Abstract
Recent studies have reported a large gap between the forceful and assertive recommendations in the guidelines and real-world practice in the use of renin-angiotensin-aldosterone inhibitors (RAASi) therapies. A comprehensive, retrospective analysis of a large database of electronic medical records (>7 million patients) was undertaken to evaluate 3 pivotal concerns: (i) whether RAASi are being prescribed according to treatment guidelines, (ii) what happens to RAASi prescriptions after hyperkalemia events, and (iii) what the clinical outcomes are in patients whose RAASi are discontinued or who are prescribed at doses lower than the guidelines recommend. The results indicate that a substantial gap exists between guideline recommendations and real-world prescribing patterns for RAASi. Among patients with cardiorenal comorbidities for which RAASi are recommended by the guidelines, more than one-half were prescribed lower-than-recommended doses, and approximately 14% to 16% discontinued RAASi therapy. RAASi prescribing patterns may be altered by the development of hyperkalemia. Moderate-to-severe hyperkalemia events were followed by down-titration or discontinuation of RAASi therapy in nearly one-half of all patients on maximal dose and by discontinuation in nearly one-third of patients on submaximal dose. This analysis highlights the challenge behind RAASi prescribing decisions, balancing the risk of provoking hyperkalemia with the benefits to reducing cardiorenal morbidity and mortality. Patients who are known to derive the greatest benefit from these drugs (chronic kidney disease patients with concomitant diabetes mellitus or heart failure) are the same patients who are at highest risk of developing hyperkalemia. These observations constitute a “call to action” to develop newer treatment modalities to lower serum potassium and to achieve and sustain normokalemia long-term.
Keywords: “big-data analytics”, cardiorenal comorbidities, chronic kidney disease, guideline recommendations for RAASi treatment, hyperkalemia, MR antagonism, prescribing patterns for RAASi, renin-angiotensin inhibition
Hyperkalemia has long constituted an electrolyte abnormality of interest to clinicians and physiologists. As detailed in the contributions by Kovesdy1 and Campese and Adenuga,2 hyperkalemia represents a most important acute electrolyte abnormality due to its potential for causing life-threatening arrhythmias. Hyperkalemia remains a vexing and challenging problem for clinicians, particularly in the management of patients with chronic kidney disease (CKD) and cardiovascular disease, especially congestive heart failure (CHF).
The renin-angiotensin-aldosterone system (RAAS) is central to the pathogenesis of cardiovascular disease and renal disease. RAAS inhibition confers multiple beneficial effects: it can reduce blood pressure, and it can prevent target organ damage in hypertension, diabetes mellitus (DM), CHF, and resistant hypertension.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Importantly, RAAS inhibition has been clearly demonstrated to improve outcomes in patients with these clinical disorders.15 Recent reports have focused on steps to enhance the appropriate implementation of RAAS inhibition with an emphasis on optimization of dosing.
Since the publication of the CONSENSUS (Cooperative North Scandinavian Enalapril Survival Study) trial 20 years ago,16 the implementation of RAAS inhibitor (RAASi) therapy has been widely adopted, with multiple strategies of RAAS inhibition ranging from single-drug optimization to implementation of combination therapies. The 3 main classes of RAASi currently used in clinical practice are angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs); with a fourth class of agents—the direct renin inhibitors—under active investigation. The observed differences in lipid solubility, plasma protein binding, bioavailability, plasma half-life, and systemic elimination for these agents influence their onset, duration of action, and efficacy in blocking tissue renin-angiotensin system, which affects their cardiovascular protection profiles.17
Table 1 briefly summarizes disease state–specific guideline recommendations for RAASi, with their corresponding level of recommendation and their strength of evidence.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 As is evident, RAASi are guideline-recommended therapies that reduce morbidity, slow the progression of renal disease, or prolong survival in several disease states (Table 1). Because of their established benefits in the treatment of cardiovascular disease and their widespread adoption by clinicians, and because these drugs increase the risk of hyperkalemia, especially in CKD patients or those treated with multiple RAASi,3, 4 the clinical landscape of hyperkalemia has changed markedly in both prevalence and severity.
Table 1.
