Abstract
There are major gaps between our growing knowledge of effective treatments for chronic kidney disease (CKD), and the delivery of evidence-based therapies to populations around the world. Although there remains a need for new, effective therapies, current evidence suggests that many patients with CKD are yet to fully realize the benefits of blood pressure–lowering drugs (with and without reducing proteinuria with renin-angiotensin system blockade), wider use of statins to reduce atherosclerotic cardiovascular disease events, and better glycemic control in both type 1 and type 2 diabetes. There are many barriers to optimizing evidence-based nephrology care around the world, including access to health care, affordability of treatments, consumer attitudes and circumstances, the dissemination of appropriate knowledge, the availability of expertise and structural impediments in the delivery of health care. Further investment in implementation science that addresses the major barriers to effective care in a cost-effective manner could yield both local and global benefits.
Keywords: chronic kidney disease, implementation, treatment gap
Although there are many unanswered questions on how best to manage patients with chronic kidney disease (CKD),1 some strategies and treatments have been shown to be effective at reducing morbidity and mortality. Despite substantial evidence gaps, the fastest and most efficient way to improve kidney outcomes is to fully implement therapies with proven benefit. Specific strategies shown to improve CKD patient outcomes include blood pressure lowering,2, 3 reduction of proteinuria,2, 4 use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers,5 and the use of statins to reduce atherosclerotic events.6, 7 Glycemic control in people with both type 1 and type 2 diabetes also improves outcomes,8, 9 and newer agents such as sodium-glucose cotransporter 2 inhibitors may have the additional benefit of reducing albuminuria, cardiovascular outcomes, and progression of CKD in diabetics.10, 11, 12 There are also recent studies suggesting therapies targeted at cause-specific CKD, for example, glomerulonephritis and polycystic kidney disease,13 may be of long-term benefit.
The implementation of established therapies is variable within and between regions for a variety of reasons. Physician, patient, and health care system factors may all play a role. Access to care or therapies is often restricted by poor availability, expense, or limited access to nephrology care.14 Physicians may fail to adopt best practices or lack the tools to ensure the delivery of optimal care. There is a clear motivation to reduce the variability in the implementation of guideline-indicated therapies, and optimizing the delivery of care presents a clear and efficient opportunity for improving health outcomes.
This report describes the deliberations of the Working Group of a meeting organized by the International Society of Nephrology: the first Global Kidney Health Summit held on July 26 to 28, 2016 in Vancouver, Canada. This article expands on the recently published International Society of Nephrology CKD Roadmap,15 which is a result from the Summit. Our article describes current knowledge gaps and suggests goals for evaluating and implementing evidence-based treatment options for people with CKD.
Knowledge Gaps
Evidence-practice gaps
Evidence-practice gaps occur when evidence-based therapy is withheld or suboptimally delivered. For many evidence-based therapies, clinical practice guidelines exist, and evidence-practice gaps may occur due to the failure in implementing these guidelines. However, not all therapies for which there is sound evidence are covered by up-to-date guidelines. There will always be patients with genuine contraindications or preferences regarding specific therapies who represent an obligate evidence-practice gap. However, the real concern for health services is the myriad of potentially reversible causes of evidence-practice gap, including patient, provider, health system, and socioeconomic factors.
