Abstract
BACKGROUND:
Angiogenic factors are important in granuloma formation and serve as biomarkers in pulmonary tuberculosis (PTB). The relationship between these markers and tuberculous lymphadenitis (TBL) is not known.
OBJECTIVE AND DESIGN:
To examine the association of vascular endothelial growth factor (VEGF) and angiopoietin (Ang) family molecules in TBL, we measured systemic levels of VEGF-A, C, D, R1 (VEGF-receptor 1), R2, R3, Ang-1, Ang-2 and TIE2 (tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2) levels in TBL, latent tuberculous infection (LTBI) and lymph node culture supernatants (VEGF-A, C and Ang-2) of the same TBL patients.
RESULTS:
Circulating levels of VEGF-A and VEGF-C were significantly diminished, whereas VEGF-R2, R3, Ang-2 and TIE2 levels were significantly increased, in TBL. Likewise, VEGF-A, C and Ang-2 levels were significantly increased in lymph node supernatants compared with plasma in individuals with TBL. Receiver operating characteristic curve analysis showed that VEGF-C and VEGF-R2 markers clearly distinguished TBL from LTBI. Following treatment, VEGF-C and Ang-1 levels were significantly altered. No association was observed between angiogenic factors and culture grade or lymph node size, except for VEGF-A. VEGF-A was also significantly decreased in multiple lymph nodes compared with single lymph nodes.
CONCLUSIONS:
Our data suggest that altered levels of circulating angiogenic factors in TBL might reflect underlying vasculo-endothelial dysfunction. Reversal of angiogenic markers after anti-tuberculosis treatment suggests that these angiogenic markers may serve as biomarkers of disease severity or response to treatment in TBL.
Keywords: tuberculous lymphadenitis, ELISA, VEGF, Angiopoietin, circulating biomarker
RÉSUMÉ
CONTEXTE:
Les facteurs angiogéniques sont importants dans la formation du granulome et servent de biomarqueurs dans la tuberculose pulmonaire. L’association de ces marqueurs avec la lymphadénite tuberculeuse (TBL) est inconnue.
OBJECTIFET SCHÉMA:
Examiner l’association entre le facteur de croissance de l’endothélium vasculaire (VEGF) et des molécules de la famille Ang dans la TBL. Nous avons mesuré les niveaux systémiques de VEGF-A, C, D, R1, R2, R3, Ang-1,2 et TIE2 (tyrosine kinase avec des domaines de type facteur de croissance épidermique et de type immunoglobuline 2) dans la TBL, l’infection tuberculeuse latente (LTBI) et les surnageants de culture de ganglions (VEGF-A, C et Ang-2) des meˆmes patients TBL.
RÉSULTATS:
Les niveaux circulants de VEGF-A et de VEGF-C ont été significativement diminués, tandis que les niveaux de VEGF-R2, R3, Ang-2 et TIE2 ont été significativement élevés dans la TBL. De même, les niveaux VEGF-A,C et Ang-2 ont és;té significativement élevés dans les surnageants de ganglion lymphatique quand on les compare au plasma de patients atteints de TBL. L’analyse courbe de la fonction d’efficacité du récepteur a révélé que les marqueurs VEGF-C et VEGF-R2 avaient clairement distingué la TBL de la LTBI. Après la chimiothérapie, les niveaux de VEGF-C et Ang-1 ont été significativement altérés. Aucune association n’a été observée en ce qui concerne les facteurs angiogéniques comparés au grade de la culture et a` la taille du ganglion lymphatique, sauf pour VEGF-A. VEGF-A a également été significativement diminué dans les ganglions lymphatiques multiples en comparaison avec les ganglions lymphatiques uniques.
CONCLUSION:
Nos données révèlent que l’altération des niveaux de facteurs angiogéniques circulants dans la TBL pourraient refléter une dysfonction vasculo-endothéliale sous-jacente. L’inversion des marqueurs angiogéniques après le traitement anti-tuberculeux révèle que ces marqueurs angiogéniques pourraient servir de biomarqueurs de gravité de la maladie ou de réponse a` la chimiothérapie dans la TBL.
RESUMEN
MARCO DE REFERENCIA:
Los factores angiógenos son importantes en la formación del granuloma y sirven como biomarcadores de la tuberculosis pulmonar. No se conoce la correlación entre estos marcadores y la linfadenitis tuberculosa (TBL).
OBJETIVO Y MÉTODOS:
Por esta razón, en el presente estudio se examinóla relación de las moléculas de la familia de los factores de crecimiento vascular endotelial (VEGF) y la familia de las angiopoyetinas (Ang) con la TBL. Se midieron las concentraciones sistémicas de VEGF-A, C, D, R1, R2, R3, Ang-1, Ang-2 y del receptor TIE2 (tirosina cinasa con dominios tipo inmunoglobulina y similares al factor de crecimiento epidérmico 2) en casos de TBL, infección tuberculosa latente (LTBI) y en los sobrenadantes del cultivo de nódulos linfáticos (VEGF-A, C y Ang-2) de las mismas personas con TBL.
RESULTADOS:
Se observóuna concentración muy disminuida de VEGF-A y VEGF-C circulantes y una concentración notablemente alta de VEGF-R2, R3, Ang2 y TIE2 en los casos de TBL. De igual manera, las concentraciones de VEGF-A, C y Ang-2 fueron muy altas en los sobrenadantes del cultivo de ganglios linfá ticos, en comparación con su concentración plasmática en las personas con TBL. El análisis ROC (característica operativa del receptor) revelóque los marcadores VEGF-C y VEGF-R2 pueden diferenciar claramente la TBL de la LTBI. Tras el tratamiento médico, las concentraciones de VEGF-C y Ang-1 se modificaron de manera considerable. No se observóninguna correlación al comparar los factores angiógenos con el grado de clasificación del cultivo y el taman˜o de los ganglios linfáticos, a excepción del VEGF-A. Además, el VEGF-A estaba muy disminuido en los casos de afectación de ganglios linfáticos múltiples en comparación con la afectación de ganglios linfáticos únicos.
