Abstract
Integrated mechanisms controlling the maintenance of potassium homeostasis are well established and are defined by the classic “feedback control” of potassium balance. Recently, increasing investigative attention has focused on novel physiological paradigms that increase the complexity and precision of homeostasis. This review briefly considers the classic and well-established feedback control of potassium and then considers subsequent investigations that inform on an intriguing and not widely recognized complementary paradigm: the “feed-forward control of potassium balance.” Feed-forward control refers to a pathway in a homeostatic system that responds to a signal in the environment in a predetermined manner, without responding to how the system subsequently reacts (i.e., without responding to feedback). Studies in several animal species, and recently in humans, have confirmed the presence of a feed-forward control mechanism that is capable of mediating potassium excretion independent of changes in serum potassium concentration and aldosterone. Knowledge imparted by this update of potassium homeostasis hopefully will facilitate the clinical management of hyperkalemia in patients with chronic and recurrent hyperkalemia. Awareness of this updated integrative control mechanism for potassium homeostasis is more relevant today when the medical community is increasingly focused on leveraging and expanding established renin-angiotensin-aldosterone system inhibitor treatment regimens and on successfully coping with the challenges of managing hyperkalemia provoked by renin-angiotensin-aldosterone system inhibitors. These new insights are relevant to the future design of clinical trials delineating renal potassium handling.
Keywords: hyperkalemia, potassium homeostasis, RAAS inhibitors, renal potassium handling
The integrated mechanisms controlling the maintenance of potassium homeostasis are well established and are defined by the classic “feedback control” of potassium balance. In recent years, increasing investigative attention has focused on novel physiological paradigms that increase the complexity as well as the precision of homeostasis. In this review, we briefly consider the classic and well-established feedback control of potassium and then consider subsequent investigations that inform on an intriguing and not widely recognized complementary paradigm: the “feed-forward control of potassium balance.” Awareness of this updated integrative control mechanism for potassium homeostasis is more relevant today when the medical community is increasingly focused on the challenges of managing the hyperkalemia provoked by renin-angiotensin-aldosterone system inhibitors (RAASi).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11
As detailed elsewhere in this supplement (Epstein12), it is well established that RAASi confer substantive benefits, such as reducing cardiovascular events and retarding the progression of renal disease in several disease states, including congestive heart failure, chronic kidney disease, and diabetes.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 Regretfully, treatment with RAASi is complicated by hyperkalemia, which is a frequent side effect of RAASi therapy.1, 2, 3, 4, 9, 10, 11 Indeed, the problem will only become increasingly prominent and frequent, because hyperkalemia will remain an issue with newly introduced drugs such as neprilysin inhibitors (LCZ-696, now known as valsartan/sacubitril, brand name Entresto; Novartis Pharmaceuticals Corporation, East Hanover, NJ), as reported in PARADIGM-HF (Prospective Comparison of ARNI [Angiotensin Receptor–Neprilysin Inhibitor] with ACEI [Angiotensin-Converting–Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial).25 Furthermore, as detailed by Epstein,12 the wide gap between RAASi prescribing guidelines and reality is widely thought to be attributable to hyperkalemia.1, 2, 3, 4, 9, 10, 11 Consequently, we require a greater knowledge of the complexities of the regulatory mechanisms that subserve potassium homeostasis. Finally, as detailed in Weir26 in this supplement, with the recent approval of a potassium-binding polymer and the imminent approval of another potassium binder,2, 4, 5, 6, 7, 9 it is essential to understand how these binders mediate their effects. A thorough understanding of potassium homeostasis is a requirement if we are to avail ourselves of the maximum benefits from these newly available agents.
Normal potassium balance and renal potassium excretion
To establish the context and foundation for considering newer—albeit poorly recognized—adaptive mechanisms for subserving potassium homeostasis, we will first summarize key concepts of steady-state potassium handling. Normal persons who consume a typical Western diet ingest approximately 70–80 mmol of potassium per day.27, 28 The intestine absorbs virtually all of the ingested potassium and delivers it to the liver by means of the hepatoportal circulation, where the ingested potassium is extracted by the liver. In normal circumstances, minimal amounts of potassium are excreted in the feces.
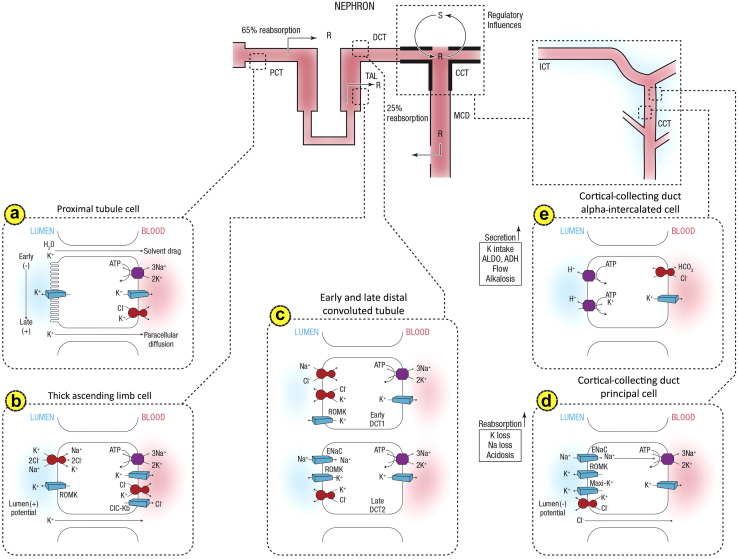
The principal defense against chronic potassium imbalances is renal potassium excretion, which depends on free filtration at the glomerulus, extensive proximal tubule reabsorption, and a highly regulated secretory process in the distal convoluted tubule and segments of the collecting duct in the cortex and outer medulla (the cortical collecting duct and the outer medullary collecting duct, respectively). The more predominant of the 2 types of collecting duct cells are the principal cells, which comprise approximately 75% of collecting duct cells. They mediate sodium reabsorption and potassium secretion and also constitute targets for angiotensin II,29, 30, 31 aldosterone, mineralocorticoid receptor antagonists, and potassium-sparing diuretics (Figure 1).
