Abstract
Hyperkalemia is associated with life-threatening cardiac arrhythmias and increased mortality. Hyperkalemia is most often observed in patients with chronic kidney disease and/or in those with congestive heart failure being treated with drugs that limit renal potassium excretion, especially drugs that inhibit the renin-angiotensin-aldosterone system. Treatment of hyperkalemia may be either acute, as needed during rapid changes in serum potassium, which are associated with cardiac arrhythmia, or chronic, which stabilizes serum potassium levels and limits the development of life-threatening arrhythmias. There are a number of both acute and chronic treatments available for the treatment of hyperkalemia, but some are limited by complex administration requirements and/or serious side effects. Hyperkalemia remains a vexing problem for clinicians, particularly in the care of patients with chronic kidney disease and cardiovascular disease.
Keywords: chronic kidney disease, congestive heart failure, hyperkalemia, patiromer, RAAS inhibitors, ZS-9
Hyperkalemia is a common electrolyte disorder with numerous etiologies. Potassium is primarily an intracellular ion whose absorption and secretion occurs in distinct areas of the nephron and gastrointestinal tract.1, 2 The kidneys are the most important site of excretion.2, 3 However, with progressive impairment of kidney function, colonic excretion assumes increasing importance.1, 2, 3 Consequently, patients with chronic kidney disease (CKD) or cardiovascular disease who often are receiving drugs that block the renin-angiotensin-aldosterone system (RAAS) are at greatest risk for hyperkalemia.4, 5, 6, 7 The reader is referred to the in-depth review by Epstein in the fourth article of this supplement.8 A 5-year database of the prevalence of hyperkalemia illustrated that as CKD progresses from stage 3A to 3B to 4, the frequency of hyperkalemia defined as ≥5.1 mEq/l increased from 23.5% to 33% to 47.7%, respectively.9 Moreover, clinical and epidemiological data link higher levels of serum potassium (above 5.1 mEq/l) with increased risk of death. Given the frequency and significance of hyperkalemia in patients with CKD and cardiovascular disease,10, 11, 12, 13, 14 it is common for health care providers to underdose or to not use these drugs at all, if there is even a threat of hyperkalemia.
Increases in serum potassium of 0.4 to 0.5 mEq/l are common when a RAAS blocker is used. However, increases can be much more substantial with the concomitant use of nonsteroidal anti-inflammatory drugs, salt substitutes, or nutritional supplements, or if large amounts of fruits containing potassium—such as cantaloupe or watermelon—are consumed.1, 2 Thus, there is an important need for a well-tolerated and simple means of controlling serum potassium in a safe range, even in patients with CKD or cardiovascular disease who require the therapeutic advantage of drugs that block the RAAS.
Acute hyperkalemia
There are 3 different strategies to treat acute elevations in serum potassium leading to cardiac cell membrane instability and potentially lethal arrhythmias.1, 5, 9, 12, 15, 16, 17
The first of these strategies is stabilization of membrane potentials by raising the threshold of the cardiac action potential or altering (flattening) the slope of membrane depolarization as the main physiological considerations.18, 19, 20, 21, 22, 23 Calcium gluconate salts, when acutely administered, can raise serum calcium levels, which elevate the threshold for the cardiac action potential for a brief period and allow time for other measures to be implemented. This is critical because higher extracellular potassium levels facilitate more rapid membrane depolarization.24, 25
Next, the physician can facilitate potassium redistribution into cells by administering either glucose and insulin or beta-agonists. However, the latter approach can be problematic, especially in people with cardiovascular disease.