CONCLUSIÓN:
Los resultados del estudio revelan que la alteración de las concentraciones de los factores angiógenos circulantes en la TBL podría corresponder a una disfunción subyacente del endotelio vascular. La recuperación de los marcadores angiógenos después del tratamiento antituberculoso pone de manifiesto que estos factores pueden constituir biomarcadores de la gravedad de la enfermedad o de la respuesta al tratamiento médico en los casos de TBL.
ANGIOGENESIS is an obligatory biological process in healthy and diseased states.1 Vascular endothelial growth factors (VEGFs) are a family of secreted polypeptides that are crucial for regulating the formation of vessels during embryonic growth. These anti-parallel homodimers, characterised by an eight cysteine-knot superfamily of growth factors, are key ‘governors’ of angiogenesis and lymphangiogenesis.2 These VEGFs bind to their endothelial transmembrane receptors with varying specificities.3 The biological function of VEGF isoforms as well as their receptors has been described; any defect in the former can lead to various disorders.1,4
Angiopoietins (Angs) are also essential for vascular growth and angiogenesis. Specifically, Ang-2 is involved in promoting blood vessel growth and sprouting in concert with VEGF, whereas Ang-1 helps to stabilise blood vessels through TIE2 (tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2) receptors and promotes vascular maturation.5 Angiogenesis and lymphangiogenesis may therefore play important roles in the pathogenesis of tuberculosis (TB).
TB is characterised by the formation of granulomatous inflammation.6 Tuberculous lymphadenitis (TBL) is the most common form of the extra-pulmonary manifestation of TB, comprising 2–5% of all TB cases and characterised by the local manifestation of systemic disease. The most common appearance is the involvement of the cervical chain, reported in 45– 70% of cases, with 12–26% in the supraclavicular region; 20% of cases are bilateral. TBL has a high prevalence due to poor immune responses and dissemination of mycobacteria after repeated initial infection via the tonsils or lung parenchyma. TBL is more common in females and is widespread in Asian Our research team recently analysed the circulating TB patients. The prevalence of lymphadenitis is higher if infection is associated with human immunodeficiency virus (HIV) infection.7 Although a wide array of specific and non-specific host-immune responses contribute to the differential outcomes of TB, substantial reports on the severity of extra-pulmonary tuberculosis (EPTB) are not available.
Our research team recently analysed the circulating levels of angiogenic factors in pulmonary TB (PTB).8 Increased levels of VEGF-A have been reported in the sputum and peripheral blood of individuals with PTB.8–10 The present study aimed to analyse circulating angiogenic makers in TBL patients, pre- and post-treatment, and in individuals with latent tuberculous infection (LTBI).
MATERIALS AND METHODS
Ethics statement
All the study volunteers enrolled were examined according to the clinical research protocol (NIR-TIEC2010007) approved by the Institutional Ethical Committee Review Board of the National Institute for Research in Tuberculosis (NIRT), Chennai, India. Written informed consent was provided by all study participants.
Study population
A total of 44 platelet-poor plasma samples from individuals with TBL, along with 23 samples taken during follow-up visits, and samples from 44 individuals with LTBI, were collected in Chennai. Likewise, 22 TBL lymph node culture supernatants with matched plasma samples were also used. Lymph nodes were processed and culture supernatants obtained as described elsewhere.11 It has been reported that VEGF is released from platelets during platelet aggregation;12 samples were therefore collected from sodium-heparin whole blood, as described previously.13
The enrolled study participants did not consume drugs that interfere with platelet activation or aggregation. TBL was diagnosed on the basis of bacteriological culture (performed on homogenised lymph node tissue) positivity. Lymph node culture grades were used to identifybacterialburdenandclassifiedas 1+, 2+and 3+. During enrolment, none of the active TBL patients had any proof of previous exposure to TB disease or intake ofanti-tuberculosismedication. Noneoftheindividuals with TBL had PTB. LTBI status was diagnosed basedon tuberculin skin test positivity and Quanti FERON® TB Gold In-Tube (Qiagen, Hilden, Germany) enzyme-linked immunosorbent assay (ELISA), lack of chest radiograph abnormalities or pulmonary symptoms and negative sputum smear. All study participants were bacille Calmette-Guérin-vaccinated, non-diabetic and HIV-negative, and had a normal body mass index. The demographics of the study groups and their baseline characteristics are given in the Table. Standard anti-tuberculosis treatment was administered, and blood samples were collected again at the end of treatment.
Table.
Demographics of the study population
| Item | TBL pre-treatment | TBL post-treatment | LTBI |
|---|---|---|---|
| Subjects recruited, n | 44 | 23 | 44 |
| Sex: male/female | 16/28 | 10/13 | 20/24 |
| Age, years, median (range) | 30 (18–59)* | 27 (18–59) | 38 (21–60)* |
| Lymph node culture grade: 0/1+/2+/3+ | 9/23/10/2 | Not done | Not done |
No significant difference was observed between the groups (Mann-Whitney test). TBL = tuberculous lymphadenitis; LTBI = latent tuberculous infection.
