Abstract
The International Society of Nephrology has adopted a proactive approach to defining the current state of kidney care and unmet needs through a multifaceted Closing the Gaps initiative. As part of this initiative, the International Society of Nephrology convened a meeting of experts to develop an approach to tackle acute kidney injury and chronic kidney disease (CKD). This manuscript expands on the recently published International Society of Nephrology CKD Roadmap and reports on the discussions of the working group assigned to the task of reviewing the global impact of complication of CKD. The working group defined the following goals:
Goal 1: Optimize the management of anemia and endocrine and metabolic abnormalities associated with CKD. The impact of these conditions at a global level is not well understood, particularly in regions where renal replacement therapy is not readily available. Some treatment regimens may be affordable in low- and middle-income countries and if implemented, could have an impact on the burden of suffering associated with CKD.
Goal 2: Improve the prevention and management of cardiovascular complications linked to CKD. Most research on cardiovascular complications of CKD has focused on atherosclerotic diseases (myocardial infarction, ischemic stroke, and peripheral gangrene). There has been growing recognition that other forms of cardiovascular diseases, such as heart failure, valvular disease and arrhythmias, have a major impact on patient outcomes. Much less is known about the mechanisms and treatment of these non-atherosclerotic complications.
Goal 3: Improve the diagnosis and management of symptoms associated with CKD. Symptom management is one of the greatest challenges in the management of CKD, with limited knowledge about the mechanisms associated with the development of these common problems and how best to characterize them into usable clinical phenotypes.
Improved understanding of the complications of CKD may alleviate suffering and prolong life among millions of people worldwide both in developed countries and in regions where renal replacement therapy is not widely available.
Keywords: CKD, complications, knowledge gaps, management, mechanisms
Chronic kidney disease (CKD) is associated with several adverse clinical outcomes, such as cardiovascular events, kidney failure requiring renal replacement therapy, mortality, and poor quality of life for survivors in general.1, 2, 3, 4, 5, 6 Kidney disease amplifies the enormous burden and population health impact associated with both communicable and noncommunicable diseases.6, 7
CKD has not been included in the major chronic disease control strategies at international, regional, and/or national levels. The progressive nature of CKD, the associated cardiovascular morbidity and mortality, and the ensuing end-stage kidney disease place a considerable burden on global healthcare resources.6, 7, 8 A better understanding of the nature of CKD-related complications may help to optimize the diagnosis, prevention, and management.
The International Society of Nephrology Closing the Gaps initiative was established to define the global needs and current state of kidney care building on acute kidney injury initiatives (0by25; www.theisn.org) and focusing on CKD. The goal was to create a “blueprint” to enhance the optimal care globally through research, education, and advocacy. As part of this initiative, the International Society of Nephrology convened the first Global Kidney Health Summit on July 26 to 28, 2016 in Vancouver, Canada. This article expands on the recently published International Society of Nephrology CKD Roadmap,9 which is the result of the Summit.
CKD-Related Complications: Current State
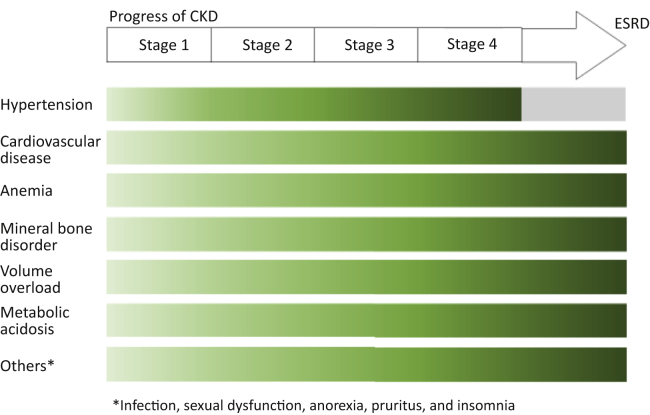
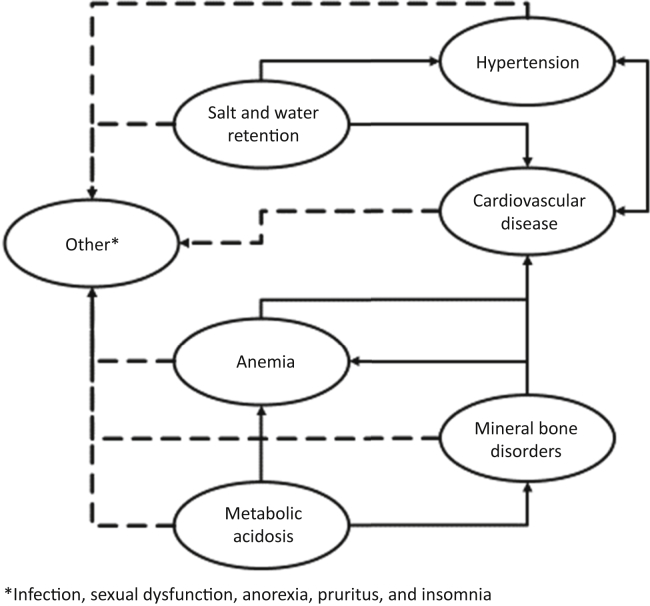
Progressive CKD is linked to several complications with higher prevalence and intensity at lower levels of kidney function, which interact with each other8, 10, 11 (Table 1, Figures 1 and 2). These complications contribute to high morbidity and mortality and poor quality of life. Some of these complications can be readily defined and quantified (cardiovascular disease, hypertension, anemia, mineral bone disorder, volume overload, electrolytes, and acid-base abnormalities) and may require a specific management approach, for example, the prescription of erythropoiesis-stimulating agents to correct anemia. Other less well-defined complications with a less distinct pathogenesis, such as anorexia, fatigue, cachexia, pruritus, nausea, and sexual dysfunction, may manifest as complex symptoms often associated with advanced CKD. The work group identified the following complications of CKD as being relevant to the global burden of poor health caused by CKD.