Guideline recommendations for RAASi treatment of heart failure, chronic kidney disease, and diabetes mellitusa
| Disease state | Recommendation | Source of recommendation | Level of recommendation | Strength of evidence |
|---|---|---|---|---|
| Heart failure with reduced ejection fraction | In patients with history of MI and reduced EF, ACEIs or ARBs should be used to prevent HF | ACC/AHA18 | I | A |
| ACEIs are recommended in patients with HFrEF (LVEF ≤40%) and current or prior symptoms, unless contraindicated, to reduce morbidity and mortality | ESC19 ACC/AHA18 |
I | A | |
| ARBs are recommended in patients with HFrEF with current or prior symptoms who are ACEI-intolerant, unless contraindicated, to reduce morbidity and mortality | ESC19 ACC/AHA18 |
I | A | |
| Addition of an ARB may be considered in persistently symptomatic patients with HFrEF who are already being treated with an ACEI and a beta-blocker in whom an aldosterone antagonist is not indicated or tolerated | ESC19 ACC/AHA18 |
IIb | A | |
| MRAs are recommended in patients with NYHA class II to IV HF and who have LVEF of ≤35%, unless contraindicated, to reduce morbidity and mortality | ESC19 ACC/AHA18 |
I | A | |
| MRAs are recommended to reduce morbidity and mortality following an acute MI in patients who have LVEF ≤40% who develop HF symptoms or who have a history of DM, unless contraindicated | ACC/AHA18 | I | A | |
| Chronic kidney disease | For prevention of CKD progression, suggest an ARB or ACEI be used in diabetic adults with CKD and UAE 30 to 300 mg/24 h | KDIGO20, 21 | 2 | D |
| For prevention of CKD progression, recommend an ARB or ACEI be used in both diabetic and nondiabetic adults with CKD and UAE >300 mg/24 h | KDIGO20, 21 | 1 | B | |
| Do not routinely discontinue RAASi (ACEI, ARB, MRA, direct renin inhibitor) in people with GFR <30 ml/min/1.73 m2 as they remain nephroprotective | KDIGO22, 23 | NA | NA | |
| In the population ≥18 years of age with CKD, initial (or add-on) antihypertensive treatment should include an ACEI or ARB to improve kidney outcomes. This applies to all CKD patients with hypertension regardless of race or diabetes status | JNC 824, 25 | Moderate recommendation | B | |
| Diabetes mellitus | Pharmacological therapy for patients with DM and HTN should comprise a regimen that includes either an ACEI or an ARB | ADA26 | B | |
| Either an ACEI or ARB is suggested for the treatment of diabetic nephropathy patients with modestly elevated UAE (30–299 mg/day) and is recommended for those with UAE >300 mg/day | ADA27 | B: UAE 30–299 mg/day A: UAE >300 mg/day |
||
| Resistant hypertension | MRAs should be considered, if no contraindication exists | ESH/ESC28 JNC 824 |
IIa | B |
ACC, American College of Cardiology; ACEI, angiotensin-converting enzyme inhibitor; ADA, American Diabetes Association; AHA, American Heart Association; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; DM, diabetes mellitus; EF, ejection fraction; ESC, European Society of Cardiology; ESH, European Society of Hypertension; GFR, glomerular filtration rate; HF, heart failure; HFrEF, HF with reduced EF; HTN, hypertension; JNC, Joint National Committee; KDIGO, Kidney Disease Improving Global Outcomes; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NA, not applicable; NYHA, New York Heart Association; RAASi, renin-angiotensin-aldosterone system inhibitor; UAE, urine albumin excretion.
Recent data suggest ACEIs are possibly superior to ARBs for kidney failure, cardiovascular death, and all-cause mortality in patients with CKD.15
Hyperkalemia rates vary from 2% to >50% in observational studies, depending on the patient population and CKD severity.5, 6 Ironically, patients with risk factors for hyperkalemia (e.g., older age, DM, CKD)1 are also those who receive the greatest absolute benefit from RAASi.7
Considerations regarding the clinical utilization of RAASi and consequent hyperkalemia
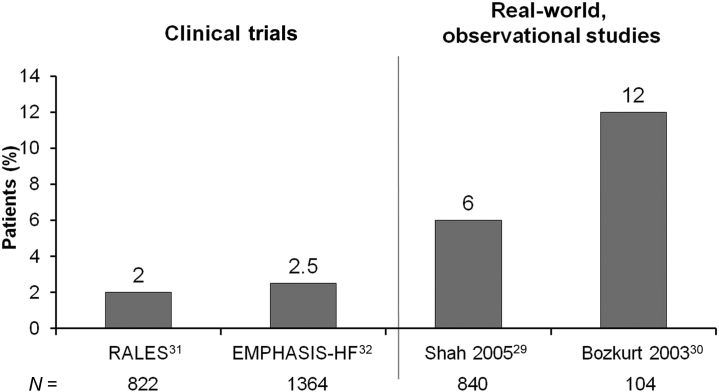
Recent reports have highlighted several important aspects of RAASi treatment regimens that have not been universally appreciated. First, the rates of hyperkalemia documented in clinical trials—including RALES (Randomized Aldactone Evaluation Study), EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study), and EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure)—do not reflect the prevalence of this complication in the real world. As shown in Figure 1, Shah et al.29 and Bozkurt et al.30 have reported rates of hyperkalemia that substantively exceed the rates reported in the original clinical trials.31, 32
Figure 1.
A comparison of hyperkalemia rates in patients with CHF in clinical trials and in the real-world setting. The left bars in the 2 panels depict hyperkalemia rates in RALES (Randomized Aldactone Evaluation Study)31 and in a clinical study after the publication of RALES.29 The right bars in the two panels depict the hyperkalemia rates in the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) study32 and an analysis following the application of the RALES trial protocol to the care of 104 patients, who were identified as being started on spironolactone for HF after prerelease of the RALES trial.30 Clearly, hyperkalemia rates are higher in congestive heart failure patients on mineralocorticoid receptor antagonist therapy in a real-world clinical setting.