Enzyme-linked immunosorbent assay
Circulating levels of VEGF-A, VEGF-C, VEGF-R1 (VEGF-receptor 1), VEGF-R2, VEGF-R3, Ang-1 and Ang-2 were measured in platelet-poor plasma using the Duoset ELISA Development System (R&D Systems, Minneapolis, MN, USA). VEGF-A, VEGF-C and Ang-2 levels were also measured in lymph node supernatants. VEGF-D and TIE2 levels were measured using a Quantikine® ELISA kit (R&D Systems). The minimum limit of detection (in pg/ml) of individual markers was: VEGF-A, 31.25; VEGF-C, 62.5; VEGF-D, 62.5; VEGF-R1, 125; VEGF-R2, 31.25; VEGF-R3, 156.25; Ang-1, 156.25; Ang-2, 93.75; also, the limit of detection for the Ang receptor (TIE2) was 0.313 ng/ml.
Statistical analysis
The geometric mean (GM) was used to determine trends. Statistically significant differences were analysed using the Mann-Whitney test, with Holm’s correction between the two study groups. Receiver operator characteristics (ROC) curves were designed to test the power of each candidate angiogenic factor to distinguish TBL from LTBI. The Wilcoxon signedrank test was performed to compare angiogenic factor concentrations before and after anti-tuberculosis treatment. All analyses were performed using Prism v6 (GraphPad, La Jolla, CA, USA).
RESULTS
Diminished vascular endothelial growth factors and increased receptor levels are observed in tuberculous lymphadenitis
To examine systemic levels of angiogenic factors associated with TBL disease, we measured the circulating levels of VEGF-A, C, D, R1, R2 and R3, Ang-1, Ang-2 and TIE2 in individual TBL plasma samples, and compared them with LTBI samples (Figure 1).
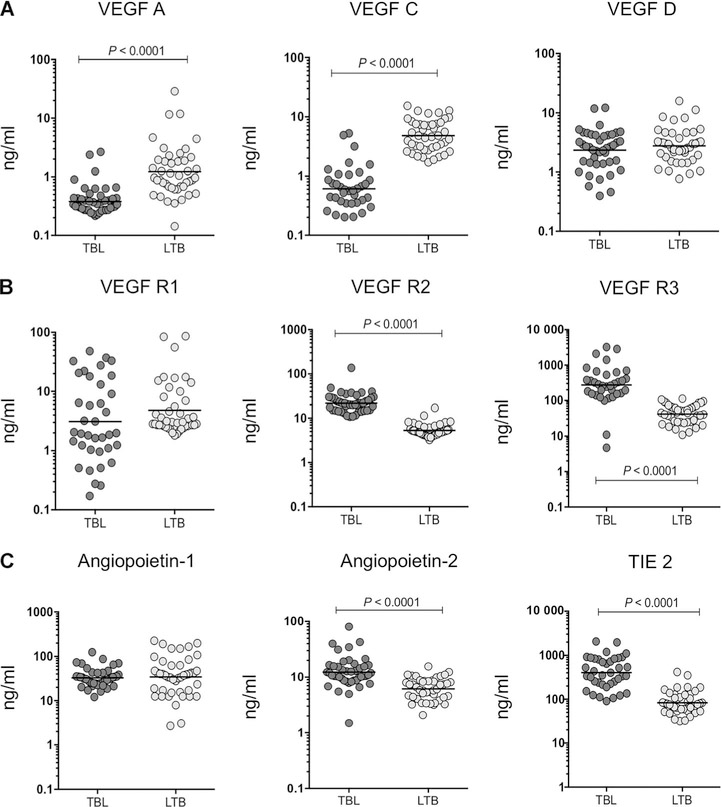
Figure 1.
Circulating angiogenic factors in TBL individuals are associated with decreased systemic levels of VEGFs and enhanced VEGF, TIE2 receptors and Ang levels. Plasma levels of A) VEGF-A, C and D, B) receptors (VEGF-R1, R2 and R3) and C) Ang-1, 2 and Tie2 were measured in individuals with TBL (n=44) and LTBI (n=44). Data are shown in scatter plots, with each circle representing a single individual GM depicted with a bar. P values were calculated using the Mann-Whitney U-test. VEGF = vascular endothelial growth factor; VEGF-R = VEGF receptor; TBL = tuberculous lymphadenitis; LTBI= latent tuberculous infection; TIE2 = tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2; GM = geometric mean.
In TBL, systemic levels were significantly lower for VEGF-A (GM 0.03798 ng/ml in TBL vs. 0.1220 ng/ml in LTBI) and VEGF-C (GM 0.06115 ng/ml in TBL vs. 0.4814 ng/ml in LTBI), and higher in VEGF-R2 (GM 2.176 ng/ml in TBL vs. 0.5299 ng/ml in LTBI), VEGF-R3 (GM 27.33 ng/ml in TBL vs. 4.184 ng/ml in LTBI), Ang-2 (GM 1.235 ng/ml in TBL vs. 0.6147 ng/ml in LTBI) and TIE2 (GM 0.4038 ng/ml in TBL vs. 0.08332 ng/ml in LTBI) than in LTBI (Figure 1A–C). However, no significant differences were observed for VEGF-D (GM 0.2348 ng/ml in TBL vs. 0.2773 ng/ml in LTBI), VEGF-R1 (GM 0.3107 ng/ml in TBL vs. 0.4812 ng/ml in LTBI) or Ang-1 (GM 3.284 ng/ml in TBL vs. 3.446 ng/ml in LTBI) (Figure 1A–C).
