenotype (Murray et al., 2014). With respect to Wallerian degeneration, Vereyken et al. (2011) showed that M(LPS-IFNγ) macrophages consumed a greater amount of fluorescent myelin than M(IL-4) macrophages, suggesting that M(LPS-IFNγ) have a greater propensity for myelin phagocytosis. Additionally, Kroner et al. (2014) reported that M(LPS) macrophages down-regulate M1 markers and upregulate M2 markers upon myelin phagocytosis, suggesting a shift from M1 to M2 may occur during Wallerian degeneration. Conversely however, Wang et al. (2015) reported that BMDMs treated with recombinant MCS-F, another M2 stimulus, followed by myelin debris led to both reduced expression of M2 markers (YM1, FIZZ-1, Arg-1, CD206) and elevated expression of M1 markers (CD86, iNOS) measured by western blot. As reviewed by Kopper and Gensel (2018), these conflicting results are likely due to differences in experimental protocol, specifically whether exposure of macrophages to myelin occurs prior or after polarization.
In assessing regeneration, Kigerl et al. (2009) were the first to describe that neurite outgrowth from dissociated DRGs grown in conditioned media (CM) from M(LPS/IFNγ) or M(IL-4) macrophages was elevated compared to media from unstimulated macrophages. Interestingly, the type of growth was different in the two groups, with M(LPS/IFNγ) eliciting shorter highly branched extensions and M(IL-4) promoting longer neurites with less branching, analogous to the elongating axonal growth seen during successful regeneration. Additionally, the authors demonstrated that unlike M(LPS/IFNγ) CM, the growth promoting effects of M(IL-4) CM were present on inhibitory substrates (chondroitin sulfate proteoglycans and myelin). However, it should be noted that the growth promoting effect of M(LPS/IFNγ) CM on permissive substrates is not seen by all investigators. While Hervera et al. (2018) corroborate neurite outgrowth induced by M(LPS/IFNγ) CM, other groups report either no change between M(LPS/IFNγ) CM and control media, or that M(LPS/IFNγ) CM actually decreases DRG neurite outgrowth and branching compared to unstimulated CM (Gaudet et al., 2016; Kroner et al., 2014). It is important to note that these discrepancies could be due to differences in methodology between these studies. They include the time DRG spent in culture prior to addition of CM (1–3 days), time spent in CM (24–48 hours), time macrophages spent under polarizing conditions (24 hours vs 7 days), or whether DRG were grown on glass coverslips or in microfluidic devices. Indeed, Smith and Skene (1997) showed early on that growth and branching patterns differed between 24 and 48 hours in culture, highlighting a need for consistency between studies.
9.3. Role of polarized macrophages in the injured PNS: In vivo studies.
In peripheral nerve injury, complete ablation of macrophages via pharmacological or genetic approaches is detrimental to both Wallerian degeneration and subsequent regeneration (Barrette et al., 2008; Bruck et al., 1996; Dailey et al., 1998; Liu et al., 2000). While not completely understood, it is thought that these processes are related to the “destroy” and “repair” stereotype historically attributed to M1 and M2 macrophages, respectively.
As previously mentioned, IFNγ and LPS are well-characterized inducers of the M1 phenotype. Genetic ablation of the LPS receptor TLR4 leads to decreased inflammatory cytokine production and macrophage recruitment, impaired Wallerian degeneration and delayed functional recovery compared to WT mice after sciatic nerve transection (Boivin et al., 2007; Hsieh et al., 2017; Wu et al., 2013). Conversely, an intra-neural injection of LPS into the transected sciatic nerve of rats not only enhanced macrophage recruitment to the distal nerve, but also accelerated myelin phagocytosis and functional recovery compared to PBS injection (Boivin et al., 2007). While loss of IFNγ on Wallerian degeneration was not assessed, Tomlinson et al. (2018) reported that mice deficient in the IFNγ receptor (Ifngr1−/−) displayed no differences in axon regeneration or functional recovery 8 weeks after sciatic nerve transection. It should be noted, however, that assessment of regeneration at 8 weeks after axotomy does not take into consideration a possible difference in the rate of regeneration which would require assessment at earlier time points (Barrette et al., 2008; Mokarram et al., 2012; Siqueira Mietto et al., 2015). Interestingly, mice deficient in secreted factors commonly associated with M1 macrophages show a similar phenotype to that of TLR4-deficient mice. Specifically, a delay in macrophage recruitment, Wallerian degeneration and structural regeneration were found in mice where Nos2, Il-1β or Tnfα activity were ablated via genetic or pharmacological mechanisms (Levy et al., 2001; Liefner et al., 2000; Perrin et al., 2005).
In respect to M2 specific stimuli, there is no evidence on how IL-4 and IL-13 ablation affects macrophage infiltration, Wallerian degeneration, and regeneration following sciatic nerve axotomy or crush. While Tomlinson et al. (2018) did report that IL-4 deficient mice had no deficits in sciatic nerve regeneration 8 weeks after injury, the 8 week time point assessed is not optimal due to reasons described above. Uceyler et al. (2007), using the chronic constriction injury (CCI) model of neuropathic pain, reported that IL-4 deficient mice experience persistent elevation of both TNFα and IL-1β in the distal sciatic nerve 4 weeks following CCI. Whether this persistent upregulation of proinflammatory cytokines caused by IL-4 deficiency affects Wallerian degeneration or regeneration requires study. In terms of M2 macrophage secreted factors, published studies have only focused on IL-10. While the authors did not assess the effect on Wallerian degeneration, Siqueira Mietto et al. (2015) reported that IL-10 deficiency resulted in prolonged M1 marker expression and downregulated M2 marker expression in macrophages, along with prolonged expression of pro-inflammatory cytokines in the distal stump of the sciatic nerve. IL-10 deficiency also resulted in both functional and structural deficits in regeneration compared to WT mice. However, Atkins et al. (2007) only reported increases in functional recovery, but not structural regeneration, 6 weeks following sciatic nerve axotomy and exogenous application of 125 ng IL-10. Assessment of regeneration at earlier time points may yield an effect on rate of regeneration as previously described. Further studies looking at macrophage specific deletion in IL-10 in these outcomes measures, as well as other M2 macrophage specific factors (e.g., TGF- β, arginase) are needed.
Overall, these data would suggest that M1 macrophages are more involved in Wallerian degeneration, while M2 macrophages are more involved in the regenerative process. However, this hypothesis cannot be confirmed until additional in vivo studies are done. First, it is still unclear whether specifically ablating M2 macrophages impairs Wallerian degeneration. Second, while ablation of multiple M1-related factors does result in decreased Wallerian degeneration and subsequent regeneration, it is unclear: 1) how the M1/M2 population is affected; 2) whether lack of Wallerian degeneration is due to loss of the M1 phenotype or to the overall impaired macrophage recruitment reported; and 3) whether lack of regeneration is simply due to decreased Wallerian degeneration.
Studies where macrophages are manipulated on a local scale suggest a regenerative effect of M2 macrophages. For example, Mokarram et al. (2012) devised a nerve conduit to fit between the two ends of the axotomized sciatic nerve and continually released IL-4. Interestingly, supplementing with IL-4 led to increased numbers of macrophages that were positive for M2 markers (CD163, CD206) compared to that of control matrix or matrix supplemented with IFNγ. This led to an increase in the number of regenerated fibers that extended the length of the conduit (Mokarram et al., 2012). In another study using a similar nerve conduit, Lv et al. (2017) demonstrated that supplementing the matrix with collagen VI also elevated numbers of M2 macrophages within the conduit, which boosted both structural and functional recovery after sciatic nerve transection.
10. Molecular mechanisms of macrophage action in the PNS.
Macrophage release of nitric oxide has been implicated in the breakdown of myelin after axotomy (Levy et al., 2001; Panthi and Gautam, 2017). Phagocytosis of myelin by macrophages includes both opsonized and non-opsonized myelin (for review see Rotshenker, 2011). Myelin opsonized by complement components is phagocytosed by binding to the complement receptor type 3 (Bruck and Friede, 1991; DeJong and Smith, 1997; Vriesendorp et al., 1995). Antibodies bound to myelin lead to myelin phagocytosis via macrophage Fc receptors (DeJong and Smith, 1997; Smith, 1999). A third receptor used by macrophages in myelin phagocytosis is the scavenger receptor-AI/II (da Costa et al., 1997).
Less is known about the molecular mechanisms underlying the regeneration-promoting effects of macrophages. Neuroinflammation within the DRG has been shown to increase the expression of certain regeneration-associated genes. Following the injection of Corynebacterium parvum into the ganglion, increases in expression of GAP-43 and cJUN were found in the sensory neurons (Lu and Richardson, 1995). The increase in GAP-43 mRNA that occurs after sciatic nerve axotomy was blocked by intrathecal administration of minocycline, perhaps by blocking macrophage infiltration (Kwon et al., 2013). As already mentioned, overexpression of CCL2 led to an increase in Lif mRNA (Niemi et al., 2016).
