Abstract
In order to change the current state of chronic kidney disease knowledge and therapeutics, a fundamental improvement in the understanding of genetic and environmental causes of chronic kidney disease is essential. This article first provides an overview of the existing knowledge gaps in our understanding of the genetic and environmental causes of chronic kidney disease, as well as their interactions. The second part of the article formulates goals that should be achieved in order to close these gaps, along with suggested timelines and stakeholders that are to be involved. A better understanding of genetic and environmental factors and their interactions that influence kidney function in healthy and diseased conditions can provide novel insights into renal physiology and pathophysiology and result in the identification of novel therapeutic or preventive targets to tackle the global public health care problem of chronic kidney disease.
Keywords: chronic kidney disease, environment, genetic kidney disease, genetics, genome-wide association studies
Background and gaps in knowledge
Understanding genetic and environmental factors influencing kidney function in healthy and diseased conditions and the interaction between genetic susceptibility factors and the environment can provide important insights into renal physiology and pathophysiology. It can reveal previously unknown or unexpected mechanisms, and consequently, research of genetic and environmental factors associated with chronic kidney disease (CKD) has the potential to identify novel therapeutic or preventive targets.
In order to identify knowledge gaps and propose and prioritize activities in CKD research, the International Society of Nephrology (ISN) held a meeting of international experts in CKD research in July 2016. The main recommendations and overview of the ISN CKD roadmap were published in The Lancet.1 This review builds on the concepts in the main article and provides a more detailed summary of the current knowledge and knowledge gaps, including a detailed discussion of environmental risk factors, consideration of issues related to understanding genetic risk factors, and suggestions on methods to improve our knowledge of genetic and environmental risk factors of CKD.
Genetic factors
Important advances in human genetics in the past decade include the sequencing of the human genome, determination of patterns of genetic variation in human populations around the globe, improvements in high-throughput genotyping and massively parallel sequencing technologies, and advances in statistical genetics and bioinformatics. These resources together have led to the discovery of many novel risk genes and disease-associated genetic variants.2, 3, 4, 5 Genome-wide association studies as well as whole-exome and whole-genome sequencing have become standard techniques to identify genetic loci in which variations associate with complex traits and diseases. They have been used successfully in nephrology to identify genetic variants associated with important CKD etiologies as well as with kidney function in healthy and diseased conditions and to detect mutations that cause monogenic kidney diseases.6, 7, 8 Several hundred genes are currently known to contain mutations that can cause single-gene disorders with a kidney phenotype, as well as dozens of genetic loci in which common genetic variants are associated with kidney function in the normal range and with complex kidney diseases.8
Although it is now possible to efficiently discover new disease genes as a basis for the translation of gene discovery into improved CKD prevention and treatment, important gaps in understanding the mechanism of action of the genetic components of CKD remain, hindering translational efforts.
First, there is limited education and awareness of the value and importance of genetic research. This is true not only for the lay public but also for clinicians, researchers, and patients. Lack of education can pose a particular challenge in clinical genetics, especially with respect to the initiation and type of genetic testing, assessment of the pathogenicity of detected genetic variants, and patient counseling. Moreover, realistic expectations and timelines for the clinical translation of genetic findings are often not well communicated.
Second, despite the fact that some indigenous populations of non-European ancestry show especially high rates of kidney disease, much of the genetic research so far has been carried out in patients and study populations of European ancestry. Previous studies have supported the existence of region-specific genetic risk factors for CKD.9, 10, 11 Current evidence is therefore unlikely to be a representative globally, which can have significant implications for research as well as clinical genetics.12
Third, genetic research, especially of but not limited to rare diseases, can reach its full potential only through widespread data sharing. This practice is currently limited and often occurs in unstandardized formats. However, comprehensive and current inventories of existing genetic datasets as well as their findability and accessibility are prerequisites to maximize the use of existing genetic evidence.
Fourth, the limited existence of tools for functional genomics research in nephrology is a major roadblock for the identification of causal genes and variants, improved mechanistic insights, and clinical translation.13
Environmental factors
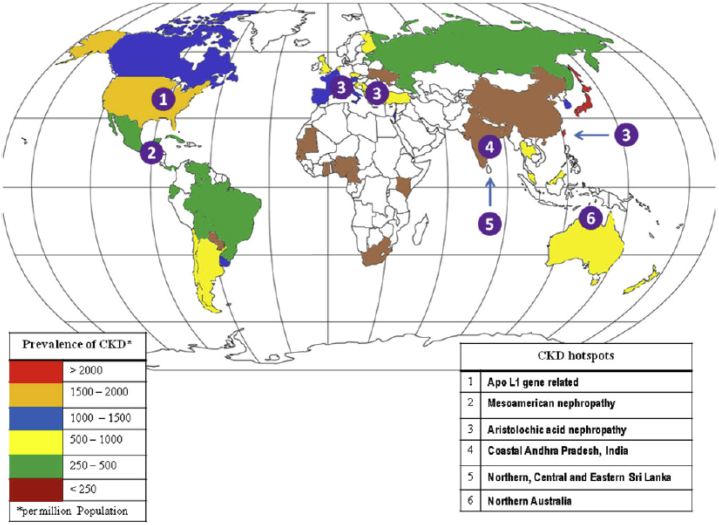
A variety of environmental factors have been associated with the development of CKD (Table 1).14, 15 Several of these factors have been implicated as potential causes of CKD in so-called CKD hotspots, which are defined as countries, regions, communities, or ethnicities with higher than an average incidence of CKD16 (Figure 117). In most CKD hotspots, CKD is not due to traditional causes such as diabetes or hypertension.15, 18 Despite the suspected causative role of environmental factors, a cause-effect relationship has not been demonstrated in most regions, and thus, CKD of unknown etiology (CKDu) and infections remain the leading causes of CKD in the majority of CKD hotspots15, 18, 19 (Table 220, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40).
Table 1.
Environmental factors potentially associated with the development of chronic kidney disease
| Factor |
|---|
| Heavy metals (lead, cadmium, arsenic, mercury, uranium) |
| Environmental chemicals |
| Agricultural chemicals |
| Industrial waste products |
| Aristolochic acid |
| Occupational exposures |
| Nonsteroidal antiinflammatory drugs |
| Counterfeit drugs |
| Traditional herbal medicines |
| Infections |
| Illegal alcohol consumption |
| Sugary beverages |
| Salty food |
CKD, chronic kidney disease.
Figure 1.
Map of chronic kidney disease hotspots and prevalence.
Reprinted from Abraham G, Varughese S, Thandavan T, et al. Chronic kidney disease hotspots in developing countries in South Asia. Clin Kidney J. 2016;9:135–141.17Copyright © The Authors 2015. Published by Oxford University Press on behalf of ERA-EDTA. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/).
