Renal cell carcinoma (RCC) is the most common malignancy of the kidney, with clear cell (ccRCC) being the subtype identified in 85% of the cases.9 After nephrectomy, 20% of these patients will experience recurrence or metastatic disease. Bone is the second most common site of distant metastatic spread, with an incidence of 35%.17 Bone metastasis (BM) is usually an aggressive lytic process related to significant morbidity and deterioration of life quality through skeletal-related events (SREs).11 SREs are defined as a pathologic fracture, surgical intervention, and requirement for palliative radiotherapy, spinal cord compression, or hypercalcemia.
The ulna is a rare site of BM because the axial skeleton is predominantly affected. Zekri et al,18 in a recent study of 103 patients with advanced RCC and metastatic bone disease, reported that the pelvis and ribs were involved in 48% of the patients, followed by the spine in 42% and the long bones and skull coming next. BM from RCC is associated with a poor prognosis, with a mean survival rate of 12 months. In some studies, however, long survival in such cases is not a rare event.12
We present a case of a 50-year-old man with an 8-year history of ccRCC who presented with a metastatic lesion of his right proximal ulna and was successfully managed with autogenous, nonvascularized fibula graft transfer after complete excision of the tumor. To our knowledge, this is the second case in the literature where a free fibular graft was used for ulnohumeral reconstruction after tumor resection at the proximal ulna.
Case report
A 50-year-old man with a history of renal cancer was admitted to our department complaining of a painful swelling around his right elbow for 3 weeks, with no history of trauma. The patient reported onset of moderate pain to his right elbow about 7 months ago, for which he had received consultation from an orthopedic surgeon in another center. Plain radiographs did not reveal any osseous pathology at that stage, and oral nonsteroidal anti-inflammatory drugs and activity modification was recommended. Pain persisted, though, and had increased during the last 4 months, being minimal at night or having other manifestations.
The patient underwent a complete left kidney nephrectomy 8 years earlier because of a lower pole neoplasm, which was diagnosed as ccRCC on the biopsy specimen. He failed to attend any follow-up meetings and had received no supplementary therapy or other consultation since then.
Our clinical examination revealed a painful swelling of the ulnar side of the right elbow, with severe restriction of motion and slight varus deformity with no neurovascular compromise. X-ray imaging showed a lytic lesion involving the entire proximal ulna, with a soap bubble appearance and destruction of the articular surface (Fig. 1). The patient underwent a complete diagnostic imaging workup, including computed tomography (CT) staging protocol (brain-chest-abdomen), 3-phase bone scintigraphy, and magnetic resonance imaging of the right elbow. No other possible metastatic lesions were identified. The magnetic resonance imaging scan demonstrated an osteolytic lesion of the proximal ulna, measuring 8.8 × 5.4 × 5.3 cm in size, involving the surrounding soft tissues but without breaching nearby radial cortex.
Figure 1.
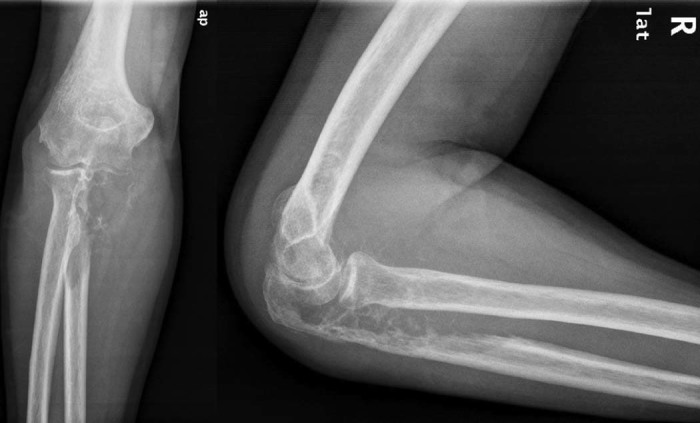
(Left) Anteroposterior (ap) and (right) lateral (lat) x-ray images of the right elbow show an osteolytic lesion of the proximal ulna involving the entire ulnohumeral articulation with soap bubble appearance.
