Abstract
Background:
IL-10 family cytokines are associated with the host immune response to pulmonary tuberculosis (PTB), but their association with host response in tuberculous lymphadenitis (TBL) is not known.
Methods:
Hence, we examined the circulating levels of the whole panel of IL-10 family cytokines in TBL (n = 44) and compared them to the levels in PTB (n = 44) and healthy control (HC, n = 44) individuals. We also assessed the pre and post-treatment cytokine levels in TBL individuals following the completion of anti-tuberculosis treatment (ATT). Next, we also compared the levels of IL-10 family cytokine in circulation versus lymph node (LN) culture supernatants in a subset of TBL individuals (n = 22). Finally, we also measured the levels of IL-10 family cytokines in tuberculosis antigen (purified protein derivative, PPD) stimulated and unstimulated LN culture supernatants.
Results:
TBL individuals exhibit significantly decreased levels of IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29 in the circulation when compared to PTB (except IL-10) and HC (except IL-20 and IL-28B) and significantly increased levels of IL-22 when compared to PTB individuals. Following ATT, TBL individuals exhibit significantly elevated levels of IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29 and significantly diminished levels of IL-26. Similarly, TBL individuals also exhibited significantly increased levels of IL-10, IL-19, IL-20, IL-24, IL-28A and IL-29 in LN culture supernatants compared to plasma and significantly decreased levels of IL-22. This was associated with enhanced levels of IL-19, IL-20, IL-24, IL-28B and IL-29 upon PPD stimulation of LN cultures.
Conclusions:
Therefore, we demonstrate that TBL is associated with significantly diminished plasma and elevated LN culture supernatant levels of most of the IL-10 family cytokines. This to our knowledge is the first comprehensive examination of IL-10 family cytokines in TBL.
Keywords: Tuberculous lymphadenitis, ELISA, Cytokines, IL-10 family, TB lymph node
1. Introduction
The interleukin-10 (IL-10) or class II family of cytokines includes IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29 which are named after the combination of three different subgroup of cytokines. IL-10 which targets leukocytes comes under the first group, whereas the IL-20 subfamily (IL-19, IL-20, IL-22 and IL-24) cytokines belongs to the second group. Type III interferon (IFN) group or distantly related cytokine members like IL-28A, IL-28B and IL-29 fall in the final or third group [1–4]. IL-10 binds to both IL-10R1 and IL-10R2 which are closely linked to IL-20 sub family cytokine (except IL-22) [1]. IL-19, IL-20 and IL-24 signal mainly via diverse heterodimer receptors (IL-20RB), whereas IL-22 signals via IL-10RB [2,4]. Broadly, these cytokines elicit numerous functions like regulation of inflammation, protection against tissue damage, tissue remodeling, homeostasis and wound healing. IL-19, IL-20 and IL-24 are predominantly produced by epithelial, myeloid and immune cells, whereas IL-22 and IL-24/ melanoma differentiation associated gene 7 (MDA-7) are produced by T cells. IL-22 is also produced by γδ T cells, activated natural killer (NK), innate lymphoid cells and involved in diverse immune responses.
Further IL-19 is also expressed by monocytes and B cells (to some extent) and lipopolysaccharide (LPS) and granulocyte macrophage colony-stimulating factor (GM-CSF) are capable of inducing IL-19 production [2,3,5–7]. IL-24 is known to act using autocrine and paracrine actions and mediates the activation of Th1 cytokines [8]. The IL-10 family of cytokines are a component of the innate immune host defense mechanisms to fight against bacterial, fungal and viral infections [6,9,10]. The IL-20 sub-family cytokines also possess pro-inflammatory properties through the involvement of cytokine and chemokine production, barrier strengthening in epithelial cells and sustaining mucosal homeostasis [6,11–13]. IL-28A (IFN-λ2), IL-28B (IFN-λ3) and IL-29 (IFN-λ1) cytokines are similar to the class I IFNs produced by leukocytes, hepatocytes and epithelial cells upon stimulation with toll like receptor (TLR) ligands. These IFNs signals through the receptors which share a common motif on the extracellular space and consist of class II cytokine receptor family (CRF2). Apart from their antiviral and anti-tumor potential, the role of type III IFNs is not well described [14–16].
Extra-pulmonary tuberculosis (EPTB) comprises about 15% of TB cases worldwide. Tuberculous lymphadenitis (TBL) or lymph node tuberculosis (LN-TB) is the most frequent form of EPTB and accounts for 30–40% cases with higher ratio in females [17]. Due to challenges in diagnosis, TBL is associated with poor treatment outcome and increased disease rate [18]. In the context of PTB, IL-24 was shown to be present at significantly lower levels in serum in comparison to healthy controls [19]. Similarly, IL-19, IL-20 and IL-24 have also been shown to be present at significantly lower concentrations in PTB compared to latent TB infection (LTB), with IL-22 serving as the sole exception [20,21]. The role of Type III IFNs in TB is not known. However, previous report shows that both IL-28A and IL-28B cytokines are capable of inhibiting ovalbumin induced allergic airway diseases [22].