1. Hypertension: Hypertension remains one of the most damaging complications of CKD and is thought to contribute to the acceleration of progressive decline in kidney function, cardiovascular diseases (CVD), and related mortality. Both detection and control of high blood pressure is frequently suboptimal and improvements could directly help patients.12 The Systolic Blood Pressure Intervention Trial provided important information about the effects of a more stringent lowering of systolic blood pressure to a target of <120 mm Hg that may be relevant to CKD patients; although, this trial excluded high-risk subjects with CKD, proteinuria, or diabetes.13 Lifestyle modifications, such as weight loss and dietary salt restriction, may also improve the blood pressure control. Such interventions can be lower in cost than pharmacological therapies and have the potential to affect outcomes, such as heart failure and stroke, in both developed health care systems and low- and middle-income countries (LMICs). Since many anti-hypertensive agents are available and affordable in LMICs, one feasible goal would be to improve the control of high blood pressure complications in CKD patients, aiming to achieve target ranges in a proportion of patients. Such a goal can be attained globally, and its impact is easily measurable.
2. Cardiovascular complications: CVD represents the leading cause of mortality in CKD patients, and the prevalence and burden of this complication increases with declining kidney function (Figures 1 and 2).8, 14 For example, the risk of mortality from CVD is 8.1-fold greater in a patient with CKD stage G5 A3 (eGFR < 15 ml/min per 1.73 m2 and urinary albumin-creatinine ratio > 300 mg/g) than in a reference population without kidney disease.4 While the risk of conventional atherosclerotic cardiovascular events increases with CKD, the majority of increased risk is attributable to non-atherosclerotic pathologies, such as left ventricular hypertrophy with diastolic and systolic dysfunction, valvular disease, and arterial calcification. These pathologies can manifest as atrial and ventricular dysrhythmias, heart failure, and sudden death.15 While it is generally accepted that the treatment of traditional cardiovascular risk factors, such as cholesterol16 and blood pressure,17 is efficacious in the CKD population, particularly in patients with CKD stages 1 to 3, there are additional risk factors to consider in CKD patients, most of which are considered to be CKD complications. For example, mineral and endocrine disturbances that reflect CKD-mineral bone disorder, such as phosphate retention, elevated levels of fibroblast growth factor 23, and disturbances in Klotho metabolism, may contribute to cardiomyopathy and vasculopathy.18 Improvements in our understanding of the factors that contribute to CVD associated with CKD and identification of additional therapeutic targets, along with efforts to control blood pressure and increased prescription of lipid-lowering therapies could ultimately lead to a global reduction in the burden of CVD attributable to CKD.19
3. Anemia: Anemia complications in CKD patients has been well characterized and treated in many parts of the world with iron and erythropoiesis-stimulating agents (ESAs). However, the optimal doses of ESAs and parenteral iron have not been established. While ESAs can provide symptomatic relief, the impact of these medications on survival remains unclear20 and may increase cardiovascular and cancer risks. The full spectrum of side effects of ESA is unknown, and the role of high hepcidin in CKD remains inadequately studied.21 There may be regional differences in resistance to ESA therapy, which renders patients more susceptible to harmful effects of these high-cost agents.22 The current management of anemia in CKD patients in many LMICs, where ESAs are variably available and prohibitively expensive, is different from developed countries where these agents are widely available. While we still need to learn more about risks and benefits of ESAs and i.v. iron, efforts to make these therapies (and blood transfusions) more readily accessible in LMICs may help to reduce the symptom burden associated with anemia in CKD.