In a previous review article, we listed a number of molecules secreted by macrophages that could be involved in promoting DRG regeneration (DeFrancesco-Lisowitz et al., 2015). The only one of these for which there is strong evidence is oncomodulin, which can be released by macrophages and neutrophils. Oncomodulin has been proposed to underlie the promotion of regeneration by axotomized retinal ganglion cells that is produced as a result of inflammation in the eye (Kurimoto et al., 2013; Leon et al., 2000; Yin et al., 2009). However, an alternate interpretation of this phenomenon has been proposed involving a role for LIF and ciliary neurotrophic factor released by retinal astrocytes (Leibinger et al., 2009). Kwon et al. (2013) reported that the stimulation of sensory neuron outgrowth seen in co-cultures with macrophages can be blocked by a neutralizing antibody to oncomodulin. In a submitted manuscript currently under review, we have shown that the conditioning lesion effect seen in wild type mice in the DRG in vivo is not seen in mice in which the gene for oncomodulin had been knocked out.
In the 1980s, Hans Thoenen’s laboratory proposed a novel function for macrophages after axotomy in addition to myelin clearance, namely that they stimulate the induction of NGF. This relationship was suggested by the finding that IL-1β released by macrophages triggers NGF induction in the sciatic nerve (Bandtlow et al., 1987; Guenard et al., 1991; Heumann et al., 1987; Lindholm et al., 1987; Thoenen et al., 1988). The induction occurs in Schwann cells and fibroblasts (Heumann et al., 1987). Cell culture experiments indicated that NGF was induced by IL-1β in fibroblasts but not in Schwann cells (Matsuoka et al., 1991). Whether macrophages alter Schwann cell expression of NGF via a different cytokine is not known.
More recently, Barrette et al. (2008) reported that reduction of circulating monocytes and granulocytes in a transgenic mouse leads to a nearly complete block of NGF induction and of the other three neurotrophins (BDNF, NT3, and NT4/5) following axotomy; however, whether it is macrophages or granulocytes that are involved in this process is unknown. In addition, in conflict with earlier findings, Barrette et al. (2008), using in situ hybridization together with an antibody to GR1 (which recognizes both a subset of monocytes/macrophages and granulocytes) reported that the neurotrophins were expressed by these myeloid cells and not by other non-neuronal cells such as Schwann cells. The basis for the discrepancy as to which cells in the nerve expressed these growth factors is unclear.
While it is often assumed that the increase in growth factors like NGF expressed in the distal nerve stump promotes regeneration that is not necessarily the case. Several studies have concluded that NGF does not promote regeneration of sensory or sympathetic neurons in vivo (e.g., Diamond et al., 1987; Diamond et al., 1992; Gloster and Diamond, 1992). In fact, treatment of rats with an antiserum against NGF has been shown to cause the expression of the regeneration-associated genes ATF3 and galanin and to produce a conditioning lesion like stimulation of neurite outgrowth in sympathetic neurons (Hyatt Sachs et al., 2007; Shadiack et al., 2001; Shadiack et al., 1998; Shoemaker et al., 2006).
In a recent article, Hervera et al. (2018) proposed a novel and complex hypothesis for how macrophages promote regeneration. According to these researchers, (1) there is a CX3CR1-dependent infiltration of macrophages into the nerve, (2) these cells release exosomes containing enzymes that produce reactive oxygen species, (3) these exosomes are endocytosed by axons and retrogradely transported to the neuronal cell body where they release reactive oxygen species, (4) these molecules oxidize and thereby inactivate phosphatase and tensin homologue (PTEN) resulting in activation of the phosphatidylinositol 3-kinase/Akt signaling pathway which promotes regeneration. In some of these studies, bone marrow-derived macrophages stimulated with LPS were used, thereby producing an M1-like macrophage.
However, this proposed mechanism is in apparent conflict with other findings. As already noted, in a previous study, no increase in expression of Cx3cl1, the sole known ligand for CX3CR1, was found in the distal sciatic nerve after axotomy (Lindborg et al., 2017). Also, as reviewed in a previous section, studies in culture have generally reported that M2-like macrophages but not M1-like macrophages promote the elongating form of neurite outgrowth in DRG neurons, which is characteristic of regeneration (Kigerl et al., 2009). In fact, Kroner et al. (2014) reported that bone marrow-derived macrophages treated with LPS decreased neurite outgrowth by DRG neurons. Further testing of the exosome hypothesis will be instructive.
11. Involvement of macrophages in diseases of the PNS: The example of Guillain Barré syndrome (GBS) and experimental autoimmune neuritis (EAN).
As noted at the beginning of this review article, neuroinflammation is sometimes referred to as a “double-edged sword”. An example in terms of macrophages is that macrophage phagocytosis of myelin during Wallerian degeneration in the PNS promotes nerve regeneration; however, in certain diseases of the PNS, macrophages attack myelin causing demyelination. For example, macrophages have been implicated in autoimmune demyelinating polyneuropathies (Kiefer et al., 1998), disorders previously thought to be caused largely by T cells (e.g., Soliven, 2014). GBS, an inflammatory peripheral neuropathy, can be divided into two subtypes: acute inflammatory demyelinating polyradiculoneuropathy and acute motor axonal neuropathy (Cashman and Hoke, 2015). Acute inflammatory demyelinating polyradiculoneuropathy is the most common variant in Western countries (Soliven, 2014) and involves both “CD4+ T cell-mediated inflammation and macrophage-induced demyelination” (Shen et al., 2018). Kiefer et al. (2001) observed that the “pathological hallmark of the demyelinating autoimmune neuropathies, mainly GBS and CIDP (authors’ note: this stands for chronic inflammatory demyelinating polyneuropathy) is a process termed macrophage-mediated demyelination, where macrophages virtually strip off the myelin sheath from the axon leaving behind a nude, demyelinated axon”.
EAN is an animal model of GBS (Shen et al., 2018; Soliven, 2012) that “duplicates the clinical, pathological and electrophysiological features of GBS in humans” (Zhu et al., 2002). EAN is produced either by injection of animals with peripheral nerve myelin or fragments of myelin protein or by adoptive transfer of T cells activated by peripheral myelin. A role for macrophages in EAN was demonstrated by depleting circulating monocytes/macrophages with clodronate liposomes (Jung et al., 1993; Katzav et al., 2013), which produced a decrease in the clinical and morphological symptoms of EAN.
An increase in CCL2 mRNA was found in lumbar and sacral spinal nerve in the preclinical stage of EAN (Fujioka et al., 1999). In addition, RANTES (Regulated on Activation Normal T cell Expressed and Secreted), which is chemotactic for T-cells and CCL3 (which is chemotactic for both macrophages and neutrophils) are expressed (Fujioka et al., 1999). Infiltration of lymphocytes, monocytes, and, to a somewhat lesser extent, neutrophils occurs (Ballin and Thomas, 1969) . Macrophages were also seen in peripheral nerves from postmortem tissue and from biopsies from GBS patients (Kiefer et al., 2001). In Wallerian degeneration, macrophages are predominantly of the M2 subtype (Nadeau et al., 2011), while it appears that it is M1 macrophages that are involved in causing EAN (e.g., Zhang et al., 2009).
12. Conclusions.
Many think of neuroinflammation only as having destructive impact on the nervous system. However, as we have reviewed here, neuroinflammation plays very constructive roles in the PNS after nerve transection or crush. In the distal nerve segment after axotomy, non-neuronal cells express chemokines for macrophages and neutrophils, and they attract these immune cells into the degenerating nerve. Both macrophages and neutrophils play important roles in clearing myelin and axonal debris and thereby facilitate subsequent regeneration. At the same time, the axotomized neuronal cell bodies also express macrophage chemokines and attract macrophages to the vicinity of these cell bodies. This latter finding raises the question of what the role of these phagocytic cells might be near neuronal cell bodies. We propose that these macrophages increase the growth capacity of the axotomized neurons. With respect to the conditioning lesion response, under conditions in which macrophages do not accumulate near axotomized neurons, the conditioning lesion is abolished. Under conditions in which macrophage accumulation near neurons is enhanced, a conditioning lesion-like effect is promoted. What the macrophages secrete to produce such an effect is not certain, but one possibility is oncomodulin. As noted at the beginning of this review, although regeneration occurs in the PNS, it is slow and incomplete. It has been proposed recently that, if the molecular mechanism underlying the conditioning-lesion effect were understood, it might be possible to elicit this response via a non-destructive means and facilitate regeneration in patients soon after a peripheral nerve injury (Senger et al., 2018).