Finally, potassium elimination can be facilitated through the use of loop diuretics to enhance kaliuresis. The physician can also administer sodium bicarbonate with loop diuretics to alkalinize the urine and facilitate kaliuresis through the provision of more sodium to exchange distally with potassium. Potassium elimination via the gastrointestinal tract can also be facilitated with the use of potassium-binding resins, such as sodium polystyrene sulfate (SPS) (Kayexalate; Covis Pharmaceuticals, Cary, NC). In patients with advanced kidney disease, acute hemodialysis may be required to acutely reduce serum potassium.26, 27
Chronic treatment strategies
Reduction of dietary potassium is an important strategy for the chronic management of hyperkalemia. However, there is incomplete knowledge of how best to avoid dietary potassium. For example, a cup of low-fat milk has nearly 11 mEq of potassium, and 1 cup of plain yogurt has almost 14 mEq of potassium. Likewise, vegetables or fruit such as raisins, watermelon, avocado, grapefruit, or cantaloupe are rich in potassium, but other fruits such as blueberries, grapes, or pineapple are quite low. Many vegetables, including squash, spinach, and brussels sprouts, are very rich in potassium, whereas lettuce and beans are much lower. Dietary education is a necessary and critical feature of the chronic management of hyperkalemia.1, 28
Sodium bicarbonate tablets may occasionally be used in hyperkalemia patients with severe acidemia. If utilized, it should be administered with loop diuretics on a chronic basis to control serum potassium. However, this may require multiple pills and numerous medication adjustments, as it is often difficult to give salt to patients with CKD or cardiovascular disease without exacerbating their volume status or blood pressure.
SPS was approved by the US Food and Drug Administration (FDA) in 1958.26, 27 This was 4 years before the requirement to prove safety and effectiveness of medications as noted in the 1962 Kefauver-Harris Drug Amendments. There is limited evidence that SPS increases fecal potassium losses in experimental animals or in humans and no evidence that adding sorbitol to the resin increases its effectiveness. SPS can be administered in doses of 50 to 60 g from 1 to 4 times per day. It is an irregular bulk gel material with sharp edges, nonuniform size, and a clay-like consistency. It is not well dissolved in water and is sodium-loaded; there are approximately 100 mg of sodium per gram of SPS.
SPS directly exchanges sodium for potassium and may be associated with increases in volume and pedal edema.27 In patients with CKD, it may be associated with increases in blood pressure. Because of the danger of acquisition of sodium in patients with severe congestive heart failure, the Heart Failure Society of America has issued a caution for the use of SPS in patients with heart failure. A systematic review of case reports noted that gastrointestinal necrosis has been reported as frequently in patients receiving SPS alone as in patients receiving SPS with sorbitol.27, 29, 30 Harel et al.29 recently identified 58 cases in 30 reports. Necrosis was reported in patients receiving SPS with (n = 41) and without (n = 17) sorbitol. Mortality due to gastrointestinal injury was reported in 33% of these cases. Consequently, SPS must be used very cautiously and sparingly, with close monitoring of volume and blood pressure.
In 2009, the FDA issued a warning noting that there were cases of colonic necrosis and other serious gastrointestinal events associated with the use of SPS.31, 32 The FDA also recommended against the use of sorbitol with SPS. The FDA also warned that only people with normal bowel function should use SPS and recommended strongly against its use in people with problems with constipation, inflammatory bowel disease, ischemic colitis, and intestinal vascular atherosclerosis.
In perspective, the chronic treatment strategies for hyperkalemia, short of discontinuing the RAAS blocker, are limited.28 The health care provider should advise patients about avoiding nonsteroidal anti-inflammatory drugs and herbal/nutritional supplements and should instruct patients on reduced dietary potassium, which, unfortunately, requires the elimination of many healthy foods. The health care provider can also adjust sodium bicarbonate tablets coupled with loop diuretics to facilitate kaliuresis. Finally, the health care provider can use SPS sparingly, preferably only in those individuals with good bowel function and no concerns about constipation.
Future treatments
Two new therapies will likely alter the landscape for the management of both acute and chronic hyperkalemia: patiromer (Veltassa; Relypsa, Redwood City, CA) and sodium zirconium cyclosilicate (ZS Pharma, San Mateo, CA).
The first agent, patiromer, was recently approved by the FDA in October 2015. The active moiety for oral suspension is a nonabsorbed polymer that binds potassium in exchange for calcium.30 It binds potassium throughout the gastrointestinal tract, but it is believed to act predominantly in the distal colon, where the concentration of free potassium is highest.30 The net result is an increase in secretion and reduction of serum potassium levels. Patiromer is stable and nonsystemically absorbed. It has been studied in a variety of populations, but primarily in people with CKD or cardiovascular disease.