4. CKD-related mineral bone disorder: The syndrome of CKD-mineral and bone disorder was defined by Kidney Disease: Improving Global Outcomes23, 24, 25 guidelines and encompasses traditional mineral biochemical abnormalities, the spectrum of renal osteodystrophy, and soft tissue calcification. Left ventricular hypertrophy may be causally linked to these abnormalities. This complex group of disorders is poorly understood and despite a considerable body of preclinical data, very few developments have been translated to clinical applications.10 High blood phosphate levels, deficiency of vitamin D, and secondary hyperparathyroidism can be monitored and treated; although, the true benefits of interventions to correct these abnormalities are unproven. The role of low-cost calcium-based phosphate binders is controversial because of the potential of these agents to exacerbate tissue calcium deposition.26, 27 A pragmatic approach based on our current level of knowledge would be to increase the availability of phosphate binders, nutritional vitamin D, and analogs of 1,25-dihydroxyvitamin D to alleviate the recognized symptoms of tertiary hyperparathyroidism.
5. Salt and water retention: In CKD stages 4 to 5, and possibly in CKD stage 3, there is loss of defense against both sodium excess and sodium depletion. In clinical practice, sodium excess with fluid retention is by far the most common, although the exact prevalence has not been determined. While the extracellular fluid volume may be expanded, the sodium balance appears to be relatively well-maintained until end-stage renal disease.28 Excess sodium and fluid contribute to not only edema, which may negatively affect quality of life, but also hypertension and thereby CVD (specifically concentric left ventricular hypertrophy, which can result in diastolic dysfunction). The mainstay of therapy is adherence to simple fluid balance (intake vs. output) concepts, restriction of dietary salt intake, and use of natriuretic agents (which may be less effective in the more advanced stages of CKD). Thiazides and loop diuretics are widely available at low cost and could be used more widely to alleviate symptomatic edema in CKD patients with the potential to improve cardiovascular outcomes.
6. Metabolic acidosis and electrolyte disorders: Metabolic acidosis is common in CKD and is caused when the acid intake and generation exceed the renal acid excretion. In early stages, it may be manifest as “acid excess with normal bicarbonate,” a state of positive acid balance without low plasma bicarbonate due to buffering and renal adaptation.29 Alkali therapy is effective but limited by mandatory sodium and/or potassium loads. Chronic metabolic acidosis contributes to skeletal muscle catabolism, insensitivity to endocrine hormones, and bone disease30 and may accelerate the progression of CKD.31 The challenge is early detection, which requires the identification of potentially harmful acid loading before a fall in serum bicarbonate occurs. The treatment of metabolic acidosis could be implemented on a global basis because the therapies are inexpensive, but the benefits of such intervention are unproven, and the sodium or potassium loading that accompanies current alkali therapy may be harmful, particularly in more advanced stages of CKD. Alternative ways of alkali delivery are needed. Non-sodium and potassium-containing alkalis are under development but the availability and affordability, particularly in LMICs, are likely to be problematic. At the present time, the more widespread use of sodium bicarbonate to treat symptomatic metabolic acidosis in advanced CKD seems appropriate in an effort to alleviate suffering.
7. Uremic symptoms: The syndrome of uremia encompasses a variety of symptoms: anorexia, fatigue, cachexia, pruritus, nausea, restless leg syndrome, sleep disturbances, and sexual dysfunction.32 Pruritus is common and can adversely affect quality of life. The causes are poorly understood but are likely to include the accumulation of specific uremic toxins in the skin. Distinguishing uremic itching from itching caused by other conditions is important because the management may be different. Topical therapy and antihistamines are accessible to LMIC. Other agents, such as gabapentin and opioid receptor modulators, are likely to be of more limited availability.33 The treatment of hyperparathyroidism and hyperphosphatemia may be effective in relieving pruritus in at least some patients. Restless leg syndrome is a related clinical diagnosis that can be debilitating.23 Although this problem is recognized in individuals with normal kidney function, it is much more prevalent in CKD and dialysis patients. Both pruritus and restless leg syndrome are associated with sleep disturbance, depression, poor quality of life, higher cardiovascular morbidity, and higher mortality. The pathophysiology is unknown but may reflect a state of general poor health. The symptoms of restless leg syndrome can be relieved by exercise, as well as by several pharmacologic agents, including gabapentin, dopaminergic modulators, serotonin antidepressants, and lithium. Although data on efficacy of these interventions are limited, they are accessible in many LMICs.