Hence, the role of the IL-10 family cytokines in TBL is not elucidated. Thus, we wanted to examine and analyze the whole panel of IL-10 family cytokines in TBL (plasma and LN) and compare them with PTB and HC. We demonstrate that TBL is associated with diminished plasma and elevated LN supernatant levels of most of the IL-10 family cytokines.
2. Materials and methods
2.1. Study population
The study subjects consist of 44 individuals with TBL in one group, 44 PTB individuals in second group and 44 individual healthy controls (HC) in the final group (Table 1). Similarly, in a subset of TBL individuals (n = 22,) LN culture supernatants were also used in this study. The LN supernatants were obtained as described earlier [23]. TBL individuals were selected based on positivity for fine needle aspiration cytology and culture or histology confirming tuberculosis from lymph node biopsy samples. None of the TBL individuals had PTB, as demonstrated by absence of pulmonary symptoms and normal chest Xray. Similarly, PTB subjects were diagnosed based on the positivity in clinical symptoms, chest X-ray and sputum microscopy (acid-fast bacilli [AFB]) as well as positivity for Mycobacterium tuberculosis (Mtb) in culture on Lowenstein–Jensen (LJ) medium. Over all, two sputum samples per patient were collected and examined by fluorescence microscopy. Healthy controls had no symptoms of any disease and had normal chest X-rays. All individuals were negative for HIV and did not undergo any steroid treatment during the period of sample collection. The demographics of the study populations is shown in Table 1. The TBL group were administered anti-TB treatment (ATT) for 6 months and once again the blood sample was collected at the end of treatment. Treatment resulted in cure of TBL, characterized by either complete or significant resolution of lymph node enlargement. Informed written consent form was obtained from all the study patients and the ethical approval (NIRTIEC2010007) was obtained from the Institutional Ethics Committee of the National Institute of Research in Tuberculosis (NIRT).
Table 1.
Demographics of the study population.
| Study Demographics | TBL | PTB | HC |
|---|---|---|---|
| No of subjects recruited (n) | 44 | 44 | 44 |
| Gender (M/F) | 11/33 | 14/30 | 14/30 |
| Median age (range) | 29 (18–59) | 35 (20–55) | 33 (20–59) |
| Culture grade (0/1+/2+/3+) | 18/21/4/1 | NA | NA |
2.2. Lymph node cultures
The LN were harvested in RPMI medium and processed immediately. The LN was washed twice and chopped into smaller pieces in plain RPMI. The chopped LN was then treated with Liberase (0.1 mg/ ml), DNASE (0.1 mg/ml) enzyme (Roche diagnostics) and incubated for 20–30 mins. After incubation, the cells were washed with RPMI and centrifuged at 2600 rpm for 10 min. The supernatant was discarded and the cells were further used for stimulation. The cultures were stimulated with the following conditions: unstimulated (UNS) and PPD and incubated at 37 °C in 5% CO2 for 18 h. After 18 h, the cells were transferred to sterile 50 ml falcon tubes and centrifugation was done. The culture supernatants were transferred to 2 ml screw cap tubes and stored at −80 °C.
2.3. Plasma and lymph node (LN) supernatants ELISA
The levels of different IL-10 family cytokines in plasma and lymph node (LN) culture supernatants were measured using ELISA and the experiment was performed according to the manufacturer’s protocol instructions. The IL-10 family cytokines analyzed were IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29. All the kits were purchased from R&D Systems (DuoSet) except IL-26 which was purchased from my BioSource. The threshold detection was given as follows: IL-10 −31.25 pg/ml, IL-19 −31.2 pg/ml; IL-20 −1.2 pg/ml; IL-22 −31.25 pg/ml, IL-24 −6.5 pg/ml, IL-26 −31.25 pg/ml, IL-28A −125 pg/ml, IL-28B −12.5 pg/ml and IL-29 −12.5 pg/ml.
