Abstract
Despite the potentially life-threatening consequences of hyperkalemia, symptoms are often absent or mild. However, when hyperkalemia has been recognized, evaluation of vital signs is essential for determining hemodynamic stability and identifying the presence of cardiac arrhythmias related to the hyperkalemia. Quite commonly, and depending on the severity and rapidity of onset, hyperkalemia may be associated with substantial electrocardiographic (EKG) changes that can lead to death if proper interventions are not instituted. Through its effects on the resting membrane potential and threshold potential of excitable cells, hyperkalemia is a potentially life-threatening disorder. Symptoms and physical examination findings are often absent. Once identified, the entire clinical picture must be taken into account, including an assessment of hemodynamic stability, the presence of other electrolyte abnormalities, and an EKG evaluation. While there is a typical progression of EKG findings based on hyperkalemia severity, EKG manifestations are myriad and their evolution may be unpredictable.
Keywords: EKG, electrophysiology, hemodynamic stability, hyperkalemia, potassium
Hyperkalemia can be classified according to serum potassium (K+) levels as mild (5.5–6.5 mEq/l), moderate (6.5–7.5 mEq/l), and severe (>7.5 mEq/l). However, the severity of clinical manifestations depends not only on the serum K+ levels but also on the rapidity of onset, the presence of concomitant electrolyte abnormalities, medications, and other comorbidities.
Despite the potentially life-threatening consequences of hyperkalemia, symptoms are often absent or mild. When present, the symptoms associated with hyperkalemia are nonspecific, and include muscle pain or tightness, paresthesias, weakness, nausea, vomiting, and palpitations. Findings on physical examination are often absent or nonspecific. Vital signs are generally normal; however, bradycardia resulting from heart block or tachypnea resulting from respiratory muscle weakness may be present. Occasionally, cardiac examination may reveal extrasystoles, pauses, or bradycardia. Skeletal muscle weakness and flaccid paralysis may be present, along with depressed or absent deep tendon reflexes. Muscle tenderness may accompany muscle weakness, suggesting rhabdomyolysis. Patients with ileus may have hypoactive or absent bowel sounds. Symptoms and the physical examination may not alert the physician to the diagnosis. However, when hyperkalemia has been recognized, evaluation of vital signs is essential for determining hemodynamic stability and identifying the presence of cardiac arrhythmias related to the hyperkalemia.
EKG changes associated with hyperkalemia
Quite commonly, and depending on the severity and rapidity of onset, hyperkalemia may be associated with substantial EKG changes that can lead to death if proper interventions are not instituted. Due to the potentially life-threatening nature of hyperkalemia, careful screening in high-risk populations is paramount.
The earliest EKG manifestation associated with “mild” hyperkalemia (serum K+ = 5.5–6.5 mEq/l) may include tall, peaked, narrow-based T waves in precordial (V2–V4) leads and fascicular blocks (left anterior and left posterior fascicular blocks).1, 2 Moderate hyperkalemia (serum K+ between 6.5 and 7.5 mEq/l) may be associated with first-degree atrioventricular block, decreased P-wave amplitude followed by disappearance of the P waves, and sinus arrest. ST segment depression and sometimes ST segment elevation simulating an acute myocardial infarction have also been described.3 Severe hyperkalemia (serum K+ >7.5 mEq/l) is manifested by atypical bundle branch block, intraventricular conduction delay, ventricular tachycardia, ventricular fibrillation, idioventricular rhythm, the “sine wave,” and asystole.4 Occasionally, severe hyperkalemia may mimic a pattern of Brugada syndrome, which disappears promptly with the correction of hyperkalemia.5 Several other EKG alterations may occur, including atrioventricular arrhythmias, pacemaker dysfunction, rate-dependent bundle branch block, atrioventricular block with junctional rhythm, and pseudonormalization of inverted T waves.6, 7 Patients with pacemakers may have widening of the paced P wave and paced QRS complex.8 The effects of hyperkalemia on the heart vary depending on the tissue involved. The atrial myocardial cells are more sensitive to the effects of hyperkalemia than ventricular cells are, followed by (in decreasing order) cells in the sinoatrial node, His bundle, and interatrial pathways. The sympathetic nervous system seems to play a role in the sinus node resistance to hyperkalemia.9