Lymph node supernatants from patients with tuberculous lymphadenitis show increased levels of VEGF-A, C and Ang-2
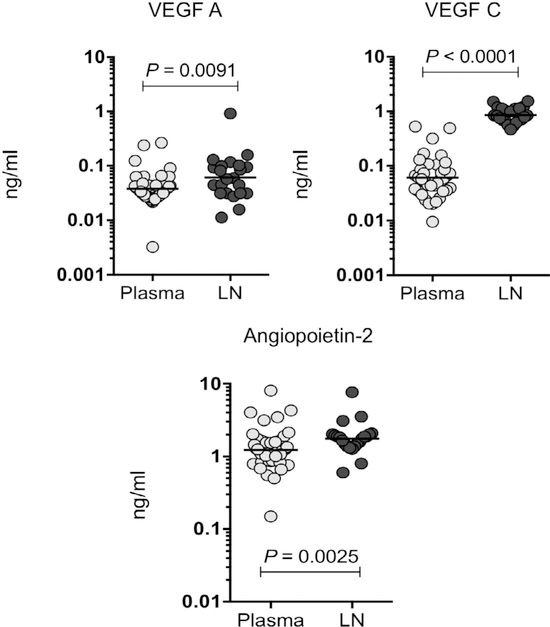
We measured the VEGF-A, C and Ang-2 levels in the plasma and lymph node supernatants of individuals with TBL (Figure 2). The levels of the angiogenic factors VEGF-A (GM 0.03798 ng/ml in plasma vs. 0.06111 ng/ml in lymph node), VEGF-C (GM 0.06115 ng/ml in plasma vs. 0.8573 ng/ml in lymph node) and Ang-2 (GM 1.235 ng/ml in plasma vs. 1.765 ng/ml in lymph node) were increased in the lymph node supernatants of TBL patients when compared with circulating plasma levels. We concluded that TBL was associated with increased levels of angiogenic factors in lymph node supernatants.
Figure 2.
Increased levels of angiogenic factors (VEGF-A, C and Ang-2) are associated with lymph node supernatants in TBL cases. VEGF-A and C and Ang-2 levels were measured in plasma and lymph node supernatants in individuals with TBL. Data are shown as scatter plots, with each circle representing a single individual GM depicted with a bar. P values were calculated using the Mann-Whitney U-test. VEGF = vascular endothelial growth factor; LN = lymph node; TBL = tuberculous lymphadenitis; GM = geometric mean.
Circulating angiogenic factors differentiate tuberculous lymphadenitis from latent tuberculous infection
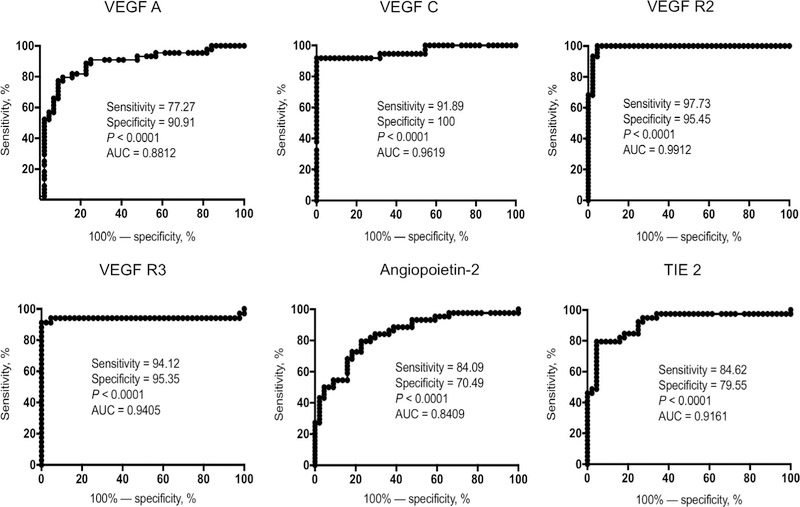
To explore the discriminatory power of circulating angiogenic markers in distinguishing TBL from LTBI patients, we performed ROC analysis of VEGF-A, C, R2 and R3, as well as Ang-2 and TIE2, for the same groups. Most of the angiogenic markers exhibited significant discriminatory power, along with high area under the curve (AUC) values, sensitivity and specificity (Figure 3). Among these, VEGF-C, R2, R3 and TIE2 shared the highest AUC, sensitivity and specificity in discriminating between differences in TBL and LTBI individuals. We therefore believe that VEGF-C and R2 could serve as biomarkers for distinguishing active TBL from LTBI.
Figure 3.
ROC curve illustrates the discriminatory power of systemic angiogenic factors in individuals with TBL. ROC curve analysis was performed to assess the sensitivity, specificity and area under the curve using systemic angiogenic factors (VEGF-A, C, VEGF-R2, R3, Ang-2 and TIE2) and to calculate the ability of these factors to differentiate TBL from individuals with LTBI. VEGF = vascular endothelial growth factor; VEGF-R= VEGF receptor; TIE2 = tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2; ROC = receiver operating characteristic; TBL = tuberculous lymphadenitis; LTBI = latent tuberculous infection.
Efficacy of anti-tuberculosis treatment was analysed using circulating angiogenic markers
To ascertain if the enhanced or diminished levels of circulating angiogenic factors are liable to change upon therapeutic intervention in TBL, we determined the levels of these angiogenic markers before and after a standard course of anti-tuberculosis treatment (pretreatment vs. post-treatment).