The patient underwent an open biopsy, and the specimen confirmed the typical morphologic and immunohistochemistry characteristics of ccRCC metastatic disease. Because this was proved to be a solitary osseous metastatic lesion, the aim of treatment was to provide a tumor-free, “functional” elbow. After careful consideration and discussion with the patient, the decision was to perform en bloc excision of the affected proximal portion of the ulna and reconstruct the ulnohumeral articulation using a nonvascularized, autogenous fibular graft. This technique has rarely been reported in the literature in cases of oncologic procedures involving the proximal ulna.
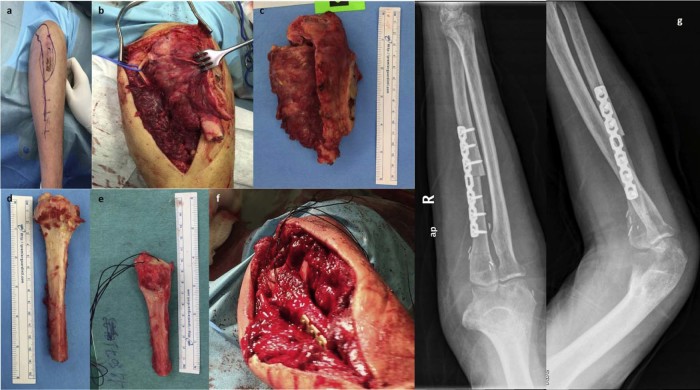
Under general anesthesia, the patient was placed supine in a radiolucent side table, and the tumor was exposed through a posterior approach including the site of the previous biopsy (Fig. 2, a). The ulnar nerve was identified and protected. During a careful microsurgical dissection, approximately 12.5 cm of the proximal ulna, including the tumor, were excised en bloc with all muscle insertions and surrounding soft tissues (Fig. 2, b and c).
Figure 2.
Intraoperative photographs: (a) the posterior approach used, including the site of the previous biopsy, has been drawn on the arm, (b and c) excision of the tumor using microsurgical technique with protection of the ulnar nerve (arrow), (d and e) the autogenous free fibular graft before and after its preparation with transosseous sutures for triceps reattachment and proper trimming to accommodate the exact length of the bone defect, and (f) the graft is shown in place. (g) Postoperative (left) anteroposterior (ap) and (right) lateral (lat) x-ray images show the reconstruction.
An autologous, nonvascularized fibular graft was used to reconstruct the bone defect and the ulnohumeral articulation. A 14-cm proximal fibular graft was harvested from the ipsilateral leg, and a small oscillating saw was used to shape the fibula to match the olecranon articular surface. In particular, the medial and lateral sides of the fibular head were trimmed to create a triangular part in line with the diaphysis. The tibial facet of the fibula was preserved, and the anterior surface was also deepened to create a shallow socket (Fig. 2, d and e).
Transosseous sutures were passed through the tip of the fibular head for reattachment of the triceps tendon, and the graft was further trimmed at its distal part to accommodate the exact length of the bone deficit. The distal part of the fibular graft was then aligned to the remaining ulnar stump, and standard compression osteosynthesis was performed using a 7-hole dynamic compression plate. (Fig. 2, f and g). The stability of the elbow joint after triceps reattachment and ulna fixation was good. Because the tumor was excised en block, no ligamentous attachments were preserved for a more stable fixation of the proximal part; thus, the patient was immobilized postoperatively in a full arm cast for 4 weeks. An elbow brace was applied thereafter for another 4 weeks, and passive assisted motion was initiated. The patient also received chemotherapy for 1 year, as suggested by the oncologists.