Table 2.
Environmental and occupational factors that are associated with kidney damage
| Heavy metals14, 15 |
Lead
|
| Other toxins |
|
| Occupational exposures |
|
| Medications |
|
| Herbal remedies |
|
| Infections |
|
AA, aristolochic acid; AAN, aristolochic acid nephropathy; AKI, acute kidney injury; APOL1, apolipoprotein L1; BEN, Balkan endemic nephropathy; CKD, chronic kidney disease; GFR, glomerular filtration rate; LMICs, low- and middle-income countries; MeN, Mesoamerican nephropathy; NSAID, nonsteroidal antiinflammatory drug.
A high incidence of CKDu in mostly poor resource settings has important implications. The lack of or very limited access to health care, reduced availability of nephroprotective medications and renal replacement therapy, limited infrastructure and funding for health care, and a shortage of trained personnel are only a few of the many challenges faced by the low- and middle-income countries (LMICs) where most CKD hotspots are located34 (Figure 117).
Table 341, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 summarizes the characteristics of some confirmed and suspected CKD hotspots, but knowledge gaps remain. Factors contributing to a research gap include a lack of detailed and comparable epidemiologic data among regions and countries that prevents the identification of true CKD hotspots16, 18; lack of or limited access to clinical care in LMICs for an accurate determination of the prevalence of CKDu34; lack of proof of causality for reported associations between environmental factors and CKDu; lack of a systematic approach to address CKDu in various regions of the world due to clinical, scientific, and funding limitations61; and complexities of the interactions between global and local factors.18 Lastly, there is a paucity of research regarding the potential role of genetic susceptibility, low birth weight, prenatal and childhood exposures, and social factors (e.g., poverty and social determinants of ill health).17, 18, 61, 62
Table 3.
Characteristics of some confirmed and suspected chronic kidney disease hotspots
| MeN |
|
| BEN |
|
| Sri Lanka nephropathy |
|
| Andhra Pradesh, India |
|
| Northern- Australian Territory and Maori and Pacific people in New Zealand |
|
| Other suspected CKD hotspots23 |
|
AA, aristolochic acid; BEN, Balkan endemic nephropathy; CKD, chronic kidney disease; CKDu, chronic kidney disease of unknown etiology; ESRD, end-stage renal disease; MeN, Mesoamerican nephropathy; NSAID, nonsteroidal antiinflammatory drug; SNP, single-nucleotide polymorphism.
SCL13A3, sodium-dependent dicarboxylate transporter member 3.
In addition to research gaps, advocacy and regulatory issues, which often have a basis in political and financial conflicts of interest, contribute to our incomplete understanding of the potential role of environmental factors in CKD,63 and noncompliance with international treaties, laws, and rules and regulations regarding the protection of the labor force’s health and control of exposure to toxins that are potentially nephrotoxic is common.63 These factors lead to the identification of what has been named Mesoamerican nephropathy in the coastal zones of El Salvador, Nicaragua, and Costa Rica, which has been hypothesized to be caused by multiple factors, including repeated episodes of dehydration and volume depletion, heat stress, and rhabdomyolysis because of the extreme labor among agricultural workers22 or by exposure to environmental toxins, industrial waste products, agrochemicals, and contaminated drinking water. Moreover, access to traditional herbal medicines and over-the-counter nonsteroidal antiinflammatory drugs is poorly regulated in both developing and developed countries, and efforts to eradicate counterfeit drugs appear to be insufficient.34
Both research and advocacy gaps lead to limitations in clinical practice and education. In combination with the knowledge gaps of specific causes of CKDu, prevention, treatment efforts, and appropriate education of health care professionals, workers, and the general public are inhibited.15 Clinical interventions are complicated by the lack of access to health care and renal replacement therapy, insufficient funding, and lack of trained personnel, as is often the case in LMICs where most CKD hotspots are located.17, 34
Gene-environment interactions
Our current understanding of the interactions between genetic and environmental CKD risk factors is incomplete. A better understanding of these interactions will provide insights into important patient subgroups and facilitate efforts aimed at the identification of targeted therapies and prevention.
One of the best examples of gene-environment (GxE) interactions is the association of the apolipoprotein L1 (APOL1) gene with the development of CKD in African Americans in the United States.38 MYH9 variants initially associated with CKD in African Americans were found to be in linkage disequilibrium with APOL1 variants.9, 64 These variants are only common in West Africa and have been shown to lyse Trypanosoma brucei rhodesiense in vitro, providing a plausible evolutionary explanation for their high frequencies. Despite their deleterious effect on the kidney, the variants persist in an environment where trypanosomes are present and infection can cause sleeping sickness. APOL1 alleles have been linked to the development of HIV nephropathy, focal and segmental glomerulosclerosis, and hypertension associated with CKD.8 A weaker association also occurs with severe lupus nephritis and sickle cell disease. APOL1 alleles explain the higher rates of nondiabetic kidney disease in African Americans than in European Americans and probably the lower allograft survival after transplantation of cadaveric donor kidneys from African ancestry donors than from European descent donors. Genetic susceptibility by the APOL1 alleles might lead to CKD, depending on modifying factors that can be considered environmental, such as HIV infection, treatment with antiretroviral medications, coinfection with non-HIV protective viruses (e.g., JC polyoma virus), as well as the potential presence of certain genetic modifiers.39
Few studies have assessed the role of genetic predisposition in CKD hotspots, which could identify further GxE interactions. It has been proposed that a gene located in 3q25 together with the instability of chromosome 3 can explain the familial nature of Balkan endemic nephropathy. However, the disease occurs in people of different ethnic backgrounds, including immigrants to the area, but it is uncommon in those who have moved away from endemic areas. Also, the incidence is reportedly decreasing even in areas where it used to be more common.50 These observations are suggestive of environmental factors or a GxE interaction. In Sri Lanka nephropathy, a single-nucleotide polymorphism in sodium-dependent dicarboxylate transporter member 3 (SCL13A3) appears to predispose to CKDu. However, further studies are needed to confirm this association.56
Epigenetic studies, in addition to genetic research, are also needed to understand the effect of genes and environment on CKD hotspots. Thorough longitudinal studies of the interaction between genetic risk factors and fetal, childhood, and adult exposures, including those via nutrition, will also be critical for understanding the development of CKD, particularly in LMICs.34, 62
Goals to increase the understanding of genetic and environmental determinants of CKD
The following paragraphs as well as Tables 4, 5, and 6 provide an overview of goals that should be achieved in order to address the previously mentioned limitations and knowledge gaps regarding genetic and environmental CKD risk factors as well as their interactions. Specific actions to achieve each of the goals are formulated, along with the identification of important partners, milestones, and possible metrics and timelines. Together, these achievements would substantially improve our knowledge of the genetic and environmental determinants of CKD as well as their interactions.
