Abstract
Radiotherapy serves as a foundational pillar for the therapeutic management of diverse solid tumors through the generation of lethal DNA damage and induction of cell death. While the direct cytotoxic effects of radiation therapy remain a cornerstone for cancer management, in the era of immunooncology there is renewed and focused interest in exploiting the indirect bystander activities of radiation, termed abscopal effects. In radioimmunobiologic terms, abscopal effects describe the radiotherapy-induced regression of cancerous lesions distant from the primary site of radiation delivery and rely upon the induction of immunogenic cell death and consequent systemic anticancer immune activation. Despite the promise of radiation therapy for awaking potent anticancer immune responses, the purposeful harnessing of abscopal effects with radiotherapy remain clinically elusive. In part, failure to fully leverage and clinically implement the promise of radiation-induced abscopal effects stems from limitations associated with existing conventional tumor models which inadequately recapitulate the complexity of malignant transformation and the dynamic nature of tumor immune surveillance. To supplement this existing gap in modeling systems, pet dogs diagnosed with solid tumors including melanoma and osteosarcoma, which are both metastatic and immunogenic in nature, could potentially serve as unique resources for exploring the fundamental underpinnings required for maximizing radiation-induced abscopal effects. Given the spontaneous course of cancer development in the context of operative immune mechanisms, pet dogs treated with radiotherapy for metastatic solid tumors might be leveraged as valuable model systems for realizing the science and best clinical practices necessary to generate potent abscopal effects with anti-metastatic immune activities.
Keywords: immunooncology, comparative oncology, abscopal, immunogenic cell death, radiation, canine, metastases
Significance of a Dog Model—Strengths and Limitations
Strengths
Domesticated dogs are second only to human beings in terms of being afflicted with naturally-occurring and inherited diseases, and the purposeful breeding of companion dogs for specific characteristics has produced lineage-specific homogeneity that mimics human demographics such as race or geographic phenotypes (1–4). Dogs acquire genetic diseases as do humans, and consequently might serve as suitable comparative models for conserved pathologies, including certain types of cancer (5, 6). Given that pet dogs often share the same environment and are exposed to similar carcinogens as people, the natural development and evolution of canine tumors can closely parallel those that afflict human beings and share comparable recurrence and metastases patterns. The compressed lifespans of dogs in comparison with humans, combined with the substantial veterinary healthcare dollars spent on pet dogs exceeding $15 billion annually (7), provide researchers with a robust population of pet dogs available to participate in studies of cancer pathogenesis and the preclinical assessment of investigational therapeutics and medical devices (8–11). Collectively, the shared genetics of specific canine cancers with their human counterparts (12–17), and the high societal value placed upon dogs as companion animals, uniquely and ethically allow pet dogs to serve as potential valuable large animal models for translational cancer research. Particularly, in the era of immuno-oncology, pet dogs might uniquely serve as ideal parallel tumor models, given the development of spontaneous cancers under competent immune surveillance mechanisms which invariably contributes to shaping of cancer cell immunogenicity and the associated immune topography of the tumor microenvironment (18, 19).
Limitations
While the recognition of comparative oncologic pathology has been existent for over 50 years (20), the establishment of comparative oncology as a health science discipline by the National Cancer Institute's Center for Cancer Research remains relatively nascent, being formalized in 2003. As such, the purposeful inclusion of pet dogs as parallel cancer models for investigational anticancer immunotherapeutic strategies has only recently begun to bear scientific results in support of the potential model value (21), and has not been maximally leveraged by the scientific cancer research committee given the existence of perceived and true barriers (9), which include heterogeneity of study populations and tumor biology, necessity to conduct adequately powered and prospective clinical trials, and limited availability of diagnostic and therapeutic tools for in-depth scientific investigations. For the study of anticancer immune responses, the diversity and number of commercially available and validated reagents for characterizing immune activation in the domestic canine remain limited in comparison to the existent murine and human reagent toolboxes (22, 23). Additionally, the nuances of immune composition and activation responses in canines is less well-annotated compared to traditional inbreed mouse strains (24–26), however, in aggregate there is sufficient data to support the comparative similarities for specific aspect of the immune system between canines and humans (27, 28).
To expedite the translation of novel immune-based strategies to people with metastatic tumor histologies, the evaluation of experimental therapies in the most highly relevant tumor models should be considered. Besides people, domesticated dogs are also large mammals that develop solid tumors spontaneously that are not only metastatic, but also immunogenic and include canine oral malignant melanoma (OMM) and appendicular osteosarcoma (OS) (29, 30). Importantly, studies demonstrate that these 2 specific solid tumors share similar genetic and histologic features as those found in humans (31–35); suggesting that pet dogs might serve as excellent predictive models for guiding the rational development of immune-based strategies in people with comparable tumor histologies (36).
Ionizing Radiation Therapy
Radiation Principles and Mechanisms of Cell Death
The biologic responses of cells exposed to radiation traditionally have been categorized into the 5 R's, being Repopulation, Reassortment, Reoxygenation, Repair, and Radiosensitivity. Understandings of these foundational cellular reactions to ionizing radiation have been leveraged to maximize the anticancer activities of radiation therapy (37, 38). The primary target for radiation cellular damage is DNA, and with low linear energy transfer radiation, such as photons and electrons, single strand DNA breaks are created, accumulate, and mimic damage similar to double strand breaks that become difficult, if not impossible, to repair. Consequently, irreparably damaged cells can no longer replicate limitlessly, and the primary cause of cellular death is mitotic catastrophe (39, 40). Irradiated cells can also undergo apoptosis rapidly following radiation exposure with this form of death most relevant to lymphoid cells (39). Other death pathways also play roles in response to radiation, including autophagy and necrosis. Autophagy involves internal degradation of organelles for the promotion of cellular survival and occurs after radiation as a survival mechanism; but can also progress to cellular death and influence inherent radiosensitivity (41, 42). Lastly, by extensive cellular stress through DNA damage, radiation can induce cellular senescence with consequent tumor cell growth arrest (43, 44).
Radiation-Induced Immunogenic Cell Death and Abscopal Effects
While anticancer activities from radiation have traditionally been ascribed to direct DNA damage to tumor cells, in the era of immunooncology, there has been focused interest to understand the indirect or “out-of-field” immunomodulatory activities induced by radiation therapy. Specifically, a unique form of radiation-induced cell killing called immunogenic cell death (ICD) holds promise for activating systemic immunity against tumor masses distant from the field of radiation delivery (45), a phenomena termed abscopal effect (46). The regressive activity of local irradiation on distant metastatic cells, constituting the abscopal effect, is attributed to an immune-mediated response (47). Given the recognized potential to amplify systemic anticancer immunogenicity following localized radiation, excitement has been garnered by the scientific community to understand and harness the promise of radioimmunotherapy (48, 49).