Table 1.
Systematic complications of chronic kidney disease and cross-links
| System | Common manifestations | Cardiovascular | Endocrine and metabolic | Gastrointestinal | Hematologic | Neurologic | Muscuskeletal | Integument |
|---|---|---|---|---|---|---|---|---|
| Cardiovascular | Atherosclerosis, HTN, cardiomyopathy | X | X | X | ||||
| Endocrine and metabolic | Menstrual disorders, sexual dysfunction, infertility, pregnancy disorders, electrolytes, and MBD | X | X | X | X | X | ||
| Gastrointestinal | Anorexia, nausea, emesis, weight loss | X | ||||||
| Hematologic | Anemia, platelets disorders, coagulopathy, low cell count and infection risk | X | X | X | ||||
| Neurologic | Neuropathy, seizures (with severe uremia), strokes | X | X | X | ||||
| Musculoskeletal | MBD, fractures, myopathy | x | x | x | x | |||
| Integument | Dry skin, dermatitis, pruritus | X | X | X | ||||
| Complex symptomsa | Fatigue, insomnia, impotence, cachexia | X | X | X | X | X | X |
HTN, hypertension; MBD, mineral bone disorder.
X denotes a crosslink between systems, e.g., MBD contributing to cardiovascular system, anemia contributing to cardiovascular system, and interplay of all systemic features causing complex symptoms.
Poorly defined disorders associated with advanced chronic kidney disease.
Figure 1.
Progressive CKD and related complications by disease stage. CKD, chronic kidney disease; ESRD, end-stage renal disease.
Figure 2.
Chronic kidney disease–related complications: cross-links and interactions.
Knowledge Gaps
Research into CKD complications over the last few decades has been largely focused on the management of endocrinological abnormalities (anemia and secondary hyperparathyroidism). Despite this effort, there have been few advances proven to improve clinical outcomes. This calls for more efforts to improve our understanding of the mechanisms by which these abnormalities impact patient-related outcomes, clinical implications of complications, and the development of more effective treatment strategies (Table 2).
Table 2.
Action plan to reduce the global impact of chronic kidney disease–related complications9
| Goal and related activities | Opportunities | Threats |
|---|---|---|
Goal 1: optimize the management of anemia and other endocrine abnormalities associated with CKD
|
Availability of globally accepted KDIGO guidelines International collaborative networks of clinicians and researchers International advocacy support, such as ISN, and other regional and national nephrology associations Patient advocacy groups and kidney foundations Willing and supportive industry partners |
Lack of proven efficacy of the current management arsenals (e.g., erythropoietin, vitamin D) to positively impact patient outcomes beyond changes in laboratory metrics Limited access and affordability issues of available medications in developing nations Limited or unavailable diagnostic tools across all settings Language barriers in guidelines disseminations (though KDIGO is making significant efforts to eliminate this barrier, it remains a challenge due to the multiplicity of languages across countries) |
Goal 2: improve prevention and management of CVD complications linked to CKD
|
As above CVD recognition as the most common adverse endpoint in CKD |
As above Very limited evidence as care approach remains an extrapolation based on studies in general population |
Goal 3: improve the diagnosis and management of symptoms associated with CKD
|
Growing interests in the nephrology community to focus research in this area Important area of patients’ priority with increasing attention that gives voice to both researchers and their funders to develop interest in this area |
Lack of standard taxonomy to define disorders Significant heterogeneity in the manifestation of these disorders Limited fundamental knowledge of disorders in terms of pathophysiological mechanisms Change the management from what practitioners and researchers are accustomed to |
CKD, chronic kidney disease; CVD, cardiovascular disease; ISN, International Society of Nephrology; KDIGO, Kidney Disease: Improving Global Outcomes; PTH, parathyroid hormone.