Table 4.
Recommendations for an action plan to improve the understanding of the genetic determinants of chronic kidney disease1
| Goals | Activities | Category |
Partners | Special aspects in LMICs | Possible deliverables | Commentary | |||
|---|---|---|---|---|---|---|---|---|---|
| Research | Clinical care | Education | Advocacy | ||||||
| 1.Increase awareness about the value and importance of genetics for understanding and treating CKD | |||||||||
| Educate clinicians and researchers about the value and importance of clinical genomics and genetic research for CKD, including challenges (e.g., ethical aspects, limitations in variant interpretation), opportunities, and realistic timelines for mechanistic understanding and translation | x | Nephrology fellows, medical schools, geneticists, professional organizations, patient advocacy groups, pharmaceuticals, technology and biotechnology companies; pairing with other organizations | Information relevant to LMICs in order to facilitate the understanding and awareness of the importance of clinical genomics and genetic research alongside more basic health care needs | Inventory of existing training and educational programs; double the number of programs in 5 years; offer training programs in nephrogenetics at international nephrology meetings or as standalone meetings | LT deliverable: Improved understanding of the importance of genetics by all clinicians and patients (consent to participate in genetic research) | ||||
| Educate patients and the public about the value of clinical genomics and genetic research | x | Geneticists, professional organizations, patient advocacy groups, journals, mass media, pharmaceuticals; pairing with other organizations | Information relevant to LMICs in order to facilitate the understanding and awareness of the importance of clinical genomics and genetic research alongside more basic health care needs | Increase media coverage in the next 2–5 yr | LT deliverable: Improved understanding of the importance of genetics by all clinicians and patients (consent to participate in genetic research) | ||||
| Educate clinicians and researchers about findings from large-scale sequencing projects of nephrology patients and asymptomatic individuals that provide adjusted estimates of prevalence and penetrance of presumed pathogenic variants necessary for counseling and risk prediction | x | x | x | Medical schools, teaching hospitals, nephrology divisions, professional societies, patient advocacy groups, pharmaceuticals, nephrology journals | Has implications in LMICs for genetic variants that are region or ancestry specific or are specific to a CKDu hotspot | Research reports and review articles in the next 2 yr, including discussion of potential implications for counseling | LT deliverable: Improved understanding of the importance of genetics by all clinicians and patients (consent to participate in genetic research) | ||
| Educate clinicians about diverse clinical presentations of genetic kidney disease and revise genetic testing accordingly | x | x | Medical schools, teaching hospitals, professional societies, patient advocacy groups, pharmaceuticals, journals, clinical sequencing laboratories | Has implications in LMICs for genetic variants that are region or ancestry specific or are specific to a CKDu hotspot; gene panels for certain presentations may vary for different parts of the world | Research reports and reviews on the spectrum of clinical presentations for kidney disease genes Published recommendations about which genes to sequence for which presentation Development of standard gene panels for different nephrologic diseases (tubular disease, FSGS, etc.) with region-specific content |
||||
| 2.Increase the diversity of genotyped populations | |||||||||
| Protect indigenous populations, rare disease groups, ethnic minorities, small communities, family-heritage beliefs in order to enable their inclusion in genetic analysis and increase the diversity of genotyped populations | x | x | x | Communities, governments, regulators, IRBs | Information relevant to LMICs in order to facilitate genotyping of ethnically diverse individuals | Inventory of genotyped populations and their diversity in CKD hotspots, review of existing protocol-policy recommendations, and publication of recommendations on where to focus genotyping efforts in the next 2 yr | LT deliverable: Improved understanding of the importance of genetics by all clinicians and patients (consent to participate in genetic research) | ||
| Improve SNP diversity on commercially available chips; improve imputation reference panels for ethnically diverse populations | x | Genotyping companies (Affymetrix, Illumina), computational biologists (for enhanced and diverse imputation platforms) | Information relevant to LMICs to enable targeted genotyping of high-risk populations | Development of affordable genotyping for worldwide populations, provision of improved genotype imputation for non-European ancestry populations, and promote comprehensive SNP array genotyping for CKDu in CKD hotspots in order to identify a potential major gene effects and research reports | |||||
| Educate groups, patients, populations, and other stakeholders about the value of genetic research in diverse populations | x | x | In addition to the above, media | APOL1 and HIV infection can illustrate the concept | Increase media and journal coverage regarding the value of genetic research in diverse populations over the next 2–5 yr | ||||
| 3.Increase the accessibility of genetic data | |||||||||
| Increase the accessibility and usability of existing and future datasets by promoting standardized format, broad data sharing, and enhanced use | x | x | iNET CKD, journal editors, technology companies, CKDGen, biobanks and biorespositories, dbGaP, BD2K initiative, AMP portal, CHARGE consortium, NHGRI GWAS catalog (now EMBL) | Information relevant to LMICs as this can make existing data more accessible to resource-poor regions where primary data generation can be more challenging | Development and publication of position statement on standardized format for data sharing, tracking of the number of publications and the number of requests for data, review of cataloged resources, and reduction of redundancies | ||||
| Develop data mining tools and search functions to catalog existing datasets | x | Computational scientists, pharmaceuticals | Share tools (e.g., search functions) to investigate publicly available data; research publications based on existing datasets (secondary use) | ||||||
| Promote common data elements/phenotypes/standards in existing and future datasets (e.g., age, sex, SCr, UACR, and ethnicity). Improve renal phenotype harmonization and laboratory assays used to measure renal function parameters. Develop search tools for renal patients’ electronic health records | x | Clinical chemists, epidemiologists, laboratory assay developers | Need a minimum set of affordable laboratory assays to allow for LMIC participation | Over the next 2 yr, establishment of a consensus on a set of core nephrologic parameters to enable kidney disease genetics research and consensus of how to identify CKD patients using electronic medical records | LT deliverable: More focused research in genetics within the renal space | ||||
| Create incentives for data sharing | x | x | Journals, pharmaceuticals | Development of journal guidelines that require data sharing for publication. Sponsor platforms/portals/infrastructure to share data |
LT deliverable: More focused research in genetics within the renal space | ||||