2.4. Statistical analysis
GraphPad PRISM version 7 (GraphPad Software Inc., San Diego, CA) was used to analyze the data. Geometric means were used as the measure of central tendency. Kruskal-Wallis with Dunn multiple comparisons were used to analyze the significance between three different groups of individuals. Similarly, non-parametric Mann-Whitney U test were used to analyze the significance between the TBL plasma and LN supernatants. Finally, pre-and post-treatment and the LN supernatants cytokine levels were analyzed using Wilcoxon signed rank test. All the graphs were shown in log scale and the statistically significant values were shown as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
3. Results
3.1. TBL is characterized by decreased circulating levels of IL-10, IL-19, IL-24 and IL-29 cytokines
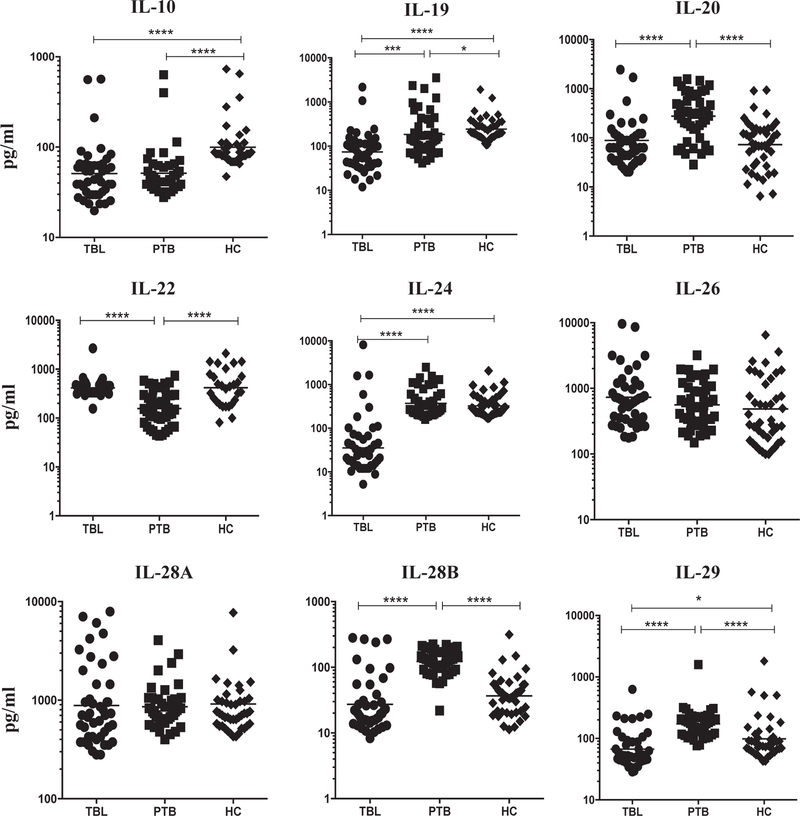
We measured the circulating levels of IL-10 family cytokines, IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29 in three different (TBL, PTB and HC) groups of individuals. As shown in Fig. 1, IL-10 (geometric mean (GM) of 50.97 pg/ml in TBL compared to 51.3 pg/ ml in PTB and 99.69 pg/ml in HC), IL-19 (GM of 73.93 pg/ml in TBL compared to 184.1 pg/ml in PTB and 242.1 pg/ml in HC), IL-24 (GM of 35.54 pg/ml in TBL compared to 372.2 pg/ml in PTB and 326.3 pg/ml in HC) and IL-29 (GM of 66.84 pg/ml in TBL compared to 172.9 pg/ml in PTB and 98.41 pg/ml in HC) levels were significantly diminished in TBL when compared to both PTB (except for IL-10) and HC. Similarly, IL-20 (GM of 88.46 pg/ml in TBL compared to 276 pg/ml in PTB and 72.67 pg/ml in HC) and IL-28B (GM of 27.24 pg/ml in TBL compared to 122.3 pg/ml in PTB and 36.69 pg/ml in HC) levels were significantly reduced in TBL compared to PTB but not with HC. In contrast, IL-22 (GM of 414.8 pg/ml in TBL compared to 156.5 pg/ml in PTB and 417.0 pg/ml in HC) levels were significantly elevated in TBL when compared to PTB and no difference with HC. Finally, no significance was observed for IL-26 (GM of 732.3 pg/ml in TBL compared to 560.8 pg/ml in PTB and 485.4 pg/ml in HC) and IL-28A (GM of 884.3 pg/ml in TBL compared to 858.8 pg/ml in PTB and 915.4 pg/ml in HC) cytokine levels among the different groups analyzed. Hence, we demonstrate that TBL is associated with diminished circulating plasma levels of most IL-10 family cytokines.
Fig. 1.
