Dear editor,
Several types of tumors affect adolescents. Despite being rare, the Ewing sarcoma family of tumors represents the second most common primary bone malignancy in children and adolescents. Paraneoplastic Cushing syndrome is a well-known condition characterized by an ectopic secretion of ACTH (or CRH), and in turn of cortisol by tumor cells. Paraneoplastic Cushing syndrome is however very rare in Ewing sarcoma, and to our knowledge, it was only described once.
Diagnosis, treatment and adherence to treatment may be especially difficult in children and adolescents. Here we describe a case of severe paraneoplastic Cushing syndrome in a young adult with Ewing sarcoma. It was associated with psychosis and complicated by hemodynamic instability due to the unexpectedly rapid apparition of iatrogenic adrenal insufficiency.
CASE
A 20-year-old man with metastatic Ewing sarcoma was admitted to our university hospital for acute psychosis. He had been diagnosed with sarcoma of the 8th costal rib at the age of 13 and treated with surgery and chemotherapy (EURO-EWING 99 protocol with vincristine, actinomycine and ifosfamide). Five years later, he relapsed with pulmonary and costal lesions. The sarcoma progressed despite new surgery (resection by excision of the 8th and 9th left ribs), radiotherapy and two lines of chemotherapy (temodal-irinotecan and ifosfamide-etoposide).
Two years after this relapse he developed truncal obesity, facial and leg edema associated with hypokaliemia (2.7 mmol/L). A paraneoplastic Cushing syndrome was diagnosed by elevated basal cortisol (1,366 nmol/L, normal 170–675 nmol/L), urinary cortisol excretion (487 nmol/L, normal 9.5–111 nmol/L) and ACTH (106 nmol/L, normal 10–60 ng/L) levels. A cerebral MRI excluded a pituitary adenoma. Metyrapone and oral potassium were initiated, as well as pentamidine aerosols for Pneumocystis prophylaxyis due to a low CD4 count (86 cells/mm3).
Upon admission (day 1), four months after the diagnosis of paraneoplastic Cushing syndrome, the patient complained of asthenia and anasarca. He reported an occasional cough, exertional dyspnea and an episode of hemoptysia. His family was particularly worried about increasing episodes of agitation and confusion. On clinical examination we measured blood pressure of 188/124 mmHg, heart rate of 109/minute, oxygen saturation of 93%, and temperature of 36.4 °C. We noticed agitation and confusion without any focal neurologic defect, left basis crackles upon pulmonary auscultation, as well as typical morphological changes compatible with a severe form of Cushing disease (Figure 1). Blood analyses showed that sodium 146 mmol/L, potassium 2.7 mmol/L, calcium 2.06 mmol/L, glucose 7.5 mmol/L, CRP 1 mg/L, hemoglobin 104 g/L, leukocytes 18.1 g/L, and thrombocytes 354 g/L. Basal cortisol was markedly elevated at 3,396 nmol/L. Thoracic computed tomography excluded a pulmonary embolism but displayed bilateral ground-glass opacities suspicious for an infectious process. We added ketoconazole (300 mg, tid) to metyrapone (5 grams daily). Parenteral potassium and antibiotherapy with ceftriaxone were started.
Figure 1.
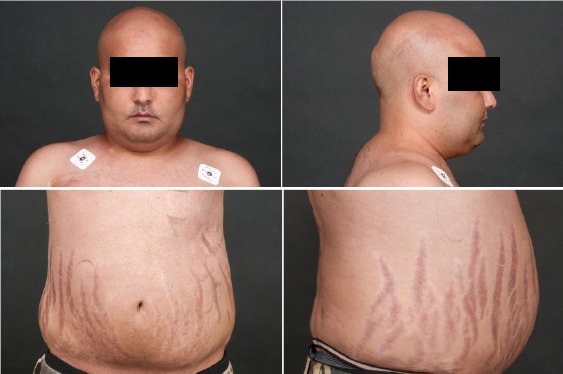
Morphological changes caused by Cushing syndrome.
On day 3 the patient presented a decreased level of consciousness (Glasgow Coma Scale 13/15, confused response, eye opening upon pain stimulation only), a fever of 38.2 °C, a respiratory rate of 35 breaths/minute, an oxygen saturation of 88%, a heart rate of 110 beats/minute, and a blood pressure of 106/60 mmHg. His only complaint was diffuse myalgia. Clinical examination revealed symmetric reactive pupils and no meningism. Blood analyses showed mild respiratory alcalosis (pH 7.45, PO2 97 mmHg, PCO2 36 mmHg, base excess 1.6, lactic acid 0.9 mmol/L), CRP 56 mg/L, leukocytes 17 g/L, hemoglobin 101 g/L, sodium 147 mmol/L, potassium 3.2 mmol/L, glucose 5.4 mmol/L. Given the rapid deterioration, we suspected pulmonary sepsis: large-spectrum antibiotherapy with piperacilin-tazobactam, clarithromycin and trimethoprim-sulfamethoxazol (to cover Pneumocystis jirovecci) was initiated. Cerebral computed tomography excluded cerebral metastases, a hemorrhage or a profound abscess. A lumbar puncture was not contributive.