Besides the serum K+ level, the severity of EKG manifestations depends on the coexistence of other electrolyte abnormalities (concentrations of calcium, magnesium, sodium, or chloride), acid–base abnormalities, body temperature, tonicity of the extracellular fluids, and comorbidities such as cardiomyopathy and the administration of digoxin or ouabain.10, 11, 12, 13, 14, 15, 16 The concomitant use of other medications may also be of importance. Mild hyperkalemia, for instance, has been associated with junctional bradycardia in patients on verapamil.17 Although typical EKG manifestations of hyperkalemia are more likely in the presence of severe hyperkalemia (Figure 1), it is important to note that the characteristic EKG changes described are not always present, even in patients with severe hyperkalemia.18, 19, 20 It is imperative when treating hyperkalemia that the whole clinical picture is taken into account, rather than just the numerical potassium values. Further, EKG changes have low sensitivity in identifying individuals with hyperkalemia or predicting severity.21, 22 Among patients with end-stage renal disease, a population at particularly high risk for hyperkalemia, differences in calcium levels may contribute to the predictive value of the EKG to detect hyperkalemia.18, 20 Older age and the presence of diabetes have also been associated with a lower likelihood of hyperkalemia-induced peaked T waves.23
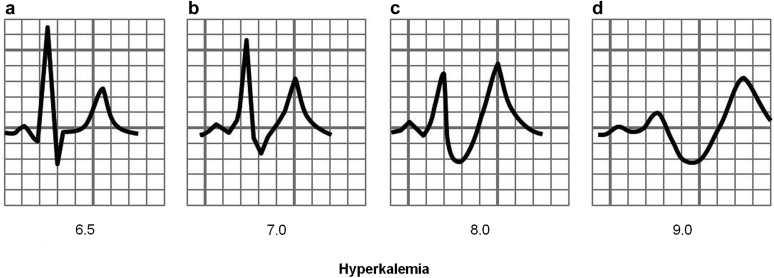
Figure 1.
Typical progression of hyperkalemia induced electrocardiogram abnormalities. The 4 panels depict (a) peaked T waves; (b,c) progressive P-wave flattening and QRS lengthening; and (d) a sine wave, a potentially terminal arrhythmia.
Factitious hyperkalemia and clinical conditions that confound diagnosis
One major limitation in the evaluation of patients with hyperkalemia is that serum levels of K+ are usually measured when the patients are fasting. Obviously, this does not take into account changes in serum K+ that can occur after ingestion of food. Normally, during feeding, even large amounts of K+ can be rapidly sequestered thanks to the action of insulin and catecholamines on cells in early stages, and to the action of aldosterone on renal tubular K+ excretion in later stages. K+ excretion is also subject to a circadian rhythm, suggesting that the timing of K+ intake and subsequent excretion is also of importance.24 However, patients who do not produce insulin or who may have reduced production of aldosterone—the “hypoaldosteronism syndrome,” particularly common among diabetic patients—could experience rapid rises in serum K+ to very dangerous levels. An additional clinical consequence of hyperkalemia includes decreased renal production of ammonium, and the presence of metabolic acidosis has been associated with prolonged duration of hyperkalemia.25 This is usually not monitored by physicians.
Neurologic derangements related to hyperkalemia
Neurologic derangements related to hyperkalemia, which may include muscle weakness and paralysis, are less frequently seen. Paralysis related to high serum K+ levels may be a recurrent phenomenon in patients with familial periodic paralysis, or may be a sporadic finding in patients with severe hyperkalemia. In a review of 119 patients reported in the literature with neurologic manifestations thought to be related to hyperkalemia, 56 (47.1%) manifested flaccid tetraparesis, 38 (31.9%) manifested ascending flaccid paralysis, 25 (21%) displayed muscular weakness, 26 (21.8%) had paresthesia or dysesthesia, 22 (18.5%) had difficulty breathing, 12 (10.1%) had lethargy, 7 (6.0%) had sensory loss, and 6 (5.1%) had dysphagia or difficulty in mastication.26 Acute quadriplegia as a manifestation of hyperkalemia has been reported.27
Electrophysiological consequences of hyperkalemia
The clinical manifestations of hyperkalemia result from substantial alterations of the excitability and conduction velocity of all excitable cells. Potassium concentration is much greater inside ([K+]i) than outside ([K+]o) the cells, and this gradient is the most important determinant and regulator of the resting membrane potential (RMP). The Nernst equation28 predicts the effects of changes in K+ gradient on RMP (referred to as Vm):
A decrease in extracellular concentration of K+ results in reduction in the conductance of the inward potassium rectifier current (Iki) and membrane permeability to K+, resulting in hyperpolarization of the RMP (RMP becomes more negative).29 Hypokalemia also results in reduction of the delayed rectifier current, which explains why the duration of the action potential is shorter at low [K+] and longer at higher [K+] concentrations.30 By contrast, hyperkalemia enhances the conductance of Iki and membrane permeability to K+, resulting in depolarization of cell membranes.