Mechanistically, ICD has been a focus of radiobiology research and requires activation of the innate immune system through the release of damage-associated molecular patterns (DAMPs) or alarmins, which are released from injured, stressed, or dying cells within the radiation field (50). Scores of different endogenous alarmins derived from cellular organelles and extracellular matrix proteins have been described (51); however, three specific molecules appear to be required for optimal dendritic cell activation and immune priming against malignant cells, specifically being membrane localization of calreticulin and the release of high mobility box group 1 (HMBG1) and adenosine triphosphate into the tumor microenvironment (52). Collectively the expression and secretion of alarmins by dying cells create a localized milieu which exert either “eat me” or “come find me” signals, and are capable of activating innate immune cells exhibiting cognate DAMPs receptors (TLR, RAGE, P2X7), which leads to the priming of cytotoxic T lymphocytes for an adaptive anticancer immune response (53). Given their immune activating properties, the purposeful induction of alarmins within the tumor microenvironment as an in-situ vaccine strategy is actively being investigated (54, 55).
While the elicitation of ICD within the primary tumor microenvironment through ionizing radiation has potential to prime the innate immune system, there remains the necessity for generating sufficient out-of-target tumor responses known as the abscopal effect, especially at sites of metastatic burden that might be unamendable to conventional localized treatment strategies. Despite the documentation of abscopal activities induced by localized radiation therapy in combination with adjunctive treatments (cytokines and chemotherapy), the fraction of human cancer patients that reliably demonstrate abscopal activities sufficient to induce macroscopic tumor regression remains <30% (56). The contextual scenarios (tumor type, host environment, therapeutic combinatorial sequencing) by which abscopal effects can be generated by radiation therapy remain incompletely defined (57, 58). As such, prospective investigations with high-value animal models could accelerate the identification of ideal circumstances to augment the proportion of human cancer patients whom might benefit from the life-extending activities of radiation-induced ICD and associated abscopal effects.
Opportunity to Optimize Radiation-Induced ICD Protocols
While several recent investigations have discussed the optimal dose and timing of radiation therapy relative to immunologic intervention, no single protocol is clearly superior to others, and the impact of dose rate is relatively unexplored. Given the non-uniformity of various therapeutic radiation regimens for the management of diverse solid tumor histologies, a significant research barrier exists for the thorough characterization of contributory radiation variables required for optimal radiation-induced ICD. While recent meta-analysis has been conducted to “standardize” immune activating potential of radiation treatment protocols through the comparison of biologic effective dose in preclinical models (59), there remains a scientific need for additional prospectively-designed studies inclusive of model systems that more faithfully recapitulate the natural progression of cancer development under immune evolutionary pressures. This “gap” in knowledge given the absence of an ideal experimental model system, is underscored by the rarity of achieving radiation-induced abscopal effects in human cancer patients (56, 60–62). As such, the consistent and reproducible generation of clinically meaningful abscopal effects in most cancer patients remains infrequent and suggests that the current state of understanding regarding radiation-induced immune activation remains incomplete and necessitates the inclusion of complementary innovative modeling systems.
One mechanism to generate new knowledge regarding the feasibility and limitations of radiation-induced ICD and associated abscopal effects could include the rational inclusion of pet dogs with solid tumors. Therapeutic management of cancer in pet dogs parallel the same modalities in human cancer patients, with the inclusion of radiation therapy for controlling localized tumor progression and associated morbidity. Importantly, the repertoire of cognate receptors including toll-like receptors responsible for detecting the presence of pathogens (pathogen associate molecular patterns) and danger signals (damage associated molecular patterns) have been recently characterized in the domestic canine (26, 63–65). With existing tools and knowledge of radiobiology and immunology in the canine species, an opportunity exists to prospectively and systemically evaluate novel radiation-induced ICD strategies in pet dogs that could be translated into life-extending abscopal activities in human cancer patients.
Relevant Solid Tumors in Pet Dogs for Optimizing Radiation Abscopal Effects
Canine Oral Malignant Melanoma (OMM)
Malignant melanoma is a metastatic solid tumor affecting both dogs and people (66), however, the anatomic locations of primary tumors differ, with oral cavity and skin being the primary sites for malignant melanoma in dogs and humans, respectively. In canines, melanoma is considered the most common oral malignancy, accounting for ~40% of all oral cancers (67). Despite differences in primary anatomic site, prominent molecular drivers of malignancy are conserved between dogs and people, including AKT and MAPK signaling pathways (31).
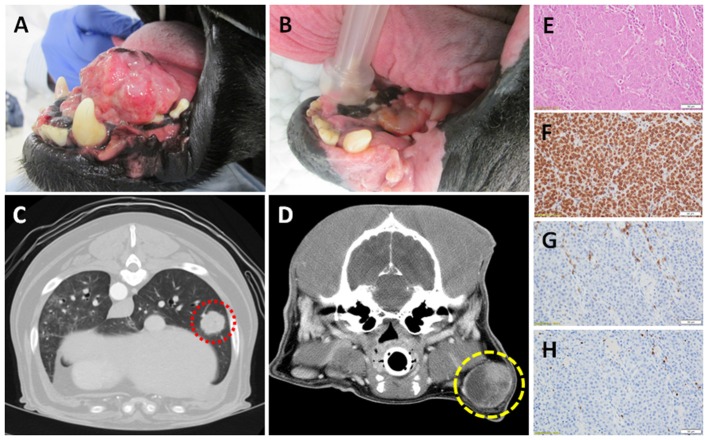
Effective management of canine OMM requires local treatment strategies, as well as systemic intervention to delay the onset and progression of regional and/or distant metastases (68–72). While surgical resection is feasible for some dogs with rostrally-confined primary tumors, most canines are diagnosed with invasive inoperable tumors, and hypofractionated ionizing radiation is instituted for local tumor control (73–75). Radiation therapy, alone or as an adjuvant to marginal resection, can achieve satisfactory local primary tumor control (Figures 1A,B), however a substantive fraction of dogs will develop metastatic progression within 6–9 months of diagnosis (67, 68, 76). While the most common site for OMM metastases are regional lymph nodes (77, 78), progression of distant metastases within the pulmonary parenchyma can become life-limiting in dogs that have achieved durable local disease control (67) (Figures 1C,D), and the institution of adjuvant cytotoxic agents does not definitively yield any survival benefit (74, 79). As such, no standard-of-care adjuvant therapy in dogs with metastatic OMM exists and creates a unique and ethical opportunity to model novel immunotherapeutic strategies that might not be otherwise possible in human patients. Importantly, commercial reagents for the assessment of immunobiologic endpoints including tumoral expression of PD-L1, tumor-infiltrating lymphocytes, and regulatory T cells have recently been validated in canine tissues (Figures 1E–H).