The full extent of evidence-practice gaps is not known for a variety of conditions and locations. A majority of evidence-practice gap studies have been conducted in high-income countries and followed a retrospective or cross-sectional design. For example, a study of 322 representative primary health care providers in Australia, a country with universal health care coverage, looked specifically at evidence-practice gaps in the management of 1845 patients with evidence of CKD in 2008. Guideline-directed management for blood pressure lowering and lipid lowering was not met in 59% and 64% of patients with CKD, respectively.16 Even the universally accepted therapeutic approach of renin-angiotensin blockade for blood pressure lowering in the presence of proteinuria was not adopted for 35% of apparently eligible people.16 A similar finding was found in a Canadian analysis in which 35% of patients with known CKD were treated by nephrology specialists and were not prescribed renin-angiotensin blockade.17 Among US Medicaid recipients, only 25% of patients were adherent after 5 years.18
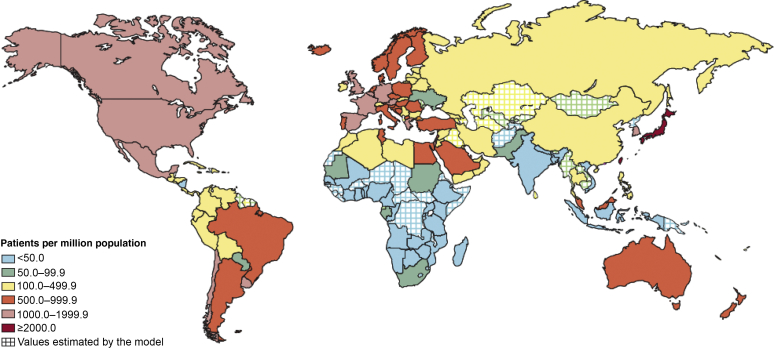
Estimates of the extent of evidence-practice gap for the delivery of established therapies for CKD in low and lower middle–income countries (LLMICs) are scant.14 It remains unclear how many are affected by no or suboptimal access to chronic disease management or acute glomerulonephritis diagnosis and treatment. Illustrations from the delivery of non-CKD and renal replacement therapies describe discrepancies in evidence-practice gap according to the country’s income. For example, the evidence-practice gap for active epilepsy is in the range of 25% to 100% across low and lower middle–income countries compared with less than 10% in high-income countries.19 Similarly, the relative availability of renal replacement therapy for end-stage kidney disease differs in higher- and lower-income countries (Figure 1). Overall, there is a large treatment gap globally for access to renal replacement therapy, conservatively estimated at 53%.20 However, the major contributor to the gap in renal replacement therapy is the excessive gap in resource-poor settings, including a gap of 96% in low-income countries, 88% in lower middle–income countries and 58% in upper middle–income countries.20
Figure 1.
Availability of renal replacement therapy according to the country.
Reproduced from Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982, with permission from Elsevier.20
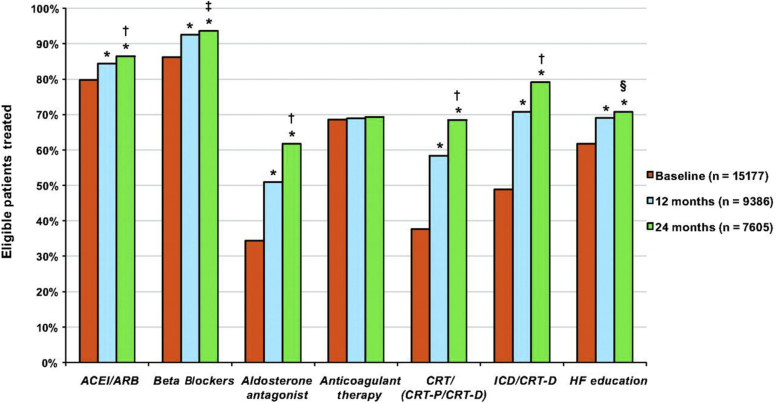
Prospective evidence-practice gap studies have provided iterative feedback to health services that potentially provides a means to both measure and reduce evidence-practice gaps. Ongoing systematic surveillance of evidence-practice gaps could monitor the success of interventions designed to close the gap, but this is not a routine component of health service delivery even in high-income countries. The potential for such an approach is illustrated by the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting’s report on the delivery of established therapies for heart failure (Figure 2).21 The prospective Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting compared the effect of cross-sectional reports (single point in time) with that of a multipronged intervention that included longitudinal, patient-level feedback reports. Improvements in the delivery of 5 of the 7 nominated quality care indicators were observed with both models but were substantially greater in the longitudinal program utilizing patient-level feedback reports. The growing use of electronic medical records and other data systems offers opportunities to efficiently monitor evidence-practice gaps, provide decision support tools, and deliver ongoing feedback to physicians and health services.