The initial phase 3 trial by Weir et al.,33 was designed to examine patients with CKD on RAAS blockers with serum potassium levels between 5.1 and <6.5 mEq/l. A total of 243 patients were randomized to receive patiromer, either 4.2 or 8.4 g, twice daily for a period of 4 weeks. The second part of the study involved 107 patients whose baseline potassium was 5.5 to <6.5 mEq/l and in whom the potassium levels decreased to 3.8 to <5.1 mEq/l with patiromer in the initial part of the study. These patients were then entered into an 8-week randomized withdrawal phase in which they either continued on patiromer or switched to placebo. The primary endpoint of the first 4-week segment of the study was the change from baseline in mean serum potassium.
In the second part of the study encompassing the randomized withdrawal over 8 weeks, the primary planned endpoint was the change in serum potassium between the 2 groups at the 4-week point. As shown in Figure 1 during the initial 4 weeks of therapy, there was a statistically significant reduction in serum potassium in the overall group of about 1.0 mEq/l (P < 0.001). Not surprisingly, there was a greater reduction in serum potassium in the group with the higher baseline potassium level. In the withdrawal phase of the study there was a statistically significant difference in change in serum potassium of 0.72 mEq/l (P < 0.001) between the group that was switched to placebo versus the group that remained on patiromer at the 4-week point. Of those patients with serum potassium >5.5 mEq/l at baseline, over 90% were able to remain on their RAAS blocker; whereas of the patients who were switched to placebo, less than one-half were able to be maintained on the RAAS-blocking drug.
Figure 1.
Serum potassium levels during the 4-week initial treatment phase of a phase 3 trial of patients with chronic kidney disease and hyperkalemia receiving renin-angiotensin-aldosterone inhibitors (RAASi). All patients received treatment with patiromer depending on baseline hyperkalemia. Patients with mild hyperkalemia (serum potassium 5.1 to <5.5 mEq/l) received 4.2 g of patiromer twice daily, and those with moderate-to-severe hyperkalemia (serum potassium 5.5 to <6.5 mEq/l) received 8.4 g of patiromer twice daily. Values are the observed mean values as measured in a central laboratory. I bars indicated standard error. Data points are staggered to make them more legible.
From Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221.33 Copyright © 2015 Massachusetts Medical Society. Reproduced with permission from Massachusetts Medical Society.
It is of interest that more than one-half the study participants had diabetes, and it is striking to note that nearly one-half of the participants in the whole study had an estimated glomerular filtration rate below 30 ml/min/1.73 m2, and nearly 40% of the patients had a history of congestive heart failure. The mean age of all patients in the study was nearly 65 years; thus, the study population was a high-risk group that was successfully maintained on a RAAS blocker. Only about 50% of the patients were on chronic diuretic support with either loop diuretics or thiazide diuretics. Patiromer was well tolerated. In the initial treatment phase, constipation was reported in 11% of patients and was the only adverse event with a reported frequency >3%.
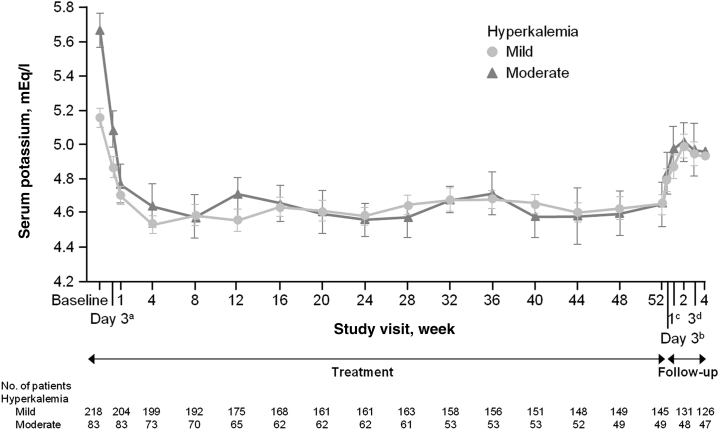
Patiromer has also been studied in a long-term clinical trial of 306 patients with type 2 diabetes and reduced glomerular filtration rate (about 40 ml/min/1.73 m2) on a RAAS blocker with a serum potassium over 5.0 mEq/l, but ≤5.5; these patients were randomized to receive 1 of 3 starting doses of patiromer: 4.2, 8.4, or 12.6 g twice a day.34 If the patient’s potassium level was >5.5 mEq/L, but <6.0,34 the range of starting doses was higher: 8.4, 12.6, or 16.8 g twice daily. The patiromer dose could be titrated to achieve and maintain a serum potassium of 5.0 mEq/l or lower. The study was designed to last for 52 weeks. The primary efficacy endpoint was mean change in serum potassium level from baseline to week 4 or prior to initiation of dose titration. The primary safety endpoint was adverse events that were collected through the 52 weeks of the clinical trial. Secondary efficacy endpoints included mean change in serum potassium level through 52 weeks. As shown in Figure 2, the mean change from baseline serum potassium demonstrates the sustained efficacy of patiromer. Note that the majority of patients were controlled to a level of 4.6 to 4.7 mEq/l with maintained stability over 52 weeks. From weeks 4 through 52, the statistically significant decreases in serum potassium levels were sustained and documented at each monthly point.