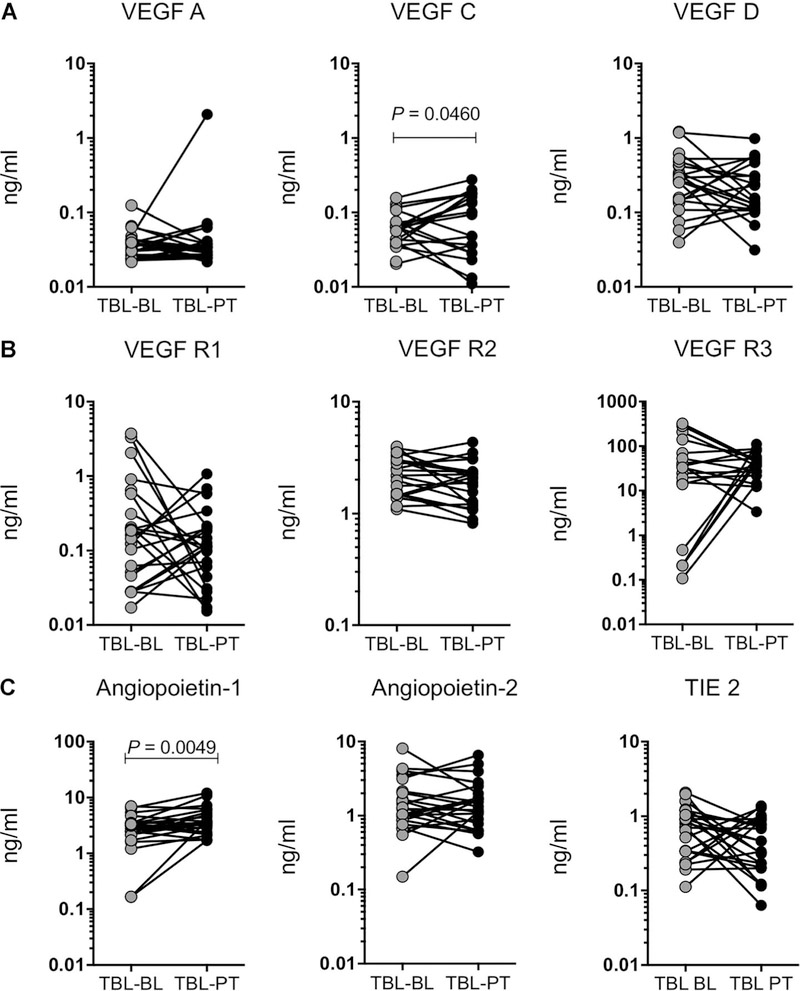
After anti-tuberculosis treatment, levels of the angiogenic markers VEGF-C (GM 0.05251 ng/ml vs. 0.05972 ng/ml) and Ang-1 (GM 2.346 ng/ml vs. 3.747 ng/ml) were increased compared with pre-treatment levels in TBL individuals (Figure 4A–C). No significant differences between pre- and post- treatment levels were observed in the other angiogenic markers analysed in TBL patients.
Figure 4.
TBL is associated with increased plasma levels of VEGF-C and Ang-1 following anti-tuberculosis treatment. The systemic plasma levels of A) VEGF (A, C and D), B) their receptors (R1, R2 and R3) and C) Ang-1, 2 and TIE2 were measured in individuals with TBL before and after standard anti-tuberculosis treatment. Data are shown as line graphs, with each line representing a single individual. P values were calculated using the Wilcoxon signed rank test. VEGF vascular endothelial growth factor; TBL tuberculous lymphadenitis; BL before treatment; PT post-treatment; VEGF-R VEGF receptor; TIE2 tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2.
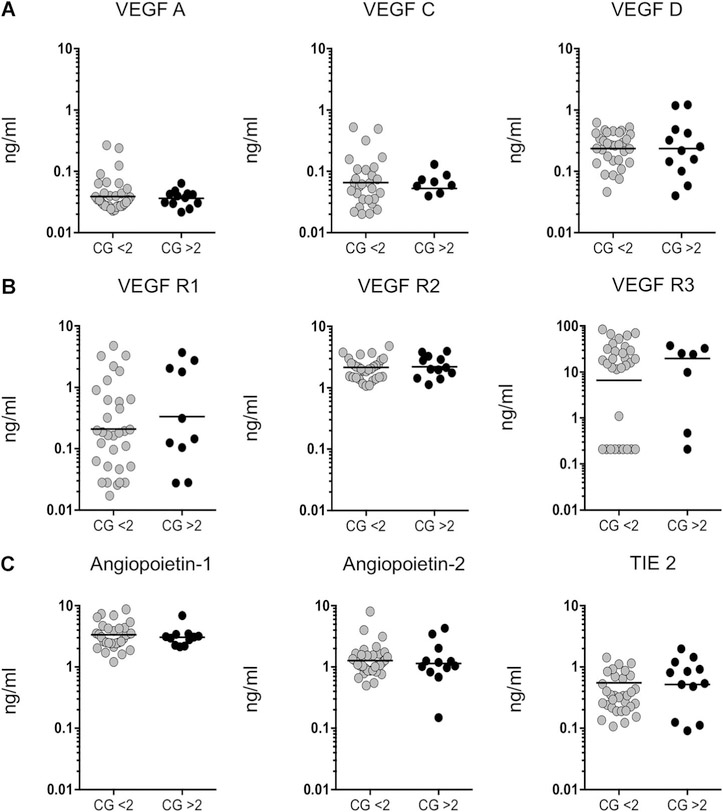
There is no association between tuberculous lymphadenitis culture grade and circulating angiogenic markers
To examine the association between systemic angiogenic marker levels and lymph node bacillary culture grade in TBL patients, we correlated culture grade values with the corresponding circulating angiogenic levels of VEGF-A, C, D, R1, R2, R3, Ang-1, Ang-2 and TIE2 (Figure 5A–C). None of the angiogenic markers showed a positive relationship with culture grade.
Figure 5.
No correlation between the systemic plasma levels of angiogenic factors and culture grade in individuals with TBL. The relationship between TBL plasma levels of A) VEGF (A, C and D), B) their receptors (R1, R2 and R3) and C) angiopoietin (Ang-1 and 2) and their receptors (TIE2) were correlated with bacillary culture grade. Results are represented in scatter plots, with each circle representing a single individual and the bars indicating the geometric means. P values were calculated using the Mann-Whitney U-test. VEGF= vascular endothelial growth factor; CG= culture grade; VEGF-R = VEGF receptor; TIE2 = tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2; TBL = tuberculous lymphadenitis.