Figure 2.
Increase in utilization of guideline-recommended therapies in an implementation project: the Atrial Fibrillation registry. Use of guideline-recommended therapies at baseline, 12 months, and 24 months in the longitudinal cohort. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker, CRT, cardiac resynchronization therapy; CRT-P, CRT with pacemaker; CRT-D, CRT with defibrillator; HF, heart failure. *P < 0.001, 12 and 24 months versus baseline. †P < 0.001, 12 versus 24 months. ‡P = 0.007, 12 versus 24 months. §P = 0.009, 12 versus 24 months.
Reproduced from Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596, http://circ.ahajournals.org/content/122/6/585.21
Causes of evidence-practice gap
Beyond quantifying the extent of evidence-practice gap, an understanding of the barriers to using established therapies is needed to inform the design of implementation strategies to close the gap. Setting-specific analyses should be undertaken as the barriers may be different in different health settings, as illustrated in other fields of medicine. For example, a systematic review of patient-reported barriers and facilitators of antiretroviral adherence in Sub-Saharan Africa identified 154 studies reporting 43 barriers and 30 facilitators across the region.22 Frequently identified barriers included those that may be universal (e.g., forgetting and side effects) and those that may be sensitive to the setting (e.g., lack of access to adequate food, lack of money to visit the HIV clinic, stigma and discrimination, being outside the house or traveling, and religious and traditional beliefs).
Financial barriers to the use of established therapies are a fundamental issue for low- and middle-income countries. Indeed, access to health care providers is limited for many people.23 Financial barriers can also affect individuals in high-income countries because even in these countries, the burden of CKD lies disproportionately on those with relatively fewer means.23 Universal health coverage or income supplementation can be of value if personal finances are limiting access to medications.24, 25 The impact of time-limited immunosuppressive medication funding support for US transplant recipients on graft outcomes has been widely discussed in the context of graft loss in individual patients and dialysis services.26, 27
The widespread availability of low-cost versions of established medications is urgently needed, particularly in low and lower middle–income countries. Polypill preparations could address cost and adherence barriers. Polypill preparations have been shown to be effective in cardiovascular prevention compared with usual care. An individual patient data meta-analysis of 3 randomized trials (Multidrug Pill In Reducing cardiovascular Events [UMPIRE], Kanyini Guidelines Adherence with the Polypill [Kanyini-GAP], and IMProving Adherence using Combination Therapy [IMPACT]) found a polypill that included generic versions of established cardioprotective therapies improved medication adherence, blood pressure, and lipid control in a high-risk population of 3140 participants from Australia, England, India, Ireland, New Zealand, and The Netherlands.28 Systematic reviews of other polypill trials for cardiovascular prevention have found similar results.29
System and regulatory barriers may also need to be addressed in each country to bring established therapies to patients who may benefit from these treatments. Despite the conceptual support of the World Health Organization and evidence of benefit, very few polypills are available for routine clinical practice.30 Authors of some of the trials identified further barriers, including the lack of market structures to fund the development of affordable preparations for noncommunicable diseases. They also point to the lack of a defined pathway to regulatory approval.30
Decision support tools
Decision support tools, including prompts and reminders, may be of value. A prompt at the point of laboratory creatinine reporting increased the utilization of renin-angiotensin blockade in elderly patients with stage 4 CKD in Alberta, Canada.31 Computerized decision support tools led to improvements in risk evaluations but did not increase prescriptions for recommended therapies in a high-risk cohort in Eastern Australia.32 More recently, the Tobacco, Exercise, and Diet Messages randomized trial found that prompts delivered directly to patients with coronary artery disease by text resulted in the improved control of lipids, blood pressure, and weight, improved physical activity, and reduced smoking rates.33
Implementation strategies
The implementation of any strategy to close evidence-practice gaps presents an opportunity for formal scientific evaluation of the immediate impact on treatment uptake and the ultimate goal of improved patient outcomes. Pragmatic trial designs, such as stepped-wedge trials, cluster-randomized trials, and/or registry-based trials lend themselves to the implementation of science evaluations. Efficient evaluations have been used to test the implementation of decision support interventions. The effect of implementing an automated “prompt” message on CKD management recommendations at the point of laboratory creatinine reporting31 was studied using a trial design that included cluster-randomization (primary care practices in Alberta, Canada) and the use of routine administrative data sources for data collection, allowing efficient outcome assessment of the prescribing behavior of 354 physicians and the clinical outcomes of around 22,000 patients. In Eastern Australia, the Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion trial evaluated the impact of a decision support tool that was compatible with medical record software widely used in existing practice.32 Again, a cluster-randomized design was used, and the clinical medical record software itself provided the de-identified data extractions needed to assess outcomes in nearly 39,000 patients.