Figure 2.
Least-square means of serum potassium levels over 52 weeks of treatment with patiromer and during posttreatment in patients with chronic kidney disease on renin-angiotensin-aldosterone system blockers (n = 306). aAt treatment day 3, there were 202 patients with mild hyperkalemia and 82 with moderate hyperkalemia. bAt follow-up day 3, there were 163 patients with mild hyperkalemia and 58 with moderate hyperkalemia. cAt follow-up week 1, there were 154 patients with mild hyperkalemia and 57 with moderate hyperkalemia. dAt follow-up week 3, there were 126 patients with mild hyperkalemia and 48 with moderate hyperkalemia.
Reproduced with permission from Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161.34 Copyright © 2015 American Medical Association. All rights reserved.
It is interesting to note that during the course of this study there were significant reductions in mean systolic and diastolic blood pressure in all starting-dose groups in both the higher as well as the lower serum potassium strata. Although there is no direct pharmacological mechanism to explain the reduction of blood pressure, it could be related to the continued use of the RAAS-blocking therapy, better adherence to antihypertensive therapy during the course of the study, reduction in serum aldosterone, or the possibility that patiromer may be associated with sodium binding.
From a safety standpoint, hypomagnesemia was the most common treatment-related adverse event (7.2%). No patient developed severe hypomagnesemia (<1.0 mg/dl). No patient had cardiac arrhythmias or neuromuscular abnormalities that were temporally associated with hypomagnesemia. Mild-to-moderate constipation occurred in 6.3% of patients and hypokalemia <3.5 mEq/l occurred in 5.6% of patients. Worsening of CKD was the most frequently reported adverse event during the trial and the most common adverse event leading to study drug discontinuation. Most of these adverse events occurred during the long-term maintenance phase, suggesting that the progression of underlying CKD was the main contributory factor. Consistent with this observation was the finding that the proportion of patients with more severe CKD (stages 4–5) at baseline was higher among those who were discontinued from the study (32.3%) than among those who completed the study (19.9%).
Patiromer has also been studied carefully to evaluate its onset of action in patients with CKD receiving RAAS-blocking drugs.35 After a 3-day potassium- and sodium-restricted diet in an inpatient general clinical research unit, 25 patients with sustained hyperkalemia (5.5 to <6.4 mEq/l) received patiromer 8.4 g twice daily. Serum potassium was assessed at baseline, 4 hours, post dose, and every 2 to 4 hours up to 48 hours. The mean baseline serum potassium was 5.93 mEq/l for the whole group, and this was reduced significantly by the 7-hour point after the first dose (–0.21 mEq/l, P = 0.004) and at all subsequent assessments through 48 hours. At 48 hours (14 hours after the last dose), the mean reduction was –0.75 mEq/l (P < 0.001). Serum potassium did not increase prior to the next dose of patiromer, nor did it increase for 24 hours after the last dose of patiromer. Overall, patiromer was well tolerated, without serious adverse events and with no withdrawals.
In interpreting the current and future clinical trials of patiromer, ZS-9, and future potassium binders, greater focus should be devoted to the differences in experimental design. As detailed in an extensive review by Epstein and Lifschitz in the second article of this supplement,36 the occurrence and magnitude of dyskalemia may be determined in part by not only the classic and well-established “feedback control” of potassium, but also not widely recognized complementary regulatory paradigm—“the feed-forward control of potassium balance.” Consequently, as detailed in this review, the conditions of fasting or the fed state may influence both the timeline and magnitude of intracellular-extracellular potassium shifts and/or ensuing gut sensor–mediated kaliuresis. Of note, all the clinical trials of patiromer were conducted with patients in the fed state.