Figure 1.
Summary of potassium transport along the nephron. Following filtration, potassium is extensively reabsorbed along the proximal tubule and the loop of Henle. Potassium is secreted along the initial and cortical collecting tubules. Net secretion can be replaced by net reabsorption in states of potassium depletion. (a) A cell model for K+ transport in the proximal tubule. K+ reabsorption in the proximal tubule primarily occurs through the paracellular pathway. Active Na+ reabsorption drives net fluid reabsorption across the proximal tubule, which in turn, drives K+ reabsorption through a solvent drag mechanism. As fluid flows down the proximal tubule, the luminal voltage shifts from slightly negative to slightly positive. The shift in transepithelial voltage provides an additional driving force favoring K+ diffusion through the low-resistance paracellular pathway. Experimental studies suggest that there may be a small component of transcellular K+ transport; however, the significance of this pathway is not known. K+ uptake through the Na+-K+-ATPase pump can exit the basolateral membrane through a conductive pathway or coupled to Cl–. An apically located K+ channel functions to stabilize the cell negative potential, particularly in the setting of Na+-coupled cotransport of glucose and amino acids, which has a depolarizing effect on cell voltage. (b) A cell model for K+ transport in the thick ascending limb of Henle. K+ reabsorption occurs by both paracellular and transcellular mechanisms. The basolateral Na+-K+-ATPase pump maintains intracellular Na+ at a low level, thus providing a favorable gradient to drive the apically located Na+-K+-2Cl– cotransporter (an example of secondary active transport). The apically located ROMK channel provides a pathway for K+ to recycle from cell to lumen and ensures an adequate supply of K+ to sustain Na+-K+-2Cl– cotransport. This movement through ROMK creates a lumen-positive voltage, providing a driving force for passive K+ reabsorption through the paracellular pathway. Some of the K+ entering the cell through the cotransporter exits the cell across the basolateral membrane, accounting for transcellular K+ reabsorption. K+ can exit the cell through a conductive pathway or in cotransport with Cl–. ClC-Kb is the primary pathway for Cl– efflux across the basolateral membrane. (c) A cell model for K+ transport in the DCT. In the early DCT, luminal Na+ uptake is mediated by the apically located thiazide-sensitive Na+-Cl– cotransporter. The transporter is energized by the basolateral Na+-K+-ATPase, which maintains low intracellular Na+ concentration, thus providing a favorable gradient for Na+ entry into the cell through secondary active transport. The cotransporter is abundantly expressed in the DCT1 but progressively declines along the DCT2. ROMK is expressed throughout the DCT and into the cortical collecting duct. Expression of the ENaC, which mediates amiloride-sensitive Na+ absorption, begins in the DCT2 and is robustly expressed throughout the downstream connecting tubule and cortical collecting duct. The DCT2 is the beginning of the ASDN as identified by the presence of both the mineralocorticoid receptor and the enzyme 11b-hydroxysteroid dehydrogenase II. This enzyme maintains the mineralocorticoid receptor free to only bind aldosterone by metabolizing cortisol to cortisone, which has no affinity for the receptor. Electrogenic-mediated K+ transport begins in the DCT2 with the combined presence of ROMK, ENaC, and aldosterone sensitivity. Electroneutral K+-Cl– cotransport is present in the DCT and collecting duct. Conditions that promote a low luminal Cl– concentration increase K+ secretion through this mechanism, which occurs with delivery of poorly reabsorbable anions, such as sulfate, phosphate, or bicarbonate. (d) The cell that is responsible for K+ secretion in the initial collecting duct and the cortical collecting duct is the principal cell. This cell possesses a basolateral Na+-K+-ATPase that is responsible for the active transport of K+ from the blood into the cell. The resultant high cell K+ concentration provides a favorable diffusion gradient for movement of K+ from the cell into the lumen. In addition to establishing a high intracellular K+ concentration, the activity of this pump lowers intracellular Na+ concentration, thereby maintaining a favorable diffusion gradient for movement of Na+ from the lumen into the cell. Both the movements of Na+ and K+ across the apical membrane occur through well-defined Na+ and K+ channels. (e) Reabsorption of HCO3 in the distal nephron is mediated by apical H+ secretion by the alpha-intercalated cell. Two transporters secrete H+, a vacuolar H+-ATPase, and an H+-K+-ATPase. The H+-K+-ATPase uses the energy derived from ATP hydrolysis to secrete H+ into the lumen and reabsorb K+ in an electroneutral fashion. The activity of the H+-K+-ATPase increases in K+ depletion and thus provides a mechanism by which K+ depletion enhances both collecting-duct H+ secretion and K+ absorption. ADH, antidiuretic hormone; ALDO, aldosterone; ASDN, aldosterone-sensitive distal nephron; ATPase, adenosine triphosphatase; CCT, cortical collecting tubule; Cl, chloride; ClC-Kb, chloride channel Kb; DCT, distal convoluted tubule; ENaC, epithelial sodium channel; H, hydrogen; HCO3, bicarbonate; ICT, initial connecting tubule; K, potassium; MCD, medullary collecting duct; Na, sodium; PCT, proximal tubule; R, reabsorption; ROMK, renal outer medullary potassium; S, secretion; TAL, thick ascending limb.