| Catalog and aggregate existing data repositories and biobanks-biospecimens to enable more rapid and accessible research | x | Computational scientists, pharmaceuticals | Information relevant to LMICs as this can make existing data more accessible to resource-poor regions where primary data generation can be more challenging | Development of a concept for centralized platforms/portals/infrastructure to share data and identify funding mechanisms | LT deliverable: More focused research in genetics within the renal space | ||||
| Link biomarkers to genetic data to determine causality (Mendelian randomization) | x | Statisticians, pharmaceuticals | Over the next 2–5 yr, development of software that facilitates Mendelian randomization analyses and make it publicly available | ||||||
| 4.Tool generation for functional genomics | |||||||||
| Develop tools for functionalization of genetic findings to identify the causal gene/variant and genetic mechanism of action to facilitate translational research. Tools should be shared broadly | x | Geneticists, bioinformaticians and computational biologists, technology companies, pharmaceuticals, funding agencies | No direct benefit to LMICs in the short term. However, these tools are critical to turn genetic findings into potential novel therapeutics | Inventory of available tools, cell types, cell lines in the next 2–5 yr and tracking of published articles with mechanism of action of genetic findings and collection in a centralized resource |
LT deliverable: Faster time from discovery to phase 1 and 2 trials in nephrology with less failure of compounds | ||||
| Promote the creation of disease-relevant cellular assays, bioinformatics pipelines, and tools for use in the scientific community | x | Geneticists, bioinformaticians and computational biologists, technology companies, pharmaceuticals, funding agencies | Published research reports elucidating the mechanism of action of newly uncovered genetic loci; development of assays that are available on request | LT deliverable: Faster time from discovery to phase 1 and 2 trials in nephrology with less failure of compounds | |||||
| Generate tools to study genetic modifiers, including epigenetic effects, to understand mutations in their genomic context and identify potential therapeutic targets | x | Geneticists, bioinformaticians and computational biologists, technology companies, pharmaceuticals, funding agencies | Creation of tools as documented in published research reports of epigenetic catalogs of different kidney cell types | LT deliverable: Faster time from discovery to phase 1 and 2 trials in nephrology with less failure of compounds | |||||
AMP, accelerated medicine partnership; APOL1, apolipoprotein L1; BD2K, Big Data to Knowledge; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CKD, chronic kidney disease; CKDGen, CKDGen Consortium; CKDu, chronic kidney disease of unknown etiology; dbGaP, database of Genotypes and Phenotypes; EMBL, European Molecular Biology Laboratory; FSGS, focal segmental glomerulosclerosis; GWAS, genome-wide association study; iNET CKD, International Network of Chronic Kidney Disease cohort studies; IRBs, institutional review boards; LMICs, low- and middle-income countries; LT, long term; NHGRI, National Human Genome Research Institute; SCr, serum creatinine; SNP, single-nucleotide polymorphism; UACR, urinary albumin-to-creatinine ratio.
Table 5.
Recommendations for an action plan to improve the understanding of environmental factors of chronic kidney disease hotspots
| Goals | Activities | Category |
Partners | Special aspects in LMICs | Milestones | Timeline | |||
|---|---|---|---|---|---|---|---|---|---|
| Research | Clinical care | Education | Advocacy | ||||||
| 1.Characterize CDK hotspots and CKDu | |||||||||
| Agree on a definition of CDK hotspots and CKDu | x | ISN, CENCAM, WHO, PAHO, universities | PAHO, CDC, and SLANH recently convened a working group to define CKDu in agricultural communities | Publications and reports | 1 yr | ||||
| Develop a registry and an interactive map of CKD hotspots and CKDu with data on CKD prevalence, incidence, and potential risk factors | x | ISN, CENCAM, WHO, PAHO, MOH, public health schools | Will require funding | Publications, reports, and the Internet | 2 yr | ||||
| 2.Improve research methods | |||||||||
| Search for the cause of CKDu | Perform studies with a lifecycle approach that considers factors such as genetic predisposition, prenatal exposures, parents’ health status, working age, and social determinants of health | x | CENCAM, ISN, WHO, PAHO, public health schools, MOH | Particularly relevant in LMICs due to the potential role of genetic, developmental, and socioeconomic factors | Publications and reports | 5–10 yr | |||
| Perform epidemiologic studies to continuously assess CKD prevalence and risk factors | x | CENCAM, ISN, WHO, PAHO, public health schools, MOH, NGOs | Particularly relevant in LMICs due to the potential role of genetic, developmental, and socioeconomic factors | Publications and reports | 5–10 yr | ||||
| Perform interventional studies when risk factors are reasonably well established | x | CENCAM, ISN, WHO, PAHO, public health schools, MOH, NGOs | Potential risk factors can be suspected but not necessarily confirmed | Publications and reports | 5–10 yr | ||||
| Standardize data collection tools in different studies in different countries to facilitate comparison of research results and accelerate progress | x | ISN, CENCAM, WHO, PAHO, public health schools | Should include global as well as local risk factors for CKD | Reports | 1 yr | ||||
| Promote international and multidisciplinary collaboration through a research consortium to link efforts around the globe | x | x | ISN, CENCAM, WHO, PAHO, public health schools, MOH, NGOs | LMICs may benefit from such international collaborations | Publications, reports, and the Internet homepage |
2 yr | |||
| Develop biomarkers for detecting early kidney disease and identifying exposure | x | Basic scientists, clinical researchers | Synergies between HICs and LMICs may be required | Publications and reports | 5–10 yr | ||||
| Perform genetic and epigenetic studies to evaluate genetic susceptibility of specific populations to different environmental factors | x | Genetic epidemiologists | Synergies between HICs and LMICs may be required | Publications and reports | 5–10 yr | ||||
| Develop a biorepository with urine samples, serum samples, and DNA to enable future analysis with advanced techniques and understanding | x | ISN, CENCAM, WHO, PAHO, public health schools | Synergies between HICs and LMICs may be required | Biorepository | 5–10 yr | ||||
| 3.Prevent CKD | |||||||||
| Implement preventive interventions | Evaluate local factors and implement preventive interventions based on confirmed or presumed risk factors for CKD, including children and adolescents if appropriate | x | WHO, PAHO, MOH, local health care authorities | Basic public health and occupational interventions may have significant impact and be relatively inexpensive | Assess CKD incidence trends | 5 yr | |||
| Develop local ongoing surveillance systems | x | CENCAM, MOH, NGOs | Essential to assess the efficacy of interventions | Publications and reports, and the Internet | 5 yr | ||||
| Reduce environmental exposure | Agrochemical and industrial chemical control | x | MOH, Ministries of Labor, corporations | Although evidence that agrochemicals cause nephrotoxicity is very limited, it causes CKD | Reports | 5–10 yr | |||