In summary, the available data with patiromer illustrate that patiromer is a well-tolerated, effective medication for controlling serum potassium in patients with CKD and/or cardiovascular disease who are concurrently receiving RAAS blockers. It has a quick onset of action and has sustained effects lasting through 52 weeks. Thus, a high proportion of patients can be maintained on their RAAS inhibitors while on this therapy without problems associated with hyperkalemia.
The other important investigational drug that is being reviewed by the FDA for the treatment of hyperkalemia is sodium zirconium cyclosilicate, also known as ZS-9.37 ZS-9 is a highly selective inorganic cation exchanger that entraps potassium in the intestinal tract in exchange for sodium and hydrogen. ZS-9 is a microporous zirconium silicate compound designed to be selective for potassium. It is insoluble, highly stable, and nonsystemically absorbed. It has 9.3 times more potassium-binding capacity than SPS and is more than 125 times more selective for potassium than SPS.
The initial large-scale trial to evaluate the use of ZS-9 was a multicenter, randomized, double-blind, placebo-controlled trial in which a total of 258 patients were dosed 3 times daily in an initial 48-hour, open-label phase.38 A total of 237 patients were randomized to once-daily ZS-9 in either a 5-, 10-, or 15-g dose, or to placebo daily for 28 days. In this trial, about 70% of the patients were on RAAS blockers, about two-thirds had diabetes, and about one-third had congestive heart failure. In the initial 48 hours, serum potassium levels declined from 5.6 mEq/l at baseline to 4.5 mEq/l at 48 hours. Median time to normalization was 2.2 hours, with 84% of patients achieving normokalemia by 24 hours and 98% by 48 hours. The proportion of patients with a mean serum potassium below 5.1 mEq/l during days 8 to 29 of the study was significantly higher in all ZS-9 groups compared with the placebo group.
Overall, adverse events were comparable between ZS-9 and placebo, although edema was more frequent in the 10-g ZS-9 cohort (5.9%) and 15-g ZS-9 group (14%) compared with the placebo group, which is probably attributable to the sodium as the cation exchanged for potassium. Hypokalemia developed in only 10% of patients in the 10-g and 15-g groups versus none in the 5-g or placebo group. Figure 3 shows the stability of control of the serum potassium during the randomized phase of the study (days 8–29).
Figure 3.
Serum potassium levels during the randomized phase (days 8–29) in patients with chronic kidney disease who received a placebo or zirconium cyclosilicate dose of 5 g, 10 g, or 15 g once daily (error bars depict 95% confidence interval).
Reproduced with permission from Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233.38 Copyright © 2014 American Medical Association. All rights reserved.
ZS-9 has also been studied in a large, multicenter trial involving 753 patients with hyperkalemia who were randomized either to receive ZS-9 at a dose of 1.25, 2.5, 5, or 10 g or to receive placebo; dosages were given 3 times daily for 48 hours.39 Patients with normokalemia (3.5 to 4.9 mEq/l) at 48 hours were randomized to remain on either ZS-9 or placebo once daily from days 3 to 14. The primary endpoint of the study was the exponential rate of change in the mean serum potassium level at 48 hours. The average baseline serum potassium was 5.3 mEq/l. For each of the active treatment groups, the serum potassium level decreased in a significant fashion (P < 0.001). For the 2.5-g group, serum potassium decreased to 4.9 mEq/l. In the 5-g group, the decrease was to 4.8 mEq/l; and in the 10-g group, the decrease was to 4.6 mEq/l. Net mean reductions were 0.5, 0.5, and 0.7 mEq/l, respectively, for each of the 3 groups. For the 1.25-g group and the placebo group, the serum potassium only decreased to 5.1 mEq/l, which was not significant. For those patients who received the 5-g and 10-g doses of ZS-9, the serum potassium levels were maintained at 4.7 and 4.5 mEq/l, respectively, during study days 3 to 15, compared with a maintained level of >5 mEq/l in the placebo group. The rates of adverse events were similar in the ZS-9 and placebo groups. Diarrhea was the most common complication in the study groups and did not differ between ZS-9 and placebo groups.