The limitations in our knowledge reflect the variety and complexity of underlying pathophysiology processes that lead to CKD complications and the heterogeneity in their presentation. This is reflected in the limited therapeutic options available. Unfortunately, the uremic symptoms described above, such as anorexia, fatigue, cathexia, etc., which matter most to patients, are the most poorly understood of all CKD complications.32
Of the other CKD complications, CVD is perhaps the most important because of its potential to increase mortality and reduce quality of life. However, as discussed above, the pathogenetic mechanisms may be somewhat different in CKD patients compared with the general population, including pathways that are complex and may involve other CKD-related complications. The pathophysiology of CVD remains incompletely understood; thus, it will be challenging to develop effective treatment strategies with a high degree of specificity.
The work group defined the following specific goals to focus future efforts to alleviate the morbidity and mortality caused by CKD complications at a global level.
Goal 1: optimize the management of anemia and other endocrine abnormalities associated with CKD
Clinical practice guidelines, such as those developed by Kidney Disease: Improving Global Outcomes guidelines, provide recommendations on the management of common CKD-related complications.24, 25 The clinical consequences of these complications in LMICs, particularly in regions where renal replacement therapy programs are not available, needs to be defined. Dissemination and implementation of Kidney Disease: Improving Global Outcomes guidelines in LMICs has been limited and requires improvement.34
To achieve this goal and close these gaps the following activities were suggested.
Activity 1: promote research into understanding the links between laboratory abnormalities (low hemoglobin, mineral disorders, such as calcium and phosphate, and elevated parathyroid hormone) and clinically relevant outcomes (CVD, progression of CKD, and uremic symptoms).Activity 2: promote consistent assessment and documentation of laboratory abnormalities in CKD populations as recommended in Kidney Disease: Improving Global Outcomes guidelines in both developing and developed nations around the world.Activity 3: promote research and education into region-specific causes of abnormalities in CKD patients
Most publications focused on the management of laboratory abnormalities in CKD populations are based on studies conducted in developed countries.11, 35, 36, 37 However, it is likely that the underlying causes of these abnormalities differ by health system characteristics and are influenced by sociocultural factors. For example, in LMICs, parasitic infection or nutritional deficiency may be a more common cause of anemia compared with erythropoietin deficiency and is not appropriately treated with ESAs.
Activity 4: promote the availability of affordable point-of-care measurement devices and treatments for endocrine abnormalities
A key challenge is the lack of adequate laboratory capacity and/or trained workers to measure common biochemical abnormalities (e.g., parathyroid hormone) due to high costs and limited availability of reagents. Effort should be targeted toward the development of affordable point-of-care testing instruments for these common abnormalities. Point-of-care devices with acceptable performance at affordable prices should be a high priority for future research and perhaps public-private sector partnerships. Data from a variety of settings that document the current availability of affordable assays (or lack thereof) would help to make the case that these innovations are worthwhile, and thus should be included in future global CKD surveys.
Goal 2: improve the prevention and management of cardiovascular complications linked to CKD
Most cardiovascular research in CKD has focused on atherosclerotic disease. Non-atherosclerotic diseases that may lead to heart failure and sudden death through arrhythmia have been less studied. Understanding regional variations in CVD phenotypes among CKD populations may offer new insights into how outcomes can be improved. In addition, much remains to be learned about fundamental aspects of cardiovascular risk reduction in CKD populations (e.g., optimal target blood pressure or benefits of aspirin in dialysis patients). Finally, continued work is needed to develop novel therapies for CVD associated with CKD.
Activity 1: develop an integrated research program to better understand nontraditional cardiovascular risk factors and the impact on patients’ outcomes in terms of mechanism, treatment, and prognosis
The current approach to CVD management largely involves the extrapolation of evidence from the general population. There is a pressing need for focused research programs that evaluate the benefits of standard treatment approaches (e.g., angiotensin receptor blockers for heart failure and implantable defibrillators to prevent sudden cardiac death) in CKD populations, as well as investigate novel interventions that might reduce the risk of cardiovascular events in people with kidney disease (e.g., intradialytic potassium profiling).
Activity 2: improve the understanding of global variation in CVD associated with CKD
The phenotype of CVD in people with CKD exhibits a potentially important regional variation. For example, Japanese hemodialysis patients appear to have a lower risk of sudden death than patients in other countries. Whether this is due to patient characteristics, environmental factors, or treatment practices is unknown. If this observation is confirmed, further study of between-country variations in risk and outcomes might lead to new insights into pathophysiology or optimal management. Careful observational studies that use common definitions to compare the epidemiology of these conditions across countries should be a high priority. There is also a paucity of data from LMICs on CVD outcomes in CKD patients, and there are pertinent geographic and racial characteristics that define these disorders in the populations from those countries.