An analysis following the application of the RALES trial protocol to the care of 104 patients who were identified as being started on spironolactone for heart failure after prerelease of the RALES trial30 clearly demonstrated hyperkalemia rates that were higher in CHF patients on MRA therapy in a real-world clinical setting. The investigators concluded that their data suggest that spironolactone was being used widely in heart failure without consideration of the New York Heart Association class and ejection fraction, and without optimization of background treatment with ACEIs and beta-blockers. Furthermore, they reported that clinical follow-up does not adhere to the RALES trial guidelines, resulting in higher complications.
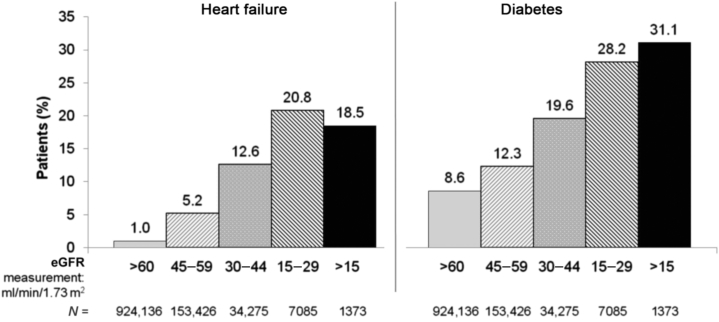
An additional consideration is the demonstration that as CKD progresses, the prevalence of HF and diabetes increases (Figure 2).33 The increase in comorbidities enhances the risk of hyperkalemia, based on the interplay of these comorbidities and the requirement for concomitant therapies.
Figure 2.
As CKD progresses, the prevalence of heart failure and diabetes increases. Longitudinal estimated glomerular filtration rate (eGFR) among 1,120,295 adults within a large integrated system between 1996 and 2000 who had not undergone dialysis or kidney transplantation. It is readily apparent that as chronic kidney disease (CKD) progresses, the prevalence of both heart failure and diabetes mellitus increases as baseline eGFR declines progressively. Consequently, the risk of hyperkalemia increases, because of both the underlying disease and concomitant therapies.
Drawn from data from Go et al.33 Courtesy of Murray Epstein, MD, FASN.
Several investigators have reported that RAASi are underutilized in patients with CKD, and they have suggested that hyperkalemia constitutes the major barrier for their initiation or sustained treatment.25, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 As an example, Yildirim et al.34 investigated the barriers that mitigated against the use of RAAS blockers in CKD patients. Patients with stages 3 to 5 CKD referred to the University Hospital Nephrology Unit during a 1-year period were evaluated for RAAS blocker use. A total of 279 patients (166 male, 113 female) were analyzed. The mean age of the patients was 56.7 ± 15.2 years, mean serum creatinine was 2.45 ± 1.44 mg/dl, and mean glomerular filtration rate (GFR) was 33.3 ± 15.1 ml/min. The mean follow-up time was 22.0 ± 21.9 months. ACEIs or ARBs were used by 68.8% of all patients and 67.7% of diabetic patients at the time of analysis. Hyperkalemia was the principal reason for not starting and also for discontinuing RAASi in patients with CKD. In 37.4% of patients, reasons for not starting RAASi were unclear. The Yildirim study demonstrated that hyperkalemia is the major barrier against the use of RAASi in patients with CKD.
In addition to conventional RAASi, including ACEIs, ARBs, and MRAs, it should be emphasized that it is probable that hyperkalemia will supervene when newer treatment regimens are introduced for the management of CHF, CKD, and resistant hypertension. In the PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor–Neprilysin Inhibitor] with ACEI [Angiotensin-Converting–Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial) study,45 study participants were selected to constitute a population at low risk for hyperkalemia prior to randomization. Patients with estimated GFR <30 ml/min/1.73 m2 were excluded. Patients with a serum potassium level of >5.2 mmol/l at screening (or >5.4 mmol/l at randomization) were also excluded. In addition, PARADIGM-HF had a run-in phase on ACEIs that excluded 6% of patients due to adverse events, then a run-in phase on LCZ-696 (now known as valsartan/sacubitril, brand name Entresto; Novartis Pharmaceuticals Corporation, East Hanover, NJ) that excluded another 6% of patients due to adverse events. Consequently, the investigators selected a population that would be at low risk for hyperkalemia. Despite these exclusions, hyperkalemia rates remained high in all patients despite the carefully selected population (>5.5 mmol/l: 16.1% LCZ-696 vs. 17.3% ACEI (P = 0.15). It must be emphasized that the elevated hyperkalemia rates are probably attributable not solely to LCZ-696, but rather to the drug regimen utilized (a combination of LCZ-696, plus beta-blockers, plus MRAs in approximately 50% of the study cohort). Consequently, the caution regarding hyperkalemia applies to newer and more complicated treatment regimens encompassing several agents that favor the development of hyperkalemia.