The results of this study indicated that ZS-9 was an effective potassium binder that corrected hyperkalemia within 48 hours of treatment. Within an hour after ZS-9 administration, declines in serum potassium were consistent and dose-dependent starting at a dose of 2.5 g 3 times daily. As anticipated, the reduction in serum potassium levels was most pronounced in patients with higher baseline levels, and ZS-9 was effective in normalizing serum potassium levels in patients whether or not they were receiving RAAS inhibitors for the treatment of CKD or cardiovascular disease.
Additional studies are in progress or ongoing with ZS-9 to ensure its safety and efficacy over longer treatment periods.
Conclusions
Patiromer and ZS-9 appear to be well tolerated and predictable medications to consistently and safely reduce serum potassium levels. The developmental plans for both of these drugs are broad in providing necessary information about how best to use them for both short-term and chronic clinical situations of hyperkalemia. The recent approval of patiromer and the likely approval of ZS-9 should allow an opportunity for health care providers to use necessary and appropriate doses of RAAS-blocking drugs in their patients with CKD and cardiovascular disease. Hopefully, this opportunity for improved control of hyperkalemia will permit wider use of RAAS blockers and will improve the overall quality of care for patients with CKD and cardiovascular disease. It may also allow a greater opportunity to utilize mineralocorticoid receptor antagonists, both in CKD and cardiovascular disease, as detailed in a recent critical review by Epstein.40 It is likely that mineralocorticoid receptor antagonist drugs may have important additive benefits with traditional RAAS-blocking drugs, including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Disclosure
Publication of this article was supported by Relypsa, Inc. MRW is a consultant for Relypsa, Inc., AstraZeneca, Merck & Co., Akebia Therapeutics, Sandoz, AbbVie, and Boston Scientific Corporation. He has received research/grant support from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute.
References
- 1.Gabow P.A., Peterson L.N. Disorders of potassium metabolism. In: Schrier R.W., editor. Renal and Electrolyte Disorders. 14th edition. Little, Brown and Company; New York, NY: 1992. pp. 231–285. [Google Scholar]
- 2.Gennari F.J., Cohen J.J. Role of the kidney in potassium homeostasis: lessons from acid-base disturbances. Kidney Int. 1975;8:1–5. doi: 10.1038/ki.1975.69. [DOI] [PubMed] [Google Scholar]
- 3.Ellison D.H., Velazquez H., Wright F.S. Mechanisms of sodium, potassium, and chloride transport by the renal distal tubule. Miner Electrolyte Metab. 1987;13:422–432. [PubMed] [Google Scholar]
- 4.DeFronzo R.A. Hyperkalemia and hyporeninemic hypoaldosteronism. Kidney Int. 1980;17:118–134. doi: 10.1038/ki.1980.14. [DOI] [PubMed] [Google Scholar]
- 5.Palmer B.F. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 6.Palmer B.F. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis. 2010;56:387–393. doi: 10.1053/j.ajkd.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo R.A., Bia M., Smith D. Clinical disorders of hyperkalemia. Annu Rev Med. 1982;33:521–554. doi: 10.1146/annurev.me.33.020182.002513. [DOI] [PubMed] [Google Scholar]
- 8.Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl. 2016;6:20–28. doi: 10.1016/j.kisu.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein M., Reaven N.L., Funk S.E. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin aldosterone system inhibitors. Am J Manag Care. 2015;21:S212–S220. [PubMed] [Google Scholar]
- 10.An J.N., Lee J.P., Jeon H.J. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16:R225. doi: 10.1186/cc11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einhorn L.M., Zhan M., Hsu V.D. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain N., Kotla S., Little B.B. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2015;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 13.Khanagavi J., Gupta T., Aronow W.S. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10:251–257. doi: 10.5114/aoms.2014.42577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCullough P.A., Beaver T.M., Bennett-Guerrero E. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev Cardiovasc Med. 2014;15:11–23. [PubMed] [Google Scholar]
- 15.Elliott M.J., Ronksley P.E., Clase C.M. Management of patients with acute hyperkalemia. CMAJ. 2010;182:1631–1635. doi: 10.1503/cmaj.100461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisberg L.S. Management of severe hyperkalemia. Crit Care Med. 2008;36:3246–3251. doi: 10.1097/CCM.0b013e31818f222b. [DOI] [PubMed] [Google Scholar]
- 17.UK Renal Association. Clinical practice guidelines: treatment of acute hyperkalaemia in adults—March 2014. Available at: http://www.renal.org/docs/default-source/guidelines-resources/joint-guidelines/treatment-of-acute-hyperkalaemia-in-adults/hyperkalaemia-guideline---march-2014.pdf?sfvrsn=2. Accessed January 11, 2016.