| Access to safe drinking water | x | Government, MOH | Basic human right | Reports | 5–10 yr | ||||
| Regulate herbal and other traditional medicines | x | Government, MOH | Herbal medicine use is very common in LMICs | Reports | 5–10 yr | ||||
| Reduce or eliminate exposure to heavy metals | x | Government, MOH, corporations | Prioritize by the level of exposure and risk of nephrotoxicity | Reports | 5–10 yr | ||||
| Regulate exposure to organic contaminations, including ongoing surveillance programs | x | Government, MOH, corporations | Prioritize by the level of exposure and risk of nephrotoxicity | Reports | 5–10 yr | ||||
| Eradicate infections potentially related to CKD | Access to safe drinking water | x | Government, MOH, WHO, PAHO | Basic human right | Reports | 5–10 yr | |||
| Sanitation control | x | Government, MOH, local health care authorities | Prioritize based on local epidemiology | Reports | 5–10 yr | ||||
| Vector control | x | MOH, local health care authorities | Prioritize based on local epidemiology | Reports | 5–10 yr | ||||
| Implement vaccination | x | MOH, local health care authorities | Prioritize based on local epidemiology | Reports | 5–10 yr | ||||
| Assess the potential role of infections and develop an action plan with infectious disease specialists and health care authorities for mass treatment programs | x | ISN, CENCAM, WHO, PAHO, MHO, public health schools | Particularly important in some LMICs | Publications and reports | 5 yr | ||||
| Improve working conditions in poor agricultural communities | Develop a legal framework for workers’ protection | x | Government, international agencies, corporations, human rights organizations | Importance of involving nonmedical parties | Reports | 2–5 yr | |||
| Enforce compliance with existing rules and regulations | x | Government, international agencies, corporations, NGOs | Importance of involving nonmedical parties | Reports | 2–5 yr | ||||
| Break the cycle of poverty, malnutrition, and death | x | x | Government, international agencies, corporations, NGOs | Importance of involving nonmedical parties | Reports | >10 yr | |||
| 4.Offer affordable CKD treatment options to affected individuals | |||||||||
| Implement CKD screening programs based on local risk factors | x | MOH, local health care authorities | Both case finding and opportunistic CKD screening | Assess CKD incidence | 5 yr | ||||
| Increase access to affordable and quality CKD care | x | ISN, MOH, local health care authorities | Will require more funding for infrastructure | Publications and reports | 5–10 yr | ||||
| Improve access to nephroprotective medications | x | WHO, PAHO, ISN, MOH, local health care authorities, industries | Use bioequivalent generic medications | Reports | 5–10 yr | ||||
| Improve access to RRT if possible | x | Government, MOH, ISN, ASN, NGOs, industries | Peritoneal dialysis may be a cheaper option | Reports | 5–10 yr | ||||
| 5.Increase funding and advocacy | |||||||||
| Support research and channel aid money to research related to prevention | x | ISN, CENCAM, WHO, PAHO, MOH, NGOs, corporations, industries | Essential to continuously search for the cause of CKDu in many regions and to know trends | Reports of research funds | 2 yr | ||||
| Develop synergies to strengthen fund raising efforts and collaborate with other parties interested in chronic conditions | x | ISN, CENCAM, WHO, PAHO, NGOs, MOH, corporations | LMICs will require national and international support | Reports | 2 yr | ||||
| 6.Improve education and awareness | |||||||||
| Improve the education of health care professionals (mainly PCPs) regarding the prevention and treatment of CKD | x | ISN, regional and local nephrology societies, NGOs | Partnerships with local renal societies may be helpful | Reports | 5 yr | ||||
| Build workforce capacity by training nephrologists | x | ISN, regional and local renal societies, hospitals | ISN may continue to play an important role by training nephrologists of developing countries | Reports | 5 yr | ||||
| Educate workers about potential preventive measures at work (i.e., hydration, etc.) | x | Government, MOH, NGOs | Basic public health and occupational interventions may have significant impact and be relatively inexpensive | Reports | 5 yr | ||||
| Educate the general population to increase the awareness about risk of herbal medicines and other nephrotoxins | x | Government, MOH, NGOs | Herbal medicine and NSAID use is very common in LMICs | Reports | 5 yr | ||||
CDC, Centers for Disease Control and Prevention; CENCAM, Consortium for the Epidemic of Nephropathy in Central America and Mexico; CKD, chronic kidney disease; CKDu, chronic kidney disease of unknown etiology; HICs, high-income countries; ISN, International Society of Nephrology; LMIC, low- and middle-income country; MOH, Ministry of Health; NGOs:, non-profit organizations; NSAID, nonsteroidal antiinflammatory drug; PAHO, Pan American Health Organization; PCPs, primary care physicians; RRT, renal replacement therapy; SLANH, Society of Latin American Nephrology and Hypertension; WHO, World Health Organization.
Table 6.
Recommendations for an action plan to improve the understanding of gene-environment interactions in chronic kidney disease
| Goals | Activities | Category |
Partners | Special aspects in LMIC | Possible deliverables | Commentary | |||
|---|---|---|---|---|---|---|---|---|---|
| Research | Clinical care | Education | Advocacy | ||||||
| 1.Better understanding of GxE interactions | |||||||||
| Leverage existing large initiatives (PMI, UKBB, etc.) to elucidate GxE interactions. | x | CKDGen, iNET CKD, biobanks and biorepositories, CKDu investigators | Information relevant to LMICs, particularly if a major gene effect or region-specific environmental factors are found in association with CKDu | Research reports | |||||
| Develop electronic health record search tools for the most common environmental CKD risk factors | x | x | Computational scientists, specialists for environmental risk factors, epidemiologists | Consensus on a small set of the potentially most important interactors and standardization of their definition and methods for data capture | |||||
| Develop renal endophenotypes to increase the power to assess GxE interactions; use more homogeneous subgroups to facilitate GxE discoveries | x | Research reports | |||||||
| Evaluate GxE interactions in CKDu hotspots, focusing on region-specific environmental interactors | x | Environmental scientists | Information relevant to LMICs, where many CKDu hotspots are located | Research reports | |||||
CKD, chronic kidney disease; CKDGen, CKDGen Consortium; CKDu, chronic kidney disease of unknown etiology; GxE, gene-environment; iNET CKD, International Network of Chronic Kidney Disease cohort studies LMIC, low- and middle-income country; PMI, Precision Medicine Initiative; UKBB, UK Biobank.
Genetic factors
The first goal is to increase awareness about the value and importance of genetics for understanding and treating CKD through improved education. DNA sequence variation can be detected reproducibly and accurately, but there is relatively little knowledge and guidance on how to interpret and what to expect of genetic findings in research, clinics, and the lay press. One reason for this is uncertainty related to variant pathogenicity,65, 66 which in addition may be ethnic dependent11 or context specific, that is, it may depend on the (epi-)genomic context and/or the environment. Another issue is the lack of communication of the importance of genetics and adequate training of professionals.