Figure 1.
Canine OMM (A) pre- and (B) post- palliative radiation therapy; note the achievement of strong partial response of primary tumor (courtesy of Dr. Michael Kent, UC Davis). Computed tomography of (C) distant pulmonary (red) and (D) regional lymph node metastases (yellow) of OMM origin; demonstrating the potential for reproducible and quantitative volumetric assessments for documentation of abscopal activities. Panel (E–H; top to bottom) represents the histologic and immunobiologic assessment of a regional lymph node effaced with amelanotic melanoma by H&E, PD-L1, CD3+ tumor-infiltrating lymphocytes, and regulatory T cells (courtesy of Dr. Jonathan Samuelson, UIUC). Magnification 400x.
Clinical Evidence for Canine OMM Immunogenicity
With conservation of certain tumor-associated antigens in both humans and dogs (80–82), canine OMM has been explored as a relevant tumor model in evaluating various immunotherapeutic strategies, in particular tumor vaccine (30). Both autologous and xenogeneic (tyrosinase) vaccines exert measurable anticancer activities in subsets of dogs treated, with objective responses being documented in patients with unsatisfactorily controlled primary tumors, as well as regression of regional and distant metastatic lesions (83–85). In addition to tyrosinase as a therapeutic target, a limited number of investigations have characterized the immunogenic targeting of xenogeneic GP100 and adenoviral CD40L transfection through vaccination strategies; demonstrating immunobiologic activities and clinical benefit in dogs with OMM (Table 1) (86, 87).
Table 1.
Summary of canine melanoma immunogenic strategies.
| Immunotherapeutic strategy | Target | Number of animals | Immunologic endpoint | Clinical benefit |
|---|---|---|---|---|
| Xenogeneic melanoma-antigen-enhanced allogenic tumor cell vaccine | Human glycoprotein 100 (hgp100) | 34 dogs | PBMC cytotoxicity Neutralizing anti-hgp100 antibody | 1 CR 5 PR 6 SD |
| Local adenovector human CD40L immunogene transfection | Human CD40L | 19 dogs | Circulating cytokines (TNFα, IL8, IL10) Neutralizing anti-human adenovirus serotype 5 antibody | 5 CR 8 PR 4 SD |
In addition to vaccines, checkpoint blockade strategies have been recently described in dogs with OMM. Initial studies identified the upregulation of PD-L1 following INF-γ exposure in immortalized canine melanoma cell lines, as well as, PD-L1 expression in 100% (8/8) of spontaneous canine OMM samples (88). A follow-up confirmatory study similarly identified 90% (36/40) OMM samples to express PD-L1, and importantly demonstrated that tumor-infiltrating lymphocytes, both CD4+ and CD8+, expressed PD-1 (89). Expressions of PD-L1 by melanoma cells and PD-1 by TILs, support the potential for melanoma cells to induce T-cell exhaustion as an immunoevasive mechanism. To confirm the functional immunosuppressive activities of PD-L1 expressions in canine OMM, an anti-PD-L1 antibody was evaluated in dogs with OMM, with suggestive evidence for survival time prolongation in four dogs with pulmonary metastasis when compared to historical controls (90). Collectively these clinical investigations support the relevancy of canine OMM as a naturally-occurring model system for testing immunotherapeutic combinations inclusive of other immunomodulatory strategies such as radiation-induced ICD and abscopal activities.
Canine Appendicular Osteosarcoma (OS)
Osteosarcoma (OS) accounts for 85% of all skeletal tumors in the dog with an estimated 10,000 dogs diagnosed each year (33, 91), and is a disease primarily afflicting the appendicular skeleton of large and giant breed dogs (33). Similarly, OS is the most common primary focal skeletal tumor in people, being the third most frequent cause of cancer in adolescents (92). The comparative similarities at genetic, molecular, and clinical levels shared between canine and pediatric OS are robust (12, 13, 33–35, 93–97); evidence that strongly emphasize the potential value for the utilization of canine OS to guide investigations related to pathogenesis and novel therapeutic discovery (98).
The biologic behavior of OS is aggressive, starting within the local bone microenvironment but then involving distant organs because of metastatic progression. Although 15% of dogs and 20% of people present with detectable lung metastases, the development of metastatic foci in the absence of chemotherapy is 90% within 1 year for dogs and 80% within 2 years for people (99, 100). While the institution of chemotherapy for OS patients has tripled the cure rate of people (20 → 65%) and doubled the survival time of dogs (130 → 270 days), no substantive improvement in long-term outcomes has been achieved for either species over the past 2 decades despite the institution of dose intensification strategies (101, 102). Given the current therapeutic ceiling, there is clinical need to explore alternative adjuvant therapies that might improve metastatic disease control.
Because the cure rate for canine OS remains <10% 3-years post diagnosis (103), the palliative management of primary tumor malignant osteolysis and associated pain is considered an acceptable treatment option in veterinary medicine (104). Similar to skeletal metastasis in humans, ionizing radiation alone or with bisphosphonates is considered effective for attenuating pathologic bone resorption and associated pain syndromes in affected dogs (105–111), and provides a durable therapeutic window of acceptable analgesia lasting from 3 to 12 months, whereby it is possible to serially monitor for the development, progression, or regression of distant pulmonary metastases. Prospective assessment of combinatorial strategies inclusive of radiation and other immunostimulatory therapies to amplify tumoral lymphocyte infiltrates such as ICD-inducing anthracyclines, toll-like receptor agonists, and checkpoint blocking antibodies which maximally generate robust abscopal effects could be leveraged to guide translational studies in human patients (Figure 2).
Figure 2.
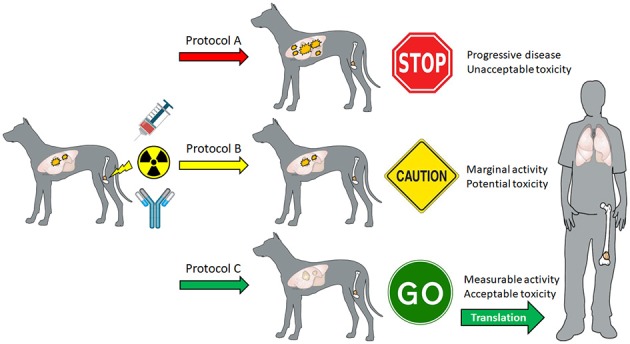
Theoretical schematic for how canine OS patients can be prospectively recruited to evaluate different immunomodulatory strategies inclusive of radiation therapy in combination with other agents such as ICD-inducing anthracyclines (mitoxantrone, doxorubicin, idarubicin), toll-like receptors (CpG ODN, Poly I/C, imiquimod), and checkpoint antibodies (PD-1, PD-L1, OX40) to generate high-value preclinical data to inform “go,” “caution,” or “no go” parallel translational studies maximizing abscopal activities in adolescents diagnosed with OS.