Highlights.
After axotomy macrophage were seen only as being involved in Wallerian degeneration.
In addition, macrophages accumulate around axotomized neuronal cell bodies.
Macrophages enter into distal nerves and ganglia in response to the chemokine CCL2.
Macrophages in peripheral ganglia play a role in the conditioning lesion response.
Progress is being made in understanding the mechanisms of these macrophage actions.
Acknowledgements.
The research in our laboratory is funded by a grant from NINDS (NS095017). We thank Jon Niemi and Aaron Talsma for helpful comments on our manuscript.
Abbreviations:
- ATF3
activating transcription factor 3
- CCL2
chemokine C-C motif ligand 2
- CCR2
C-C chemokine receptor type 2
- CNS
central nervous system
- DRG
dorsal root ganglion
- EAN
experimental autoimmune neuritis
- GAP-43
growth associated protein- 43
- GBS
Guillain Barré syndrome
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- LIF
leukemia inhibitory factor
- L
lumbar
- Ly6G
lymphocyte antigen 6 complex locus G6D
- MCP-1
monocyte chemoattractant protein-1
- NGF
nerve growth factor
- PNS
peripheral nervous system
- STAT3
signal transducer and activator of transmission 3
- TNFα
tumor necrosis factor-α
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ 2003. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl. Acad. Sci. U. S. A 100, 7947–7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram CL, Roberge GL, Hu Y, Lowell CA 2014. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 408, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T 2000. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci 20, 2602–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambron RT, Walters ET 1996. Priming events and retrograde injury signals. A new perspective on the cellular and molecular biology of nerve regeneration. Mol. Neurobiol 13, 61–79. [DOI] [PubMed] [Google Scholar]
- Arvidson B 1977. Cellular uptake of exogenous horseradish peroxidase in mouse peripheral nerve. Acta Neuropathol 37, 35–41. [DOI] [PubMed] [Google Scholar]
- Atkins S, Loescher AR, Boissonade FM, Smith KG, Occleston N, O’Kane S, Ferguson MW, Robinson PP 2007. Interleukin-10 reduces scarring and enhances regeneration at a site of sciatic nerve repair. J. Peripher. Nerv. Syst 12, 269–276. [DOI] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666670. [DOI] [PubMed] [Google Scholar]
- Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, Malissen B, Osborne LC, Artis D, Mowat AM 2014. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol 15, 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballin RH, Thomas PK 1969. Electron microscope observations on demyelination and remyelination in experimental allergic neuritis. I. Demyelination. J. Neurol. Sci 8, 1–18. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Heumann R, Schwab ME, Thoenen H 1987. Cellular localization of nerve growth factor synthesis by in situ hybridization. EMBO J 6, 891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner LR, Patterson PH 1994. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc. Natl. Acad. Sci. U. S. A 91, 7109–7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S 2008. Requirement of myeloid cells for axon regeneration. J. Neurosci 28, 9363–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batbold D, Shinoda M, Honda K, Furukawa A, Koizumi M, Akasaka R, Yamaguchi S, Iwata K 2017. Macrophages in trigeminal ganglion contribute to ectopic mechanical hypersensitivity following inferior alveolar nerve injury in rats. J. Neuroinflammation 14, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzan E, Turato G, Tinè M, Radu CM, Balestro E, Rigobello C, Biondini D, Schiavon M, Lunardi F, Baraldo S, Rea F, Simioni P, Calabrese F, Saetta M, Cosio MG 2017. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir Res 18, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi KS, Winter J, Berry M, Cohen J 1992. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not on normal peripheral nerves in vitro. Eur. J. Neurosci 4, 193–200. [DOI] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M 2012. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 31, 1350–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA 2016. New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U. S. A 113, E1738–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biguet NF, Rittenhouse AR, Mallet J, Zigmond RE 1989. Preganglionic nerve stimulation increases mRNA levels for tyrosine hydroxylase in the rat superior cervical ganglion. Neurosci. Lett 104, 189–194. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Chen S 1990. Delayed wallerian degeneration in sciatic nerves of C57BL/Ola mice is associated with impaired regeneration of sensory axons. Brain Res 530, 117–120. [DOI] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G 1968. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z. Zellforsch. Mikrosk. Anat 85, 145–157. [DOI] [PubMed] [Google Scholar]
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE 2004. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J. Neurobiol 59, 216–235. [DOI] [PubMed] [Google Scholar]
- Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S 2007. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J. Neurosci 27, 12565–12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Chensue SW, Kunkel SL, Farese RV Jr., Broxmeyer HE, Charo IF 1997. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest 100, 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Cho J 2013. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res 36, 1039–1050. [DOI] [PubMed] [Google Scholar]
- Brosius Lutz A, Chung WS, Sloan SA, Carson GA, Zhou L, Lovelett E, Posada S, Zuchero JB, Barres BA 2017. Schwann cells use TAM receptor-mediated phagocytosis in addition to autophagy to clear myelin in a mouse model of nerve injury. Proc. Natl. Acad. Sci. U. S. A 114, E8072–E8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broude E, McAtee M, Kelley MS, Bregman BS 1997. c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp. Neurol 148, 367–377. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Lunn ER, Gordon S, Heumann R 1991. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron 6, 359–370. [DOI] [PubMed] [Google Scholar]
- Bruck W 1997. The role of macrophages in Wallerian degeneration. Brain Pathol 7, 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck W, Friede RL 1991. The role of complement in myelin phagocytosis during PNS wallerian degeneration. J. Neurol. Sci 103, 182–187. [DOI] [PubMed] [Google Scholar]
- Bruck W, Huitinga I, Dijkstra CD 1996. Liposome-mediated monocyte depletion during wallerian degeneration defines the role of hematogenous phagocytes in myelin removal. J. Neurosci. Res 46, 477–484. [DOI] [PubMed] [Google Scholar]
- Bruhl H, Cihak J, Plachy J, Kunz-Schughart L, Niedermeier M, Denzel A, Rodriguez Gomez M, Talke Y, Luckow B, Stangassinger M, Mack M 2007. Targeting of Gr-1+,CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum 56, 2975–2985. [DOI] [PubMed] [Google Scholar]
- Brushart TM (2011) Nerve Repair Oxford University Press: New York. [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL 2014. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat. Neurosci 17, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SL, Frohnert PW 1998. Expression of JE (monocyte chemoattractant protein-1) is induced by sciatic axotomy in wild type rodents but not in C57BL/Wld(s) mice. J. Neuropathol. Exp. Neurol 57, 915–930. [DOI] [PubMed] [Google Scholar]
- Cashman CR, Hoke A 2015. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci. Lett 596, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M, Yekkirala A, Barrett L, Blesch A, Michaelevski I, DavisTurak J, Gao F, Langfelder P, Horvath S, He Z, Benowitz L, Fainzilber M, Tuszynski M, Woolf CJ, Geschwind DH 2016. A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron 89, 956–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM 2006. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med 354, 610–621. [DOI] [PubMed] [Google Scholar]
- Cheah TB, Geffen LB 1973. Effects of axonal injury on norepinephrine, tyrosine hydroxylase and monoamine oxidase levels in sympathetic ganglia. J. Neurobiol 4, 443–452. [DOI] [PubMed] [Google Scholar]
- Cheepudomwit T, Guzelsu E, Zhou C, Griffin JW, Hoke A 2008. Comparison of cytokine expression profile during Wallerian degeneration of myelinated and unmyelinated peripheral axons. Neurosci. Lett 430, 230–235. [DOI] [PubMed] [Google Scholar]
- Chen S, Bisby MA 1993. Long-term consequences of impaired regeneration on facial motoneurons in the C57BL/Ola mouse. J. Comp. Neurol 335, 576–585. [DOI] [PubMed] [Google Scholar]
- Chong MS, Reynolds ML, Irwin N, Coggeshall RE, Emson PC, Benowitz LI, Woolf CJ 1994. GAP-43 expression in primary sensory neurons following central axotomy. J. Neurosci 14, 4375–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, Nickerson CA, Gao F, Chandran V, Bravo-Caparros I, GonzalezCano R, Riva P, Andrews NA, Latremoliere A, Seehus CR, Perazzoli G, Nieto FR, Joller N, Painter MW, Ma CHE, Omura T, Chesler EJ, Geschwind DH, Coppola G, Rangachari M, Woolf CJ, Costigan M 2018. Mechanistic differences in neuropathic pain modalities revealed by correlating behavior with global expression profiling. Cell reports 22, 1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR 2010. Wallerian degeneration, wld(s), and nmnat. Annu. Rev. Neurosci 33, 245–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corness J, Shi TJ, Xu ZQ, Brulet P, Hokfelt T 1996. Influence of leukemia inhibitory factor on galanin/GMAP and neuropeptide Y expression in mouse primary sensory neurons after axotomy. Exp. Brain Res 112, 79–88. [DOI] [PubMed] [Google Scholar]