Diminished circulating levels of IL-10 family cytokines and elevated IL-22 levels in TBL individuals. The systemic levels of IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29 were measured by ELISA in TBL (n = 44), PTB (n = 44) and HC (n = 44) individuals. The data were shown as scatter plots with each circle or square or diamond indicates a single individual and the bar representing the geometric mean. P values were calculated using the Kruskal-Wallis test with Dunn multiple comparisons -*P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
3.2. Effect of ATT on the circulating levels of IL-10 family cytokines in TBL
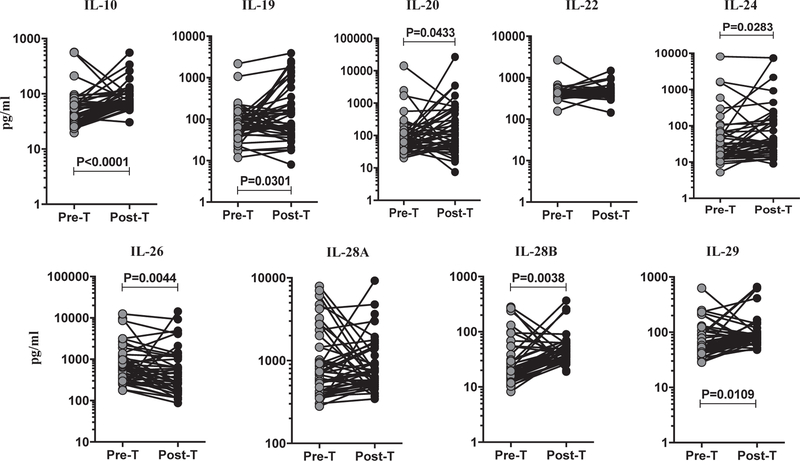
To assess the effect of ATT (pre-T versus post-T) on the circulating levels of IL-10 family cytokines (IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29) in TBL, we measured these systemic cytokine levels at pre- and post-treatment time points. Among the IL-10 family cytokines analyzed (Fig. 2), IL-10 (GM of 50.97 pg/ml in pre-T versus 81.53 pg/ml in post-T), IL-19 (GM of 73.93 pre-T pg/ml versus 127.2 pg/ml in post-T), IL-20 (GM of 88.46 pre-T pg/ml versus 176.0 pg/ml in post-T), IL-24 (GM of 35.54 pg/ml pre-T versus 69.37 pg/ml in post-T), IL-28B (GM of 27.24 pg/ml in pre-T versus 46.73 pg/ml in post-T), and IL-29 (GM of 66.84 pg/ml in pre-T versus 96.74 pg/ml in post-T) plasma levels were significantly elevated after the treatment with TBL individuals. In contrast, IL-26 (GM of 732.3 pg/ ml in pre-T versus 465.4 pg/ml in post-T) levels were significantly diminished in post-T when compared to pre-T, whereas IL-22 (GM of 414.8 pg/ml in pre-T versus 448.8 pg/ml in post-T) and IL-28A (GM of 884.3 pre-T pg/ml versus 825.2 pg/ml in post-T) did not exhibit any significant alterations following ATT. Thus, we demonstrate that TBL is associated with increased IL-10 family circulating plasma levels after anti-TB treatment.
Fig. 2.
Elevated levels of IL-10 family cytokines in TBL individuals following ATT. The systemic plasma levels of IL-10 family (IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29) cytokines in pre-T and post-T treatment responses were analyzed in TBL individuals. The data were shown as line graph with each line represents the single individual. P values were measured using the Wilcoxon signed rank test.
3.3. Elevated lymph node levels of IL-10 family cytokines in TBL individuals
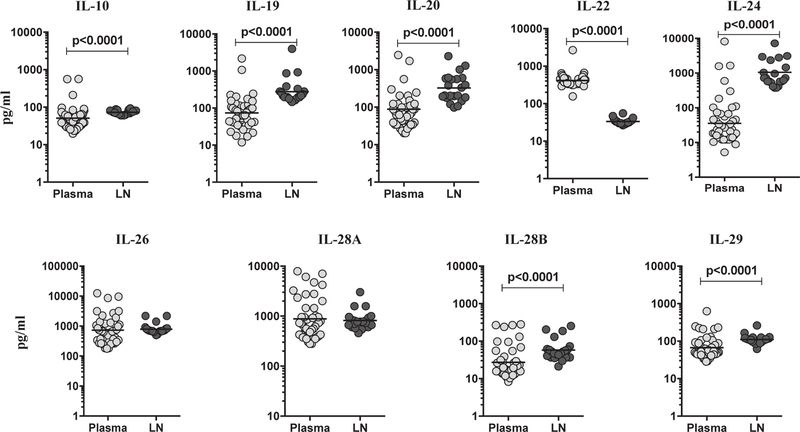
We measured the levels of IL-10 family cytokines in LN of a subset of TBL individuals and compared them to plasma levels in the same individuals (Fig. 3). As shown, IL-10 (GM of 50.97 pg/ml in plasma compared to 72.91 pg/ml in LN), IL-19 (GM of 73.93 pg/ml in plasma compared to 276.8 pg/ml in LN), IL-20 (GM of 88.46 pg/ml in plasma compared to 324.5 pg/ml in LN), IL-24 (GM of 35.54 pg/ml in plasma compared to 1055.0 pg/ml in LN), IL-28B (GM of 27.24 pg/ml in plasma compared to 57.79 pg/ml in LN) and IL-29 (GM of 66.84 pg/ml in plasma compared to 110.8 pg/ml in LN)) levels in LN was elevated in comparison to circulating plasma. In contrast, IL-22 (GM of 414.8 pg/ ml in plasma compared to 33.45 pg/ml in LN) levels were significantly lower in LN compared to plasma. However, there were no significant differences between the plasma and LN levels for IL-26 (GM of 732.3 pg/ml in plasma compared to 790.8 pg/ml in LN) and IL-28A GM of 884.3 pg/ml in plasma compared to 818.2 pg/ml in LN) cytokines. Therefore, we demonstrate that TBL LN are associated with significantly increased levels of IL-10 family cytokines.