This composite figure is modified, extended, and updated from several earlier publications including Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10:1050–106027 with permission to republish from the American Society of Nephrology conveyed through Copyright Clearance Center, Inc., and Giebisch GH. A trail of research on potassium. Kidney Int. 2002;62:1498–1512, Copyright © 2002, with permission from Elsevier. Courtesy of Murray Epstein, MD, FASN.
Principal cells exploit the electrochemical gradient established by sodium entry into the cell through a sodium channel at the luminal membrane (the molecular target of amiloride) and the basolateral membrane sodium-potassium adenosine triphosphatase (Na-K-ATPase) to drive potassium secretion through 2 classes of luminal membrane potassium channels.32 One class, the renal outer medullary potassium (also termed ROMK) channels, secretes potassium under normal tubular fluid flow conditions and is inserted into or internalized from the luminal membrane, depending on the demand for potassium secretion. The other class of potassium channels is the “big” conductance channels (known as BK channels), which are relatively inactive under normal conditions but exhibit increased activity during high tubular flow or high-potassium conditions.32 The factors that regulate principal cell potassium secretion include previous potassium intake; intracellular potassium level; sodium delivery to the cells; urine flow rate; and hormones, such as aldosterone and catecholamines.33 The other of the 2 collecting duct cell types, intercalated cells, mediate acid-base transport but up-regulate expression of luminal hydrogen-potassium adenosine triphosphates (H-K-ATPases) during potassium depletion to enhance potassium reabsorption (Figure 1).27, 34
To summarize, the kidney excretes sufficient potassium to maintain total body homeostasis. Although the proximal nephron reabsorbs the bulk of the potassium filtered at the level of the glomerulus, it is a distal site—the collecting duct that ultimately fine-tunes potassium excretion, thereby determining the final amount of potassium excreted in the urine. Consequently, the collecting duct is the major site that responds to increased potassium intake and, in turn, is subject to several regulatory influences.
Feedback control of potassium balance
The classic feedback control of potassium is an exemplar of a homeostatic system that uses the consequence, or output of a process, to “feed back” and regulate the process itself. The feedback control of potassium is defined by the following stepwise cascade. In response to a high-potassium meal that includes glucose, pancreatic insulin secretion activates skeletal muscle and liver Na-K-ATPase, which pumps potassium (Na/K exchange) from the plasma to the intracellular fluid of these cells. This mechanism minimizes the postprandial increase in plasma potassium concentration.35 With muscle activity, potassium is released into the plasma and filtered at the glomerulus. To maintain balance, the amount of potassium consumed in the meal (minus the small amount lost in the feces) is excreted into the urine.
When an increase in potassium consumption increases plasma potassium concentration sufficiently, it triggers aldosterone synthesis and release from the adrenals, which stimulates the activity and synthesis of Na-K-ATPase and luminal potassium channels in collecting duct principal cells to secrete the excess potassium (Figure 1, Figure 2).36 Aldosterone also enhances potassium excretion in the distal colon.37 This latter function can be extremely important in the adaptation that occurs when renal function is compromised.
Figure 2.
A schematic depicting the complementary roles of the classic feedback and feed-forward control mechanisms for maintaining potassium homeostasis. An increase in plasma potassium evokes an array of responses that promote a kaliuresis. In contrast, the feed-forward control mechanism is engaged when dietary potassium is sensed by K+ sensors in the gastrointestinal tract in the absence of perceptible changes in plasma potassium.
Courtesy of Murray Epstein, MD, FASN.
Conversely, if potassium intake is very low or urinary potassium excretory losses are excessive, plasma potassium concentration decreases and the feedback regulation is invoked, redistributing potassium from intracellular fluid to plasma and thereby minimizing hypokalemia. Concomitantly, skeletal muscle becomes insulin-resistant to the uptake of potassium (but not glucose). Specifically, insulin continues to mediate glucose entry into cells, whereas potassium entry into cells (which usually follows glucose) is blunted. This may be due to a decrease in sodium pump activity or number. This blunting of potassium intake occurs even before the plasma potassium concentration decreases, which teleologically acts to blunt the shift of potassium from plasma into the cell.38
If hypokalemia ensues, the expression of skeletal muscle Na-K-ATPase alpha 2 isoform decreases, which facilitates a net potassium “leak” from intracellular fluid to the plasma.39 The low plasma potassium concentration suppresses adrenal aldosterone release; as a result, the kidney can reclaim essentially all but about 1% of the filtered potassium (Figure 1). This renal potassium conservation involves down-regulation of potassium secretion by the ROMK channels in the principal cells of the cortical collecting duct. In conditions of potassium depletion, potassium reabsorption can even occur in the collecting duct. This appears to be mediated by up-regulation in the apically located H-K-ATPase on intercalated cells.34
What is of great interest is the realization that this intricate feedback mechanism for potassium regulation is not the sole mechanism for compensatory renal potassium excretion. Rather, there is a complementary regulatory mechanism; a “feed-forward” control of potassium regulation that acts in a complementary manner to subserve potassium homeostasis.