Clinical Evidence for Canine OS Immunogenicity
Scientific and clinical evidence supports OS to be immunogenic in dogs and humans (29, 112), and strategies that amplify anticancer immunity would be expected to improve long-term outcomes. In dogs, investigations have demonstrated immune activation as an effective strategy for either regressing macroscopic metastases or delaying micrometastatic disease progression. For macroscopic disease, inhalation therapy with liposome interleukin-2 demonstrated the capacity to activate immune cells with consequent regression of measurable pulmonary metastases (113, 114). In the setting of microscopic disease, dogs that develop post-operative wound infection after limb-spare surgery experience prolongation to pulmonary metastases development, with survival times being doubled in dogs that develop osteomyelitis (115, 116), and mechanistically localized infectious inflammation has been linked to NK cell and macrophage activation with consequent mediation of systemic anticancer effects (117). Similarly, L-MTP-PE, a synthetic lipophilic glycopeptide capable of activating monocytes and macrophages to a tumoricidal state, when administered to dogs with OS increases survival time, and underscores the key participation of innate immune cell activation for curbing metastatic progression (118, 119). Lastly, intravenous delivery of a genetically modified Listeria monocytogenes to OS-bearing dogs exerts promising anticancer immune activities and extends survival times (120). Collectively, these clinical investigations support the feasibility of stimulating immune effector cells to regress macroscopic and microscopic metastatic disease burdens in dogs diagnosed with OS.
Emerging Abscopal Modeling in Canine OMM and OS
While existing aggregate data for validating radiation-induced ICD and abscopal activities in pet dogs with cancer remains limited, experimental data is emerging to support the prospective evaluation of hypofractionated radiation therapy for augmenting immune responses. Recently, combinatorial strategies inclusive of ionizing radiation, hyperthermia, and intratumorally delivered virus-like nanoparticle-based therapies have been evaluated in canine OMM, and demonstrate the capacity to elicit immunogenic changes within the localized tumor microenvironment including the promotion TILs into the primary tumor (121, 122). In another investigation conducted in dogs with OMM, abscopal effects were documented in dogs treated with a combination of localized radiation therapy, intratumoral CpG ODN, and an indolamine-2,3-dioxygenase inhibitor (123). For canine OS, combining radiation and immunotherapy has been recently explored in a first-in-dog trial of autologous natural killer (NK) cells (124). In this study, OS-bearing dogs were treated with a coarsely fractionated radiation protocol consisting of 9 Gy once weekly for 4 treatments, with NK cells being harvested and expanded, and then delivered back to dogs by intratumoral injection following the completion of radiation therapy. Of the 10 dogs treated, 5 remained metastasis-free at 6 months, and one had regression of a suspicious pulmonary nodule detected at the time of diagnosis.
Future Directions and Conclusions
Dogs diagnosed with naturally-occurring cancers of comparative relevance can serve as biology-rich models of disease. If leveraged appropriately, the inclusion of pet dogs can accelerate the discovery of optimal combinations of radiation and immunotherapies which robustly and consistently elicit life-extending abscopal effects. With the availability of linear accelerator-based radiation facilities in veterinary centers analogous to human hospitals, coupled with the development of dog-specific immune-based therapies including vaccines, monoclonal antibodies, and CAR-T technologies, the purposeful inclusion of pet dogs with immunogenic tumors should be seriously contemplated as a unique strategy to aid in defining the limits and benefits of radiation-induced abscopal activities.
The scientific development and clinical assessment of novel immunotherapeutic strategies are rapidly growing areas in veterinary medicine and have demonstrated promise in the settings of canine OMM and OS. Given the conserved biology of these two immunogenic solid tumors between dogs and people, unique opportunities exist collectively for human and veterinary researchers to pilot and validate innovative immune strategies inclusive of radiation therapy in efforts to harness the promise of abscopal anticancer activities.
Author Contributions
TF and KS project conception and manuscript authorship.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to acknowledge Renee Walker and Drs. Michael Kent (UC Davis) and Jonathan Samuelson (UIUC) for technical assistance and provision of images.
Footnotes
Funding. This mini-review was supported by Morris Animal Foundation, D19CA-064.