Fig. 3.
Elevated LN supernatant levels of IL-10 (IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29) family cytokines in TBL individuals. The plasma and LN supernatants levels of IL-10 family cytokines were analyzed among TBL individuals (n = 22). The data are shown as scatter plots with each circle representing a single individual and GM were represented with bar. P values were calculated using the Mann-Whitney U test.
3.4. TBL individuals associated with elevated mycobacterial (PPD) antigen stimulated LN supernatants of IL-10 family cytokines
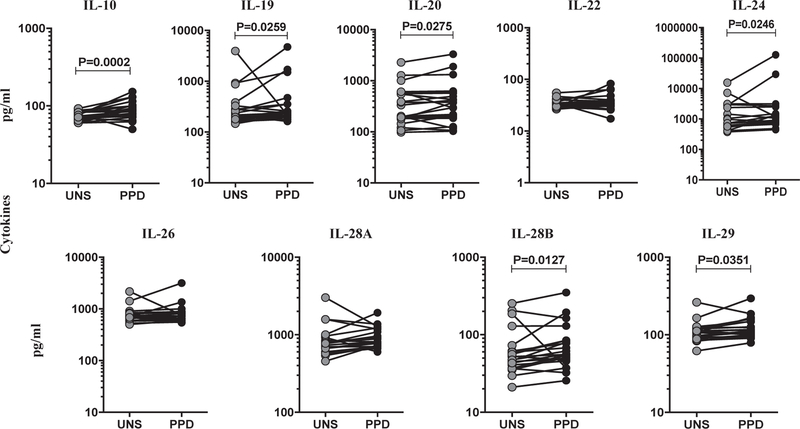
We characterized the TB antigen-stimulated levels of IL-10 family cytokines at the site of infection in TBL individuals. We measured unstimulated (UNS) and PPD-stimulated levels of IL-10 family (IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29) cytokines in LN supernatants (Fig. 4). In TBL, IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29 cytokines were significantly increased in PPD stimulated LN supernatants when compared to the UNS supernatants. However, IL-22, IL-26 and IL-28A cytokines did not exhibit any significant difference between the UNS and PPD stimulated LN culture supernatants. Thus, herein we demonstrate that TBL is associated with increased IL-10 family cytokines in LN stimulated supernatants.
Fig. 4.
TB antigen stimulated levels of IL-10 family cytokines in LN culture supernatants of TBL individuals. LN cells from TBL individuals (n = 22) were cultured with medium alone or PPD for 18 h. The baseline and antigen-stimulated (PPD) supernatant levels of whole IL-10 family cytokines were measured using ELISA. IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29 were significantly elevated in PPD stimulation when compared to unstimulated LN culture supernatants. Each line represents a single individual. P values were analyzed using the Wilcoxon signed-rank test.
4. Discussion
The immunological or host defense function of IL-10 family cytokines (IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28A, IL-28B and IL-29) in TBL has not been studied so far. However, IL-19, IL-20, IL-22 and IL-24, belonging to the IL-20 sub family cytokines, had been documented to contribute to the cytokine regulation in T cell mediated diseases [24], whereas the general role of type III IFNs is to mediate the antiviral response [15]. Similarly, the function of IL-10 with respect to inflammation has been demonstrated in different infection models [25]. Increased IL-19, IL-20 and IL-24 cytokine expression was observed in psoriasis and blockade of IL-20R signal revealed their harmful effects to the host during Staphyloccus aureus infection through inhibition of IL-1β and IL-17A [26–28]. IL-19 expression also has been reported to be increased in asthma [29,30], breast cancer [31] and other disease conditions [32]. In addition, IL-24 plays a vital role in host defense against Salmonella typhimurium infection [33]. Likewise, IL-22, the most studied cytokine in IL-20 family has a pronounced effect in mucosal immunity against pathogens like Clostridium diffcile, Toxoplasma gondii and Citrobacter rodentium [34–36]. Thus, these cytokines are thought to play either protective or harmful role in infectious disease, that are determined by the nature of infection that occurs in the respective host.
Based on this view, we expand our research to understand the role of IL-10 family cytokines in TBL individuals. Our analysis revealed an interesting observation, most of the IL-10 family cytokines such as IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29 were significantly reduced in TBL circulating plasma when compared to pulmonary TB and/or HC. This observation is in concordance with mRNA transcript levels of TB Immune reconstitution inflammatory syndrome (IRIS) patients, where they observed diminished IL-19 and IL-24 levels [37]. The significantly lower plasma levels of IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29 in our study might reflect the anti-inflammatory nature of these cytokines since TBL being a paucibacillary form of TB, might not induce pronounced pro-inflammatory effects which is essential for the control of disease pathogenesis. Additionally, the elevated levels of IL-10 family cytokines in the LN suggests that these cytokines were expressed significantly higher in the site of infection i.e in the affected lymph nodes rather than the circulation. Our results with TBL versus PTB plasma resembles to our previous study, wherein IL-19, IL-20 and IL-24 levels were significantly lower in latent TB and PTB infection associated with comorbidities [20]. Similar to our data on IL-24, Ma et al., demonstrated decreased IL-24 levels in sera of PTB individuals compared to HC [19]. It was also shown that exogenous IL-24 can able to activate CD8+ T cells which essentially produce IFNγ which counteract against TB infection.