- Corness J, Stevens B, Fields RD, Hokfelt T 1998. NGF and LIF both regulate galanin gene expression in primary DRG cultures. Neuroreport 9, 1533–1536. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ 2002. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 3, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg BG 1970. What is the signal for chromatolysis? Brain Res 23, 1–21. [DOI] [PubMed] [Google Scholar]
- Curcio M, Bradke F 2018. Axon regeneration in the central nervous system: Facing the challenges from the inside. Annu. Rev. Cell. Dev. Biol [DOI] [PubMed] [Google Scholar]
- Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS 1994. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron 12, 191–204. [DOI] [PubMed] [Google Scholar]
- da Costa CC, van der Laan LJ, Dijkstra CD, Bruck W 1997. The role of the mouse macrophage scavenger receptor in myelin phagocytosis. Eur. J. Neurosci 9, 2650–2657. [DOI] [PubMed] [Google Scholar]
- Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M 1998. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J. Neurosci 18, 6713–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansereau MA, Gosselin RD, Pohl M, Pommier B, Mechighel P, Mauborgne A, Rostene W, Kitabgi P, Beaudet N, Sarret P, Melik-Parsadaniantz S 2008. Spinal CCL2 pronociceptive action is no longer effective in CCR2 receptor antagonist-treated rats. J. Neurochem 106, 757–769. [DOI] [PubMed] [Google Scholar]
- David S, Bouchard C, Tsatas O, Giftochristos N 1990. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron 5, 463–469. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA 2013. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio 4, e00264–00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE 2015. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience 302, 174–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong BA, Smith ME 1997. A role for complement in phagocytosis of myelin. Neurochem. Res 22, 491–498. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE 2009. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res 29, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L, Holmes M, Visheau B 1987. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc. Natl. Acad. Sci. U. S. A 84, 6596–6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Foerster A, Holmes M, Coughlin M 1992. Sensory nerves in adult rats regenerate and restore sensory function to the skin independently of endogenous NGF. J. Neurosci 12, 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom A, Ekstrom PA, Tonge D 1996. Axonal outgrowth and neuronal apoptosis in cultured adult mouse dorsal root ganglion preparations: effects of neurotrophins, of inhibition of neurotrophin actions and of prior axotomy. Neuroscience 75, 1165–1174. [DOI] [PubMed] [Google Scholar]
- Enes J, Langwieser N, Ruschel J, Carballosa-Gonzalez MM, Klug A, Traut MH, Ylera B, Tahirovic S, Hofmann F, Stein V, Moosmang S, Hentall ID, Bradke F 2010. Electrical activity suppresses axon growth through Ca(v)1.2 channels in adult primary sensory neurons. Curr. Biol 20, 1154–1164. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Reading MC 1989. Macrophages, lymphocytes and major histocompatibility complex (HLA) class II antigens in adult human sensory and sympathetic ganglia. J. Neuroimmunol 23, 187–193. [DOI] [PubMed] [Google Scholar]
- Farley JE, Burdett TC, Barria R, Neukomm LJ, Kenna KP, Landers JE, Freeman MR 2018. Transcription factor Pebbled/RREB1 regulates injuryinduced axon degeneration. Proc. Natl. Acad. Sci. U. S. A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenbach DA, Sheldrake TA, Dhaliwal K, Kipari TM, Marson LP, Kluth DC, Hughes J 2012. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 82, 928–933. [DOI] [PubMed] [Google Scholar]
- Flugel A, Hager G, Horvat A, Spitzer C, Singer GM, Graeber MB, Kreutzberg GW, Schwaiger FW 2001. Neuronal MCP-1 expression in response to remote nerve injury. J. Cereb. Blood Flow Metab 21, 69–76. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Strittmatter SM 2001. Repulsive factors and axon regeneration in the CNS. Curr. Opin. Neurobiol 11, 89–94. [DOI] [PubMed] [Google Scholar]
- Freeman MR 2014. Signaling mechanisms regulating Wallerian degeneration. Curr. Opin. Neurobiol 27, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey E, Valakh V, Karney-Grobe S, Shi Y, Milbrandt J, DiAntonio A 2015. An in vitro assay to study induction of the regenerative state in sensory neurons. Exp. Neurol 263, 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka T, Kolson DL, Rostami AM 1999. Chemokines and peripheral nerve demyelination. J. Neurovirol 5, 27–31. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Mandrekar-Colucci S, Hall JC, Sweet DR, Schmitt PJ, Xu X, Guan Z, Mo X, Guerau-de-Arellano M, Popovich PG 2016. miR-155 deletion in mice overcomes neuron-intrinsic and neuron-extrinsic barriers to spinal cord repair. J. Neurosci 36, 8516–8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS 2011. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflammation 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrmann J, Monaco S, Kreutzberg GW 1991. Spinal cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor. Neurol. Neurosci 2, 181–198. [DOI] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Milbrandt J, DiAntonio A 2016. Axon self-destruction: New links among SARM1, MAPKs, and NAD+ metabolism. Neuron 89, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM 2007. Electrical stimulation promotes sensory neuron regeneration and growthassociated gene expression. Exp. Neurol 205, 347–359. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M 2016. Tissue-resident macrophage ontogeny and homeostasis. Immunity 44, 439–449. [DOI] [PubMed] [Google Scholar]
- Gloster A, Diamond J 1992. Sympathetic nerves in adult rats regenerate normally and restore pilomotor function during an anti-NGF treatment that prevents their collateral sprouting. J. Comp. Neurol 326, 363–374. [DOI] [PubMed] [Google Scholar]
- Gordon T 2016. Electrical stimulation to enhance axon regeneration after peripheral nerve injuries in animal models and humans. Neurotherapeutics 13, 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Chan KM, Sulaiman OA, Udina E, Amirjani N, Brushart TM 2009. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery 65, A132–144. [DOI] [PubMed] [Google Scholar]
- Gordon T, Sulaiman O, Boyd JG 2003. Experimental strategies to promote functional recovery after peripheral nerve injuries. J. Peripher. Nerv. Syst 8, 236250. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW 1988. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J. Neurosci. Res 21, 18–24. [DOI] [PubMed] [Google Scholar]
- Grafstein B 1975. The nerve cell body response to axotomy. Exp. Neurol 48, 32–51. [DOI] [PubMed] [Google Scholar]
- Greter M, Lelios I, Croxford AL 2015. Microglia versus myeloid cell nomenclature during brain inflammation. Front. Immunol 6, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, George R 1993. The resident macrophages in the peripheral nervous system are renewed from the bone marrow: new variations on an old theme. Lab. Invest 69, 257–260. [PubMed] [Google Scholar]
- Griffin JW, George R, Ho T 1993. Macrophage systems in peripheral nerves. A review. J. Neuropathol. Exp. Neurol 52, 553–560. [DOI] [PubMed] [Google Scholar]
- Groh J, Weis J, Zieger H, Stanley ER, Heuer H, Martini R 2012. Colonystimulating factor-1 mediates macrophage-related neural damage in a model for Charcot-Marie-Tooth disease type 1X. Brain 135, 88–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenard V, Dinarello CA, Weston PJ, Aebischer P 1991. Peripheral nerve regeneration is impeded by interleukin-1 receptor antagonist released from a polymeric guidance channel. J. Neurosci. Res 29, 396–400. [DOI] [PubMed] [Google Scholar]
- Hall SM 1993. Observations on the progress of Wallerian degeneration in transected peripheral nerves of C57BL/Wld mice in the presence of recruited macrophages. J. Neurocytol 22, 480–490. [DOI] [PubMed] [Google Scholar]
- Hanani M 2005. Satellite glial cells in sensory ganglia: from form to function. Brain Res. Brain Res. Rev 48, 457–476. [DOI] [PubMed] [Google Scholar]
- Hanani M 2010. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res. Rev 64, 304–327. [DOI] [PubMed] [Google Scholar]
- Hanz S, Fainzilber M 2006. Retrograde signaling in injured nerve--the axon reaction revisited. J. Neurochem 99, 13–19. [DOI] [PubMed] [Google Scholar]
- Hasan NA, Neumann MM, de Souky MA, So KF, Bedi KS 1996. The influence of predegenerated nerve grafts on axonal regeneration from prelesioned peripheral nerves. J. Anat 189 ( Pt 2), 293–302. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry IA (1992) Response of autonomic neurones to target deprivation: Axotomy and regeneration. In: Development, Regeneration and Plasticity of the Autonomic Nervous System pp. 415–462. Eds. Hendry IA, Hill. CE Harwood Academic: Chur. [Google Scholar]
- Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, Hutson T, Danzi MC, Perry RB, Santos CXC, Kapustin AN, Fleck RA, Del Río JA, Carroll T, Lemmon V, Bixby JL, Shah AM, Fainzilber M, Di Giovanni S 2018. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol 20, 307–319. [DOI] [PubMed] [Google Scholar]