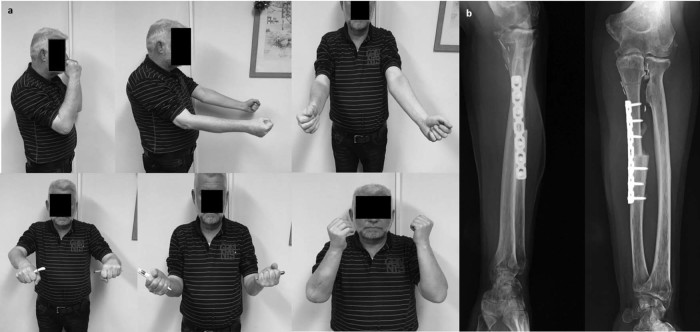
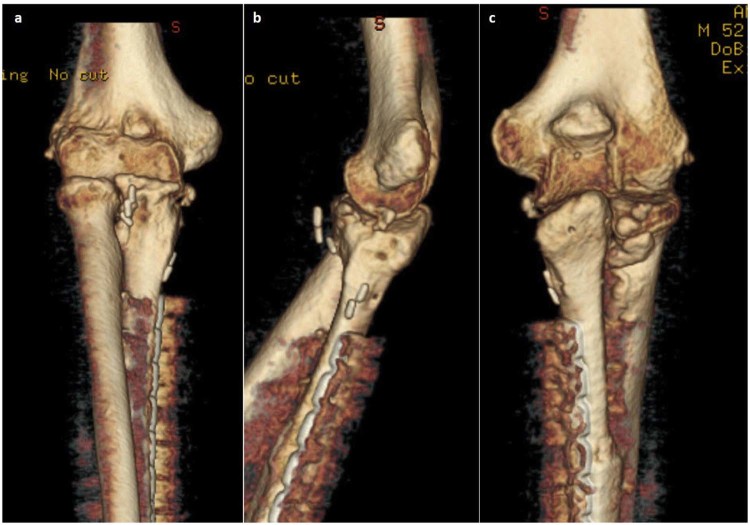
The patient had no problems from the harvest site, and at his last follow-up examination at 25 months postoperatively, he was almost pain free, having a flexion/extension range at the elbow of 20° to 110°, supination of 30°, pronation of 40°, and a solid bony union in the x-ray images (Fig. 3). A 3-dimensional CT scan of the elbow obtained together with his formal follow-up chest CT showed a congruent joint without any evidence of recurrence (Fig. 4). His Mayo Elbow Score was 75 points, analyzed as follows: mild pain, 30 of 45; motion, 15 of 20 (50°-100° arc); stability, 5 of 10 (moderate); and stability function of the elbow, 25 of 25 (5 of 5 common tasks completed). He was returned back to his normal activities of daily living and previous occupation.
Figure 3.
(a) Photographs from the last follow-up clinical evaluation show active range of flexion/extension and pronation/supination at the elbow joint. (b) Follow-up anteroposterior and lateral x-ray images of the elbow show solid union of the graft and no evidence of recurrence.
Figure 4.
Computed tomography 3-dimensional reconstruction images of the elbow 25 months postoperatively in (a) anterior, (b) lateral, (c) posterior views show good congruency of the joint and no evidence of recurrence.
Discussion
The management of large osseous defects after tumor excisions represents a difficult task for the orthopedic surgeon, especially in cases of complete resection of an articular surface. Elbow joint reconstruction after proximal ulna excision has been performed in various ways, without a clear consensus over the superiority of one treatment option over another.
Endoprosthetic elbow reconstruction has been attempted in several tumor cases of the distal humerus but has been scarcely reported in the treatment of proximal ulna oncologic procedures.6, 14, 16 This method allows for early mobilization and immediate commencement of adjuvant chemotherapy but has numerous complications, such as infection, stem loosening, and periprosthetic fracture, that can compromise long-term outcome. Except for the demanding surgical technique, this method is not well established in the setting of large proximal ulna defects.10 Sewell et al13 reported 4 patients (mean age, 17.5 years) with proximal ulna tumors treated with wide excision and total elbow arthroplasty, achieving good functional result and elbow stability. However, 1 patient underwent transhumeral amputation 1 month postoperatively due to local recurrence, and another required radial head excision 6 months postoperatively due to fixed flexion deformity.