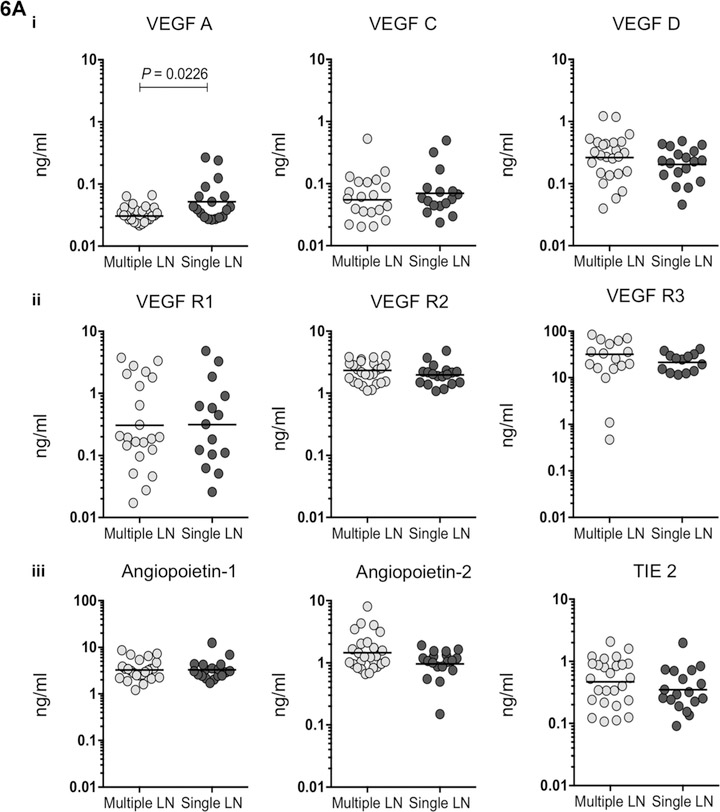
VEGF-A alone distinguishes between multiple and single lymph node status
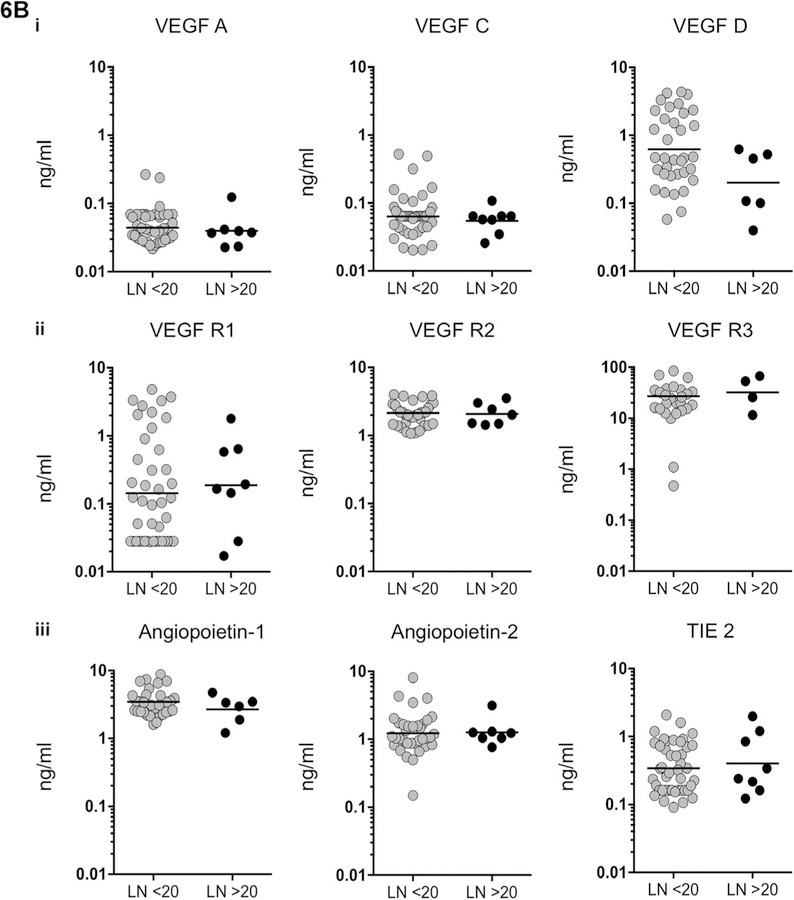
VEGF-A levels were significantly decreased in multiple lymph nodes compared with single lymph nodes in TBL patients (Figure 6A). None of the levels of other circulating angiogenic markers showed variation between single and multiple lymph nodes. No significant association was noted between circulating angiogenic factors and two clusters of lymph nodes (the size of the lymph node was calculated using a vertical and horizontal length of <20 or >20 cm) (Figure 6B).
Figure 6.
A) No association between levels of angiogenic factors and lymph node number was observed in individuals with TBL. Association between systemic plasma levels in single vs. multiple lymph nodes in: i) VEGF (A, C and D), ii) their receptors (R1, R2 and R3) and iii) angiopoietin (Ang-1, 2 and TIE2) were examined. Data are shown as scatter plots, with each circle representing a single individual. P values were calculated using the Mann-Whitney U-test. B) No association between levels of angiogenic factors and lymph node size was observed in individuals with TBL. Levels of circulating angiogenic factors are shown in two clusters of lymph nodes (the size of the lymph node calculated using vertical and horizontal length and represented as < or >20 cm). i) VEGF (A, C and D), ii) their receptors (R1, R2 and R3) and iii) angiopoietin (Ang-1, 2 and TIE2) were examined. Data are shown as scatter plots, with each circle representing a single individual. P values were calculated using the Mann-Whitney U-test. VEGF = vascular endothelial growth factor; LN = lymph node; VEGF-R = VEGF receptor; TIE2 = tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2; TBL = tuberculous lymphadenitis.