References
- 1.Karlsson EK, Lindblad-Toh K. Leader of the pack: gene mapping in dogs and other model organisms. Nat Rev Genet. (2008) 9:713–25. 10.1038/nrg2382 [DOI] [PubMed] [Google Scholar]
- 2.Parker HG, Ostrander EA. Canine genomics and genetics: running with the pack. PLoS Genet. (2005) 1:e58. 10.1371/journal.pgen.0010058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson DF. Companion animal medicine in the age of medical genetics. J Vet Intern Med. (2000) 14:1–9. 10.1111/j.1939-1676.2000.tb01492.x [DOI] [PubMed] [Google Scholar]
- 4.Wayne RK, Ostrander EA. Lessons learned from the dog genome. Trends Genet. (2007) 23:557–67. 10.1016/j.tig.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 5.Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. (2011) 17:380–8. 10.1016/j.molmed.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starkey MP, Scase TJ, Mellersh CS, Murphy S. Dogs really are man's best friend–canine genomics has applications in veterinary and human medicine! Brief Funct Genomic Proteomic. (2005) 4:112–28. 10.1093/bfgp/4.2.112 [DOI] [PubMed] [Google Scholar]
- 7.Addady M. This is How Much Americans Spend on Their Dogs. Fortune. (2016). [Google Scholar]
- 8.Carter A. Man's best friend is also a friend of cancer research. J Natl Cancer Inst. (2008) 100:984. 10.1093/jnci/djn247 [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc AK, Breen M, Choyke P, Dewhirst M, Fan TM, Gustafson DL, et al. Perspectives from man's best friend: National Academy of Medicine's Workshop on Comparative Oncology. Sci Transl Med. (2016) 8:324ps5. 10.1126/scitranslmed.aaf0746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer (2008) 8:147–56. 10.1038/nrc2273 [DOI] [PubMed] [Google Scholar]
- 11.Ranieri G, Gadaleta CD, Patruno R, Zizzo N, Daidone MG, Hansson MG, et al. A model of study for human cancer: spontaneous occurring tumors in dogs. Biological features and translation for new anticancer therapies. Crit Rev Oncol Hematol. (2013) 88:187–97. 10.1016/j.critrevonc.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 12.Angstadt AY, Thayanithy V, Subramanian S, Modiano JF, Breen M. A genome-wide approach to comparative oncology: high-resolution oligonucleotide aCGH of canine and human osteosarcoma pinpoints shared microaberrations. Cancer Genet. (2012) 205:572–87. 10.1016/j.cancergen.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 13.Angstadt AY, Motsinger-Reif A, Thomas R, Kisseberth WC, Guillermo Couto C, Duval DL, et al. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosomes Cancer (2011) 50:859–74. 10.1002/gcc.20908 [DOI] [PubMed] [Google Scholar]
- 14.Breen M, Modiano JF. Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans–man and his best friend share more than companionship. Chromosome Res. (2008) 16:145–54. 10.1007/s10577-007-1212-4 [DOI] [PubMed] [Google Scholar]
- 15.Richards KL, Motsinger-Reif AA, Chen HW, Fedoriw Y, Fan C, Nielsen DM, et al. Gene profiling of canine B-cell lymphoma reveals germinal center and postgerminal center subtypes with different survival times, modeling human DLBCL. Cancer Res. (2013) 73:5029–39. 10.1158/0008-5472.CAN-12-3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas R, Duke SE, Wang HJ, Breen TE, Higgins RJ, Linder KE, et al. 'Putting our heads together': insights into genomic conservation between human and canine intracranial tumors. J Neurooncol. (2009) 94:333–49. 10.1007/s11060-009-9877-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poorman K, Borst L, Moroff S, Roy S, Labelle P, Motsinger-Reif A, et al. Comparative cytogenetic characterization of primary canine melanocytic lesions using array CGH and fluorescence in situ hybridization. Chromosome Res. (2015) 23:171–86. 10.1007/s10577-014-9444-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science (2011) 331:1565–70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 19.Vesely MD, Schreiber RD. Cancer immunoediting: antigens, mechanisms, and implications to cancer immunotherapy. Ann NY Acad Sci. (2013) 1284:1–5. 10.1111/nyas.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulligan RM. Comparative pathology of human and canine cancer. Ann NY Acad Sci. (1963) 108:642–90. 10.1111/j.1749-6632.1963.tb13414.x [DOI] [PubMed] [Google Scholar]
- 21.Paoloni MC, Tandle A, Mazcko C, Hanna E, Kachala S, Leblanc A, et al. Launching a novel preclinical infrastructure: comparative oncology trials consortium directed therapeutic targeting of TNFalpha to cancer vasculature. PLoS ONE (2009) 4:e4972. 10.1371/journal.pone.0004972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JS, Withers SS, Modiano JF, Kent MS, Chen M, Luna JI, et al. Canine cancer immunotherapy studies: linking mouse and human. J Immunother Cancer (2016) 4:97. 10.1186/s40425-016-0200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chabanne L, Marchal T, Kaplanski C, Fournel C, Magnol JP, Monier JC, et al. Screening of 78 monoclonal antibodies directed against human leukocyte antigens for cross-reactivity with surface markers on canine lymphocytes. Tissue Antigens (1994) 43:202–5. 10.1111/j.1399-0039.1994.tb02324.x [DOI] [PubMed] [Google Scholar]
- 24.Bergeron LM, McCandless EE, Dunham S, Dunkle B, Zhu Y, Shelly J, et al. Comparative functional characterization of canine IgG subclasses. Vet Immunol Immunopathol. (2014) 157:31–41. 10.1016/j.vetimm.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 25.Summerfield A, Auray G, Ricklin M. Comparative dendritic cell biology of veterinary mammals. Annu Rev Anim Biosci. (2015) 3:533–57. 10.1146/annurev-animal-022114-111009 [DOI] [PubMed] [Google Scholar]
- 26.Turin L, Riva F. Toll-like receptor family in domestic animal species. Crit Rev Immunol. (2008) 28:513–38. 10.1615/CritRevImmunol.v28.i6.30 [DOI] [PubMed] [Google Scholar]
- 27.Felsburg PJ. Overview of immune system development in the dog: comparison with humans. Hum Exp Toxicol. (2002) 21:487–92. 10.1191/0960327102ht286oa [DOI] [PubMed] [Google Scholar]
- 28.Cobbold S, Metcalfe S. Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW). Tissue Antigens (1994) 43:137–54. 10.1111/j.1399-0039.1994.tb02315.x [DOI] [PubMed] [Google Scholar]
- 29.Wycislo KL, Fan TM. The immunotherapy of canine osteosarcoma: a historical and systematic review. J Vet Intern Med. (2015) 29:759–69. 10.1111/jvim.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atherton MJ, Morris JS, McDermott MR, Lichty BD. Cancer immunology and canine malignant melanoma: a comparative review. Vet Immunol Immunopathol. (2016) 169:15–26. 10.1016/j.vetimm.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 31.Simpson RM, Bastian BC, Michael HT, Webster JD, Prasad ML, Conway CM, et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigment Cell Melanoma Res. (2014) 27:37–47. 10.1111/pcmr.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillard M, Cadieu E, De Brito C, Abadie J, Vergier B, Devauchelle P, et al. Naturally occurring melanomas in dogs as models for non-UV pathways of human melanomas. Pigment Cell Melanoma Res. (2014) 27:90–102. 10.1111/pcmr.12170 [DOI] [PubMed] [Google Scholar]