Radius neck-to-humerus trochlea transposition has been proposed as another method of elbow reconstruction after proximal ulna excision but with somewhat unsatisfactory functional results in forearm rotation and muscle strength. Duncan et al3 reported acceptable functional outcomes in 2 patients treated with this technique, achieving a elbow range of motion (ROM) between 20° and 130° in 1 patient and between 20° and 140° in the other but with poor forearm rotation. Chen et al1 performed the same operation in an 80-year-old man with metastatic disease of the proximal ulna and reported decreased elbow ROM (10°-90°) and slightly impaired forearm supination and pronation, without any additional information.
Elbow allografts have been also used as an alternative to biologic reconstruction but have been implicated to a high risk of infection and joint degeneration along with other complications, including fracture, instability, and nonunion.10 Dean et al2 reported 23 patients treated with allograft reconstruction after massive bone loss at the elbow. Complications occurred in 16 patients (70%), prompting the authors to recommend this operation for salvage purposes only.
Free fibular grafting is an established technique to restore long osseous defects but has been rarely used in joint reconstruction.4, 8, 10 Vascularized fibula autografts have the potential of remodeling and hypertrophy under mechanical load and may behave better in union and growth, especially in the young immature skeleton. Kimura8 reported excellent functional result in an 8-year-old boy with Ewing sarcoma at 4 years of follow-up. Active elbow ROM was 5° to 150o, pronation and supination were unrestricted, and there was no deformity of the growing upper extremity.
Reconstruction of the proximal ulna after wide tumor resection using a nonvascularized fibular autograft has been reported in only 3 patients.5, 7, 15 Goyal et al5 treated a 15-year-old child with desmoplastic fibroma of the ulna, preserving the proximal half of the olecranon for firm fixation to the graft. At 2 years of follow-up, the active ROM at the elbow was 40° to 130°. Similarly, Wang et al15 performed a subtotal resection of the proximal ulna and subsequent reconstruction of the proximal portion of the intraosseous membrane with a hernia mesh in a 29-year-old patient with Ewing sarcoma. They reported an excellent functional outcome, with 0° to 135° elbow ROM and forearm pronation of 35° and supination of 85°at 2 years of follow-up. The preservation of the proximal olecranon process and normal triceps insertion in both previous patients gave not only the chance for better internal fixation of the graft but also conserved some of the joint's integrity.
The proximal ulna in our patient was totally resected, and the triceps tendon had to be reinserted to the fibular graft to preserve the extensor apparatus of the elbow. There is only 1 report in the literature where a nonvascularized fibula autograft was used to reconstruct the ulnohumeral articulation after complete excision of the proximal ulna. Kalaiah et al7 treated a 30-year-old patient with giant cell tumor of the proximal ulna using this method, covering also the fibular head with tensor fascia lata to act as an articular surface. They reported functional and stable elbow at 2 years of follow-up but did not provide any specific details on elbow ROM or forearm rotation. In the present patient, no additional ligamentous or other soft tissue repair was performed except from triceps tendon reinsertion in the preshaped fibular head.
Conclusion
Nonvascularized, fibula autograft is an excellent source for ulnohumeral joint reconstruction after complete resection of the proximal ulna in tumor cases. Achieving the correct graft length and shape to provide adequate tension for the triceps tendon provides a suitable articular surface for the humeral trochlea and can restore a functional ROM and adequate stability at the elbow joint.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional Review Board approval was not required for this case report. The patient gave informed consent for his case to be published.