Feed-forward control of potassium balance
Although feedback loops are commonly recognized as regulators of biological systems, feed-forward loops of various sorts are also important biological regulators.40, 41 Feed-forward control refers to a pathway in a homeostatic system that responds to a signal in the environment in a predetermined manner, without responding to how the system subsequently reacts (that is, without responding to feedback). A widely recognized example of feed-forward control is the conditioned salivation of Pavlov’s dogs in anticipation of food.40 The Pavlovian conditioned salivary reflex is an example of a feed-forward regulatory loop in that a stimulus temporally associated with food (input) triggers salivation (output) in advance of the presentation of food.40
With this theoretic construct as context, we now consider the formulation of another recent example of feed-forward control that is highly relevant and of great immediacy: the feed-forward control of potassium, which modulates potassium homeostasis. Thus, even minor changes in dietary potassium intake that are insufficient to alter plasma concentrations of either potassium42 or aldosterone43 and are consequently insufficient to activate feedback control are capable of evoking rapid changes in renal potassium excretion through feed-forward mechanisms.
Almost 30 years ago, Rabinowitz et al.44 conducted a series of elegant experiments in sheep that demonstrated that potassium intake in food or potassium placed into the rumen (sheep stomach) was associated with a large and significant increase in urinary potassium excretion. Although others45, 46, 47, 48 had earlier demonstrated that feeding was associated with an increase in urinary potassium excretion, Rabinowitz et al.44 designed a set of 13 experiments to explore the known factors that regulate urinary potassium excretion to determine which of them might contribute to this effect. As detailed in Table 1, the experiments showed that the increase in urinary potassium excretion was not related to an increase in serum potassium or glomerular filtration rate and thus was not a consequence of an increase in filtered potassium, but rather of tubular potassium excretion. The researchers demonstrated that aldosterone was not responsible for this by (i) showing that there was no change in plasma aldosterone concentration, (ii) infusing aldosterone and showing that it did not alter the effect, or (iii) giving an early aldosterone antagonist (potassium canrenoate), which also did not alter the effect. Similarly, when either urine flow rate, sodium excretion, or urine pH was altered, the effect persisted (Table 1). Rabinowitz et al.44 concluded, “The efferent factors involved do not appear to be aldosterone, urine flow, sodium excretion, or acid/base status, nor do changes in plasma potassium appear to be necessary or sufficient to produce the changes in potassium excretion associated with meal intake or fasting.” They concluded that their studies were “compatible with the presence of receptors located at some point prior to the systemic circulation, which sense enteric potassium levels and influence renal potassium excretion.”
Table 1.
Determinants of the increase in urinary potassium excretion associated with feeding
|
Numerous studies have subsequently been conducted (i) to confirm these findings in additional species, including humans, (ii) to try and determine the location for this putative potassium sensor; and (iii) to evaluate potential signals that might increase renal potassium excretion.
Studies in different species to confirm the effect that potassium administered directly into the gastrointestinal tract leads to an increase in renal potassium excretion
Lee et al.,47 Oh et al.,48 and Morita et al.49 conducted studies in anesthetized rats with findings consistent with those of Rabinowitz et al.44 Caló et al.42 found qualitatively similar findings in humans. In these human studies, during a water diuresis, intake of potassium led to an increase in urinary potassium excretion within 20 minutes, at a time where neither plasma potassium nor aldosterone had increased.
Recently, Preston et al.50 conducted studies to further delineate this gastrointestinal (GI)–renal kaliuretic signaling axis in 32 normal subjects in a clinical research unit while on a 20-mmol sodium and 60-mmol potassium diet. The serum potassium concentration, potassium excretion, aldosterone, and insulin were measured following either a 35-mmol oral potassium load, a potassium- and sodium-deficient complex meal, or a potassium-deficient complex meal plus 35-mmol potassium. This experimental design facilitated determination of the component effects on potassium handling of the meal and on potassium load separately. The meal-plus-potassium test was repeated following aldosterone blockade achieved with eplerenone treatment to specifically evaluate the role of aldosterone. In response to the potassium-deficient meal plus 35-mmol potassium, the serum potassium did not increase but the hourly mean potassium excretion increased sharply. This kaliuresis persisted following aldosterone blockade with eplerenone, thereby suggesting independence from aldosterone. The investigators concluded that this experiment further substantiated the existence of a “GI–renal kaliuretic” signaling axis in humans that is capable of mediating potassium excretion independent of changes in the serum potassium concentration and aldosterone.50
Studies designed to determine the location for the GI potassium sensor
The original studies by Rabinowitz et al.43, 44 involved direct administration of potassium into the rumens of sheep. Morita et al.49 and Tsuchiya et al.51 suggested that the sensor might be in the hepatoportal area, whereas Lee et al.47 indicated that the stomach, and not the portal circulation, was more important. In the studies by Morita et al.49 and Tsuchiya et al.,51 intraportal infusion of potassium led to a greater renal potassium excretion than did an intravenous infusion. Cutting the periarterial hepatic nervous plexus diminished the increase in urinary potassium excretion. In addition, potassium infused directly into the hepatoportal circulation stimulated hepatic afferent nerve activity and increased urinary potassium excretion without changes in plasma potassium.
Studies designed to determine the potential signal(s) that might increase renal potassium excretion
Oh et al.48 evaluated a number of GI hormones to test whether they might be responsible for the increase in renal potassium excretion. These investigators evaluated guanylin, uroguanylin, glucagon-like peptide, and extra-intestinal hormones such as arginine vasopressin, alpha melanocyte-stimulating hormone, gamma melanocyte stimulating hormone, and aldosterone. Their data do not support a role for these hormones in this phenomenon, leading them to suggest that there might be “previously unknown humoral factors that stimulate renal K+ excretion during dietary K+ intake.”