- Heumann R, Korsching S, Bandtlow C, Thoenen H 1987. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J. Cell Biol 104, 1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heylmann D, Rodel F, Kindler T, Kaina B 2014. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta 1846, 121–129. [DOI] [PubMed] [Google Scholar]
- Hilton BJ, Bradke F 2017. Can injured adult CNS axons regenerate by recapitulating development? Development 144, 3417–3429. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Zhang X, Wiesenfeld-Hallin Z 1994. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci 17, 22–30. [DOI] [PubMed] [Google Scholar]
- Holmes FE, Mahoney S, King VR, Bacon A, Kerr NC, Pachnis V, Curtis R, Priestley JV, Wynick D 2000. Targeted disruption of the galanin gene reduces the number of sensory neurons and their regenerative capacity. Proc. Natl. Acad. Sci. U. S. A 97, 11563–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XE, Lundmark K, Dahlstrom AB 1998. Cellular reactions to axotomy in rat superior cervical ganglia includes apoptotic cell death. J. Neurocytol 27, 441–451. [DOI] [PubMed] [Google Scholar]
- Hsieh CH, Rau CS, Kuo PJ, Liu SH, Wu CJ, Lu TH, Wu YC, Lin CW 2017. Knockout of toll-like receptor impairs nerve regeneration after a crush injury. Oncotarget 8, 80741–80756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Tsai M, Winter J, Emson PC, Woolf CJ 1994. Neurite outgrowth and GAP-43 mRNA expression in cultured adult rat dorsal root ganglion neurons: effects of NGF or prior peripheral axotomy. J. Neurosci. Res 39, 634–645. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM 2002. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience 112, 23–38. [DOI] [PubMed] [Google Scholar]
- Hu P, McLachlan EM 2004. Inflammation in sympathetic ganglia proximal to sciatic nerve transection in rats. Neurosci. Lett 365, 39–42. [DOI] [PubMed] [Google Scholar]
- Hume DA 2011. Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. J. Leukoc. Biol 89, 525–538. [DOI] [PubMed] [Google Scholar]
- Hume DA, MacDonald KP 2012. Therapeutic applications of macrophage colonystimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820. [DOI] [PubMed] [Google Scholar]
- Hyatt Sachs H, Schreiber RC, Shoemaker SE, Sabe A, Reed E, Zigmond RE 2007. Activating transcription factor 3 induction in sympathetic neurons after axotomy: response to decreased neurotrophin availability. Neuroscience 150, 887–897. [DOI] [PubMed] [Google Scholar]
- Inoue T, Plieth D, Venkov CD, Xu C, Neilson EG 2005. Antibodies against macrophages that overlap in specificity with fibroblasts. Kidney Int 67, 2488–2493. [DOI] [PubMed] [Google Scholar]
- Jenkins SJ, Hume DA 2014. Homeostasis in the mononuclear phagocyte system. Trends Immunol 35, 358–367. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R 2016a. The repair Schwann cell and its function in regenerating nerves. J. Physiol 594, 3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R 2016b. The repair Schwann cell and its function in regenerating nerves. J Physiol 594, 3521–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson IP, Sears TA 2013. Target-dependence of sensory neurons: an ultrastructural comparison of axotomised dorsal root ganglion neurons with allowed or denied reinnervation of peripheral targets. Neuroscience 228, 163–178. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ 2008. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J. Neurochem 104, 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Huitinga I, Schmidt B, Zielasek J, Dijkstra CD, Toyka KV, Hartung HP 1993. Selective elimination of macrophages by dichlormethylene diphosphonate-containing liposomes suppresses experimental autoimmune neuritis. J. Neurol. Sci 119, 195–202. [DOI] [PubMed] [Google Scholar]
- Katzav A, Bina H, Aronovich R, Chapman J 2013. Treatment for experimental autoimmune neuritis with clodronate (Bonefos). Immunol. Res 56, 334–340. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Langnaese K, Wolf G, Fansa H 2007. Inhibiting effect of minocycline on the regeneration of peripheral nerves. Dev. Neurobiol 67, 1382–1395. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Schild L, Fansa H 2008. Minocycline protects Schwann cells from ischemia-like injury and promotes axonal outgrowth in bioartificial nerve grafts lacking Wallerian degeneration. Exp. Neurol 212, 189–200. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Danielsen N, Holmquist B, Kanje M, Lundborg G 1993. The influence of predegeneration on regeneration through peripheral nerve grafts in the rat. Exp. Neurol 122, 28–36. [DOI] [PubMed] [Google Scholar]
- Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G 2010. Macrophage plasticity in experimental atherosclerosis. PLoS One 5, e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer R, Kieseier BC, Bruck W, Hartung HP, Toyka KV 1998. Macrophage differentiation antigens in acute and chronic autoimmune polyneuropathies. Brain 121 ( Pt 3), 469–479. [DOI] [PubMed] [Google Scholar]
- Kiefer R, Kieseier BC, Stoll G, Hartung HP 2001. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog. Neurobiol 64, 109–127. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci 29, 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Cho S 2016. Microglia and monocyte-derived macrophages in stroke. Neurotherapeutics 13, 702–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Patzko A, Schreiber D, van Hauwermeiren A, Baier M, Groh J, West BL, Martini R 2015. Targeting the colony stimulating factor 1 receptor alleviates two forms of Charcot-Marie-Tooth disease in mice. Brain 138, 3193–3205. [DOI] [PubMed] [Google Scholar]
- Komori T, Morikawa Y, Inada T, Hisaoka T, Senba E 2011. Site-specific subtypes of macrophages recruited after peripheral nerve injury. Neuroreport 22, 911–917. [DOI] [PubMed] [Google Scholar]
- Kopper TJ, Gensel JC 2018. Myelin as an inflammatory mediator: Myelin interactions with complement, macrophages, and microglia in spinal cord injury. J. Neurosci. Res 96, 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsching S, Thoenen H 1985. Treatment with 6-hydroxydopamine and colchicine decreases nerve growth factor levels in sympathetic ganglia and increases them in the corresponding target tissues. J. Neurosci 5, 1058–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Graham JB, Muir D 2002. Metalloproteinasedependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts. J. Neurosci 22, 10408–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S 2014. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83, 1098–1116. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Bitsch A, Stadelmann C, Siebert H, Bruck W 2001. Macrophages are eliminated from the injured peripheral nerve via local apoptosis and circulation to regional lymph nodes and the spleen. J. Neurosci 21, 3401–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto T, Yin Y, Habboub G, Gilbert HY, Li Y, Nakao S, Hafezi-Moghadam A, Benowitz LI 2013. Neutrophils express oncomodulin and promote optic nerve regeneration. J. Neurosci 33, 14816–14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, Kim J, Shin H, Jeong SR, Kang YM, Choi JY, Hwang DH, Kim BG 2013. Contribution of macrophages to enhanced regenerative capacity of dorsal root ganglia sensory neurons by conditioning injury. J. Neurosci 33, 15095–15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MJ, Shin HY, Cui Y, Kim H, Thi AH, Choi JY, Kim EY, Hwang DH, Kim BG 2015. CCL2 mediates neuron-macrophage interactions to drive proregenerative macrophage activation following preconditioning injury. J. Neurosci 35, 15934–15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley JN 1897. On the regeneration of pre-ganglionic and of post-ganglionic visceral nerve fibres. J Physiol 22, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Shi XQ, Fan A, West B, Zhang J 2018. Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Mol. Pain 14, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Monkkonen J, Rogers MJ, Azhayev A, Vaananen HK, Hassinen IE 2002. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol 61, 1255–1262. [DOI] [PubMed] [Google Scholar]
- Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D 2009. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci 29, 14334–14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI 2000. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci 20, 4615–4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C, Muller M, Hickey WF, Ringelstein EB, Kiefer R 2002. Lesion response of long-term and recently immigrated resident endoneurial macrophages in peripheral nerve explant cultures from bone marrow chimeric mice. Eur. J. Neurosci 16, 1654–1660. [DOI] [PubMed] [Google Scholar]
- Levy D, Kubes P, Zochodne DW 2001. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J. Neuropathol. Exp. Neurol 60, 411–421. [DOI] [PubMed] [Google Scholar]
- Li Q, Barres BA 2017. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol 18, 225–242. [DOI] [PubMed] [Google Scholar]
- Lieberman AR 1969. Absence of ultrastructural changes in ganglionic neurons after supranodose vagotomy. J. Anat 104, 49–54. [PMC free article] [PubMed] [Google Scholar]
- Lieberman AR (1971) The axon reaction: a review of the principle features of perikaryal responses to axon injury. In: Int. Rev. Neurobiol pp. 49–124. Eds. Pfeiffer CC, Smythies JR. Academic Press: New York. [DOI] [PubMed] [Google Scholar]
- Liefner M, Siebert H, Sachse T, Michel U, Kollias G, Bruck W 2000. The role of TNF-alpha during Wallerian degeneration. J. Neuroimmunol 108, 147–152. [DOI] [PubMed] [Google Scholar]