Suggested Goals and Activities
Goal 1: enhance global access to strategies and agents that retard progression of CKD (Table 1)
Table 1.
Goal 1: enhance global access to strategies and agents that retard the progression of chronic kidney disease
| Activities | Partners | Possible deliverables | Commentary |
|---|---|---|---|
| Development of early-stage CKD toolkit | Non-nephrology health care providers and health care politicians | CKD toolkits for different countries:
|
Needs to be adaptable in different workforces, health systems, and agents |
Work toward global access to:
|
WHO and regional health care providers | Monitor the availability of the 4 treatments and evaluate the implementation delta and publicize results Extend the GKHA36 project to include this monitoring Increase the availability of these agents or polypills for at risk populations |
|
| Develop and implement decision support tools | Create an inventory of existing decision support tools for early CKD by country Extend the GKHA36 project |
BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular disease; GKHA, Global Kidney Health Atlas; WHO, World Health Organization.
Activity 1: develop an early-stage CKD “tool kit”
Worldwide, the majority of people with early-stage CKD with access to medical care receive it in primary and general health settings rather than specialist nephrology services. Non-nephrologist services therefore care for the vast majority of people at risk and have the greatest opportunity to intervene in the course of progression of CKD. A ‘tool kit’ that provides simple advice regarding medications and goals of care aimed at slowing the common causes of progression of CKD could reduce the global burden of CKD. To promote uptake and utility, the recommended approaches should be generalizable and adaptable for different work forces and countries.
Activity 2: work toward global access to treatments
These treatments include (i) affordable blood pressure–lowering drugs, (ii) glucose-lowering drugs, (iii) renin-angiotensin system blockade for proteinuric diabetic kidney disease, and (iv) statins for CVD prevention.
Some therapeutic approaches have been well established in large randomized trials and endorsed by guideline bodies. These include medications for controlling BP and blood sugar, the blockade of the renin-angiotensin system to slow the progression of CKD and statin-based therapy for preventing CV events in the CKD population. Closing the evidence-practice gap between patients appropriate for therapies and those receiving them has the potential to reduce the progression of CKD in an intermediate term. Understanding the evidence-practice gap in different health care settings should include understanding its magnitude, as well as its predictors and associations. The knowledge generated from these observational studies should be used to inform the design of interventions to reduce evidence-practice gaps. Affordable versions of proven medications should be available in all health settings, particularly in low- and middle-income countries. The renal community should advocate for the widespread uptake of the World Health Organization Model List of Essential Medicines,34 which will also help to achieve this objective.
Activity 3: develop and implement decision support tools
Decision support tools, for example, guideline-based advice that is automatically generated from the entry of routine clinical data in laboratory systems or electronic medical records, promote the use of evidence-based medicine. These tools are particularly useful in assisting non-specialists with the delivery of care, and coupled with the tool kit, decision support tools would facilitate the identification of patients at risk, the timing of interventions, and changes in medications. Decision support tools are often developed for multiple jurisdictions in parallel, reflecting a possible duplication of effort. Although some adaptation for local settings may be appropriate, an inventory of available decision support tools is an achievable short-term goal that will increase the efficiency and reduce costs. A longer-term goal is the development of tools to meet identified gaps, particularly tools that are readily accessible in resource-constrained countries.