Although not a GI hormone, the renal kallikrein-kinin system is activated by a high K+ diet or by acute K+ loading, leading to an increase in urinary tissue kallikrein.52 El Moghrabi et al.53 showed, in mice, that renal tissue kallikrein can lead to an increase in renal potassium excretion by both activating the epithelial Na channel to increase K+ excretion by principal cells and inhibiting H-K-ATPase in intercalated cells to decrease K+ reabsorption. Renal K+ excretion increased in concert with tissue kallikrein excretion following a single meal. The investigators further showed that in tissue kallikrein knockout mice, there was a greater increase in serum potassium following a meal, suggesting that tissue kallikrein plays a role in renal potassium excretion. Thus, although renal tissue kallikrein is not a GI-derived signal, it could play a role in this phenomenon at the level of the kidney.
The studies of Sorensen et al.54 may be relevant in further considering mechanisms that are operative at the level of the kidney. These investigators studied mice and found that an acute intragastric potassium load led to rapid dephosphorylation of the renal sodium chloride cotransporter, which was associated, as would be predicted, with an increase in sodium and potassium excretion. Sorensen et al.54 showed that this effect was independent of aldosterone.
Although glucagon is also a circulating hormone that acts on the kidney to increase electrolyte excretion, including potassium, it is unlikely to play a role. Glucagon acts at the level of the proximal tubule, and its potential role as a mediator of the kaliuresis invoked by an oral potassium load has not been delineated.
Figure 3 summarizes the integrated roles of the kidney, extrarenal mechanisms, and gastrointestinal effectors in modulating potassium homeostasis. It demonstrates that the undamped increase in plasma K+ in response to potassium administration is progressively attenuated by the adaptive responses by the kidney, by a hierarchy of nonrenal mechanisms including participation by insulin and glucose, and by gastrointestinal mechanisms evoked by GI potassium sensors.
Figure 3.

A summary of the integrated roles of the kidney, extrarenal mechanisms, and gastrointestinal effectors in modulating potassium homeostasis. It demonstrates that the undamped increase in plasma K+ in response to potassium administration is progressively attenuated by the adaptive responses by the kidney, by a hierarchy of nonrenal mechanisms, including participation by insulin and glucose, and by gastrointestinal mechanisms evoked by gastrointestinal potassium sensors.
Courtesy of Murray Epstein, MD, FASN.
Additional determinants of renal potassium excretion
Both circadian rhythm and posture also constitute determinants of potassium excretion.
Circadian rhythm of potassium excretion
The presence of circadian rhythms that characterize renal function and excretion has been documented for more than 60 years.55, 56 Kidney parameters with such rhythms include glomerular filtration rate, renal plasma flow, and tubular reabsorption and secretion for most of the major urinary electrolytes. Of interest for the focus of this review is the demonstration of a circadian rhythm that characterizes renal potassium excretion in humans, with a peak in the middle of the day.57 This pattern is independent of activity, posture, and dietary intake, and this circadian rhythm persists for days in individuals and is isolated from most external cues.
It is interesting to note that this pattern of renal potassium excretion was originally thought to be driven by factors mainly external to the kidney. Recent studies, however, have demonstrated rhythms within the tubule that would account for many of these changes. Steele et al.58 suggested that transtubular potassium gradients could be the driving force for the cycles in potassium excretion. Zuber et al.59 demonstrated rhythmicity in transcripts from cells of the rat distal nephron (distal convoluted tubule/connecting tubule, and cortical collecting duct) in potassium channels such as ROMK1; potassium channel, 2-pore domain subfamily K, member 1 (KCNK1); and potassium channel, inward rectifying subfamily J, member 10 (KCNJ10). Their finding of ROMK1 cycling by about 30% at the mRNA level might explain part of the circadian variation observed in renal potassium excretion. Finally, because aldosterone is acknowledged to play a critical role in renal potassium excretion, the demonstration of a circadian rhythm in aldosterone secretion60 and its consequent effects at the level of the tubule61 allow for the possibility that cycles in renal potassium excretion could be controlled both by cycles outside the kidney, such as aldosterone, as well as cycles within the kidney tubules themselves.
Posture and potassium excretion
In contrast to circadian rhythm, studies conducted in the 1950s found little or no effect of posture on renal potassium excretion.62 “Posture had comparatively little prolonged effect on absolute potassium excretion when allowance was made for diurnal rhythmic variations.”63 “Change of posture usually produced less disturbance of the diurnal rhythm in potassium excretion, and changes in potassium excretion were usually small in comparison with those in sodium output.”64 In summary, posture can exert a considerable effect on urine flow rate and sodium excretion, but the available data suggest that changes in potassium excretion are relatively small.
GI potassium absorption and excretion
As indicated earlier, stool potassium usually averages about 10% of ingested potassium. Thus, on a standard Western diet with approximately 80 mEq of potassium, this would regularly lead to approximately 8 mEq of potassium in the stool.65 In normal individuals, the large fraction of ingested potassium is absorbed by the small intestine, and the contribution of the normal colon to net potassium absorption or secretion is small.65 Potassium transport in the duodenum, jejunum, ileum, and colon has been characterized as passive absorption, although the colon also demonstrates passive secretion.66 Colonic potassium secretion exists in mammals67 and has been shown to play a role in potassium homeostasis in patients on dialysis.68 Administration of mineralocorticoids leads to small but significant increases in stool potassium excretion.65, 69 Factors that might lead to an increase in stool potassium have been reviewed65 and are briefly discussed in the following paragraphs.