- Lindborg JA, Mack M, Zigmond RE 2017. Neutrophils are critical for myelin removal in a peripheral nerve injury model of Wallerian degeneration. J. Neurosci 37, 10258–10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindborg JA, Niemi JP, Howarth MA, Liu KW, Moore CZ, Mahajan D, Zigmond RE 2018. Molecular and cellular identification of the immune response in peripheral ganglia following nerve injury. J Neuroinflammation 15, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Heumann R, Meyer M, Thoenen H 1987. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330, 658–659. [DOI] [PubMed] [Google Scholar]
- Lingappa JR, Zigmond RE 2013. Limited recovery of pineal function after regeneration of preganglionic sympathetic axons: evidence for loss of ganglionic synaptic specificity. J. Neurosci 33, 4867–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Luan S, OuYang H, Huang Z, Wu S, Ma C, Wei J, Xin W 2016. Upregulation of CCL2 via ATF3/c-Jun interaction mediated the Bortezomibinduced peripheral neuropathy. Brain. Behav. Immun 53, 96–104. [DOI] [PubMed] [Google Scholar]
- Liu T, van Rooijen N, Tracey DJ 2000. Depletion of macrophages reduces axonal degeneration and hyperalgesia following nerve injury. Pain 86, 25–32. [DOI] [PubMed] [Google Scholar]
- London A, Cohen M, Schwartz M 2013. Microglia and monocyte-derived macrophages: functionally distinct populations that act in concert in CNS plasticity and repair. Front. Cell. Neurosci 7, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ 1998. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med 187, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Richardson PM 1991. Inflammation near the nerve cell body enhances axonal regeneration. J. Neurosci 11, 972–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Richardson PM 1993. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J. Neurocytol 22, 334–341. [DOI] [PubMed] [Google Scholar]
- Lu X, Richardson PM 1995. Changes in neuronal mRNAs induced by a local inflammatory reaction. J. Neurosci. Res 41, 8–14. [DOI] [PubMed] [Google Scholar]
- Lund H, Pieber M, Harris RA 2017. Lessons learned about neurodegeneration from microglia and monocyte depletion studies. Front. Aging Neurosci 9, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S 1989. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur. J. Neurosci 1, 27–33. [DOI] [PubMed] [Google Scholar]
- Lv D, Zhou L, Zheng X, Hu Y 2017. Sustained release of collagen VI potentiates sciatic nerve regeneration by modulating macrophage phenotype. Eur. J. Neurosci 45, 1258–1267. [DOI] [PubMed] [Google Scholar]
- Lyu C, Lyu GW, Martinez A, Shi TS 2017. Effect of nerve injury on the number of dorsal root ganglion neurons and autotomy behavior in adult Bax-deficient mice. J. Pain Res 10, 2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TC, Willis DE 2015. What makes a RAG regeneration associated? Front. Mol. Neurosci 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M, Cihak J, Simonis C, Luckow B, Proudfoot AE, Plachy J, Bruhl H, Frink M, Anders HJ, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D 2001. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol 166, 4697–4704. [DOI] [PubMed] [Google Scholar]
- Mahar M, Cavalli V 2018. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci 19, 323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka I, Meyer M, Thoenen H 1991. Cell-type-specific regulation of nerve growth factor (NGF) synthesis in non-neuronal cells: comparison of Schwann cells with other cell types. J. Neurosci 11, 3165–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MR, Nelson VH 1975. Detachment of structurally intact nerve endings from chromatolytic neurones of rat superior cervical ganglion during the depression of synaptic transmission induced by post-ganglionic axotomy. J. Physiol 245, 91–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MR, Raisman G 1972. A light and electron microscopic study of the cellular response to axonal injury in the superior cervical ganglion of the rat. Proc. R. Soc. Lond. B. Biol. Sci 181, 43–79. [DOI] [PubMed] [Google Scholar]
- Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, Lohmeyer J 2002. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am. J. Respir. Crit. Care Med 166, 268–273. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG 1978. The effect of a conditioning lesion on the regeneration of motor axons. Brain Res 152, 597–602. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B 1973. Axon outgrowth enhanced by a previous nerve injury. Arch. Neurol 29, 53–55. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B, Dreyfus CF, Gershon MD 1978. Regeneration of adrenergic axons in rat sciatic nerve: effect of a conditioning lesion. Brain Res 141, 21–34. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B, Gershon MD 1977. Axonal regeneration in the rat sciatic nerve: effect of a conditioning lesion and of dbcAMP. Brain Res 132, 443–453. [DOI] [PubMed] [Google Scholar]
- Mildner A, Marinkovic G, Jung S 2016. Murine monocytes: Origins, subsets, fates, and functions. Microbiology spectrum 4. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164, 6166–6173.10843666 [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV 2012. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33, 8793–8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco S, Gehrmann J, Raivich G, Kreutzberg GW 1992. MHC-positive, ramified macrophages in the normal and injured rat peripheral nervous system. J. Neurocytol 21, 623–634. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T 2002. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care 8, 101–105. [DOI] [PubMed] [Google Scholar]
- Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R 2003. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab. Invest 83, 175–185. [DOI] [PubMed] [Google Scholar]
- Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, Kiefer R 2001. Rapid response of identified resident endoneurial macrophages to nerve injury. Am. J. Pathol 159, 2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Leonhard C, Krauthausen M, Wacker K, Kiefer R 2010. On the longevity of resident endoneurial macrophages in the peripheral nervous system: a study of physiological macrophage turnover in bone marrow chimeric mice. J. Peripher. Nerv. Syst 15, 357–365. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Grondin J, Altares M, Richardson PM 1995. Induction of interleukin-6 in axotomized sensory neurons. J. Neurosci 15, 5130–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ 2017. Macrophage polarization. Annu. Rev. Physiol 79, 541–566. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA,Vogel SN, Wynn TA 2014. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S, Filali M, Zhang J, Kerr BJ, Rivest S, Soulet D, Iwakura Y, de Rivero Vaccari JP, Keane RW, Lacroix S 2011. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1beta and TNF: implications for neuropathic pain. J. Neurosci 31, 12533–12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y, Ando M, Takahama K, Iwata M, Hori S, Kato K 1987. Retrograde transport of endogenous nerve growth factor in superior cervical ganglion of adult rats. J. Neurochem 49, 296–302. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ 2007. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med 204, 3037–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito M, Umeda S, Takahashi K, Shultz LD 1997. Macrophage differentiation and granulomatous inflammation in osteopetrotic mice (op/op) defective in the production of CSF-1. Mol. Reprod. Dev 46, 85–91. [DOI] [PubMed] [Google Scholar]
- Navarro X, Kennedy WR 1990. The effect of a conditioning lesion on sudomotor axon regeneration. Brain Res 509, 232–236. [DOI] [PubMed] [Google Scholar]
- Neukomm LJ, Burdett TC, Seeds AM, Hampel S, Coutinho-Budd JC, Farley JE, Wong J, Karadeniz YB, Osterloh JM, Sheehan AE, Freeman MR 2017. Axon death pathways converge on axundead to promote functional and structural axon disassembly. Neuron 95, 78–91 e75. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Sanes JR, Lichtman JW 2002. Pre-existing pathways promote precise projection patterns. Nat. Neurosci 5, 861–867. [DOI] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Cregg JM, Howarth M, Zigmond RE 2016. Overexpression of the monocyte chemokine CCL2 in dorsal root ganglion neurons causes a conditioning-like increase in neurite outgrowth and does so via a STAT3 dependent mechanism. Exp. Neurol 275 Pt 1, 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE 2013. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci 33, 1623616248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JP, Filous AR, DeFrancesco A, Lindborg JA, Malhotra NA, Wilson GN, Zhou B, Crish SD, Zigmond RE 2017. Injury-induced gp130 cytokine signaling in peripheral ganglia is reduced in diabetes mellitus. Exp. Neurol 296, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nja A, Purves D 1978. The effects of nerve growth factor and its antiserum on synapses in the superior cervical ganglion of the guinea-pig. J. Physiol 277, 53–75. [PMC free article] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ 1984. A conditioning lesion of the peripheral axons of dorsal root ganglion cells accelerates regeneration of only their peripheral axons. J. Neurosci 4, 1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfors A 1980. Macrophages in peripheral nerves. An ultrastructural and enzyme histochemical study on rats. Acta Neuropathol 49, 43–49. [DOI] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH Jr., Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, Freeman MR 2012. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337, 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthi S, Gautam K 2017. Roles of nitric oxide and ethyl pyruvate after peripheral nerve injury. Inflammation and regeneration 37, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin FE, Lacroix S, Aviles-Trigueros M, David S 2005. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain 128, 854–866. [DOI] [PubMed] [Google Scholar]