- 33.Withrow SJ, Powers BE, Straw RC, Wilkins RM. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res. (1991) 270:159–68. [PubMed] [Google Scholar]
- 34.Fan TM, Khanna C. Comparative aspects of osteosarcoma pathogenesis in humans and dogs. Vet Sci. (2015) 2:210–30. 10.3390/vetsci2030210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenger JM, London CA, Kisseberth WC. Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J. (2014) 55:69–85. 10.1093/ilar/ilu009 [DOI] [PubMed] [Google Scholar]
- 36.Klingemann H. Immunotherapy for dogs: running behind humans. Front Immunol. (2018) 9:133. 10.3389/fimmu.2018.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steel GG, McMillan TJ, Peacock JH. The 5Rs of radiobiology. Int J Radiat Biol. (1989) 56:1045–8. 10.1080/09553008914552491 [DOI] [PubMed] [Google Scholar]
- 38.Trott KR. Experimental results and clinical implications of the four R's in fractionated radiotherapy. Radiat Environ Biophys. (1982) 20:159–70. 10.1007/BF01325465 [DOI] [PubMed] [Google Scholar]
- 39.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th Edn Philadelphia, PA: Lippincott Williams & Wilkins; (2006). [Google Scholar]
- 40.Roninson IB, Broude EV, Chang BD. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist Updat. (2001) 4:303–13. 10.1054/drup.2001.0213 [DOI] [PubMed] [Google Scholar]
- 41.Tang L, Wei F, Wu Y, He Y, Shi L, Xiong F, et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J Exp Clin Cancer Res. (2018) 37:87. 10.1186/s13046-018-0758-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xin Y, Jiang F, Yang C, Yan Q, Guo W, Huang Q, et al. Role of autophagy in regulating the radiosensitivity of tumor cells. J Cancer Res Clin Oncol. (2017) 143:2147–57. 10.1007/s00432-017-2487-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: an emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. (2008) 76:947–57. 10.1016/j.bcp.2008.06.024 [DOI] [PubMed] [Google Scholar]
- 44.Lehmann BD, McCubrey JA, Jefferson HS, Paine MS, Chappell WH, Terrian DM. A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle (2007) 6:595–605. 10.4161/cc.6.5.3901 [DOI] [PubMed] [Google Scholar]
- 45.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. (2013) 31:51–72. 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 46.Golden EB, Formenti SC. Is tumor (R)ejection by the immune system the “5th R” of radiobiology? Oncoimmunology (2014) 3:e28133. 10.4161/onci.28133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer (2016) 40:10–24. 10.1016/j.currproblcancer.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 48.Rodel F, Frey B, Multhoff G, Gaipl U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. (2015) 356:105–13. 10.1016/j.canlet.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 49.Wang R, Zhou T, Liu W, Zuo L. Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget (2018) 9:18637–47. 10.18632/oncotarget.24746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev. (2017) 280:41–56. 10.1111/imr.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. (2005) 17:359–65. 10.1016/j.coi.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 52.Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology (2014) 3:e28518. 10.4161/onci.28518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radogna F, Diederich M. Stress-induced cellular responses in immunogenic cell death: implications for cancer immunotherapy. Biochem Pharmacol. (2018) 153:12–23. 10.1016/j.bcp.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 54.Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. (2012) 84:879–80. 10.1016/j.ijrobp.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. (2015) 1:1325–32. 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 56.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. (2015) 16:795–803. 10.1016/S1470-2045(15)00054-6 [DOI] [PubMed] [Google Scholar]
- 57.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. (2013) 105:256–65. 10.1093/jnci/djs629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. (2014) 2:831–8. 10.1158/2326-6066.CIR-14-0069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marconi R, Strolin S, Bossi G, Strigari L. A meta-analysis of the abscopal effect in preclinical models: is the biologically effective dose a relevant physical trigger? PLoS ONE (2017) 12:e0171559. 10.1371/journal.pone.0171559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi F, Wang X, Teng F, Kong L, Yu J. Abscopal effect of metastatic pancreatic cancer after local radiotherapy and granulocyte-macrophage colony-stimulating factor therapy. Cancer Biol Ther. (2017) 18:137–41. 10.1080/15384047.2016.1276133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. (2012) 366:925–31. 10.1056/NEJMoa1112824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okuma K, Yamashita H, Niibe Y, Hayakawa K, Nakagawa K. Abscopal effect of radiation on lung metastases of hepatocellular carcinoma: a case report. J Med Case Rep. (2011) 5:111. 10.1186/1752-1947-5-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Friedenberg SG, Strange HR, Guillaumin J, VanGundy ZC, Crouser ED, Papenfuss TL. Effect of disrupted mitochondria as a source of damage-associated molecular patterns on the production of tumor necrosis factor alpha by splenocytes from dogs. Am J Vet Res. (2016) 77:604–12. 10.2460/ajvr.77.6.604 [DOI] [PubMed] [Google Scholar]
- 64.Jalilian I, Spildrejorde M, Seavers A, Curtis BL, McArthur JD, Sluyter R. Functional expression of the damage-associated molecular pattern receptor P2X7 on canine kidney epithelial cells. Vet Immunol Immunopathol. (2012) 150:228–33. 10.1016/j.vetimm.2012.09.040 [DOI] [PubMed] [Google Scholar]
- 65.Heilmann RM, Allenspach K. Pattern-recognition receptors: signaling pathways and dysregulation in canine chronic enteropathies-brief review. J Vet Diagn Invest. (2017) 29:781–7. 10.1177/1040638717728545 [DOI] [PubMed] [Google Scholar]
- 66.Nishiya AT, Massoco CO, Felizzola CR, Perlmann E, Batschinski K, Tedardi MV, et al. Comparative aspects of canine melanoma. Vet Sci. (2016) 3:E7. 10.3390/vetsci3010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergman PJ. Canine oral melanoma. Clin Tech Small Anim Pract. (2007) 22:55–60. 10.1053/j.ctsap.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 68.Tuohy JL, Selmic LE, Worley DR, Ehrhart NP, Withrow SJ. Outcome following curative-intent surgery for oral melanoma in dogs: 70 cases (1998-2011). J Am Vet Med Assoc. (2014) 245:1266–73. 10.2460/javma.245.11.1266 [DOI] [PubMed] [Google Scholar]