DISCUSSION
As the vascular endothelium can interact with and report to its environment, infection or injury leads to abnormal endothelial activation and angiogenesis. The VEGF family is the chief arbitrator of angiogenesis and lymphangiogenesis, with expression mainly observed in activated macrophages, neutrophils and hepatocytes. Hypoxia and tissue inflammation seem to be important in enhancing this expression.4,14,15 Studying the role of peripheral blood biomarkers of endothelial activation/dysfunction in infectious diseases is clinically helpful in understanding the severity of disease progression.16 Increased VEGF and Ang levels have been reported in PTB, malignancies, inflammatory disorders, inflammatory lung disease and asthma.5,8–10,17,18.
Mycobacterial trehalose 6,6’-dimycolate (cord factor) enhances neovascularisation through VEGF production by neutrophils and macrophages during granuloma formation in mice. The zebra fish model was recently used to study the pathogenesis of TB granuloma. The latter was found to be intimately associated with the enhanced angiogenesis and transcriptional induction of the pro-angiogenic molecule VEGF-A, as well as the generation of local hypoxia. The introduction of anti-angiogenic therapies along with standard anti-tuberculosis treatment has shown a reduction in infection burden and bacillary dissemination in animal models.19,20 Nevertheless, the basic mechanism of the systemic response to angiogenesis in human TBL is not known.
In the present study, we reported diminished systemic levels of VEGF-A, C and D in TBL compared with LTBI, with VEGF-C having the highest sensitivity and specificity. VEGF-A levels were not increased even in TBL cases with multiple node involvement. Only VEGF-C levels became normalised following anti-tuberculosis treatment. VEGF-A and C levels were significantly higher in lymph node supernatants than in circulating plasma. This finding suggests that in TBL, unlike PTB, high levels of angiogenic marker expression occur at the site of infection rather than in the circulation.
VEGF receptors are expressed in endothelial cells where VEGF-R1/FLT-1 (fms-like tyrosine kinase) and VEGF-R2 KDR/FLK-1 (foetal liver kinase) are involved in angiogenesis and FLT-4/VEGF-R3 is associated with haematopoiesis and lymphogenesis.21,22 VEGF receptors are prone to inducing inflammation; increased levels of VEGF-R2 and R3 thus reflect enhanced angiogenesis and lymph angiogenesis.3 These higher receptor levels might act as ‘decoys’ in lowering the corresponding VEGF levels. A decoy mechanism involving the formation of inactive tumour necrosis factor alpha (TNF-α)/TNFR2 complexes has been reported in Mycobacterium tuberculosis H37Rv (Mtb H37Rv) infection.23
The Ang/TIE2 circuit is very important for the growth of blood vessels in the later stages of TB disease.24 These factors have been reported to act as biomarkers for monitoring normal and abnormal function of the vascular endothelium in infectious and non-infectious conditions25. Ang-2 was recently recognised to be a major and sole predictor of early and late stages of critical illness in adults.26 Decreased Ang-1 and increased Ang-2 levels have been associated with sepsis and worst-case prediction in patients infected with Plasmodium vivax.27,28 The binding of Ang-1 to TIE2 generally promotes vessel integrity and endothelial cell survival, inhibits vascular leakage and suppresses inflammatory gene expression.29–31 The binding of antagonistic Ang-2 completely disrupts protective Ang-1-TIE2 signalling, which results in endothelial activation, increased inflammation and vascular barrier breakdown.32,33 In our study, systemic levels of Ang-2 and TIE2 factors were increased in TBL in comparison with LTBI. A combination of increased Ang-2 and TIE2 and decreased VEGFs reflects the possibility of ongoing disrupted angiogenesis in TBL granulomatous lesions. Ang-2 was also present at high levels in lymph node supernatants compared with plasma, which supports the hypothesis stated above. Researchers have reported higher systemic Ang-2 and TIE2 levels in PTB than in LTBI, which is in line with our results.17
The increase in Ang-1 and VEGF-C levels after anti-tuberculosis treatment also indicates a reversal of the disrupted vasculo-endothelial system in TBL granulomas to an integrated system. In this context, these two markers could be used to monitor post-treatment recovery, while Ang-2 and TIE2 levels may serve as indicators of disease severity. Furthermore, higher VEGF-C levels increase lymphangiogenesis through VEGF-R3 in TBL granulomas, which in turn could promote the generation of systemic T-cell responses to TB antigens.34,35 A surprising observation was the lack of correlation between angiogenic marker levels and bacillary culture grade. This observation is in direct contrast to our previous findings with respect to PTB.8
In conclusion, our study is the first to report on systemic levels of VEGF and Angs in TBL pre- and post-treatment. Further studies are needed to explore and understand the role of these factors in disease severity. Future studies with larger study cohorts could further elucidate the role of angiogenic and lymphangiogenic molecules in TBL pathogenesis.
Acknowledgements
The authors thank the Indian Council of Medical Research (ICMR; New Delhi, India) for the ICMR-PDF award to KGR; V R Kumar of the National Institutes of Health-National Institute for Research in Tuberculosis-International Centre for Excellence in Research (NIRT-ICER) and the staff of the Department of Clinical Research, NIRT and Government Stanley Hospital, Government General Hospital and Government Kilpauk Medical Hospital, Chennai, India, for valuable assistance in recruiting patients for the study; and N P Kumar, R Anuradha and J George of NIH-NIRT-ICER for technical assistance.
The Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA, supported this work. This work was also partly supported by an ICMR Post-Doctoral Fellowship (PDF) 14th Batch-2016 (Ref no. 3/1/3/PDF (14)/2016-HRD dated 14.10.2016).
Footnotes
Conflicts of interest: none declared.
References
- 1.Folkman J Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DI, Zachary I. The vascular endothelial growth factor (VEGF) family: angiogenic factors in health and disease. Genome Biol 2005; 6: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeltsch M, Leppanen VM, Saharinen P, Alitalo K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol 2013; 5: a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9: 669–676. [DOI] [PubMed] [Google Scholar]
- 5.Hashizume H, Falcón BL, Kuroda T, et al. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res 2010; 70: 2213–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramakrishnan L Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol 2012; 12: 352–366. [DOI] [PubMed] [Google Scholar]
- 7.Fontanilla JM, Barnes A, Reyn CFV. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis 2011; 53: 555–562. [DOI] [PubMed] [Google Scholar]
- 8.Kumar NP, Banurekha VV, Nair D, Babu S. Circulating angiogenic factors as biomarkers of disease severity and bacterial burden in pulmonary tuberculosis. PLOS ONE 2016; 11: e0146318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alatas F, Alatas O, Metintas M, Ozarslan A, Erginel S, Yildirim H. Vascular endothelial growth factor levels in active pulmonary tuberculosis. Chest 2004; 125: 2156–2159. [DOI] [PubMed] [Google Scholar]
- 10.Matsuyama W, Hashiguchi T, Matsumuro K, et al. Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis. Am J Respir Crit Care Med 2000; 162: 1120–1122. [DOI] [PubMed] [Google Scholar]
- 11.Kathamuthu GR, Moideen K, Baskaran D, et al. Tuberculous lymphadenitis is associated with enhanced baseline and antigen-specific induction of type 1 and type 17 cytokines and reduced interleukin-1b (IL-1b) and IL-18 at the site of infection. Clin Vaccine Immunol 2017; 24: e00045–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb NJ, Bottomley MJ, Watson CJ, Brenchley PE. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 1998; 94: 395–404. [DOI] [PubMed] [Google Scholar]
- 13.Franco D, Franco T, Schettino AM, Filho JM, Vendramin FS. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg 2012; 36: 1254–1259. [DOI] [PubMed] [Google Scholar]
- 14.Taichman NS, Young S, Cruchley, Taylor P, Paleolog E. Human neutrophils secrete vascular endothelial growth factor. J Leukoc Biol 1997; 62: 397–400. [DOI] [PubMed] [Google Scholar]
- 15.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011; 437: 169–183. [DOI] [PubMed] [Google Scholar]
- 16.Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 2013; 4: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar NP, Velayutham B, Nair D, Babu S. Angiopoietins as biomarkers of disease severity and bacterial burden in pulmonary tuberculosis. Int J Tuberc Lung Dis 2017; 21: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hantera M, Abd El-Hafiz H, Abdelnaby AY. Serum levels of angiopoietin-2 and vascular endothelial growth factor in severe refractory asthma. Egyptian J Chest Dis Tuberc 2014; 63: 751–754. [Google Scholar]
- 19.Datta M, Via LE, Kamoun WS, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci USA 2015; 112: 1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oehlers SH, Cronan MR, Scott NR, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature 2015; 517: 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407: 242–248. [DOI] [PubMed] [Google Scholar]
- 22.Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev 2000; 82: 673–700. [DOI] [PubMed] [Google Scholar]
- 23.Balcewicz-Sablinska MK, Keane J, Kornfeld H, Remold HG. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-alpha. J Immunol 1998; 61: 2636–2641. [PubMed] [Google Scholar]
- 24.Partanen J, Puri MC, Schwartz L, Fischer KD, Bernstein A, Rossant J. Cell autonomous functions of the receptor tyrosine kinase TIE in a late phase of angiogenic capillary growth and endothelial cell survival during murine development. Development 1996; 122: 3013–3021. [DOI] [PubMed] [Google Scholar]
- 25.Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res 2013; 319: 1271–1280. [DOI] [PubMed] [Google Scholar]
- 26.Kumpers P, Hafer C, David S, et al. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med 2010; 36: 462–470. [DOI] [PubMed] [Google Scholar]
- 27.Giuliano JS Jr, Lahni PM, Harmon K, et al. Admission angiopoietin levels in children with septic shock. Shock 2007; 28: 650–654. [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes LT, Alves-Junior ER, Rodrigues-Jesus C, Nery AF, Gasquez-Martin TO, Fontes CJ. Angiopoietin-2 and angiopoietin-2/angiopoietin-1 ratio as indicators of potential severity of Plasmodium vivax malaria in patients with thrombocytopenia. PLOS ONE 2014; 9: e109246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mammoto T, Parikh SM, Mammoto A, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 2007; 282: 23910–23918. [DOI] [PubMed] [Google Scholar]
- 30.Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation 2005; 111: 97–105. [DOI] [PubMed] [Google Scholar]
- 31.Brindle NPJ, Saharinen P, Alitalo K. Signalling and functions of angiopoietin-1 in vascular protection. Circ Res 2006: 98: 1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 2006; 27: 552–558. [DOI] [PubMed] [Google Scholar]
- 33.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med 2000; 6: 460–463. [DOI] [PubMed] [Google Scholar]
- 34.Coso S, Bovay E, Petrova TV. Pressing the right buttons: signaling in lymphangiogenesis. Blood 2014; 123: 2614–2624. [DOI] [PubMed] [Google Scholar]
- 35.Harding J, Ritter A, Rayasam A, Fabry Z, Sandor M. Lymph angiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. Am J Pathol 2015; 185: 432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]