Recent observational and retrospective studies
Recently, several observational and retrospective studies have reported a large gap between the forceful and assertive recommendations in the above-cited guidelines and real-world practice in the use of RAASi therapies.15, 16, 46, 47, 48, 49 A retrospective analysis of data from the American Heart Association’s Get With the Guidelines: Coronary Artery Disease database reported that <10% of eligible HF patients hospitalized for myocardial infarction were prescribed an aldosterone antagonist at discharge.48
Additional data derive from the ESC-HF (European Society of Cardiology Heart Failure) Long-Term Registry, a prospective, observational study conducted in 211 cardiology centers of 21 European and Mediterranean countries by members of the European Society of Cardiology.49 From May 2011 to April 2013, a total of 12,440 patients were enrolled, 40.5% with acute HF and 59.5% with chronic HF. Whereas 67% to 92% of hospitalized HF patients were prescribed the recommended RAASi therapy, <30% were up-titrated to the recommended target dose.
Underutilization of RAASi is not limited to CHF and coronary artery disease cohorts and it complicates the management of patients with CKD. In a recent report, Shirazian et al.50 examined variables associated with the prescription of renin-angiotensin system blockers in patients with CKD. They reviewed the electronic medical records of 627 patients with moderate-to-severe CKD and an indication for renin-angiotensin system blockade. Of these patients, 225 (36%) were not prescribed renin-angiotensin system blockade. For the majority, there was no provider-documented reason explaining why these medications were not prescribed. Among documented reasons, hyperkalemia or a history of acute kidney injury were the most common. The strength of the Shirazian study50 lies in the exhaustive electronic medical record review going back 10 or more years to evaluate for RAASi documentation. In addition, this study included a large population of patients with moderate-to-severe CKD, including 237 patients with stage 4 disease, the group that is at a high risk for progressing to end-stage renal disease.
RAASi therapy in the CHF patient with estimated GFR <30 ml/min
Will this cohort of patients with advanced CKD constitute the “low-hanging fruit”?
Recent publications have proposed that patients with CHF plus concomitant severe renal dysfunction should also be treated with RAASi therapy. Edner et al.51 have analyzed Swedish Heart Failure Registry data collected between 2000 and 2013 from patients with markedly diminished renal function (creatinine concentration >221 μmol/l or creatinine clearance <30 ml/min [Cockcroft-Gault method]). The carefully designed study by Edner et al.51 provides strong support for the postulate that the benefits of both renin-angiotensin inhibition and MRA treatment still occur in patients with concomitant severe renal dysfunction, even those with stages 4 to 5 CKD. The relative risk reduction in mortality throughout the 5-year follow-up was 24%, and the number needed to treat for 1 year to save 1 life was 10. Consequently, it is highly probable that CHF patients with markedly diminished renal function will no longer be excluded from clinical trials of CHF, which is appropriate and indeed exciting. However, it is reasonable to anticipate that RAASi treatment of this cohort will result in even more frequent and more severe hyperkalemia.
Studies applying big-data analytics to delineating the role of RAASi-induced hyperkalemia in disrupting utilization of guideline-mandated RAASi treatment
To better elucidate this apparent treatment gap, Epstein et al.52 recently undertook a “big-data analytics” approach—a comprehensive analysis of a large database of electronic medical records (>7 million patients) to evaluate 3 pivotal concerns: (i) whether RAASi are being prescribed according to treatment guidelines, (ii) what happens to RAASi prescriptions after hyperkalemia events, and (iii) what the clinical outcomes are in patients whose RAASi are discontinued or prescribed at doses lower than recommended in guidelines.
Deidentified medical records (2007–2012) for outpatient, in-clinic, and/or in-hospital patients in the United States with ≥2 potassium readings were obtained from Humedica, a large database of electronic health records (www.humedica.com).
Inclusion criteria required ≥1 outpatient RAASi prescription and 12 months of data prior to July 1, 2009 (the index date). RAASi included ACEIs, ARBs, direct renin inhibitors, and select MRAs. Inclusion also required evidence of patient engagement with the health care provider prior to, and continued up to, the index date.
Patients were excluded from the analysis of dose distribution and outcomes if they had a diagnosis of end-stage renal disease or CKD stage 5 or acute kidney injury at the index date. The response to hyperkalemia events was evaluated for each hyperkalemia event in the data (2007–2012) without restriction by patient comorbidity status.
Classification of patient comorbidity and RAASi dose category
Patients were classified by disease comorbidity (CKD stages 3–4 and/or HF or DM [types 1 and 2]) and age (<65 vs. ≥65 years) prior to the index date, using International Classification of Diseases, Ninth Revision diagnosis codes as well as results available on estimated GFR, left ventricular ejection fraction, hemoglobin A1c, and prescriptions for antidiabetes medications.
RAASi prescriptions were classified by dose level using the following dose categories: “supramaximum”—any RAASi dose above the labeled dose; “maximum”—the labeled dose; “submaximum”—any RAASi dose lower than the labeled dose; or “discontinued”—the absence of RAASi prescriptions for a period >390 days subsequent to prior prescription. The 390-day period allows 360 days plus an additional 30 days for patients to see or contact their health care providers for a refill.