Goal 2: enhance global capabilities to treat glomerular diseases (Table 2)
Table 2.
Goal 2: enhance global capabilities to treat glomerular diseases15
| Activities | Partners | Possible deliverables | Commentary |
|---|---|---|---|
| Establish best practices and indications for biopsy procedures and sample handling, enhance capacity for trained pathologists to interpret specimens, and establish key accessible medications for the treatment of common GN | Pathologists and laboratory supporters | Inventory of current capacity and potential capacity, identification of barriers, and work plans to address these barriers Extend the GKHA36 project |
GKHA, Global Kidney Health Atlas; GN, glomerulonephritis.
Activity 1: establish best practices and indications for biopsy procedures and sample handling; enhance capacity for trained pathologists to interpret specimens; and establish key accessible medications for the treatment of common glomerulonephritis
Primary glomerular diseases are the third most common cause of end-stage kidney disease, are more likely to affect young and working people, have a faster rate of progression, and may be associated with other systemic complications over the long term.35 Unlike most other causes of CKD, some primary glomerular diseases are potentially curable with relatively short periods of treatment, saving individuals and health services from the longer-term burden of renal replacement therapy. Identifying individuals who may benefit from intensive immunosuppressive treatments is key. Centers of expertise could potentially service a relatively wide area. The access to diagnosis and appropriate management of glomerular diseases will be enhanced by the optimization of biopsy procedures and systems to support the reach of expert pathology services. Access to kidney biopsy and a renal pathologist are critical. Particularly in low and lower-middle income countries, there may be an unmet need for safe and effective biopsy practices. Where access to pathology services is limited, an interim step may be the establishment of centers of excellence for biopsy specimen preparation and reading that can service wide areas. An inventory of current and potential capacities for diagnosis and treatment is an important short-term goal to define unmet needs and inform health services planning. The identification and characterization of disease is only valuable if treatments are accessible, making the widespread availability of low-cost immunosuppressive medications paramount.
Goal 3: sustain and enhance development, dissemination, and awareness of clinical practice guidelines (Table 3)
Table 3.
Goal 3: sustain and enhance development, dissemination, and awareness of clinical practice guidelines15
| Activities | Partners | Possible deliverables | Commentary |
|---|---|---|---|
| Continue to develop, update, and adapt clinical practice guidelines pertinent to CKD on a global scale | KDIGO and other guideline organizations | New guidelines and guideline updates Adequate guideline development capacity and advancement of guideline development methods Infrastructure and processes to coordinate global CKD guideline development |
Guidelines should cover pertinent topics and be kept current |
| Facilitate the implementation of guidelines into practice | KDIGO and other guideline organizations | Include guideline implementation champions in guideline groups and facilitate inclusion of considerations for guideline adaptation (optimal and alternative choices for specific settings), as well as recommendations for implementation and performance measurement Develop guideline commentaries and adaptations |
Guidelines should address the applicability of recommendations in low- and middle-income countries and other specific settings and offer suggestions for implementation |
| Promote guideline dissemination and education | KDIGO and other guideline organizations | Ensure global access to guidelines including generalists who provide CKD care Conference sessions on guidelines International Ambassador programs to incorporate guideline education Task force to survey non-nephrology guidelines, establish contacts with non-nephrology guideline organizations, and work toward inclusion and integration of CKD-related recommendations in future updates Inclusion of guidelines in CME Programs and dissemination of Nephrology Guidelines to other specialty and generalist guideline groups Increase number of individuals being treated according to recommendations (including in low- and middle-income countries) |
CKD care guidelines should be globally accessible, widely disseminated, adopted, and integrated |
CKD, chronic kidney disease; CME, continuing medical education; KDIGO, Kidney Disease: Improving Global Outcomes.