Colonic potassium handling
Relatively recent studies in rodents, over the past 2 decades, have further defined the intricacies of mammalian colonic K+ handling.70 One important basic aspect of K+ transport in the colon is its segmental difference. Under normal conditions, the proximal colon performs net K+ secretion, whereas net K+ absorption is observed in the distal colon. The proximal colon does not absorb K+ actively. Active K+ absorption in the distal colon occurs via the transcellular route and involves a primary active K+ entry step across the apical membrane. In the mammalian distal colon, this active transport is conducted by the nongastric H-K-ATPase localized in surface cells of the distal colon. Mice with this H-K-ATPase knocked out maintain a normal serum K+ when fed a normal diet, but become hypokalemic on a low-K+ diet. Thus, this H-K-ATPase plays a critical role in colonic K+ absorption.
Net K+ secretion is found in both the proximal colon and distal colon in animals fed a high-K+ diet. Present evidence suggests that net K+ secretion is effected by K+ channels. Although there are several potential K+ channels that could perform this function, BK channels seem to be the only functionally significant K+ secretory ion channel in the apical membrane of the distal colon.70 The role of the colon in clinically modulating K+ excretion is not adequately recognized. As mentioned earlier, normal individuals on a normal diet absorb virtually all of their ingested K+, and most of this ingested K+ is excreted in the urine. Importantly, in the setting of chronic kidney disease—and in particular, chronic dialysis patients—colonic K+ secretion is greatly enhanced and becomes an important accessory K+ excretory pathway.67 The recent approval of the postassium-binding polymer patiromer (Veltassa; Relypsa, Redwood City, CA) and the imminent availability of the potassium binder sodium zirconium cyclosilicate (also known as ZS-9; ZS Pharma, San Mateo, CA), both of which modulate their effects in large part in the colon, has further emphasized the importance of increasing our understanding of the physiology of colonic potassium handling.
Potassium binding in the GI tract
The role of potassium binding resins in hyperkalemia
As we have already detailed, the majority of potassium is renally excreted, but approximately 5% to 10% is secreted in the colon. Potassium is regularly excreted in the stool, and that process could potentially be augmented by cation exchange resins, which could then lead to a consequent increase in stool potassium excretion. In the final article in this supplement, Weir examines the potential role and drawbacks of the most widely used exchange resin at present: sodium polystyrene sulfonate (Kayexalate; Clovis Pharmaceuticals, Cary, NC, and others).26
These considerations set the stage for rationally taking advantage of the newer efficacious and “patient-friendly” potassium binders—patiromer and ZS-9. As reviewed by Weir,26 one of these new agents, patiromer, was approved by the US Food and Drug Administration on October 21, 2015. The other agent, ZS-9, is in the late stages of clinical development for the treatment of hyperkalemia and, with the US Food and Drug Administration’s acceptance of the ZS-9 New Drug Application, has an anticipated review date of May 26, 2016.
Summary
We have comprehensively reviewed the integrated mechanisms controlling the maintenance of potassium homeostasis, including its modulation by the classic “feedback control” of potassium balance. We also reviewed recent studies that have focused attention on novel physiological paradigms that increase the complexity but also the precision of potassium homeostasis, that is, the complementary paradigm—the feed-forward control of potassium balance. Awareness of this updated integrative control mechanism for potassium homeostasis is more relevant today when the medical community is increasingly focused on leveraging and expanding established RAASi treatment regimens that have been documented to confer substantive benefits, such as reducing cardiovascular events and retarding the progression of renal disease in several disease states, including congestive heart failure, chronic kidney disease, and diabetes,13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 but regretfully, with consequent hyperkalemia. We hope that the knowledge imparted by this update of potassium homeostasis will facilitate the clinical management of hyperkalemia in patients with chronic and recurrent hyperkalemia.
Disclosure
Publication of this article was supported by Relypsa, Inc. ME is a consultant for Relypsa, Inc., Bayer HealthCare Pharmaceuticals, OPKO Health, Inc., and Novartis Pharmaceuticals. MDL declares no competing interests.
Acknowledgments
The authors thank Lisa Savitt for expert editorial services and Christine Kittler for accessing many of the articles.