Activity 3: determine barriers to dissemination and implementation of existing guidelines on dyslipidemia and hypertension management to reduce cardiovascular risk in CKD and implement strategies to overcome those barriers
Although new research is certainly needed, knowledge translation (rather than knowledge generation) should be the key priority in other areas. For instance, there is little controversy about the merits of controlling blood pressure, blood sugar, and dyslipidemia in people with less advanced CKD, yet many people worldwide do not have access to these treatments. Knowledge transfer and advocacy efforts should focus on the implementation of global guidelines in CKD populations, especially in LMICs.
Activity 4: develop new therapeutic approaches to reduce CVD risk in CKD patients
In addition to a high burden of traditional risk factors, CVD in CKD appears to be driven by novel (CKD-specific) risk factors. For example, abnormalities in phosphate, fibroblast growth factor 23, and Klotho all appear to contribute to CVD in CKD populations. Continued work is needed to translate discoveries from biomedical science into novel therapies that address these risk factors and mitigate the burden of CVD.
Activity 5: promote further research into optimal therapeutic targets for cardiovascular risk factor management (e.g., blood pressure control)
Key knowledge gaps exist in fundamental aspects of CVD management and prevention in CKD populations, especially in dialysis patients. Clinical trials are needed to examine the risks and benefits of treatments like aspirin, renin-angiotensin system interruption, and spironolactone in patients with advanced kidney disease and kidney failure. Since these trials are unlikely to be funded by industry partners, their success depends on cooperation between public research funds from different countries.
Goal 3: improve the diagnosis and management of symptoms associated with CKD
These present the greatest challenge in the management of CKD-related complications, stemming from limited knowledge on the mechanisms associated with the development of these common problems and how best to characterize them into usable clinical phenotypes. The nephrology research community should focus on understanding these common, but often neglected, complications (for example, fatigue, insomnia, sexual dysfunction, etc.) that may matter most to CKD patients.
Activity 1: develop a taxonomy of symptoms (clinical phenotypes) associated with CKD and their impact on quality of life and functional status
The clinical and epidemiologic characteristics associated with the presence, severity, onset, and remission of CKD-related symptoms are poorly described. How symptoms (individually and collectively) impact quality of life and other patient outcomes, such as employability and functional status, has not been completely studied. In addition, the relative importance of each symptom to the total symptom burden has not been quantified. This information is required to characterize the impact of symptoms on patients’ well-being (thus building the case for action) and to identify the symptoms and patient populations that should be the highest priority for immediate study.
Activity 2: enhance the understanding of the pathophysiology and mechanistic pathways of key symptoms to guide diagnostic, prognostic, and therapeutic decisions
Because mechanisms underlying uremic symptoms are poorly understood, no specific treatments are available. Multidisciplinary research efforts should capitalize on new technologies, such as metabolomics and proteomics, to link uremic toxins with symptoms and identify the pathophysiology that causes or exacerbates symptom burden. Consideration should be given to the study of potentially related symptoms (e.g., pain and pruritus, which have a similar neurobiology). The collaboration of scientists from multiple disciplines, communication with patients, and communication with industry partners may help to ensure the maximum potential for clinical impact and facilitate commercialization.
Activity 3: building and enhancing effective symptom management strategies
Regulatory authorities have approved few, if any, drugs for the treatment of uremic symptoms, and there is often very little evidence to support the common off-label use of therapies. Summarizing what is known about available therapies and evaluating the best candidates in well-designed clinical trials should be a high priority. This could include therapies for similar symptoms associated with other conditions (e.g., chemotherapy-associated nausea), as well as therapies targeted at uremic-specific conditions (e.g., phototherapy for pruritus). Depending on the findings of Activity 2, new candidate treatments should undergo clinical trials.
Conclusion
Progressive CKD is linked to several complications, which occur with higher frequency and greater severity in the advance stages of the disease. These complications lead to high morbidity, mortality, and poor quality of life. We have outlined 3 key goals (supported by sets of activities) targeted at reducing the population health impact of CKD-related complications. Although there has been considerable progress in defining CKD-related complications across regions and countries, significant gaps in knowledge still remain and optimal ways to specifically close these gaps remain undefined. This is the first attempt to develop a blueprint for a concerted approach to better understand these disorders. This involves improving our understanding of the spectrum of pathophysiology and mechanistic pathways, clinical presentations and phenotypes, as well as the development of practice and policy guidelines for optimal care. Under this International Society of Nephrology initiative, we have developed an action plan to enhance our understanding of which uremic symptoms most impact the quality of life of CKD patients, the cause of these symptoms, and the best management strategies to alleviate them.