Varying levels of hyperkalemia exert different effects on the RMP and the threshold potential (TP). This concept is well illustrated in the experiments outlined in Table 1. Mild to moderate levels of hyperkalemia decrease the RMP (making it less negative) more than the TP, resulting in a reduced difference between the two and an increase in excitability and conduction velocity. Severe hyperkalemia is associated with an increase in the difference between the RMP and the TP, leading to a decrease in excitability and conduction velocity. The effects of K+ on RMP depend not only on its extracellular versus intracellular concentration, but also on the rapidity of change.8
Table 1.
Effect of extracellular potassium concentration ([K+]o) on resting membrane potential (RMP), threshold potential (TP), and maximum velocity (Vmax) in ventricular muscle fibers of guinea pigs
| [K+]o (mM) |
RMP (mV) |
TP (mV) |
RMP – TP (mV) |
Vmax (V/s) |
|---|---|---|---|---|
| 2.0 | 99.4 | 72.7 | 26.7 | 236 |
| 5.4 | 83.0 | 65.1 | 17.9 | 219 |
| 10.0 | 65.8 | 51.4 | 14.4 | 178 |
| 11.5 | 62.8 | 45.6 | 17.2 | 154 |
| 13.0 | 60.7 | 41.9 | 18.8 | 103 |
| 16.2 | 55.4 | 34.7 | 20.7 | 45 |
Adapted with permission from Kishida H, Surawicz B, Fu LT. Effects of K+ and K+-induced polarization on (dV/dt)max, threshold potential, and membrane input resistance in guinea pig and cat ventricular myocardium. Circ Res. 1979;44:800–814.31
Conclusion
Through its effects on the RMP and TP of excitable cells, hyperkalemia is a potentially life-threatening disorder. Symptoms and physical examination findings are often absent. Once identified, the entire clinical picture must be taken into account, including an assessment of hemodynamic stability, the presence of other electrolyte abnormalities, and an EKG evaluation. While there is a typical progression of EKG findings based on hyperkalemia severity, EKG manifestations are myriad and their evolution may be unpredictable.
Disclosure
Publication of this article was supported by Relypsa, Inc. All the authors declared no competing interests.
References
- 1.Diercks D.B., Shumaik G.M., Harrigan R.A. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med. 2004;27:153–160. doi: 10.1016/j.jemermed.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Chew H.C., Lim S.H. Electrocardiographical case. A tale of tall T's. Hyperkalaemia. Singapore Med J. 2005;46:429–432. [PubMed] [Google Scholar]
- 3.Pothiawala S.E. Hyperkalemia induced pseudo-myocardial infarction in septic shock. J Postgrad Med. 2014;60:338–340. doi: 10.4103/0022-3859.138828. [DOI] [PubMed] [Google Scholar]
- 4.Slovis C., Jenkins R. ABC of clinical electrocardiography: conditions not primarily affecting the heart. BMJ. 2002;324:1320–1323. doi: 10.1136/bmj.324.7349.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recasens L., Merono O., Ribas N. Hyperkalemia mimicking a pattern of Brugada syndrome. Rev Esp Cardiol. 2013;66:309. doi: 10.1016/j.rec.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Manohar N., Young M.L. Rate dependent bundle branch block induced by hyperkalemia. Pacing Clin Electrophysiol. 2003;26:1909–1910. doi: 10.1046/j.1460-9592.2003.00291.x. [DOI] [PubMed] [Google Scholar]
- 7.Yu A.S. Atypical electrocardiographic changes in severe hyperkalemia. Am J Cardiol. 1996;77:906–908. doi: 10.1016/s0002-9149(97)89197-7. [DOI] [PubMed] [Google Scholar]
- 8.El-Sherif N., Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18:233–245. [PubMed] [Google Scholar]
- 9.Vassalle M., Greineder J.K., Stuckey J.H. Role of the sympathetic nervous system in the sinus node resistance to high potassium. Circ Res. 1973;32:348–355. doi: 10.1161/01.res.32.3.348. [DOI] [PubMed] [Google Scholar]