On day 4, due to persistent lethargy and hypotension, basal plasma cortisol was reassessed and measured at 120 nmol/L. Both metyrapone and ketokonazole were stopped and hydrocortisone was administered, with rapid reversibility of symptoms and reappearance of agitation. All microbiology samples were negative during follow-up, which argued against a septic shock. We therefore concluded that the patient’s rapid deterioration was caused by an iatrogenic adrenal insufficiency.
The patient was able to leave hospital a few days after stabilization of the cortisolemia with metyrapone 1,000 mg 3 times a day. One month later, due to progression of the sarcoma metastases, regorafenib (an oral tyrosin kinase receptor inhibitor) was initiated for compassionate use as a fourth line of chemotherapy. Unfortunately, the patient’s condition gradually deteriorated so the regorafenib was stopped. Thereafter, the patient displayed a flare-up of the disease and died 3 months later of fulminant hepatic insufficiency due to new liver metastases.
DISCUSSION
Paraneoplastic Cushing’s disease is a well-known condition characterized by an ectopic secretion of ACTH (or CRH), and in turn of cortisol by tumor cells, most often by pulmonary carcinomas or carcinoid tumors.[1,2] This complication has been described only once in individuals suffering of Ewing sarcoma.[3] Treatment includes management of the primary tumor and/or inhibition of cortisol synthesis. Metyrapone and ketoconazole are two inhibitors of distinct enzymes involved in adrenal steroidogenesis and thereby reduce cortisol production synergically. Biologic adrenal insufficiency during monitoring of paraneoplastic Cushing syndrome has been reported after 1 to 4 weeks of this combined treatment, but without any associated clinical repercussion.[4]
This case highlights an unexpectedly rapid and potentially life-threatening effect of this treatment despite the chronicity and severity of the ectopic cortisol synthesis.
Symptoms compatible with a possible sepsis delayed our correct diagnosis. Retrospectively a more precise interpretation of the evolution of the serum potassium level (inversely correlated with both cortisolemia and neuropsychological symptoms) could have helped us obtain a more rapid and correct diagnosis and limit the number of additional diagnostic procedures.
In our case, the surprising rapidity and severity of adrenal insufficiency was also possibly related to an initial overestimation of the patient’s adherence to his long-term metyrapone treatment. This induced us to start directly with a high dose of ketoconazole. Indeed, treatment adherence can be complicated during long-term follow-up of cancer,[5] especially in children and adolescents. Moreover, neuropsychological manifestations of Cushing syndrome can further reduce it.
CONCLUSION
Adrenal insufficiency is a potentially fatal condition that can occur even in patients suffering from a severe form of paraneoplastic Cushing syndrome. It should be considered in the differential diagnosis of patients treated for Cushing syndrome who develop hemodynamic instability and electrolytic imbalance. The treatment is simple and the number of additional diagnostic procedures should be limited. When treatment with metyrapone and/or ketokonazole is initiated or increased, the monitoring of clinical (hemodynamic, state of vigilance) and paraclinical (serum cortisol and potassium levels) factors is very important, even more so in chronically ill adolescents. These procedures are cheap and simple and can be life-saving.
Footnotes
Funding: None.
Ethical approval: Not needed.
Conflicts of interest: No any benefits have been received from a commercial party related directly or indirectly to the study.
Contributors: CGD led the design of the study and data collection, and drafted the manuscript. All authors contributed to the interpretation of the results and the critical revision of the manuscript.
REFERENCES
- 1.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9):838–54. doi: 10.4065/mcp.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015;386(9996):913–27. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 3.Preeyasombat C, Sirikulchayanonta V, Mahachokelertwattana P, Sriphrapradang A, Boonpucknavig S. Cushing's syndrome caused by Ewing's sarcoma secreting corticotropin releasing factor-like peptide. Am J Dis Child. 1992;146(9):1103–5. doi: 10.1001/archpedi.1992.02160210105034. [DOI] [PubMed] [Google Scholar]
- 4.Corcuff JB, Young J, Masquefa-Giraud P, Chanson P, Baudin E, Tabarin A. Rapid control of severe neoplastic hypercortisolism with metyrapone and ketoconazole. Eur J Endocrinol. 2015;172(4):473–81. doi: 10.1530/EJE-14-0913. [DOI] [PubMed] [Google Scholar]
- 5.Kondryn HJ, Edmondson CL, Hill J, Eden TOB. Treatment non-adherence in teenage and young adult patients with cancer. Lancet Oncol. 2011;12(1):100–8. doi: 10.1016/S1470-2045(10)70069-3. [DOI] [PubMed] [Google Scholar]