In terms of TBL disease, the diminished levels of IL-10 family cytokines might have some major implications. IL-24 is produced by monocytes, T cells and plays an important role in host defense [38]. Also, we have previously reported that reduced IL-1β in plasma and quantiferon supernatants in TBL in comparison to LTB [39]. Since IL-1β is one of the important monocyte markers, diminished levels might result in impaired activation of effective innate immunity which are again crucial for controlling the TB infection. Further IL-10 family cytokines can stimulate the immune system in order to express the secondary cytokines like TNFα, IFNγ and IL-1, which are again crucial in controlling the infection [40]. Similarly, PBMC cells treated with IL-24 results in expression of different cytokines such as IL-6, TNF-α, IL-12, IFNγ, IL-1β and GM-CSF. Overall the diminished IL-24 plasma levels in our study indicates the non-protective nature against the TBL disease perhaps by suppressing the production of important immune mediated cytokine responses [41]. Along with IL-24, IL-19 can also modulate (increase or decrease) T cell function. Therefore, reduced IL-19 levels in our study determines that this cytokine possibly may not be able to induce the protective T cell immune cytokine responses against TBL infection.
Our study also reveals an important association of IL-22 with TBL, wherein we report elevated levels of IL-22 plasma and decreased antigen stimulated LN supernatants in TBL. This confirms our previous data on plasma levels of IL-22 in TBL individuals [39]. IL-22 is thought to play an important role in innate pathogen defense, T-lymphocyte disease and acute phase responses. Further the cytokine had also shown increased expression in skin, digestive and respiratory system [42,43]. Elevated IL-22 levels were observed in the stimulated PBMC cultures with M. tuberculosis antigens in tuberculosis-IRIS patients compared to the individuals not having the IRIS disease [37] and with PTB-DM (diabetes mellitus) individuals [20]. Thus, our data also reveal an important association of IL-22 with the immune response in TBL. Further reduced IL-26 protein levels were reported in TB infections, whereas our study reveals no differences in plasma and LN (PPD stimulation) supernatants. Similarly, reduced IL-28B and IL-29 cytokine in plasma and increased LN supernatants again reiterates the poor control of TBL infection, and the mechanism needs to be elucidated. Similarly, most of the IL-10 family (IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29) cytokines were elevated after ATT with IL-26 being the sole exception, which reiterates that the alteration in IL-10 family cytokine levels were driven by the bacterial burdens and lymph node pathology, both of which decrease after ATT. It was reported earlier that treatment with rAd-mIL-28B significantly diminished the frequency of regulatory T cells (Tregs) after 4 weeks of immunization but did not boost the Th1 type immune responses [44]. In contrast, elevated type III IFNs are observed in chronic hepatitis C virus (HCV) infection with enhanced Treg population [45]. Our study data reveal an important association of Type III IFN, especially IL-28B and IL-29 with TBL disease. Finally, our PPD stimulation data provide a mechanistic explanation for the differential cytokine levels in LN versus plasma of TBL. LN cells are capable of upregulating IL-10 family cytokine stimulation upon TB antigen stimulation and hence, have higher levels of these cytokines in the milieu of ongoing infection. It would be interesting to examine, in future experiments, the TB antigen driven response in a non-infected LN.
Thus, our data clearly provides evidence about the regulation of IL-10 family cytokines (IL-10, IL-19, IL-20, IL-24, IL-28B and IL-29), which might possibly be involved in the immune modulation of TBL infection. It is thus important to examine the association of these cytokines in TBL by studying their specific immune mediated pathways and their functional roles. Overall, we have shown an important association of the IL-10 cytokine family in the host immune response in TBL disease.
Acknowledgments
The first author (Gokul Raj Kathamuthu) immensely thanks the Indian Council of Medical Research (ICMR) for providing the ICMR Post-Doctoral Fellowship (PDF 14th batch). The research work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID) and partially by ICMR Post Doctoral Research Fellowship (PDF)-14th batch”-2016 (Ref no. 3/1/3/PDF (14)/2016-HRD dated 14.10.2016). The authors also thank V. Rajesh Kumar of NIH-NIRT-ICER and the staffs from Department of Clinical Research, NIRT, Government Stanley Hospital, Government General Hospital and Government Kilpauk Medical Hospital, Chennai, Tamil Nadu for providing the assistance in patient recruitment for this study. We also thank R. Anuradha of the NIH-NIRT-ICER for their technical assistance.
Footnotes
Disclosures
All the authors have stated no conflicts of interest.