We endeavored to identify CKD-related complications with the greatest impact on population and/or individual patient survival and quality of life. We identified 7 systemic complications of CKD and examined how they evolve and interact with each other across the spectrum of CKD symptoms (Table 1, Figures 1 and 2). We defined the current state of knowledge and existing gaps and developed a set of goals with specific activities and deliverables. We also defined potential threats and opportunities toward achieving such goals (Table 2). We deliberately focused on 3 key goals that have immediate deliverables and the potential to impact health at the global level.
Disclosure
RCR declared consulting fees from AbbVie and AstraZeneca, lecture fees from AstraZeneca and Roche, and grant support from AbbVie. RK declared lecture fees from Baxter. AK declared consulting fees and grant support from AstraZeneca and US patent 8,722,338. MN declared consulting fees from Kyowa Hakko Kirin, Daiichi Sankyo, Astellas, Chugai, GSK, Tanabe Mitsubishi, Takeda, Taisho, and Ono and lecture fees from Kyowa Hakko Kirin, JT Pharmaceuticals Inc., Tanabe Mitsubishi, MSD, Takeda, AstraZeneca, Boehringer, Kowa, Bayer, Otsuka, Alexion, Mochida, SanwaKagaku, Torii, Kissei, and Toyamakagaku. NP declared consulting fees from Patient Centered Outcomes Research Institute, Methodology Committee, Parkland Center for Clinical Innovation, Parkland Hospital, Healthwise and Informed Medical Decision Making Foundation and grant support from Centers for Disease Control and Prevention. DCW declared consulting fees from Amgen, Boehringer Ingelheim, Akebia, Union Chimique Belge Celltech, Bristol-Myers Squibb, Vifor Fresenius, Otsuka, Janssen, Alberta Innovates Health Solutions, AstraZeneca, Bio Nano; lecture fees from Fresenius, Amgen, Janssen, ZS Pharma, and Vifor Fresenius; and grant support from the British Heart Foundation, Healthcare Quality Improvement Partnership, Kidney Research UK, National Institute for Health Research, Australian National Health & Medical Research Council. OM declared consulting fees from Allena and Adelyx and grant support from the National Institutes of Health, American Heart Association, and Department of Defense and is named as the co-inventor of Effervescent calcium magnesium citrate and synthetic anti-Klotho antibodies. All the other authors have declared no competing interests.
Publication of this article was supported by the International Society of Nephrology.
Acknowledgments
The manuscript emerged as an individual product of the Global Kidney Health Summit held in Vancouver, Canada in July 2016. Support of the Summit was made possible through unrestricted grants from various organizations in addition to the International Society of Nephrology. These include the following (in alphabetical order): AbbVie Inc., Akebia Therapeutics Inc., Amgen, AstraZeneca LP, Boehringer Ingelheim-Lilly, Danone Nutricia Research, Janssen Canada, Merck Global, and Regulus Therapeutics Inc.
References
- 1.Couser W.G., Remuzzi G., Mendis S., Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 2.James M.T., Hemmelgarn B.R., Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296–1309. doi: 10.1016/S0140-6736(09)62004-3. [DOI] [PubMed] [Google Scholar]
- 3.Meguid El, Nahas A., Bello A.K. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 4.Levey A.S., de Jong P.E., Coresh J. The definition, classification, and prognosis of chronic kidney disease: a KDIGO controversies conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 5.Yuan J., Zou X.R., Han S.P. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: results from the Chinese cohort study of chronic kidney disease (C-STRIDE) BMC Nephrol. 2017;18:23. doi: 10.1186/s12882-017-0441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal N., Katz R., Robinson-Cohen C. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community-based cohort studies. JAMA Cardiol. 2017;2:314–318. doi: 10.1001/jamacardio.2016.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kent S., Schlackow I., Lozano-Kuhne J. What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol. 2015;16:65. doi: 10.1186/s12882-015-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox C.S., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy [e-pub ahead of print]. Lancet. 10.1016/S0140-6736(17)30788-2. Accessed May 1, 2017. [DOI] [PubMed]
- 10.Fujii H., Joki N. Mineral metabolism and cardiovascular disease in CKD. Clin Exp Nephrol. 2017;21(suppl 1):53–63. doi: 10.1007/s10157-016-1363-8. [DOI] [PubMed] [Google Scholar]