Results
Study population characteristics
A total of 205,108 patients met the inclusion criteria, and, of these, 66,862 (32.8%) experienced ≥1 hyperkalemia event (58,520 [28.5%] experienced ≥1 mild hyperkalemia event and 30,912 [15.1%] experienced ≥1 moderate-to-severe hyperkalemia event). After excluding patients with end-stage renal disease, 201,655 patients were included in the outcomes analyses. After further exclusion of patients with CKD stage 5 and acute kidney injury, 195,327 patients were included in the dose distribution study.
Dose distribution study
A patient-level analysis was performed to examine RAASi dose distribution as of the index date. RAASi dose level was similarly distributed irrespective of patient comorbidity status. Maximum doses were prescribed in 19% to 26% of patients, over one-half of the patients (58%–65%) were prescribed submaximum doses, and 14% to 16% of patients discontinued treatment with RAASi as of the index date.
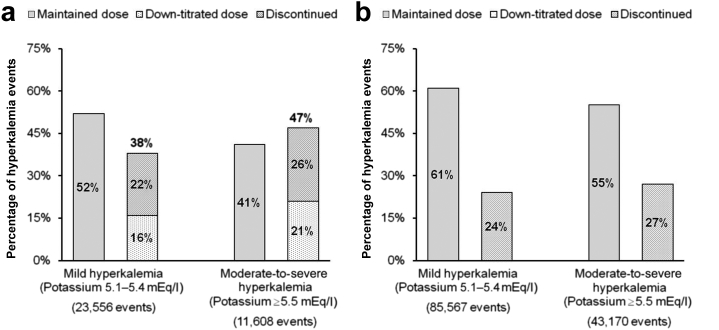
RAASi dosing subsequent to hyperkalemia events
An event-level analysis was used to examine RAASi dose changes following the occurrence of hyperkalemia (defined as any serum potassium measurement >5.0 mEq/l). Laboratory records from 66,862 patients included 218,813 hyperkalemia events: 144,800 mild (5.1–5.4 mEq/l) and 74,013 moderate-to-severe (≥5.5 mEq/l).
Analysis of RAASi prescription status before and after these hyperkalemia events revealed that a substantial proportion of patients had changes in their dose following elevated serum potassium, with dose changes occurring more frequently after moderate-to-severe hyperkalemia events. Patients on maximum-dose RAASi were down-titrated to submaximum dose or discontinued nearly one-half of the time (47%) after moderate-to-severe hyperkalemia events and 38% of the time after mild events (Figure 3a). Among patients on submaximum doses of RAASi, moderate-to-severe hyperkalemia events were followed by submaximum dose maintenance in 55% of patients and discontinuation in 27% of patients, compared with dose maintenance after 61% of mild hyperkalemia events and discontinuation after 24% of mild events (Figure 3b). In the remaining events, the data period following the hyperkalemia event was insufficient to determine subsequent RAASi dose level.
Figure 3.
Changes in RAASi dose subsequent to hyperkalemia events. Among patients on renin-angiotensin-aldosterone system inhibitor (RAASi) at (a) maximum dose and (b) submaximum dose.
Reproduced with permission from Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 suppl):S212–S220.52
Cardiorenal outcomes and mortality by RAASi dose
In the patient-level analysis, differences in clinical outcomes between patients with submaximum or discontinued RAASi versus those remaining on maximum doses were evaluated in the total study population as well as within disease categories (CKD stages 3–5, HF, or DM). Adverse outcomes evaluated were CKD progression and progression to end-stage renal disease, stroke and acute myocardial infarction, coronary artery bypass and percutaneous coronary intervention, and all-cause mortality.
Endpoints included a composite measure of any adverse outcome or mortality and mortality alone. For the composite endpoint, the most frequently prescribed dose level of RAASi was identified for each patient during the postindex period. RAASi dose category was re-evaluated and adjusted for patients who experienced an adverse outcome to ensure that the dominant dose category did not reflect data occurring after the adverse outcome; this adjustment resulted in a higher and lower RAASi dose category for 1.6% and 3.5% of patients, respectively. For the endpoint of mortality alone, all patients were classified according to their last RAASi dose level in the data.