References
- 1.Epstein M. Hyperkalemia as a constraint to therapy with combination renin-angiotensin system blockade: the elephant in the room. J Clin Hypertens (Greenwich) 2009;11:55–60. doi: 10.1111/j.1751-7176.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitt B., Anker S.D., Bushinsky D.A. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–828. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein M., Reaven N.L., Funk S.E. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care. 2015;21(suppl):S212–S220. [PubMed] [Google Scholar]
- 4.Kosiborod M., Rasmussen H.S., Lavin P. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 5.Packham D.K., Rasmussen H.S., Lavin P.T. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 6.Weir M.R., Bakris G.L., Bushinsky D.A., for the OPAL-HK Investigators Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 7.Bakris G.L., Pitt B., Weir M.R., for the AMETHYST-DN Investigators Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161. doi: 10.1001/jama.2015.7446. [DOI] [PubMed] [Google Scholar]
- 8.Edner M., Benson L., Dahlström U., Lund L.H. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: a prospective propensity score-matched cohort study. Eur Heart J. 2015;36:2318–2326. doi: 10.1093/eurheartj/ehv268. [DOI] [PubMed] [Google Scholar]
- 9.Rassi A.N., Cavender M.A., Fonarow G.C. Temporal trends and predictors in the use of aldosterone antagonists post-acute myocardial infarction. J Am Coll Cardiol. 2013;61:35–40. doi: 10.1016/j.jacc.2012.08.1019. [DOI] [PubMed] [Google Scholar]
- 10.Maggioni A.P., Anker S.D., Dahlström U., for the Heart Failure Association of the ESC Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12,440 patients of the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2013;15:1173–1184. doi: 10.1093/eurjhf/hft134. [DOI] [PubMed] [Google Scholar]
- 11.Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol. 2015;3:993–1003. doi: 10.1016/S2213-8587(15)00289-2. [DOI] [PubMed] [Google Scholar]
- 12.Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl. 2016;6:20–28. doi: 10.1016/j.kisu.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 14.Lewis E.J., Hunsicker L.G., Clarke W.R., for the Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 15.Pitt B., Zannad F., Remme W.J., for the Randomized Aldactone Evaluation Study Investigators The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 16.Pitt B., Remme W., Zannad F. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 17.Zannad F., McMurray J.J., Krum H., for the EMPHASIS-HF Study Group Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 18.McMurray J.J., Adamopoulos S., Anker S.D. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology: developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 19.The National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Available at: http://www2.kidney.org/professionals/KDOQI/guidelines_bp/guide_11.htm. Accessed November 9, 2015. [PubMed]
- 20.National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. Available at: http://www.nice.org.uk/guidance/cg182. Accessed October 4, 2015. [PubMed]
- 21.American Diabetes Association Standards of medical care in diabetes–2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg R., Yusuf S., for the Collaborative Group on ACE Inhibitor Trials Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA. 1995;273:1450–1456. [PubMed] [Google Scholar]
- 23.Maschio G., Alberti D., Janin G., for the Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 24.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 25.McMurray J.J., Packer M., Desai A.S., for the PARADIGM-HF Investigators and Committees Angiotensin-neprilysin inhibition versus enalapril in heart failure. New Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 26.Weir M.R. Current and future treatment options for managing hyperkalemia. Kidney Int Suppl. 2016;6:29–34. doi: 10.1016/j.kisu.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer B.F. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015;10:1050–1060. doi: 10.2215/CJN.08580813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott P., Dyer A., Stamler R., for the INTERSALT Co-operative Research Group The INTERSALT study: results for 24 hour sodium and potassium, by age and sex. J Hum Hypertens. 1989;3:323–330. [PubMed] [Google Scholar]
- 29.Subramanya A.R., Ellison D.H. Distal convoluted tubule. Clin J Am Soc Nephrol. 2014;9:2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peti-Peterdi J., Warnock D.G., Bell P.D. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y., Zavilowitz B., Satlin L.M., Wang W.H. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem. 2007;282:6455–6462. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sansom S.C., Welling P.A. Two channels for one job. Kidney Int. 2007;72:529–530. doi: 10.1038/sj.ki.5002438. [DOI] [PubMed] [Google Scholar]
- 33.Field M.J., Stanton B.A., Giebisch G.H. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J Clin Invest. 1984;74:1792–1802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DuBose T.D., Jr., Codina J., Burges A., Pressley T.A. Regulation of H(+)-K(+)-ATPase expression in kidney. Am J Physiol. 1995;269:F500–F507. doi: 10.1152/ajprenal.1995.269.4.F500. [DOI] [PubMed] [Google Scholar]
- 35.DeFronzo R.A., Felig P., Ferrannini E., Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am J Physiol. 1980;238:E421–E427. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 36.Wang W. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 37.Giebisch G., Krapf R., Wagner C. Renal and extrarenal regulation of potassium. Kidney Int. 2007;72:397–410. doi: 10.1038/sj.ki.5002288. [DOI] [PubMed] [Google Scholar]
- 38.McDonough A.A., Youn J.H. Role of muscle in regulating extracellular [K+] Semin Nephrol. 2005;25:335–342. doi: 10.1016/j.semnephrol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 39.McDonough A.A., Thompson C.B., Youn J.H. Skeletal muscle regulates extracellular potassium. Am J Physiol Renal Physiol. 2002;282:F967–F974. doi: 10.1152/ajprenal.00360.2001. [DOI] [PubMed] [Google Scholar]
- 40.Domjan M., Cusato B., Villarreal R. Pavlovian feed-forward mechanisms in the control of social behavior. Behav Brain Sci. 2000;23:235–249. doi: 10.1017/s0140525x00002430. [DOI] [PubMed] [Google Scholar]