Professional organizations, including ISN, patient advocacy groups, scientific journals, mass media, and the pharmaceutical industry, are important partners to improve patients’ and the public’s education about the value of clinical genomics and genetic research through increased media coverage and educational meetings in the next 2 to 5 years. These activities should include discussion of the challenges of genetic research such as privacy concerns, cultural and ethnic issues (particularly in unique populations), and patient preferences related to reporting incidental findings, as well as highlight the benefits of genetic research, such as the discovery of novel pathophysiological mechanisms and the development of new therapies. Educational activities should also include the communication of realistic timelines for the translation of genetic findings into a mechanistic understanding and into the clinical practice. Finally, patients, clinicians, researchers, and the lay public must be made aware of the potential for translational applications following a better understanding of CKD genetics.
For researchers and clinicians, educational activities should include additional aspects. For example, findings from large-scale sequencing projects of CKD patients and healthy individuals67, 68, 69, 70, 71 provide adjusted estimates of the prevalence and penetrance of presumably pathogenic variants and give insights into the phenotypic presentation spectrum for variants in a given gene. Inclusion of diverse populations reduces the risk of including estimates that are not pathogenic in diverse populations.12 Knowledge of these estimates has important implications such as informed decisions about which variants to pursue through experimental studies and the timing and scope of genetic testing, pharmacogenomics testing, and patient counseling. Therefore, important milestones over the next 2 years are to generate research reports related to prevalence, penetrance, and phenotypic spectrum of kidney disease risk variants and to develop standard gene panels for different clinical presentations in nephrology (e.g., a standard nephrotic syndrome gene panel), which can contain region-specific content. Although genetic studies for clinical research and medical care differ in aims and ethical issues,72 improving education is equally important for both areas. The need for improved education extends to LMICs, where lack of training in genome science has been one of the main reasons why LMICs have so far not been able to benefit from advances in translational genomics.73
Clinical genetics currently comprises only a small part of the medical school and clinical training curricula. We therefore recommend quantifying the number of training programs and doubling this number over the next 5 years. Continued training in nephrogenetics through workshops at international nephrology meetings or as standalone meetings can help to further improve education in this field. Several ongoing projects investigate issues related to improving genetic counseling in research and clinics, including how to communicate the results of genetic tests to patients, the need for and scope of educational activities, and the ethical and legal implications. Challenges and potential solutions identified from these efforts, such as those from the Clinical Sequencing Exploratory Research Consortium,72, 74 can provide a basis for specific recommendations about how medical training and counseling should best be expanded and delivered.
The second goal is to increase the diversity of genotyped populations beyond the European ancestry populations. The identification of APOL1 as a major kidney disease susceptibility gene in individuals of African ancestry9, 64 illustrates that kidney disease-related important ancestry-specific findings exist. Genetic research in indigenous populations with high rates of CKD may therefore be particularly informative about additional susceptibility genes and/or important GxE interactions, in addition to aiding a better understanding of allelic diversity. An additional advantage of genetic investigations in high-risk CKD populations is that they can address the question whether kidney function variants identified in the general population75, 76, 77, 78 translate to the setting of endemic or advanced CKD.
It is necessary to generate an inventory of genotyped populations in CKD hotspots to enable additional genetic research in diverse populations. Existing study protocols and policies need to be reviewed to ensure the protection of indigenous populations, rare disease groups, ethnic minorities, small communities, as well as family-heritage beliefs and to allow for culturally sensitive genetic research. Additional activities to increase the diversity of genotyped populations include working with genotyping companies, such as chip manufacturers, and computational scientists to provide affordable and comprehensive genotyping for worldwide populations as well as improved imputation reference panels for non-European ancestry populations. The value of and challenges related to genotyping ethnically diverse populations should not only be communicated in the scientific community but also include the education of patients, populations, and other stakeholders. The option of “buy-in” or ownership of genetic data by patients may facilitate such efforts. In order to engage communities worldwide and to enable the generation of comprehensive knowledge, culturally sensitive methods must be developed and applied.
The third goal is to increase the accessibility and usability of existing and future datasets of nephrogenetic studies by promoting broad data sharing and enhanced usage. The FAIR (findability, accessibility, interoperability, reusability) guidelines79 state that individual and aggregate datasets should be available with limited barriers for access in a useful, standardized format. A first step related to this effort is the development or adaptation of standard formats for data sharing and the tracking of available data and their use, which can be assisted by the development of new search tools80 and can be measured by publications that result from the use of existing nephrogenetic datasets. Vital to this effort is the development of common data elements, renal phenotypes, and standards in existing and future datasets, for example, the definition of a core set of renal parameters that will be collected and documented in each study or algorithms for the identification of CKD patients from electronic medical records. Comparable measurements and definitions maximize the potential for interoperability and full use of existing data as well as for the combination of different datasets. This is true especially for projects that rely on the combination of data such as large gene discovery studies and Mendelian randomization studies, although methods exist to combine unstandardized data, such as different definitions of a phenotype. Important partners in this process can be existing international research collaborations such as the CKDGen Consortium, CKD Prognosis Consortium, and International Network of CKD studies for the definitions of common renal phenotypes, journals, and data scientists who develop and maintain data sharing resources such as the Database of Genotypes and Phenotypes or the National Human Genome Research Institute genome-wide association studies Catalogue81 for the establishment of data sharing formats, and government funders and/or the pharmaceutical industry to support the infrastructure for data sharing such as for platforms generated as part of the Big Data to Knowledge project82 or the accelerated medicines partnership Project for type 2 diabetes.83 Although many of these resources are generated in high-income countries, research in LMICs will also benefit from these activities because of the improved accessibility of existing data in resource-poor regions where primary data generation can be more challenging.
The fourth goal is to generate tools for functional genomics that can be used to enable the translation of newly discovered genetic risk loci for CKD. Discovery genetics is the first step in a long process of mechanism elucidation.13 This is crucial because discovery genetics is an associative science, and often, the resolution of the data is insufficient to identify the causal gene or single-nucleotide polymorphism. Functional genomic tools are used to determine causal genes and variants and to clarify their mechanisms of action. Although challenging,65, 66 the determination of variant pathogenicity is necessary to prioritize the most promising findings for clinical translation.
Moreover, recent technologies such as epigenomics,84 metagenomics, metabolomics,85, 86 and proteomics have started to generate additional information that can enable the interpretation of genetic data. The resource generated by the Genotype-Tissue Expression Project is presently limited by a lack of kidney cell types. Thus, the development of libraries of kidney cell types with epigenetic maps will be necessary to realize the potential of current discoveries in kidney disease genetics. In addition, robust cellular assays in disease and cell-type relevant models will be necessary in order to translate the findings from human genetics. While short-term deliverables for this goal include tracking of published datasets and accessible tools (Table 4), the ultimate goal of this work is to gain insights into biological pathways and novel biomarkers to enable the development of new drugs and prevention. In support of this strategy, drug targets with underlying human genetic support are twice as likely to be ultimately approved compared to drug targets without underlying human support.87 In addition, there are already drug designs based on the knowledge of human mutations, such as ivacaftor for the treatment of cystic fibrosis and PCSK9 inhibitors for familial hypercholesterolemia. Because of the complexity of the work necessary to drive drug discovery and development, private-public partnerships, particularly between academia and industry, will be necessary to realize the full impact of human genetics on CKD.