References
- [1].Ouyang W, Rutz S, Crellin NK, et al. , Regulation and functions of the IL-10 family of cytokines in inflammation and disease, Annu. Rev. Immunol 29 (2011) 71–79. [DOI] [PubMed] [Google Scholar]
- [2].Rutz S, Wang X, Ouyang W, The IL-20 subfamily of cytokines from host defence to tissue homeostasis, Nat. Rev. Immunol 14 (12) (2014) 783–795. [DOI] [PubMed] [Google Scholar]
- [3].Sa SM, Valdez PA, Wu J, et al. , The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis, J. Immunol 178 (4) (2007) 2229–2240. [DOI] [PubMed] [Google Scholar]
- [4].Pestka S, Krause CD, Sarkar D, et al. , Interleukin-10 and related cytokines and receptors, Annu. Rev. Immunol 22 (2004) 929–979. [DOI] [PubMed] [Google Scholar]
- [5].Gallagher G, Dickensheets H, Eskdale J, et al. , Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10), Genes Immun 1 (7) (2000) 442–450. [DOI] [PubMed] [Google Scholar]
- [6].Eidenschenk C, Rutz S, Liesenfeld O, et al. , Role of IL-22 in microbial host defense, Curr. Top. Microbiol. Immunol 80 (2014) 213–236. [DOI] [PubMed] [Google Scholar]
- [7].Colonna M, Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity, Immunity 31 (1) (2009) 15–30. [DOI] [PubMed] [Google Scholar]
- [8].Ma Y, Chen H, Wang Q, et al. , IL-24 protects against Salmonella typhimurium infection by stimulating early neutrophil Th1 cytokine production, which in turn activates CD8+ T cells, Eur. J. Immunol 39 (12) (2009) 3357–3368. [DOI] [PubMed] [Google Scholar]
- [9].Commins S, Steinke JW, Borish L, The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29, J. Allergy Clin. Immunol 121 (5) (2008) 1108–1111. [DOI] [PubMed] [Google Scholar]
- [10].Sabat R, IL-10 family of cytokines, Cytok. Growth Factor Rev 21 (5) (2010) 315–324. [DOI] [PubMed] [Google Scholar]
- [11].Liang SC, Tan XY, Luxenberg DP, et al. , Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides, J. Exp. Med 203 (10) (2006) 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wolk K, Witte E, Wallace E, et al. , IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis, Eur. J. Immunol 36 (5) (2006) 1309–1323. [DOI] [PubMed] [Google Scholar]
- [13].Boniface K, Bernard FX, Garcia M, et al. , IL-22 inhibits epidermal differentiation and induces pro inflammatory gene expression and migration of human keratinocytes, J. Immunol 174 (6) (2005) 3695–3702. [DOI] [PubMed] [Google Scholar]
- [14].Uze G, Monneron D, IL-28 and IL-29: newcomers to the interferon family, Biochimie 89 (2007) 729–734. [DOI] [PubMed] [Google Scholar]
- [15].Kotenko SV, Gallagher G, Baurin VV, et al. , IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex, Nat. Immunol 4 (2003) 69–77. [DOI] [PubMed] [Google Scholar]
- [16].Donnelly RP, Kotenko SV, Interferon-Lambda: a new addition to an old family, J. Interf. Cytok. Res 30 (8) (2010) 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mehraj J, Khan ZY, Saeed DK, et al. , Extrapulmonary tuberculosis among females in South Asia-gap analysis, Int. J. Mycobacteriol 5 (2016) 392–399. [DOI] [PubMed] [Google Scholar]
- [18].Huebner RE, Castro KG, The changing face of tuberculosis, Annu. Rev. Med 46 (1995) 47–55. [DOI] [PubMed] [Google Scholar]
- [19].Ma Y, Chen HD, Wang Y, et al. , Interleukin 24 as a novel potential cytokine immunotherapy for the treatment of Mycobacterium tuberculosis infection, Microbes Infect 13 (12–13) (2011) 1099–1110. [DOI] [PubMed] [Google Scholar]
- [20].Kumar NP, Banurekha VV, Nair D, et al. , Type 2 diabetes – tuberculosis comorbidity is associated with diminished circulating levels of IL-20 subfamily of cytokines, Tuberculosis (Edinb) 95 (6) (2015) 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kumar NP, Sridhar R, Banurekha VV, et al. , Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other pro-inflammatory cytokines, Ann. Am. Thorac. Soc 10 (5) (2013) 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yan B, Chen F, Xu L, et al. , Interleukin-28B dampens airway inflammation through up-regulation of natural killer cell-derived IFN-γ, Sci. Rep 7 (2017) 3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kathamuthu GR, Moideen K, Baskaran D, et al. , Tuberculous lymphadenitis is associated with enhanced baseline and antigen-specific induction of type 1 and type 17 cytokines and reduced interleukin-1β (IL-1β) and IL-18 at the site of infection, Clin. Vaccine Immunol 24 (5) (2017) e00045–e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mege JL, Meghari S, Honstettre A, et al. , The two faces of interleukin-10 in human infectious diseases, Lancet infect. Dis 6 (9) (2006) 557–569. [DOI] [PubMed] [Google Scholar]