Activity 1: continue to develop, update, and enhance global CKD clinical practice guidelines
The adoption of best practice medicine will promote the delivery of quality health care and efficient expenditure of health service resources. The ongoing development of clinical practice guidelines should continue with the aim of covering major CKD management issues. The guideline impact will be enhanced if the guideline development is coupled with support for local guideline adaptation in different settings, in particular to health care delivery in low- and middle-income countries.
Activity 2: promote guideline dissemination and education
Efficient nephrology care requires the dissemination of guidelines relevant to people with kidney disease in formats that cater to different levels of nephrology expertise (Goal 1). An intermediate goal is to increase active guideline dissemination, such as by promoting the incorporation of guideline sessions in conference programs and other educational activities and ensuring affordable global access to guidelines. The long-term goal is to increase the proportion of people being treated according to guideline recommendations.
Goal 4: develop implementation science expertise in nephrology (Table 4)
Table 4.
Goal 4: develop implementation science expertise in nephrology15
| Activities | Partners | Possible deliverables | Commentary |
|---|---|---|---|
| Develop and expand implementation science infrastructure within the nephrology community | WHO | Task force to explore opportunities and develop a concrete plan, taking into account experiences in other fields; possibly supported by workshops and/or a consensus conference Developing expertise through expert group, educational meetings, and training mechanisms; tools and curriculum/plan Funding for international and country specific fellowships and Ambassadors Regional presentations and collaboration on specific projects |
Recognition of CKD in WHO (chronic tools) should be enhanced, including commentary on the guideline implementation and evaluation of interventional programs |
| Evaluate implementation strategies pertinent to CKD in clinical trials, tailor effective trial design to local circumstances, and scale/spread successful dissemination strategies for maximum global impact | Government health ministries, industry partners, and funding agencies | Conduct studies to evaluate pre-intervention use versus short-term and long-term effects of intervention; tailor effective study designs for local circumstances (e.g., comparative effectiveness and step-wedge trials) Partner with government and health services to embed research in clinical care; facilitation of comparative effectiveness studies when previously unused therapies are introduced to ensure focus of resources on high-yielding interventions |
CKD, chronic kidney disease; WHO, World Health Organization.
Activity 1: develop and expand implementation science infrastructure in nephrology
Implementation science receives relatively little attention in nephrology. A better understanding of effective implementation will lead to more effective adoption of evidence-based therapies. Benefits will include expansion of the number of people receiving currently established therapies, reduction in the time to uptake of new therapies as they become established, and potentially increased efficiencies for health service providers. An implementation science capacity that is nephrology-specific should be developed. Achievable short-term targets for building nephrology-specific capacity in implementation science include formulating curricula and creating training positions, including within nephrology residency and fellowship programs.
Activity 2: inclusion of implementation considerations in guideline development
The cost and availability of treatments covered in guidelines vary immensely. While the scope of guidelines covers the extent of available evidence, not all guideline-endorsed therapies are equally accessible across health settings. Thus, guideline bodies should consider incorporating LLMIC-specific adaptation and implementation considerations.14 For example, guidelines could distinguish the highest level of evidence-based therapies from a (lower cost) minimum acceptable approach.
Activity 3: evaluate implementation strategies to enhance clinical trials in CKD
This includes tailoring effective trial design to local circumstances and developing successful dissemination strategies for maximum global impact. The introduction or dissemination of any therapy represents a potential opportunity to evaluate implementation methods and conduct comparative effectiveness studies. The introduction of new therapies should be accompanied wherever possible by a rigorous evaluation. Such evaluations should preferably contain a randomized aspect.
Conclusion
Historically, much of the effort to reduce the burden of CKD has focused on discovering new therapies and improving treatment modalities. The yield from this investment will be maximized by a similar investment in ensuring the effective dissemination of established therapies to all those who will potentially benefit. There are many barriers to delivering effective treatments in CKD populations, including cost, inadequate health care infrastructures, and access to technical expertise. However, there are also many potential solutions to overcoming these barriers. In particular, studies that can determine the effectiveness of implementation strategies are most likely to benefit not only local participants but also patients with CKD throughout the world.