Environmental factors in CKD hotspots
We identified six goals: better characterization of CKD hotspots and CKDu, improvement in research methods, reduction of new CKD cases through prevention, increased access to affordable treatments for already affected individuals, increased funding and advocacy for research and clinical care, and improved education and awareness among health care professionals, workers, and the general public (Table 5).
The first goal is a better characterization of CKD hotspots and CKDu by revising current definitions. Additionally, a registry or shared database should be developed with interactive maps of CKD hotspots indicating the prevalence and incidence, possible risk factors, and access to CKD care.16 A working group was recently convened under the sponsorship of several organizations to develop a consensus definition of confirmed clinical cases of CKD of nontraditional causes that could be used by surveillance programs of Mesoamerican nephropathy.88 Other ongoing initiatives such as the Chronic Kidney Disease Multinational Inventory could be instrumental in developing registries and interactive maps of CKD hotspots to promote collaboration and advancement in CKDu.89
The second goal of improving research methods is primarily intended to produce a more efficient search strategy for specific causes of CKDu. Proposed activities include performing studies with a lifecycle approach that take into account factors such as genetic predisposition, prenatal exposures, parents’ health status, working age, and social determinants of health.61 The role of genetic susceptibility in some diseases primarily attributed to environmental factors remains to be elucidated through well-designed genetic and epigenetic studies in different populations.62 The potential contribution of low birth weight and prenatal and childhood exposures needs to be taken into account in such investigations.90, 91 Often CKD precursors start at a young age, masking unidentified factors that predispose to adult onset of disease triggered by environmental or occupational exposures. Social determinants of ill health are yet another poorly studied factor that contributes significantly to CKDu. Poverty, malnutrition, lack of access to health care, and disparities in environmental and occupational exposures to heavy metals are highly prevalent in minorities and disadvantaged populations, contributing to the excess risks of kidney disease.34, 61, 90, 92
Other actions that may improve research methodology include epidemiologic studies that follow the Good Epidemiological Practice guidelines to assess the prevalence and risk factors in CKD hotspots, followed by intervention studies for CKD if applicable.61 Standardization of data collection tools across studies will facilitate the comparison of research results and accelerate progress. Other actions will require substantial funding: first, the development of biomarkers for the early identification of a kidney disease and exposure to risk factors and second, the development of a biorepository with urine, serum, and DNA samples for future analyses.15, 18, 61
To achieve this second goal of improving research methodology, international and multidisciplinary collaboration through a research consortium that links efforts around the globe will be required.
Regarding the third and fourth goals of improving access to CKD prevention and treatment, it is essential to build capacities of infrastructure, financial, and human resources. When El Salvador responded to the epidemics of Mesoamerican nephropathy by increasing access to health care services in affected areas, hospitals were soon overwhelmed by a large number of patients and a lack of infrastructure and trained personnel.63 Successful examples of synergies between government and private sources that have led to significant improvements in access to health care and stabilization of renal replacement therapy rates exist, for example, among Aboriginal Australians of the Northern Territory. Targeted interventions include the spread of chronic disease management protocols to primary care through a more intensive use of electronic medical records, simplified and inexpensive targeted CKD screening, and increased access to renal replacement therapy through free-standing and mobile dialysis units. Furthermore, targeted interventions include the widespread availability of generic medications free of cost and building of local capacities, including human resources and community-controlled health services.59 A collaborative effort between developing and developed countries might leverage resources and facilitate dissemination of low-cost protocols. Moreover, ISN programs intended to build sustainable capacities such as the Sister Renal Centers, Continuing Medical Education, Educational Ambassador, Clinical Research, Fellowship, and ISN-American Nephrologists of Indian Origin programs can help improve CKD prevention and treatment around the world. The American Society of Nephrology’s Kidney Disease XPRIZE Challenge and the Kidney Health Initiative are additional examples of initiatives intended to develop new and affordable methods of providing renal replacement therapy in low-resource settings and other treatments for patients with kidney disease.
Because it is ethically imperative to address an epidemic and not to postpone it until its causes have been fully elucidated, CKD preventive interventions should be considered based on confirmed or presumed local risk factors.63 Examples of such preventive interventions include the reduction of exposure to environmental toxins, eradication of infections potentially related to CKD, and improvement of working conditions in poor agricultural communities. Reduction of exposure to environmental toxins requires enforcement of rules and regulations regarding (i) agrochemical use, (ii) access to herbal medicines, (iii) use of potentially nephrotoxic medications such as nonsteroidal antiinflammatory drugs, and (iv) elimination of exposure to heavy metals based on the level of exposure and risk of nephrotoxicity.21, 30 Measures to eradicate infections potentially related to CKD include sanitation and vector control, access to safe drinking water, vaccination campaigns, and mass treatment programs based on local epidemiology.19, 34, 36 Improvement of working conditions in poor agricultural communities requires the development of a legal framework for workers’ health protection, enforcement of compliance with relevant international treaties and existing laws and regulations, and measures to break the cycle of poverty, malnutrition, and death. The Worker Health and Efficiency Program in El Salvador is an example of specific actions taken to improve worksite conditions for local sugarcane cutters and to reduce potential risk factors for CKD.48
The fifth goal is the increase in funding. More financial resources are needed to improve research methods and reduce current shortcomings in the provision of clinical care. Advocacy is necessary to achieve this goal by focusing on CKD hotspots in both national and international forums and by developing synergies with other parties interested in chronic conditions. Support is needed to promote compliance with international treaties that protect the labor force’s health, control agrochemical use, reduce exposure to environmental toxins and organic contaminants, regulate herbal medicine use, eradicate counterfeit drugs, control infections, and improve access to safe water, among others.42 This broad range of activities requires the participation and commitment of many parties, as well as coordination and communication among them to strengthen their advocacy efforts.15, 93 Several agencies, including the World Health Organization, Pan American Health Organization, ISN, Consortium for the Epidemic of Nephropathy in Central America and Mexico, Kidney Health Initiative of the American Society of Nephrology, and Kidney Health Australia, have already taken actions. The collaboration of these agencies with regional academic societies, local health care authorities, other governmental bodies, local foundations, and industries related to environmental factors potentially associated with CKD is likely to improve the current situation.