- [25].Redford PS, Murray PJ, O’Garra A, The role of IL-10 in immune regulation during M. tuberculosis infection, Mucosal Immunol 4 (3) (2011) 261–270. [DOI] [PubMed] [Google Scholar]
- [26].Tian Y, Sommerville LJ, Cuneo A, et al. , Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia, Am. J. Pathol 173 (3) (2008) 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kumari S, Bonnet MC, Ulvmar MH, et al. , Tumor necrosis factor receptor signaling in keratincytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice, Immunity 39 (5) (2013) 899–911. [DOI] [PubMed] [Google Scholar]
- [28].Myles IA, Fontecilla NM, Valdez PA, et al. , Signaling via the IL-20 receptor inhibits cutaneous production of IL-1beta and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus, Nat. Immunol 14 (2013) 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liao SC, Cheng YC, Wang YC, et al. , “IL-19 induced Th2 cytokines and was upregulated in asthma patients, J. Immunol 173 (11) (2004) 6712–6718. [DOI] [PubMed] [Google Scholar]
- [30].Kragstrup TW, Otkjaer K, Holm C, et al. , The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy, Cytokine 41 (1) (2008) 16–23. [DOI] [PubMed] [Google Scholar]
- [31].Hsing CH, Cheng HC, Hsu YH, et al. , “Upregulated IL-19 in breast cancer promotes tumor progression and affects clinical outcome, Clin. Cancer Res 18 (3) (2012) 713–725. [DOI] [PubMed] [Google Scholar]
- [32].Gallagher G, Interleukin-19: multiple roles in immune regulation and disease, Cytok. Growth Factor Rev 21 (5) (2010) 345–352. [DOI] [PubMed] [Google Scholar]
- [33].Ma Y, Chen H, Wang Q, et al. , IL-24 protects against Salmonella typhimurium infection by stimulating earl neutrophil Th1 cytokine production, which in turn activates CD8+ T cells, Eur. J. Immunol 39 (12) (2009) 3357–3368. [DOI] [PubMed] [Google Scholar]
- [34].Dudakov JA, Hanash AM, Van den Brink MR, Interleukin-22: 349 immunobiology and pathology, Annu. Rev. Immunol 33 (2015) 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hasegawa M, Yada S, Liu MZ, et al. , Interleukin-22 regulates the complement system to promote resistance against pathobionts after pathogen-induced intestinal damage, Immunity 41 (4) (2014) 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Muñoz M, Eidenschenk C, Ota N, et al. , Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection, Immunity 42 (2) (2015) 321–331. [DOI] [PubMed] [Google Scholar]
- [37].Tadokera R, Wilkinson KA, Meintjes GA, et al. , Role of the interleukin 10 family of cytokines in patients with immune reconstitution inflammatory syndrome associated with HIV infection and tuberculosis, JID 207 (7) (2013) 1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu B, Huang C, Kato-Maeda M, et al. , IL-24 modulates IFN-gamma expression in patients with tuberculosis, Immunol. Lett 117 (2008) 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kathamuthu GR, Moideen K, Baskaran D, et al. , Reduced systemic and mycobacterial antigen stimulated concentrations of IL-1beta and IL-18 in tuberculous lymphadenitis, Cytokine 325 (90) (2017) 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Caudell EG, Mumm JB, Poindexter N, et al. , The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immune stimulatory activity and is designated IL-24, J. Immunol 168 (12) (2002) 6041–6046. [DOI] [PubMed] [Google Scholar]
- [41].Wolk K, Kunz S, Asadullah K, et al. , Cutting edge: immune cells as sources and targets of the IL-10 family members? J. Immunol 168 (2002) 5397–5402. [DOI] [PubMed] [Google Scholar]
- [42].Mege JL, Meghari S, Honstettre A, et al. , The two faces of interleukin 10 in human infectious diseases, Lancet Infect. Dis 6 (9) (2006) 557–569. [DOI] [PubMed] [Google Scholar]
- [43].Wolk K, Kunz S, Witte E, et al. , IL-22 increases the innate immunity of tissues, Immunity 21 (2) (2004) 241–254. [DOI] [PubMed] [Google Scholar]
- [44].Luo Y, Ma X, Liu X, et al. , IL-28B down-regulates regulatory T cells but does not improve the protective immunity following tuberculosis subunit vaccine immunization, Int. Immunol 28 (2) (2016) 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dolganiuc A, Kodys K, Marshall C, et al. , Type III interferons, IL-28 and IL-29, are increased in chronic HCV infection and induce myeloid dendritic cell-mediated FoxP3+ regulatory T cells, PLoS One 7 (10) (2012) e44915. [DOI] [PMC free article] [PubMed] [Google Scholar]