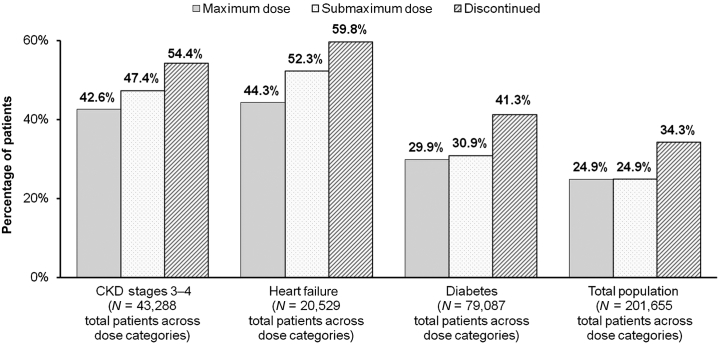
Patients on submaximum or discontinued RAASi dose levels showed consistently worse outcomes than did patients on maximum dose, irrespective of comorbidity status (Figure 4) or patient age. Over 50% of patients with CKD stages 3 to 4 who were discontinued from RAASi experienced an adverse outcome or died, compared with 47.4% of patients on submaximum dose and 42.6% of patients on maximum dose (Figure 4). Nearly 60% of patients with HF who were discontinued from RAASi experienced an adverse outcome or mortality, compared with 52.3% of patients on submaximum dose and 44.3% of patients on maximum dose (Figure 4). Patients with DM had better outcomes than patients in the HF or CKD stages 3 to 4 comorbidity groups. A total of 41.3% of diabetes patients who discontinued RAASi experienced an adverse outcome or mortality, compared with 30.9% of patients on submaximum dose and 29.9% of patients on maximum dose (Figure 4). A comparison of patients <65 years of age versus patients ≥65 years (data not shown) suggested that patients on submaximum dose or discontinued RAASi had consistently worse outcomes than did patients on maximum dose regardless of age group, with the exception of patients with DM who were <65 years of age, in whom maximum and submaximum RAASi doses were associated with similar levels of adverse outcomes or mortality (20.5% and 19.8%, respectively).
Figure 4.
Percentage of patients who experienced adverse outcomes or mortality by prior RAASi dose. Patients on submaximum doses or who discontinued renin-angiotensin-aldosterone system inhibitor (RAASi) therapy demonstrated consistently worse outcomes compared with patients on maximum doses, irrespective of comorbidity status. CKD, chronic kidney disease.
Reproduced with permission from Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 suppl):S212–S220.52
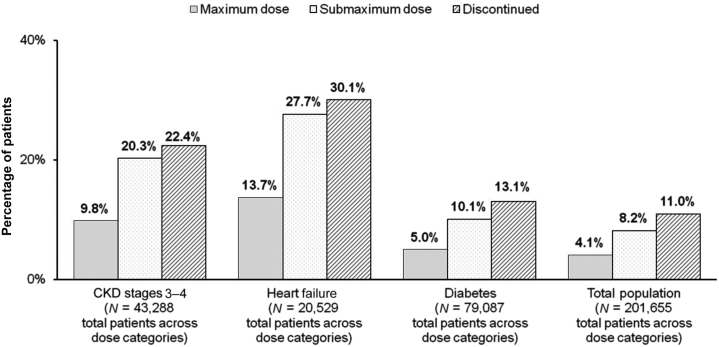
The association between discontinuation of RAASi and mortality was striking. Patients who were on submaximum dose or who discontinued RAASi died twice as frequently as patients on maximum dose, irrespective of comorbidity status (Figure 5) or patient age. Mortality was recorded for 9.8% of patients with CKD stages 3 to 4 on maximum dose of RAASi compared with 20.3% of patients on submaximum dose and 22.4% of patients who discontinued therapy.
Figure 5.
Percentage of mortality by prior RAASi dose. Patients on submaximum dose or who discontinued renin-angiotensin-aldosterone system inhibitor (RAASi) died twice as frequently as patients on maximum dose irrespective of comorbidity status. CKD, chronic kidney disease.
Reproduced with permission from Epstein M, Reaven NL, Funk SE, et al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 suppl):S212–S220.52
Among patients with HF, mortality was recorded for 13.7% of patients on maximum-dose RAASi compared with 27.7% on submaximum dose and 30.1% of patients who discontinued. Surprisingly, patients in the DM category had the lowest mortality rates, with mortality recorded for 5.0% of patients on maximum dose, 10.1% of patients on submaximum dose, and 13.1% of patients who discontinued RAASi therapy.
Lessons learned and implications for next steps
What insights and lessons can we derive from this comprehensive big-data analytics study delineating the role of RAASi-induced hyperkalemia in disrupting the utilization of guideline-mandated RAASi treatment? Overall, the results of these analyses clearly indicate that there is a substantial gap between the recommendations in treatment guidelines and the real-world prescribing patterns for RAASi. Among patients with cardiorenal comorbidities for which RAASi are recommended by the guidelines, this retrospective analysis showed that more than one-half were prescribed lower-than-recommended doses, and approximately 14% to 16% had been discontinued from RAASi therapy.52 The results suggest that the prescribing patterns for RAASi may be altered by the development of hyperkalemia. Moderate-to-severe hyperkalemia events (serum potassium ≥5.5 mEq/l) were followed by down-titration or discontinuation of RAASi therapy in nearly one-half of all patients on maximal dose, and by discontinuation in nearly one-third of patients on submaximal dose.
An extremely important observation of this study is that patients who were on submaximum doses, or who discontinued RAASi, had worse cardiorenal outcomes and higher mortality than patients on maximum doses. Taken together, these results highlight the extraordinary challenge behind RAASi prescribing decisions, attempting to balance the risk of provoking hyperkalemia with the benefits to cardiorenal morbidity and mortality. A great irony is the fact that those patients who are known to derive the greatest benefit from these drugs (CKD patients with concomitant DM or HF) are the same patients who are at highest risk of developing hyperkalemia.