- Perry VH (1994) Macrophages and the Nervous System R. G. Landes: Austin, TX. [Google Scholar]
- Perry VH, Brown MC, Gordon S 1987. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J. Exp. Med 165, 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Lunn ER, Tree P, Gordon S 1990. Evidence that very slow Wallerian degeneration in C57BL/Ola mice is an intrinsic property of the peripheral nerve. Eur. J. Neurosci 2, 802–808. [DOI] [PubMed] [Google Scholar]
- Perry VH, O’Connor V 2010. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro 2, e00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Tsao JW, Fearn S, Brown MC 1995. Radiation-induced reductions in macrophage recruitment have only slight effects on myelin degeneration in sectioned peripheral nerves of mice. Eur. J. Neurosci 7, 271–280. [DOI] [PubMed] [Google Scholar]
- Pilar G, Landmesser L 1972. Axotomy mimicked by localized colchicine application. Science 177, 1116–1118. [DOI] [PubMed] [Google Scholar]
- Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ 2003. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am. J. Pathol 163, 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunet W, Kwon BK, Tetzlaff W 2002. Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J. Neurosci. Res 68, 1–6. [DOI] [PubMed] [Google Scholar]
- Power CA, Meyer A, Nemeth K, Bacon KB, Hoogewerf AJ, Proudfoot AE, Wells TN 1995. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J. Biol. Chem 270, 19495–19500. [DOI] [PubMed] [Google Scholar]
- Purves D 1975. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J. Physiol 252, 429–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Nja A 1976. Effect of nerve growth factor on synaptic depression after axotomy. Nature 260, 535–536. [DOI] [PubMed] [Google Scholar]
- Purves D, Thompson W 1979. The effects of post-ganglionic axotomy on selective synaptic connexions in the superior cervical ganglion of the guinea-pig. J. Physiol 297, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Filbin MT 2000. Glial inhibition of nerve regeneration in the mature mammalian CNS. Glia 29, 166–174. [PubMed] [Google Scholar]
- Ramon y Cajal S (1928) Degeneration and Regeneration of the Nervous System Oxford University Press: New York. [Google Scholar]
- Ransohoff RM 2009. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity 31, 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Liu L, Cardona AE 2007. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int. Rev. Neurobiol 82, 187–204. [DOI] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RE, Brulet P, Landis SC 1993. Leukemia inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron 11, 1175–1185. [DOI] [PubMed] [Google Scholar]
- Rollins BJ 1997. Chemokines. Blood 90, 909–928. [PubMed] [Google Scholar]
- Rőszer T 2015. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015, 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S 2011. Wallerian degeneration: the innate-immune response to traumatic nerve injury. J. Neuroinflammation 8, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheib J, Hoke A 2013. Advances in peripheral nerve regeneration. Nat. Rev. Neurol 9, 668–676. [DOI] [PubMed] [Google Scholar]
- Scheidt P, Friede RL 1987. Myelin phagocytosis in Wallerian degeneration. Properties of millipore diffusion chambers and immunohistochemical identification of cell populations. Acta Neuropathol 75, 77–84. [DOI] [PubMed] [Google Scholar]
- Schiefer J, Kampe K, Dodt HU, Zieglgansberger W, Kreutzberg GW 1999. Microglial motility in the rat facial nucleus following peripheral axotomy. J. Neurocytol 28, 439–453. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Krivacic K, Kirby B, Vaccariello SA, Wei T, Ransohoff RM, Zigmond RE 2001. Monocyte chemoattractant protein (MCP)-1 is rapidly expressed by sympathetic ganglion neurons following axonal injury. Neuroreport 12, 601–606. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Shadiack AM, Bennett TA, Sedwick CE, Zigmond RE 1995. Changes in the macrophage population of the rat superior cervical ganglion after postganglionic nerve injury. J. Neurobiol 27, 141–153. [DOI] [PubMed] [Google Scholar]
- Schreiber RC, Vaccariello SA, Boeshore K, Shadiack AM, Zigmond RE 2002. A comparison of the changes in the non-neuronal cell populations of the superior cervical ganglia following decentralization and axotomy. J. Neurobiol 53, 68–79. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH 1993. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J. Neurobiol 24, 959–970. [DOI] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F 2012. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90. [DOI] [PubMed] [Google Scholar]
- Schwab ME 1996. Molecules inhibiting neurite growth: a minireview. Neurochem. Res 21, 755–761. [DOI] [PubMed] [Google Scholar]
- Senger JB, Verge VMK, Chan KM, Webber CA 2018. The nerve conditioning lesion - a strategy to enhance nerve regeneration. Ann. Neurol 83, 691–702. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ 2003. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain. Behav. Immun 17, 393–402. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Sun Y, Zigmond RE 2001. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J. Neurosci 21, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadiack AM, Vaccariello SA, Sun Y, Zigmond RE 1998. Nerve growth factor inhibits sympathetic neurons’ response to an injury cytokine. Proc. Natl. Acad. Sci. U. S. A 95, 7727–7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Chu F, Lang Y, Geng Y, Zheng X, Zhu J, Liu K 2018. Beneficial or harmful role of macrophages in Guillain-Barre Syndrome and experimental autoimmune neuritis. Mediators Inflamm 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker SE, Sachs HH, Vaccariello SA, Zigmond RE 2005. A conditioning lesion enhances sympathetic neurite outgrowth. Exp. Neurol 194, 432–443. [DOI] [PubMed] [Google Scholar]
- Shoemaker SE, Sachs HH, Vaccariello SA, Zigmond RE 2006. Reduction in nerve growth factor availability leads to a conditioning lesion-like effect in sympathetic neurons. J. Neurobiol 66, 1322–1337. [DOI] [PubMed] [Google Scholar]
- Shokouhi BN, Wong BZ, Siddiqui S, Lieberman AR, Campbell G, Tohyama K, Anderson PN 2010. Microglial responses around intrinsic CNS neurons are correlated with axonal regeneration. BMC Neurosci 11, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W 2000. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J. Neuroimmunol 110, 177–185. [DOI] [PubMed] [Google Scholar]
- Siqueira Mietto B, Kroner A, Girolami EI, Santos-Nogueira E, Zhang J, David S 2015. Role of IL-10 in resolution of inflammation and functional recovery after peripheral nerve injury. J. Neurosci 35, 16431–16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Skene JH 1997. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci 17, 646–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME 1999. Phagocytosis of myelin in demyelinative disease: a review. Neurochem. Res 24, 261–268. [DOI] [PubMed] [Google Scholar]
- Smith RP, Lerch-Haner JK, Pardinas JR, Buchser WJ, Bixby JL, Lemmon VP 2011. Transcriptional profiling of intrinsic PNS factors in the postnatal mouse. Mol. Cell. Neurosci 46, 32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliven B 2012. Autoimmune neuropathies: insights from animal models. J. Peripher. Nerv. Syst 17 Suppl 2, 28–33. [DOI] [PubMed] [Google Scholar]
- Soliven B 2014. Animal models of autoimmune neuropathy. ILAR journal 54, 282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam FJ, MacGillavry HD, Armstrong NJ, de Gunst MC, Zhang Y, van Kesteren RE, Smit AB, Verhaagen J 2007. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur. J. Neurosci 25, 3629–3637. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W 2005. Minocycline as a neuroprotective agent. Neuroscientist 11, 308–322. [DOI] [PubMed] [Google Scholar]
- Stoll G, Griffin JW, Li CY, Trapp BD 1989. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J. Neurocytol 18, 671–683. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M 2002. Detrimental and beneficial effects of injuryinduced inflammation and cytokine expression in the nervous system. Adv. Exp. Med. Biol 513, 87–113. [DOI] [PubMed] [Google Scholar]
- Subang MC, Richardson PM 2001. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur. J. Neurosci 13, 521–528. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH 2000. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur. J. Neurosci 12, 457–466. [DOI] [PubMed] [Google Scholar]