- 18.Mahoney B.A., Smith W.A., Lo D.S. Emergency intervention for hyperkalaemia. Cochrane Database Syst Rev. 2005;18:CD003235. doi: 10.1002/14651858.CD003235.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahee P.P., Crowe A.V. The management of hyperkalaemia in the emergency department. J Accid Emerg Med. 2000;17:188–191. doi: 10.1136/emj.17.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyirenda M.J., Tang J.I., Padfield P.L., Seckl J.R. Hyperkalaemia. BMJ. 2009;339:b4114. doi: 10.1136/bmj.b4114. [DOI] [PubMed] [Google Scholar]
- 21.Alfonzo A., Isles C., Geddes C., Deighan C. Potassium disorders—clinical spectrum and emergency management. Resuscitation. 2006;70:10–25. doi: 10.1016/j.resuscitation.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Davis T.R., Young B.A., Eisenberg M.S. Outcome of cardiac arrests attended by emergency medical services staff at community outpatient dialysis centers. Kidney Int. 2008;73:933–939. doi: 10.1038/sj.ki.5002749. [DOI] [PubMed] [Google Scholar]
- 23.Ostermann M. Cardiac arrests in haemodialysis patients: an ongoing challenge. Kidney Int. 2008;73:907–908. doi: 10.1038/ki.2008.40. [DOI] [PubMed] [Google Scholar]
- 24.Ray K., Dorman S., Watson R. Severe hyperkalaemia due to the concomitant use of salt substitutes and ACE inhibitors in hypertension: a potentially life threatening interaction. J Hum Hypertens. 1999;13:717–720. doi: 10.1038/sj.jhh.1000890. [DOI] [PubMed] [Google Scholar]
- 25.Doorenbos C.J., Vermeij C.G. Danger of salt substitutes that contain potassium in patients with renal failure. BMJ. 2003;326:35–36. doi: 10.1136/bmj.326.7379.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepage L., Dufour A.C., Doiron J. Randomized clinical trial of sodium polystyrene sulfonate for the treatment of mild hyperkalemia in CKD. Clin J Am Soc Nephrol. 2015;10:2136–2142. doi: 10.2215/CJN.03640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterns R.H., Rojas M., Bernstein P., Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21:733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- 28.Kovesdy C.P. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 29.Harel Z., Harel S., Shah P.S. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med. 2013;126:264.e9–264.e24. doi: 10.1016/j.amjmed.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Buysse J.M., Huang I.Z., Pitt B. PEARL-HF: prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol. 2012;8:17–28. doi: 10.2217/fca.11.71. [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration. Kayexalate (sodium polystyrene sulfonate) powder: safety labeling changes approved by FDA Center for Drug Evaluation and Research (CDER)—September 2009. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm186845.htm. Accessed September 15, 2015.
- 32.Watson M.A., Baker T.P., Nguyen A. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60:409–416. doi: 10.1053/j.ajkd.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 33.Weir M.R., Bakris G.L., Bushinsky D.A., for the OPAL-HK Investigators Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 34.Bakris G.L., Pitt B., Weir M.R. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 35.Bushinsky D.A., Williams G.H., Pitt B. Patiromer induces rapid and sustained potassium lowering in patients with chronic kidney disease and hyperkalemia. Kidney Int. 2015;88:1427–1433. doi: 10.1038/ki.2015.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Epstein M., Lifschitz M.D. Potassium homeostasis and dyskalemias: the respective roles of renal, extrarenal, and gut sensors in potassium handling. Kidney Int Suppl. 2016;6:7–15. doi: 10.1016/j.kisu.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stavros F., Yang A., Leon A., Nuttall M., Rasmussen H.S. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One. 2014;9:e114686. doi: 10.1371/journal.pone.0114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosiborod M., Rasmussen H.S., Lavin P. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 39.Packham D.K., Rasmussen H.S., Lavin P.T. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 40.Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol. 2015;3:993–1003. doi: 10.1016/S2213-8587(15)00289-2. [DOI] [PubMed] [Google Scholar]