In concert, these observations constitute a “call to action” to develop newer treatment modalities to lower serum potassium and to achieve and—even more importantly—sustain normokalemia on a long-term basis.
In the following article,53 Weir reviews current and emerging treatments for hyperkalemia. He describes the advent of 2 newly developed potassium-binding drugs, patiromer (Veltassa, Relypsa, Redwood City, CA) and sodium zirconium cyclosilicate (also known as ZS-9; ZS Pharma, San Mateo, CA), that have recently reported positive clinical trial results for the management of hyperkalemia.44, 54, 55, 56 Hopefully, these new drugs can represent a much-needed advancement in the treatment of hyperkalemia and can consequently act as “enablers” to facilitate maintaining patients on optimal doses of RAASi as recommended in the current treatment guidelines.
Disclosure
Publication of this article was supported by Relypsa, Inc. ME is a consultant for Relypsa, Inc., Bayer HealthCare Pharmaceuticals, OPKO Health, Inc., and Novartis Pharmaceuticals.
Acknowledgments
The author thanks Lisa Savitt for expert editorial services and Christine Kittler for accessing many of the articles.
References
- 1.Kovesdy C.P. Epidemiology of hyperkalemia: an update. Kidney Int Suppl. 2016;6:3–6. doi: 10.1016/j.kisu.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campese V.M., Adenuga G. Electrophysiological and clinical consequences of hyperkalemia. Kidney Int Suppl. 2016;6:16–19. doi: 10.1016/j.kisu.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrario C.M., Strawn W.B. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol. 2006;98:121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 4.Shearer F., Lang C.C., Struthers A.D. Renin-angiotensin-aldosterone system inhibitors in heart failure. Clin Pharmacol Ther. 2013;94:459–467. doi: 10.1038/clpt.2013.135. [DOI] [PubMed] [Google Scholar]
- 5.Hsu T.W., Liu J.S., Hung S.C. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 2014;174:347–354. doi: 10.1001/jamainternmed.2013.12700. [DOI] [PubMed] [Google Scholar]
- 6.Dzau V.J., Antman E.M., Black H.R. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease) Circulation. 2006;114:2850–2870. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 7.Ferrario C.M. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant S.L., Griendline K.K. Vascular effects of angiotensin II and AT1 receptor blockade. In: Epstein M., Brunner H.R., editors. Angiotensin II Receptor Antagonists. Hanley & Belfus, Inc.; Philadelphia, PA: 2000. pp. 235–256. [Google Scholar]
- 9.Gavras I., Gavras H. Cardiac effects of angiotensin II and AT1 receptor blockade. In: Epstein M., Brunner H.R., editors. Angiotensin II Receptor Antagonists. Hanley & Belfus, Inc.; Philadelphia, PA: 2000. pp. 215–222. [Google Scholar]
- 10.Fabiani M.E., Johnston C.I. AT1 receptor antagonists as antihypertensive agents. In: Epstein M., Brunner H.R., editors. Angiotensin II Receptor Antagonists. Hanley & Belfus, Inc.; Philadelphia, PA: 2000. pp. 263–278. [Google Scholar]
- 11.Morgan T.O., Griffiths C.D., Delbridge L.M.D. Wall stress, angiotensin II, and left ventricular hypertrophy. In: Epstein M., Brunner H.R., editors. Angiotensin II Receptor Antagonists. Hanley & Belfus, Inc.; Philadelphia, PA: 2000. pp. 223–234. [Google Scholar]
- 12.Schiffrin E.L., Hayoz D. Role of AT1 angiotensin receptors in vascular remodeling in hypertension. In: Epstein M., Brunner H.R., editors. Angiotensin II Receptor Antagonists. Hanley & Belfus; Inc.; Philadelphia, PA: 2000. pp. 279–294. [Google Scholar]
- 13.Gansevoort R.T., Mimran A., de Zeeuw D. AT1 receptor antagonists and the kidney. In: Epstein M., Brunner H.R., editors. Angiotensin II Receptor Antagonists. Hanley & Belfus; Inc.; Philadelphia, PA: 2000. pp. 295–316. [Google Scholar]
- 14.Kasal D.A., Schiffrin E.L. Angiotensin II, aldosterone, and anti-inflammatory lymphocytes: interplay and therapeutic opportunities. Int J Hypertens. 2012;2012:829786. doi: 10.1155/2012/829786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials [e-pub ahead of print]. Am J Kidney Dis. 10.1053/j.ajkd.2015.10.011, accessed December 15, 2015. [DOI] [PubMed]
- 16.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 17.Israili Z.H. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension. J Hum Hypertens. 2000;14(suppl 1):S73–S86. doi: 10.1038/sj.jhh.1000991. [DOI] [PubMed] [Google Scholar]
- 18.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 19.McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 20.Kidney Disease Improving Global Outcomes (KDIGO) Working Group KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414. [Google Scholar]
- 21.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 22.Kidney Disease Improving Global Outcomes (KDIGO) Lipid Work Group KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259–305. [Google Scholar]