- 69.Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, et al. Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol. (2000) 37:597–608. 10.1354/vp.37-6-597 [DOI] [PubMed] [Google Scholar]
- 70.Freeman KP, Hahn KA, Harris FD, King GK. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987-1997). J Vet Intern Med. (2003) 17:96–101. 10.1111/j.1939-1676.2003.tb01329.x [DOI] [PubMed] [Google Scholar]
- 71.Dank G, Rassnick KM, Sokolovsky Y, Garrett LD, Post GS, Kitchell BE, et al. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Vet Comp Oncol. (2014) 12:78–84. 10.1111/j.1476-5829.2012.00338.x [DOI] [PubMed] [Google Scholar]
- 72.Rassnick KM, Ruslander DM, Cotter SM, Al-Sarraf R, Bruyette DS, Gamblin RM, et al. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989-2000). J Am Vet Med Assoc. (2001) 218:1444–8. 10.2460/javma.2001.218.1444 [DOI] [PubMed] [Google Scholar]
- 73.Blackwood L, Dobson JM. Radiotherapy of oral malignant melanomas in dogs. J Am Vet Med Assoc. (1996) 209:98–102. [PubMed] [Google Scholar]
- 74.Proulx DR, Ruslander DM, Dodge RK, Hauck ML, Williams LE, Horn B, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound (2003) 44:352–9. 10.1111/j.1740-8261.2003.tb00468.x [DOI] [PubMed] [Google Scholar]
- 75.Theon AP, Rodriguez C, Madewell BR. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. J Am Vet Med Assoc. (1997) 210:778–84. [PubMed] [Google Scholar]
- 76.Kawabe M, Mori T, Ito Y, Murakami M, Sakai H, Yanai T, et al. Outcomes of dogs undergoing radiotherapy for treatment of oral malignant melanoma: 111 cases (2006-2012). J Am Vet Med Assoc. (2015) 247:1146–53. 10.2460/javma.247.10.1146 [DOI] [PubMed] [Google Scholar]
- 77.Skinner OT, Boston SE, Souza CHM. Patterns of lymph node metastasis identified following bilateral mandibular and medial retropharyngeal lymphadenectomy in 31 dogs with malignancies of the head. Vet Comp Oncol. (2017) 15:881–9. 10.1111/vco.12229 [DOI] [PubMed] [Google Scholar]
- 78.Williams LE, Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987-2001). J Am Vet Med Assoc. (2003) 222:1234–6. 10.2460/javma.2003.222.1234 [DOI] [PubMed] [Google Scholar]
- 79.Brockley LK, Cooper MA, Bennett PF. Malignant melanoma in 63 dogs (2001-2011): the effect of carboplatin chemotherapy on survival. N Z Vet J. (2013) 61:25–31. 10.1080/00480169.2012.699433 [DOI] [PubMed] [Google Scholar]
- 80.Sulaimon S, Kitchell B, Ehrhart E. Immunohistochemical detection of melanoma-specific antigens in spontaneous canine melanoma. J Comp Pathol. (2002) 127:162–8. 10.1053/jcpa.2002.0576 [DOI] [PubMed] [Google Scholar]
- 81.Berrington AJ, Jimbow K, Haines DM. Immunohistochemical detection of melanoma-associated antigens on formalin-fixed, paraffin-embedded canine tumors. Vet Pathol. (1994) 31:455–61. 10.1177/030098589403100408 [DOI] [PubMed] [Google Scholar]
- 82.Bergman PJ, McKnight J, Novosad A, Charney S, Farrelly J, Craft D, et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin Cancer Res. (2003) 9:1284–90. [PubMed] [Google Scholar]
- 83.Bergman PJ, Camps-Palau MA, McKnight JA, Leibman NF, Craft DM, Leung C, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine (2006) 24:4582–5. 10.1016/j.vaccine.2005.08.027 [DOI] [PubMed] [Google Scholar]
- 84.Grosenbaugh DA, Leard AT, Bergman PJ, Klein MK, Meleo K, Susaneck S, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. (2011) 72:1631–8. 10.2460/ajvr.72.12.1631 [DOI] [PubMed] [Google Scholar]
- 85.Verganti S, Berlato D, Blackwood L, Amores-Fuster I, Polton GA, Elders R, et al. Use of Oncept melanoma vaccine in 69 canine oral malignant melanomas in the UK. J Small Anim Pract. (2017) 58:10–6. 10.1111/jsap.12613 [DOI] [PubMed] [Google Scholar]
- 86.Alexander AN, Huelsmeyer MK, Mitzey A, Dubielzig RR, Kurzman ID, Macewen EG, et al. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol Immunother. (2006) 55:433–42. 10.1007/s00262-005-0025-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Westberg S, Sadeghi A, Svensson E, Segall T, Dimopoulou M, Korsgren O, et al. Treatment efficacy and immune stimulation by AdCD40L gene therapy of spontaneous canine malignant melanoma. J Immunother. (2013) 36:350–8. 10.1097/CJI.0b013e31829d8a1b [DOI] [PubMed] [Google Scholar]
- 88.Maekawa N, Konnai S, Ikebuchi R, Okagawa T, Adachi M, Takagi S, et al. Expression of PD-L1 on canine tumor cells and enhancement of IFN-gamma production from tumor-infiltrating cells by PD-L1 blockade. PLoS ONE (2014) 9:e98415. 10.1371/journal.pone.0098415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maekawa N, Konnai S, Okagawa T, Nishimori A, Ikebuchi R, Izumi Y, et al. Immunohistochemical analysis of PD-L1 expression in canine malignant cancers and PD-1 expression on lymphocytes in canine oral melanoma. PLoS ONE (2016) 11:e0157176. 10.1371/journal.pone.0157176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maekawa N, Konnai S, Takagi S, Kagawa Y, Okagawa T, Nishimori A, et al. A canine chimeric monoclonal antibody targeting PD-L1 and its clinical efficacy in canine oral malignant melanoma or undifferentiated sarcoma. Sci Rep. (2017) 7:8951 10.1038/s41598-017-09444-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schiffman JD, Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond B Biol Sci. (2015) 370:20140231. 10.1098/rstb.2014.0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. (2009) 152:3–13. 10.1007/978-1-4419-0284-9_1 [DOI] [PubMed] [Google Scholar]
- 93.Paoloni M, Davis S, Lana S, Withrow S, Sangiorgi L, Picci P, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics (2009) 10:625. 10.1186/1471-2164-10-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simpson S, Dunning MD, de Brot S, Grau-Roma L, Mongan NP, Rutland CS. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. (2017) 59:71. 10.1186/s13028-017-0341-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott MC, Sarver AL, Gavin KJ, Thayanithy V, Getzy DM, Newman RA, et al. Molecular subtypes of osteosarcoma identified by reducing tumor heterogeneity through an interspecies comparative approach. Bone (2011) 49:356–67. 10.1016/j.bone.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rankin KS, Starkey M, Lunec J, Gerrand CH, Murphy S, Biswas S. Of dogs and men: comparative biology as a tool for the discovery of novel biomarkers and drug development targets in osteosarcoma. Pediatr Blood Cancer (2012) 58:327–33. 10.1002/pbc.23341 [DOI] [PubMed] [Google Scholar]