- 10.Latorre R., Oberhauser A., Labarca P. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- 11.Hodgkin A.L., Katz B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milionis H.J., Alexandrides G.E., Liberopoulos E.N. Hypomagnesemia and concurrent acid-base and electrolyte abnormalities in patients with congestive heart failure. Eur J Heart Fail. 2002;4:167–173. doi: 10.1016/s1388-9842(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff U., Vogel W., Safronov B.V. Na+-activated K+ channels in small dorsal root ganglion neurones of rat. J Physiol. 1998;510:743–754. doi: 10.1111/j.1469-7793.1998.743bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hume J.R., Harvey R.D. Chloride conductance pathways in heart. J Physiol. 1991;261:C399–C412. doi: 10.1152/ajpcell.1991.261.3.C399. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W., Takeuchi H., Kurono M. Ouabain-sensitive K(+)-dependent outward current caused by threo-beta-hydroxy-L-glutamic acid on a snail neuron. Gen Pharmacol. 1997;29:625–632. doi: 10.1016/s0306-3623(96)00313-8. [DOI] [PubMed] [Google Scholar]
- 16.Fraser J.A., Wong K.Y., Usher-Smith J.A. Membrane potentials in Rana temporaria muscle fibres in strongly hypertonic solutions. J Muscle Res Cell Motil. 2006;27:591–606. doi: 10.1007/s10974-006-9091-4. [DOI] [PubMed] [Google Scholar]
- 17.Hegazi M.O., Aldabie G., Al-Mutairi S. Junctional bradycardia with verapamil in renal failure—care required even with mild hyperkalaemia. J Clin Pharm Ther. 2012;37:726–728. doi: 10.1111/j.1365-2710.2012.01352.x. [DOI] [PubMed] [Google Scholar]
- 18.Szerlip H.M., Weiss J., Singer I. Profound hyperkalemia without electrocardiographic manifestations. Am J Kidney Dis. 1986;7:461–465. doi: 10.1016/s0272-6386(86)80185-8. [DOI] [PubMed] [Google Scholar]
- 19.Montague B.T., Ouellette J.R., Buller G.K. Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol. 2008;3:324–330. doi: 10.2215/CJN.04611007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslam S., Friedman E.A., Ifudu O. Electrocardiography is unreliable in detecting potentially lethal hyperkalaemia in haemodialysis patients. Nephrol Dial Transplant. 2002;17:1639–1642. doi: 10.1093/ndt/17.9.1639. [DOI] [PubMed] [Google Scholar]
- 21.Acker C.G., Johnson J.P., Palevsky P.M. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med. 1998;158:917–924. doi: 10.1001/archinte.158.8.917. [DOI] [PubMed] [Google Scholar]
- 22.Wrenn K.D., Slovis C.M., Slovis B.S. The ability of physicians to predict hyperkalemia from the ECG. Ann Emerg Med. 1991;20:1229–1232. doi: 10.1016/s0196-0644(05)81476-3. [DOI] [PubMed] [Google Scholar]
- 23.Green D., Green H.D., New D.I., Kalra P.A. The clinical significance of hyperkalaemia-associated repolarization abnormalities in end-stage renal disease. Nephrol Dial Transplant. 2013;28:99–105. doi: 10.1093/ndt/gfs129. [DOI] [PubMed] [Google Scholar]
- 24.Gumz M.L., Rabinowitz L., Wingo C.S. An integrated view of potassium homeostasis. N Engl J Med. 2015;373:60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanagavi J., Gupta T., Aronow W.S. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10:251–257. doi: 10.5114/aoms.2014.42577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanson G., Russo S., Iudicello A. Tetraparesis and failure of pacemaker capture induced by severe hyperkalemia: case report and systematic review of available literature. J Emerg Med. 2015;48:555–561.e3. doi: 10.1016/j.jemermed.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Gil D., Reina-Lora V., Bravo-Monge R. [Acute quadriparesis as a form of presentation of a severe hyperkalemia] Rev Clin Esp. 2012;212:512. doi: 10.1016/j.rce.2012.06.008. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 28.Sheu S.S., Korth M., Lathrop D.A. Intra- and extracellular K+ and Na+ activities and resting membrane potential in sheep cardiac purkinje strands. Circ Res. 1980;47:692–700. doi: 10.1161/01.res.47.5.692. [DOI] [PubMed] [Google Scholar]
- 29.Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang T., Roden D.M. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93:407–411. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]
- 31.Kishida H., Surawicz B., Fu L.T. Effects of K+ and K+-induced polarization on (dV/dt)max, threshold potential, and membrane input resistance in guinea pig and cat ventricular myocardium. Circ Res. 1979;44:800–814. doi: 10.1161/01.res.44.6.800. [DOI] [PubMed] [Google Scholar]