- 23.Inker L.A., Astor B.C., Fox C.H. KDOQI US Commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 24.James P.A., Oparil S., Carter B.L. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 25.Taler S.J., Agarwal R., Bakris G.L. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62:201–213. doi: 10.1053/j.ajkd.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association Cardiovascular disease and risk management. Diabetes Care. 2016;39(suppl):S60–S71. doi: 10.2337/dc16-S011. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Microvascular complications and foot care. Diabetes Care. 2016;39(suppl 1):S72–S80. doi: 10.2337/dc16-S012. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G., Fagard R., Narkiewicz K. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 29.Shah K.B., Rao K., Sawyer R., Gottlieb S.S. The adequacy of laboratory monitoring in patients treated with spironolactone for congestive heart failure. J Am Coll Cardiol. 2005;46:845–849. doi: 10.1016/j.jacc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Bozkurt B., Agoston M.D., Knowlton A.A. Complications of inappropriate use of spironolactone in heart failure: when an old medicine spirals out of new guidelines. J Am Coll Cardiol. 2003;41:211–214. doi: 10.1016/s0735-1097(02)02694-3. [DOI] [PubMed] [Google Scholar]
- 31.Pitt B., Zannad F., Remme W.J., for the Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 32.Zannad F., McMurray J.J., Krum H., for the EMPHASIS-HF Study Group Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 33.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 34.Yildirim T., Arici M., Piskinpasa S. Major barriers against renin-angiotensin-aldosterone system blocker use in chronic kidney disease stages 3–5 in clinical practice: a safety concern? Ren Fail. 2012;34:1095–1099. doi: 10.3109/0886022X.2012.717478. [DOI] [PubMed] [Google Scholar]
- 35.Palmer B.F. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 36.Lazich I., Bakris G.L. Prediction and management of hyperkalemia across the spectrum of chronic kidney disease. Semin Nephrol. 2014;34:333–339. doi: 10.1016/j.semnephrol.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Raebel M.A. Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30:e156–e166. doi: 10.1111/j.1755-5922.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 38.Sarafidis P.A., Blacklock R., Wood E. Prevalence and factors associated with hyperkalemia in predialysis patients followed in a low-clearance clinic. Clin J Am Soc Nephrol. 2012;7:1234–1241. doi: 10.2215/CJN.01150112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain N., Kotla S., Little B.B. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 40.Kovesdy C.P. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10:653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 41.Einhorn L.M., Zhan M., Hsu V.D. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perazella M.A., Mahnensmith R.L. Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. J Gen Intern Med. 1997;12:646–656. doi: 10.1046/j.1525-1497.1997.07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weir M.R., Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 44.Bakris G.L., Pitt B., Weir M.R., the AMETHYST-DN Investigators Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN Randomized Clinical Trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 45.McMurray J.J., Packer M., Desai A.S., for the PARADIGM-HF Investigators and Committees Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 46.Krantz M.J., Ambardekar A.V., Kaltenbach L., for the Get With the Guidelines Steering Committee and Hospitals Patterns and predictors of evidence-based medication continuation among hospitalized heart failure patients (from Get With the Guidelines—Heart Failure) Am J Cardiol. 2011;107:1818–1823. doi: 10.1016/j.amjcard.2011.02.322. [DOI] [PubMed] [Google Scholar]
- 47.Albert N.M., Yancy C.W., Liang L. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–1665. doi: 10.1001/jama.2009.1493. [DOI] [PubMed] [Google Scholar]
- 48.Rassi A.N., Cavender M.A., Fonarow G.C. Temporal trends and predictors in the use of aldosterone antagonists post-acute myocardial infarction. J Am Coll Cardiol. 2013;61:35–40. doi: 10.1016/j.jacc.2012.08.1019. [DOI] [PubMed] [Google Scholar]
- 49.Maggioni A.P., Anker S.D., Dahlström U., for the Heart Failure Association of the ESC Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15:1173–1184. doi: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 50.Shirazian S., Grant C.D., Mujeeb S. Underprescription of renin-angiotensin system blockers in moderate to severe chronic kidney disease. Am J Med Sci. 2015;349:510–515. doi: 10.1097/MAJ.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 51.Edner M., Benson L., Dahlström U., Lund L.H. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: a prospective propensity score-matched cohort study. Eur Heart J. 2015;36:2318–2326. doi: 10.1093/eurheartj/ehv268. [DOI] [PubMed] [Google Scholar]
- 52.Epstein M., Reaven N.L., Funk S.E. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(11 suppl):S212–S220. [PubMed] [Google Scholar]
- 53.Weir M.R. Current and future treatment options for managing hyperkalemia. Kidney Int Suppl. 2016;6:29–34. doi: 10.1016/j.kisu.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weir M.R., Bakris G.L., Bushinsky D.A., OPAL-HK Investigators Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 55.Kosiborod M., Rasmussen H.S., Lavin P. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 56.Packham D.K., Rasmussen H.S., Lavin P.T. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]