- 11.Mathew R.O., Bangalore S., Lavelle M.P. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: a review. Kidney Int. 2017;91:797–807. doi: 10.1016/j.kint.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 12.Muntner P., Anderson A., Charleston J. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SPRINT Research Group. Wright J.T., Jr., Williamson J.D. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Eng J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 15.Wanner C., Amann K., Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388:276–284. doi: 10.1016/S0140-6736(16)30508-6. [DOI] [PubMed] [Google Scholar]
- 16.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie X., Atkins E., Lv J. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 18.Hu M.C., Shiizaki K., Kuro-o M., Moe O.W. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go A.S. Cardiovascular disease consequences of CKD. Semin Nephrol. 2016;36:293–304. doi: 10.1016/j.semnephrol.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer M.A., Burdmann E.A., Chen C.Y. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Eng J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 21.Panwar B., Gutierrez O.M. Disorders of iron metabolism and anemia in chronic kidney disease. Semin Nephrol. 2016;36:252–261. doi: 10.1016/j.semnephrol.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Badve S.V., Beller E.M., Cass A. Interventions for erythropoietin-resistant anaemia in dialysis patients. Cochrane Database Syst Rev. 2013:CD006861. doi: 10.1002/14651858.CD006861.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak M., Winkelman J.W., Unruh M. Restless legs syndrome in patients with chronic kidney disease. Semin Nephrol. 2015;35:347–358. doi: 10.1016/j.semnephrol.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Ketteler M., Elder G.J., Evenepoel P. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2015;87:502–528. doi: 10.1038/ki.2014.425. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith D.J., Covic A., Fouque D. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guidelines: a European Renal Best Practice (ERBP) commentary statement. Nephrol Dial Transplant. 2010;25:3823–3831. doi: 10.1093/ndt/gfq513. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith D., Ritz E., Covic A. Vascular calcification: a stiff challenge for the nephrologist: does preventing bone disease cause arterial disease? Kidney Int. 2004;66:1315–1333. doi: 10.1111/j.1523-1755.2004.00895.x. [DOI] [PubMed] [Google Scholar]
- 27.Goldsmith D.J., Covic A. Calcium and the saga of the binders: accumulating controversy, or building consensus? Int Urol Nephrol. 2008;40:1009–1014. doi: 10.1007/s11255-008-9477-x. [DOI] [PubMed] [Google Scholar]
- 28.Khan S., Floris M., Pani A., Rosner M.H. Sodium and volume disorders in advanced chronic kidney disease. Adv Chronic Kidney Dis. 2016;23:240–246. doi: 10.1053/j.ackd.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Kraut J.A., Madias N.E. Metabolic acidosis of CKD: an update. Am J Kidney Dis. 2016;67:307–317. doi: 10.1053/j.ajkd.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Wiederkehr M., Krapf R. Metabolic and endocrine effects of metabolic acidosis in humans. Swiss Med Wkly. 2001;131:127–132. doi: 10.4414/smw.2001.09666. [DOI] [PubMed] [Google Scholar]
- 31.Gaggl M., Sliber C., Sunder-Plassmann G. Effect of oral alkali supplementation on progression of chronic kidney disease. Curr Hypertens Rev. 2014;10:112–120. doi: 10.2174/1573402111666141231123314. [DOI] [PubMed] [Google Scholar]
- 32.Urquhart-Secord R., Craig J.C., Hemmelgarn B. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am J Kidney Dis. 2016;68:444–454. doi: 10.1053/j.ajkd.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 33.Combs S.A., Teixeira J.P., Germain M.J. Pruritus in kidney disease. Seminars Nephrol. 2015;35:383–391. doi: 10.1016/j.semnephrol.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jha V., Arici M., Collins A.J. Understanding kidney care needs and implementation strategies in low- and middle-income countries: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int. 2016;90:1164–1174. doi: 10.1016/j.kint.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Kim R.B., Morse B.L., Djurdjev O. Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney Int. 2016;89:1144–1152. doi: 10.1016/j.kint.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Levin A., Rigatto C., Barrett B. Biomarkers of inflammation, fibrosis, cardiac stretch and injury predict death but not renal replacement therapy at 1 year in a Canadian chronic kidney disease cohort. Nephrol Dial Transplant. 2014;29:1037–1047. doi: 10.1093/ndt/gft479. [DOI] [PubMed] [Google Scholar]
- 37.Mizobuchi M., Ogata H., Koiwa F. Research on kidney and mineral metabolism in Japan: past, present, and future. Clin Exp Nephrol. 2017;21(suppl 1):4–8. doi: 10.1007/s10157-016-1366-5. [DOI] [PubMed] [Google Scholar]