Disclosure
MJJ declared consulting fees from Baxter and Boehringer Ingelheim; lecture fees from Amgen; and grant support from Baxter, Amgen, Eli Lilley, and Merck. DA declared current grant support from the National Institutes of Health. DJO declared consulting fees from Vifor and lecture fees from Janssen. NRP declared consulting fees from Patient Centered Outcomes Research Institute, Methodology Committee, Parkland Center for Clinical Innovation, Parkland Hospital, and Healthwise and Informed Medical Decision Making Foundation and grant support from Centers for Disease Control and Prevention. CR declared a speaking fee from Fresenius Medical Care paid to her employer, International Consortium for Health Outcomes Measurement. KU declared stock options in Keryx Pharmaceuticals and is a current employee. All the other authors have declared no competing interests.
Publication of this article was supported by the International Society of Nephrology.
Acknowledgments
This manuscript emerged as an individual product of the Global Kidney Health Summit held in Vancouver, Canada in July 2016. Support of the Summit was made possible through unrestricted grants from various organizations in addition to the International Society of Nephrology. These include the following (in alphabetical order): AbbVie Inc., Akebia Therapeutics Inc., Amgen, AstraZeneca LP, Boehringer Ingelheim-Lilly, Danone Nutricia Research, Janssen Canada, Merck Global, and Regulus Therapeutics Inc.
References
- 1.Stevens P.E., Levin A., Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 2.Lv J., Ehteshami P., Sarnak M.J. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949–957. doi: 10.1503/cmaj.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv J., Neal B., Ehteshami P. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001293. doi: 10.1371/journal.pmed.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkovic V., Verdon C., Ninomiya T. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008;5:e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X., Liu Y., Perkovic V. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Hou W., Lv J., Perkovic V. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur Heart J. 2013;34:1807–1817. doi: 10.1093/eurheartj/eht065. [DOI] [PubMed] [Google Scholar]
- 7.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer I.H., Sun W., Cleary P.A. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong M.G., Perkovic V., Chalmers J. Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39:694–700. doi: 10.2337/dc15-2322. [DOI] [PubMed] [Google Scholar]
- 10.Wanner C., Inzucchi S.E., Lachin J.M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 11.Heerspink H.J., Desai M., Jardine M. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28:368–375. doi: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zinman B., Wanner C., Lachin J.M. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Torres V.E., Gansevoort R.T., Czerwiec F.S. Tolvaptan in autosomal dominant polycystic kidney disease. N Engl J Med. 2013;368:1259. doi: 10.1056/NEJMc1300762. [DOI] [PubMed] [Google Scholar]
- 14.Jha V., Arici M., Collins A.J. Understanding kidney care needs and implementation strategies in low and middle income countries: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;90:1164–1174. doi: 10.1016/j.kint.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy [e-pub ahead of print]. Lancet. 10.1016/S0140-6736(17)30788-2. Accessed May 1, 2017. [DOI] [PubMed]
- 16.Razavian M., Heeley E.L., Perkovic V. Cardiovascular risk management in chronic kidney disease in general practice (the AusHEART study) Nephrol Dial Transplant. 2012;27:1396–1402. doi: 10.1093/ndt/gfr599. [DOI] [PubMed] [Google Scholar]
- 17.Tonelli M., Gill J., Pandeya S. Barriers to blood pressure control and angiotensin enzyme inhibitor use in Canadian patients with chronic renal insufficiency. Nephrol Dial Transplant. 2002;17:1426–1433. doi: 10.1093/ndt/17.8.1426. [DOI] [PubMed] [Google Scholar]