References
- 1.Chen F., Xia J., Wei Y., Wang S., Wu J., Huang G. Radius neck-to-humerus trochlea transposition elbow reconstruction after proximal ulnar metastatic tumor resection: case and literature review. Eur J Med Res. 2012;17:23. doi: 10.1186/2047-783X-17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean G.S., Holliger E.H., Urbaniak J.R. Elbow allograft for reconstruction of the elbow with massive bone loss. Long term results. Clin Orthop Relat Res. 1997;341:12–22. [PubMed] [Google Scholar]
- 3.Duncan S.F., Athanasian E.A., Healey J.H. Radius neck–to–humerus trochlea transposition for elbow reconstruction after resection of the proximal ulna: report of 2 cases. J Hand Surg Am. 2008;33:1384–1387. doi: 10.1016/j.jhsa.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Gianoutsos M.P., Marsden F.W., McCarthy S.W., Lee K.K. Ulnar adamantinoma: en bloc excision and fibular osteoseptocutaneous free flap reconstruction. J Hand Surg Am. 1994;19:495–499. doi: 10.1016/0363-5023(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 5.Goyal T., Rastogi S., Tripathy S. Desmoplastic fibroma of ulna: excision and reconstruction of olecranon with a fibular graft. Indian J Orthop. 2013;47:207. doi: 10.4103/0019-5413.108928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo W., Tang S., Yang R., Ji T. [Total elbow arthroplasty after resection of tumors at the elbow] Chinese Journal of Surgery. 2008;46:1734–1737. doi: 10.3321/j.issn:0529-5815.2008.22.017. [DOI] [PubMed] [Google Scholar]
- 7.Kalaiah K., Thejaswi S.G., Siddappa M. Reconstruction of elbow by free fibular graft in a case of osteoclastoma of proximal ulna: a rare case report. Case Rep Med. 2015;2015:1–3. doi: 10.1155/2015/429309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura K., Tatezaki S., Ishii T., Yonemoto T., Shigehara T., Takenouchi T. Hemiarthroplasty of the elbow with a vascularized fibular graft after excision of Ewing's sarcoma of the proximal ulna: a case report. Jpn J Clin Oncol. 2002;32:430–434. doi: 10.1093/jjco/hyf088. [DOI] [PubMed] [Google Scholar]
- 9.Murai M., Oya M. Renal cell carcinoma: etiology, incidence and epidemiology. Curr Opin Urol. 2004;14:229–233. doi: 10.1097/01.mou.0000135078.04721.f5. [DOI] [PubMed] [Google Scholar]
- 10.Ogose A., Hotta T., Shibata M., Kawashima H., Endo N. Combined use of free vascularised fibula graft and extracorporeally irradiated osteochondral graft for osteosarcoma of the proximal ulna. Oncol Lett. 2010;1:133–135. doi: 10.3892/ol_00000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahi C., Knox J.J., Clemons M., Joshua A.M., Broom R. Renal cell carcinoma bone metastases: clinical advances. Ther Adv Med Oncol. 2010;2:75–83. doi: 10.1177/1758834009358417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoni M., Conti A., Procopio G., Porta C., Ibrahim T., Barni S. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J Exp Clin Cancer Res. 2015;34:10. doi: 10.1186/s13046-015-0122-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sewell M.D., Hanna S.A., Pollock R.C., Aston W.J., Skinner J.A., Blunn G.W. Proximal ulna endoprosthetic replacement for bone tumours in young patients. Int Orthop. 2012;36:1039–1044. doi: 10.1007/s00264-012-1483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling J.W., Pritchard D.J., Morrey B.F. Total elbow arthroplasty after resection of tumors at the elbow. Clin Orthop Relat Res. 1999;367:256–261. [PubMed] [Google Scholar]
- 15.Wang C., Lin N. Ewing's sarcoma of the ulna treated with sub-total resection and reconstruction using a non-vascularized, autogenous fibular graft and hernia mesh: a case report. Oncol Lett. 2015;10:2067–2070. doi: 10.3892/ol.2015.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber K.L., Lin P.P., Yasko A.W. Complex segmental elbow reconstruction after tumor resection. Clin Orthop Relat Res. 2003;415:31–44. doi: 10.1097/01.blo.0000093894.12372.53. [DOI] [PubMed] [Google Scholar]
- 17.Woodward E., Jagdev S., McParland L., Clark K., Gregory W., Newsham A. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48:160–166. doi: 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Zekri J., Ahmed N., Coleman R.E., Hancock B.W. The skeletal metastatic complications of renal cell carcinoma. Int J Oncol. 2001;19:379–382. doi: 10.3892/ijo.19.2.379. [DOI] [PubMed] [Google Scholar]