- 97.Varshney J, Scott MC, Largaespada DA, Subramanian S. Understanding the osteosarcoma pathobiology: a comparative oncology approach. Vet Sci. (2016) 3:3. 10.3390/vetsci3010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khanna C, Fan TM, Gorlick R, Helman LJ, Kleinerman ES, Adamson PC, et al. Toward a drug development path that targets metastatic progression in osteosarcoma. Clin Cancer Res. (2014) 20:4200–9. 10.1158/1078-0432.CCR-13-2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janeway KA, Walkley CR. Modeling human osteosarcoma in the mouse: from bedside to bench. Bone (2010) 47:859–65. 10.1016/j.bone.2010.07.028 [DOI] [PubMed] [Google Scholar]
- 100.Spodnick GJ, Berg J, Rand WM, Schelling SH, Couto G, Harvey HJ, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978-1988). J Am Vet Med Assoc. (1992) 200:995–9. [PubMed] [Google Scholar]
- 101.Chun R, Garrett LD, Henry C, Wall M, Smith A, Azene NM. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc. (2005) 41:382–7. 10.5326/0410382 [DOI] [PubMed] [Google Scholar]
- 102.Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. (2016) 17:1396–408. 10.1016/S1470-2045(16)30214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Selmic LE, Burton JH, Thamm DH, Withrow SJ, Lana SE. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. (2014) 28:554–63. 10.1111/jvim.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan TM. Pain management in veterinary patients with cancer. Vet Clin North Am Small Anim Pract. (2014) 44:989–1001. 10.1016/j.cvsm.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 105.Fan TM, Charney SC, de Lorimier LP, Garrett LD, Griffon DJ, Gordon-Evans WJ, et al. Double-blind placebo-controlled trial of adjuvant pamidronate with palliative radiotherapy and intravenous doxorubicin for canine appendicular osteosarcoma bone pain. J Vet Intern Med. (2009) 23:152–60. 10.1111/j.1939-1676.2008.0221.x [DOI] [PubMed] [Google Scholar]
- 106.Boston SE, Vinayak A, Lu X, Larue S, Bacon NJ, Bleedorn JA, et al. Outcome and complications in dogs with appendicular primary bone tumors treated with stereotactic radiotherapy and concurrent surgical stabilization. Vet Surg. (2017) 46:829–37. 10.1111/vsu.12669 [DOI] [PubMed] [Google Scholar]
- 107.Kubicek L, Vanderhart D, Wirth K, An Q, Chang M, Farese J, et al. Association between computed tomographic characteristics and fractures following stereotactic radiosurgery in dogs with appendicular osteosarcoma. Vet Radiol Ultrasound. (2016) 57:321–30. 10.1111/vru.12351 [DOI] [PubMed] [Google Scholar]
- 108.Covey JL, Farese JP, Bacon NJ, Schallberger SP, Amsellem P, Cavanaugh RP, et al. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet Surg. (2014) 43:174–81. 10.1111/j.1532-950X.2014.12082.x [DOI] [PubMed] [Google Scholar]
- 109.Farese JP, Milner R, Thompson MS, Lester N, Cooke K, Fox L, et al. Stereotactic radiosurgery for treatment of osteosarcomas involving the distal portions of the limbs in dogs. J Am Vet Med Assoc. (2004) 225:1567–72. 10.2460/javma.2004.225.1567 [DOI] [PubMed] [Google Scholar]
- 110.Coomer A, Farese J, Milner R, Liptak J, Bacon N, Lurie D. Radiation therapy for canine appendicular osteosarcoma. Vet Comp Oncol. (2009) 7:15–27. 10.1111/j.1476-5829.2008.00177.x [DOI] [PubMed] [Google Scholar]
- 111.Ramirez O III, Dodge RK, Page RL, Price GS, Hauck ML, LaDue TA, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs.Vet Radiol Ultrasound (1999) 40:517–22. 10.1111/j.1740-8261.1999.tb00385.x [DOI] [PubMed] [Google Scholar]
- 112.Roberts SS, Chou AJ, Cheung NK. Immunotherapy of childhood sarcomas. Front Oncol. (2015) 5:181. 10.3389/fonc.2015.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khanna C, Anderson PM, Hasz DE, Katsanis E, Neville M, Klausner JS. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer (1997) 79:1409–21. [DOI] [PubMed] [Google Scholar]
- 114.Khanna C, Hasz DE, Klausner JS, Anderson PM. Aerosol delivery of interleukin 2 liposomes is nontoxic and biologically effective: canine studies. Clin Cancer Res. (1996) 2:721–34. [PubMed] [Google Scholar]
- 115.Lascelles BD, Dernell WS, Correa MT, Lafferty M, Devitt CM, Kuntz CA, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. (2005) 12:1073–83. 10.1245/ASO.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 116.Liptak JM, Dernell WS, Ehrhart N, Lafferty MH, Monteith GJ, Withrow SJ. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg. (2006) 35:518–33. 10.1111/j.1532-950X.2006.00185.x [DOI] [PubMed] [Google Scholar]
- 117.Sottnik JL, U'Ren LW, Thamm DH, Withrow SJ, Dow SW. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother. (2010) 59:367–78. 10.1007/s00262-009-0755-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MacEwen EG, Kurzman ID, Rosenthal RC, Smith BW, Manley PA, Roush JK, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome-encapsulated muramyl tripeptide. J Natl Cancer Inst. (1989) 81:935–8. 10.1093/jnci/81.12.935 [DOI] [PubMed] [Google Scholar]
- 119.Kurzman ID, MacEwen EG, Rosenthal RC, Fox LE, Keller ET, Helfand SC, et al. Adjuvant therapy for osteosarcoma in dogs: results of randomized clinical trials using combined liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res. (1995) 1:1595–601. [PubMed] [Google Scholar]
- 120.Mason NJ, Gnanandarajah JS, Engiles JB, Gray F, Laughlin D, Gaurnier-Hausser A, et al. Immunotherapy with a HER2-targeting listeria induces HER2-specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin Cancer Res. (2016) 22:4380–90. 10.1158/1078-0432.CCR-16-0088 [DOI] [PubMed] [Google Scholar]
- 121.Hoopes PJ, Wagner RJ, Duval K, Kang K, Gladstone DJ, Moodie KL, et al. Treatment of canine oral melanoma with nanotechnology-based immunotherapy and radiation. Mol Pharm. (2018) 15:3717–22. 10.1021/acs.molpharmaceut.8b00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoopes PJ, Moodie KL, Petryk AA, Petryk JD, Sechrist S, Gladstone DJ, et al. Hypo-fractionated radiation, magnetic nanoparticle hyperthermia and a viral immunotherapy treatment of spontaneous canine cancer. Proc SPIE Int Soc Opt Eng. (2017) 10066:1006605. 10.1117/12.2256213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Monjazeb AM, Kent MS, Grossenbacher SK, Mall C, Zamora AE, Mirsoian A, et al. Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res. (2016) 22:4328–40. 10.1158/1078-0432.CCR-15-3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Canter RJ, Grossenbacher SK, Foltz JA, Sturgill IR, Park JS, Luna JI, et al. Radiotherapy enhances natural killer cell cytotoxicity and localization in pre-clinical canine sarcomas and first-in-dog clinical trial. J Immunother Cancer (2017) 5:98. 10.1186/s40425-017-0305-7 [DOI] [PMC free article] [PubMed] [Google Scholar]