- Sun Y, Rao MS, Zigmond RE, Landis SC 1994. Regulation of vasoactive intestinal peptide expression in sympathetic neurons in culture and after axotomy: the role of cholinergic differentiation factor/leukemia inhibitory factor. J. Neurobiol 25, 415–430. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE 1996a. Involvement of leukemia inhibitory factor in the increases in galanin and vasoactive intestinal peptide mRNA and the decreases in neuropeptide Y and tyrosine hydroxylase mRNA in sympathetic neurons after axotomy. J. Neurochem 67, 1751–1760. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zigmond RE 1996b. Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur. J. Neurosci 8, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Svensson M, Mattsson P, Aldskogius H 1994. A bromodeoxyuridine labelling study of proliferating cells in the brainstem following hypoglossal nerve transection. J. Anat 185 ( Pt 3), 537–542. [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Nasu M, Kanazawa T, Takahashi M, Shimazu Y 2017. Chemokine ligand 2/chemokine receptor 2 signaling in the trigeminal ganglia contributes to inflammatory hyperalgesia in rats. Neurosci. Res 128, 25–32. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M 2004. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci. Res 48, 463–469. [DOI] [PubMed] [Google Scholar]
- Taskinen HS, Roytta M 1997. The dynamics of macrophage recruitment after nerve transection. Acta Neuropathol 93, 252–259. [DOI] [PubMed] [Google Scholar]
- Taskinen HS, Roytta M 2000. Increased expression of chemokines (MCP-1, MIP1alpha, RANTES) after peripheral nerve transection. J. Peripher. Nerv. Syst 5, 75–81. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, Thompson SW, Marchand F, McMahon SB 2009. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur. J. Pain 13, 263–272. [DOI] [PubMed] [Google Scholar]
- Thoenen H, Bandtlow C, Heumann R, Lindholm D, Meyer M, Rohrer H 1988. Nerve growth factor: cellular localization and regulation of synthesis. Cell. Mol. Neurobiol 8, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews AD, Barrett C, Morell P 1998. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J. Neurosci. Res 53, 260–267. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Patterson PH, Jessen KR, Mirsky R 2002. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J. Neurosci 22, 6696–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson JE, Žygelytė E, Grenier JK, Edwards MG, Cheetham J 2018. Temporal changes in macrophage phenotype after peripheral nerve injury. J. Neuroinflammation 15, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton BH, Chen Q, Gaina G, Tucureanu C, Georgescu A, Strungaru C, Flonta ML, Sah D, Ristoiu V 2013. Activation profile of dorsal root ganglia Iba-1 (+) macrophages varies with the type of lesion in rats. Acta Histochem 115, 840–850. [DOI] [PubMed] [Google Scholar]
- Turkiew E, Falconer D, Reed N, Hoke A 2017. Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. J. Peripher. Nerv. Syst 22, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uceyler N, Tscharke A, Sommer C 2007. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain. Behav. Immun 21, 553–560. [DOI] [PubMed] [Google Scholar]
- Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M, Hoeksema MA, de Vos AF, de Winther MP 2016. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep 17, 684–696. [DOI] [PubMed] [Google Scholar]
- van Rooijen N 1989. The liposome-mediated macrophage ‘suicide’ technique. J. Immunol. Methods 124, 1–6. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Kors N, vd Ende M, Dijkstra CD 1990. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res 260, 215–222. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, van Nieuwmegen R 1984. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res 238, 355–358. [DOI] [PubMed] [Google Scholar]
- Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B, Mauborgne A, Dansereau MA, Kitabgi P, Sarret P, Pohl M, Melik Parsadaniantz S 2011. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J. Neurosci 31, 5865–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas ME, Barres BA 2007. Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci 30, 153–179. [DOI] [PubMed] [Google Scholar]
- Varol C, Mildner A, Jung S 2015. Macrophages: development and tissue specialization. Annu. Rev. Immunol 33, 643–675. [DOI] [PubMed] [Google Scholar]
- Vass K, Hickey WF, Schmidt RE, Lassmann H 1993. Bone marrow-derived elements in the peripheral nervous system. An immunohistochemical and ultrastructural investigation in chimeric rats. Lab. Invest 69, 275–282. [PubMed] [Google Scholar]
- Vega-Avelaira D, Geranton SM, Fitzgerald M 2009. Differential regulation of immune responses and macrophage/neuron interactions in the dorsal root ganglion in young and adult rats following nerve injury. Mol. Pain 5, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereyken EJ, Heijnen PD, Baron W, de Vries EH, Dijkstra CD, Teunissen CE 2011. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. J. Neuroinflammation 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD 2013. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflammation 10, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriesendorp FJ, Flynn RE, Malone MR, Pappolla MA 1998. Systemic complement depletion reduces inflammation and demyelination in adoptive transfer experimental allergic neuritis. Acta Neuropathol 95, 297–301. [DOI] [PubMed] [Google Scholar]
- Vriesendorp FJ, Flynn RE, Pappolla MA, Koski CL 1995. Complement depletion affects demyelination and inflammation in experimental allergic neuritis. J. Neuroimmunol 58, 157–165. [DOI] [PubMed] [Google Scholar]
- Vriesendorp FJ, Flynn RE, Pappolla MA, Koski CL 1997. Soluble complement receptor 1 (sCR1) is not as effective as cobra venom factor in the treatment of experimental allergic neuritis. Int. J. Neurosci 92, 287–298. [DOI] [PubMed] [Google Scholar]
- Waller AV 1850. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations of the alternatives produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. B Biol. Sci 140, 423–429. [Google Scholar]
- Wang Q, Zhang S, Liu T, Wang H, Liu K, Wang Q, Zeng W 2018. Sarm1/Myd88–5 Regulates Neuronal Intrinsic Immune Response to Traumatic Axonal Injuries. Cell reports 23, 716–724. [DOI] [PubMed] [Google Scholar]
- Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, Guo L, Bai P, Sun D, Fan J, He X, Young W, Ren Y 2015. Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia 63, 635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson WE 1974. Cellular responses to axotomy and to related procedures. Br. Med. Bull 30, 112–115. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ 2005. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc. Natl. Acad. Sci. U. S. A 102, 14092–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodham P, Anderson PN, Nadim W, Turmaine M 1989. Satellite cells surrounding axotomised rat dorsal root ganglion cells increase expression of a GFAP-like protein. Neurosci. Lett 98, 8–12. [DOI] [PubMed] [Google Scholar]
- Wu SC, Rau CS, Lu TH, Wu CJ, Wu YC, Tzeng SL, Chen YC, Hsieh CH 2013. Knockout of TLR4 and TLR2 impair the nerve regeneration by delayed demyelination but not remyelination. J. Biomed. Sci 20, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM 2014. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med 211, 1533–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ydens E, Cauwels A, Asselbergh B, Goethals S, Peeraer L, Lornet G, AlmeidaSouza L, Van Ginderachter JA, Timmerman V, Janssens S 2012. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J. Neuroinflammation 9, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, Zack DJ, Benowitz LI 2009. Oncomodulin links inflammation to optic nerve regeneration. Proc. Natl. Acad. Sci. U. S. A 106, 19587–19592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S, Zhou H, Wang X, Busuttil RW, Kupiec-Weglinski JW, Zhai Y 2017. Prolonged ischemia triggers necrotic depletion of tissue-resident macrophages to facilitate inflammatory immune activation in liver ischemia reperfusion injury. J. Immunol 198, 3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, Dougherty PM 2016. Dorsal root ganglion infiltration by macrophages contributes to Paclitaxel chemotherapy-induced peripheral neuropathy. J. Pain 17, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Schluesener HJ 2009. Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J. Immunol 183, 3081–3091. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Deneris E, Zigmond RE 1998. Differential regulation of levels of nicotinic receptor subunit transcripts in adult sympathetic neurons after axotomy. J. Neurobiol 34, 164–178. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Deneris E, Zigmond RE 2001. Nicotinic acetylcholine receptor subunit proteins alpha7 and beta4 decrease in the superior cervical ganglion after axotomy. J. Neurobiol 46, 178–192. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bao L, Zhu S, Chen Z, van der Meide P, Nennesmo I, Winblad B, Ljunggren HG, Zhu J 2002. CD4 and CD8 T cells, but not B cells, are critical to the control of murine experimental autoimmune neuritis. Exp. Neurol 177, 314–320. [DOI] [PubMed] [Google Scholar]
- Zigmond RE 1997. LIF, NGF, and the cell body response to axotomy. Neuroscientist 3, 176–185. [Google Scholar]
- Zigmond RE 2001. Can galanin also be considered as growth-associated protein 3.2? Trends Neurosci 24, 494–496. [DOI] [PubMed] [Google Scholar]
- Zigmond RE 2012. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front. Mol. Neurosci 4, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Ben-Ari Y 1977. Electrical stimulation of preganglionic nerve increases tyrosine hydroxylase activity in sympathetic ganglia. Proc. Natl. Acad. Sci. U. S. A 74, 3078–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]