- 18.Benner J.S., Glynn R.J., Mogun H. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 19.Newton C.R., Garcia H.H. Epilepsy in poor regions of the world. Lancet. 2012;380:1193–1201. doi: 10.1016/S0140-6736(12)61381-6. [DOI] [PubMed] [Google Scholar]
- 20.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 21.Fonarow G.C., Albert N.M., Curtis A.B. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010;122:585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 22.Croome N., Ahluwalia M., Hughes L.D., Abas M. Patient-reported barriers and facilitators to antiretroviral adherence in Sub-Saharan Africa: a systematic review. AIDS. 2017;31:995–1007. doi: 10.1097/QAD.0000000000001416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Garcia G., Jha V. Chronic kidney disease in disadvantaged populations. Indian J Nephrol. 2015;25:65–69. doi: 10.4103/0971-4065.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels N., Kennedy B.P., Kawachi I. Why justice is good for our health: the social determinants of health inequalities. Daedalus. 1999;128:215–251. [PubMed] [Google Scholar]
- 25.Wagstaff A. Poverty and health sector inequalities. Bull World Health Organ. 2002;80:97–105. [PMC free article] [PubMed] [Google Scholar]
- 26.Tanriover B., Stone P.W., Mohan S. Future of Medicare immunosuppressive drug coverage for kidney transplant recipients in the United States. Clin J Am Soc Nephrol. 2013;8:1258–1266. doi: 10.2215/CJN.09440912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon E.J. The ethics of medicare policy: increasing transplant access and survival. De Paul Law Rev. 2006;55:1045–1066. [PMC free article] [PubMed] [Google Scholar]
- 28.Webster R., Patel A., Selak V. Effectiveness of fixed dose combination medication ('polypills') compared with usual care in patients with cardiovascular disease or at high risk: a prospective, individual patient data meta-analysis of 3140 patients in six countries. Int J Cardiol. 2016;205:147–156. doi: 10.1016/j.ijcard.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Elley C.R., Gupta A.K., Webster R. The efficacy and tolerability of 'polypills': meta-analysis of randomised controlled trials. PLoS One. 2012;7:e52145. doi: 10.1371/journal.pone.0052145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster R., Rodgers A. Polypill treatments for cardiovascular diseases. Expert Opin Drug Deliv. 2016;13:1–6. doi: 10.1517/17425247.2016.1111869. [DOI] [PubMed] [Google Scholar]
- 31.Manns B., Tonelli M., Culleton B. A cluster randomized trial of an enhanced eGFR prompt in chronic kidney disease. Clin J Am Soc Nephrol. 2012;7:565–572. doi: 10.2215/CJN.12391211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peiris D., Usherwood T., Panaretto K. Effect of a computer-guided, quality improvement program for cardiovascular disease risk management in primary health care: the treatment of cardiovascular risk using electronic decision support cluster-randomized trial. Circ Cardiovasc Qual Outcomes. 2015;8:87–95. doi: 10.1161/CIRCOUTCOMES.114.001235. [DOI] [PubMed] [Google Scholar]
- 33.Chow C.K., Redfern J., Hillis G.S. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA. 2015;314:1255–1263. doi: 10.1001/jama.2015.10945. [DOI] [PubMed] [Google Scholar]
- 34.Hall B.M., Tiller D.J., Hardie I. Comparison of three immunosuppressive regimens in cadaver renal transplantation: long-term cyclosporine, short-term cyclosporine followed by azathioprine and prednisolone, and azathioprine and prednisolone without cyclosporine. N Engl J Med. 1988;318:1499–1507. doi: 10.1056/NEJM198806093182304. [DOI] [PubMed] [Google Scholar]
- 35.Floege J., Amann K. Primary glomerulonephritides. Lancet. 2016;387:2036–2048. doi: 10.1016/S0140-6736(16)00272-5. [DOI] [PubMed] [Google Scholar]
- 36.Bello A.K., Levin A., Tonelli M. Assessment of global kidney health care status. JAMA. 2017;317:1864–1881. doi: 10.1001/jama.2017.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]