- 41.Re R.N. A mechanism for mineralocortcoid participation in renal disease and heart failure. J Am Soc Hypertens. 2015;9:586–591. doi: 10.1016/j.jash.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Caló L., Borsatti A., Favaro S., Rabinowitz L. Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron. 1995;69:253–258. doi: 10.1159/000188466. [DOI] [PubMed] [Google Scholar]
- 43.Rabinowitz L., Denham S.C., Gunther R.A. Aldosterone and postprandial renal excretion of sodium and potassium in sheep. Am J Physiol. 1977;233:F213–F216. doi: 10.1152/ajprenal.1977.233.3.F213. [DOI] [PubMed] [Google Scholar]
- 44.Rabinowitz L., Green D.M., Sarason R.L., Yamauchi H. Homeostatic potassium excretion in fed and fasted sheep. Am J Physiol. 1988;254:R357–R380. doi: 10.1152/ajpregu.1988.254.2.R357. [DOI] [PubMed] [Google Scholar]
- 45.Dewhurst J.K., Harrison F.A., Keynes R.D. Renal excretion of potassium in the sheep. J Physiol. 1968;195:609–621. doi: 10.1113/jphysiol.1968.sp008476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarelius I.H., Greenway R.M. Rhythmic fluctuations in the urine composition of sheep: separation of food-dependent from other rhythms. Pflugers Arch. 1975;355:243–259. doi: 10.1007/BF00583687. [DOI] [PubMed] [Google Scholar]
- 47.Lee F.N., Oh G., McDonough A.A., Youn J.H. Evidence for gut factor in K homeostasis. Am J Physiol Renal Physiol. 2007;293:F541–F547. doi: 10.1152/ajprenal.00427.2006. [DOI] [PubMed] [Google Scholar]
- 48.Oh K.S., Oh Y.T., Kim S.W. Gut sensing of dietary K+ intake increases renal K+ excretion. Am J Physiol Regul Integr Comp Physiol. 2011;301:R421–R429. doi: 10.1152/ajpregu.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morita H., Fujiki N., Miyahara T. Hepatoportal bumetanide-sensitive K(+)-sensor mechanism controls urinary K(+) excretion. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1134–R1139. doi: 10.1152/ajpregu.2000.278.5.R1134. [DOI] [PubMed] [Google Scholar]
- 50.Preston R.A., Afshartous D., Rodco R. Evidence for a gastrointestinal-renal kaliuretic signaling axis in humans. Kidney Int. 2015;88:1383–1391. doi: 10.1038/ki.2015.243. [DOI] [PubMed] [Google Scholar]
- 51.Tsuchiya Y., Nakashima S., Banno Y. Effect of high-NaCl or high-KCl diet on hepatic Na+- and K+-receptor sensitivity and NKCC1 expression in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R591–R596. doi: 10.1152/ajpregu.00559.2003. [DOI] [PubMed] [Google Scholar]
- 52.Vio C.P., Figueroa C.D. Evidence for a stimulatory effect of high potassium diet on renal kallikrein. Kidney Int. 1987;31:1327–1334. doi: 10.1038/ki.1987.146. [DOI] [PubMed] [Google Scholar]
- 53.El Moghrabi S., Houillier P., Picard N. Tissue kallikrein permits early renal adaptation to potassium load. Proc Natl Acad Sci U S A. 2010;107:13526–13531. doi: 10.1073/pnas.0913070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sorensen M.V., Grossmann S., Roesinger M. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 55.Gumz M.L., Rabinowitz L., Wingo C.S. An integrated view of potassium homeostasis. N Engl J Med. 2015;373:60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mills J.N., Stanbury S.W. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol. 1952;117:22–37. [PMC free article] [PubMed] [Google Scholar]
- 57.Moore Ede M.C., Brennan M.F., Ball M.R. Circadian variation of intercompartmental potassium fluxes in man. J Appl Physiol. 1975;38:163–170. doi: 10.1152/jappl.1975.38.1.163. [DOI] [PubMed] [Google Scholar]
- 58.Steele A., deVeber H., Quaggin S.E. What is responsible for the diurnal variation in potassium excretion? Am J Physiol. 1994;267:R554–R560. doi: 10.1152/ajpregu.1994.267.2.R554. [DOI] [PubMed] [Google Scholar]
- 59.Zuber A.M., Centeno G., Pradervand S. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A. 2009;106:16523–16528. doi: 10.1073/pnas.0904890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doi M., Takahashi Y., Komatsu R. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16:67–74. doi: 10.1038/nm.2061. [DOI] [PubMed] [Google Scholar]
- 61.Susa K., Sohara E., Isobe K. WNK-OSR1/SPAK-NCC signal cascade has circadian rhythm dependent on aldosterone. Biochem Biophys Res Commun. 2012;427:743–747. doi: 10.1016/j.bbrc.2012.09.130. [DOI] [PubMed] [Google Scholar]
- 62.Viar W.N., Oliver B.B., Eisenberg S. The effect of posture and of compression of the neck on excretion of electrolytes and glomerular filtration: further studies. Circulation. 1951;3:105–115. doi: 10.1161/01.cir.3.1.105. [DOI] [PubMed] [Google Scholar]
- 63.Thomas S. Some effects of changes of posture on water and electrolyte excretion by the human kidney. J Physiol. 1957;139:337–352. doi: 10.1113/jphysiol.1957.sp005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas S. Effects of change of posture on the diurnal renal excretion rhythm. J Physiol. 1959;148:489–506. doi: 10.1113/jphysiol.1959.sp006302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Relman A.S., Schwartz W.B. The effect of DOCA on electrolyte balance in normal man and its relation to sodium chloride intake. Yale J Biol Med. 1952;24:540–558. [PMC free article] [PubMed] [Google Scholar]
- 66.Michell A.R., Debnam E.S., Unwin R.J. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu Rev Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- 67.Kliger A.S., Binder H.J., Bastl C., Hayslett J.P. Demonstration of active potassium transport in the mammalian colon. J Clin Invest. 1981;67:1189–1196. doi: 10.1172/JCI110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandle G.I., Gaiger E., Tapster S., Goodship T.H. Evidence for large intestinal control of potassium homoeostasis in uraemic patients undergoing long-term dialysis. Clin Sci (Lond) 1987;73:247–252. doi: 10.1042/cs0730247. [DOI] [PubMed] [Google Scholar]
- 69.Agarwal R., Afzalpurkar R., Fordtran J.S. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology. 1994;107:548–571. doi: 10.1016/0016-5085(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 70.Sorensen M.V., Matos J.E., Praetorius H.A., Leipziger J. Colonic potassium handling. Pflugers Arch. 2010;459:645–656. doi: 10.1007/s00424-009-0781-9. [DOI] [PubMed] [Google Scholar]