The sixth goal is education and awareness improvement, with a focus on educating health care professionals, mainly primary care physicians, about the prevention and treatment of CKD by taking local risk factors into account. Simultaneously, the workforce capacity should be built by training more nephrologists. Extensive education of workers about potential preventive measures (i.e., adequate hydration, rest periods, avoidance of sugary beverages) and of the general population about risks of herbal medicines and other nephrotoxins is important.
GxE interactions
A better understanding of GxE interactions (Table 6) builds on each of its components and is a major goal in itself. Environmental factors not only interact with genetic variants to modify their risk but also influence the evolution of disease, as shown for some renal conditions that are related to the immune response in infection. Examples include IgA nephropathy risk and the intestinal immune response to helminthic infections94 and APOL1-associated kidney disease and trypanosomiasis.9, 95
An improved understanding of GxE interactions is necessary for understanding complex diseases, which by definition originate from a combination of genetic and environmental risk factors. Examples of such interactors include diabetes mellitus and hypertension,96, 97 the main causes of CKD in many regions of the world, and other types of yet unknown environmental risk factors (i.e., those reported in CKD hotspots, mostly in LMICs).29, 51 However, unraveling the effects of genes and environment can be challenging when their interaction is required to cause disease or when GxE interactions are of small effect.
Specific activities that may improve our goal to understand GxE interactions in CKD include genotyping of cohorts with CKDu. If there is a genetic underpinning to CKDu in a given population, it is imperative that the tools be in place to detect it. Necessary elements include the availability of inexpensive high-throughput genotyping chips that are representative of diverse populations (Table 4, goal 2), and leveraging data from existing large-scale initiatives and/or making use of electronic health records to identify individuals exposed to certain environmental factors. The development of standardized data collection tools is an important prerequisite to refine the identification of global and local environmental risk factors18 and to develop additional renal endophenotypes. Improved phenotyping can increase the power of detecting GxE interactions and allows for the conduct of genetic studies in more homogeneous subgroups (i.e., those exposed to a certain environmental factor), which should enhance our ability to identify CKD risk genes.98 Tracking of these activities will occur by monitoring the published literature and presentations at major scientific meetings.
Conclusion
An improved understanding of the genetic and environmental factors that influence kidney function in healthy and diseased conditions, as well as GxE interactions, has the potential to provide important insights into renal physiology and pathophysiology as a basis for the development of novel therapeutic or preventive targets. Achieving the goals outlined in this article will represent major steps along this road.
Disclosure
GTO declared consulting fees from Boerhringer-Ingelheim, Akebia Therapeutics, General Electric Health Care do Brasil, and Janssen; lecture fees from Amgen Mexico; and grant support from Fundación Río Arronte and Janssen. MN declared consulting fees from Kyowa Hakko Kirin, Daiichi Sankyo, Astellas, Chugai, GlaxoSmithKline, Tanabe Mitsubishi, Takeda, Taisho, and Ono and lecture fees from Kyowa Hakko Kirin, Japan Tobacco, Tanabe Mitsubishi, Merck Sharp & Dohme, Takeda, AstraZeneca, Boehringer, Kowa, Bayer, Otsuka, Alexion, Mochida, SanwaKagaku, Torii, Kissei, and Toyamakagaku. RP-F declared consulting fees from AstraZeneca and Janssen; lecture fees from AstraZeneca, Novartis, and Janssen; and research grants from Fresenius Medical Care and Baxter Healthcare. CP declared consulting fees from Janssen Cliag, MSD, Otsuka, and Boehringer Ingelheim. JR declared equity ownership/stock options with Amgen and Thrasos Therapeutics and patents related to the treatment of kidney diseases. RC-R declared consulting fees from AbbVie and AstraZeneca, lecture fees from AstraZeneca and Roche, and grant support from AbbVie. PS declared consulting fees from Akebia, Boehringer Mannheim, AstraZeneca, Baxter, Abbott, and Keryx; lecture fees from Pfizer, MSD, AstraZeneca, Baxter, Bayer, and Roche; and grant support from AstraZeneca and Bayer. RW declared grant support from Health Research Council of New Zealand, and Otago Medical Research Foundation. CSF declared stock options in Merck and is a current employee. AK declared consulting fees and grant support from AstraZeneca, and US patent 8,722,338. All the other authors declared no competing interests.
Publication of this article was supported by the International Society of Nephrology.
Acknowledgments
The manuscript emerged as an individual product of the Global Kidney Health Summit held in Vancouver, Canada in July 2016. Support of the summit was made possible through unrestricted grants from various organizations in addition to the International Society of Nephrology. These include (in alphabetical order): AbbVie Inc., Akebia Therapeutics Inc., Amgen, AstraZeneca LP, Boehringer Ingelheim-Lilly, Danone Nutricia Research, Janssen Canada, Merck Global, and Regulus Therapeutics Inc.
The work of AK was supported by the CRC 1140 Initiative and by a Heisenberg Professorship (KO 3598/3-1) of the German Research Foundation.
The work of MN was partly supported by a grant from the Japanese Ministry of Health and Labor (H28-Menneki-Shitei-001). UTS was funded by a grant from the Else Kröner-Fresenius-Stiftung (2013_Kolleg.03), Bad Homburg, Germany.
References
- 1.Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy [e-pub ahead of print]. Lancet. 10.1016/S0140-6736(17)30788-2. Accessed May 1, 2017. [DOI] [PubMed]
- 2.Manolio T.A. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–176. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 3.Bamshad M.J., Ng S.B., Bigham A.W. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–755. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 4.Visscher P.M., Brown M.A., McCarthy M.I., Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong J.X., Buckingham K.J., Jhangiani S.N. The genetic basis of Mendelian phenotypes: discoveries, challenges, and opportunities. Am J Hum Genet. 2015;97:199–215. doi: 10.1016/j.ajhg.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Seaghdha C.M., Fox C.S. Genome-wide association studies of chronic kidney disease: what have we learned? Nat Rev Nephrol. 2011;8:89–99. doi: 10.1038/nrneph.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivante A., Hildebrandt F. Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol. 2016;12:133–146. doi: 10.1038/nrneph.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuttke M., Kottgen A. Insights into kidney diseases from genome-wide association studies. Nat Rev Nephrol. 2016;12:549–562. doi: 10.1038/nrneph.2016.107. [DOI] [PubMed] [Google Scholar]
- 9.Genovese G., Friedman D.J., Ross M.D. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson C.F., Simmons D., Collins J.F., Cecil A. Predisposition to nephropathy in Polynesians is associated with family history of renal disease, not diabetes mellitus. Diabet Med. 2001;18:40–46. doi: 10.1046/j.1464-5491.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- 11.Stewart J.H., McCredie M.R., McDonald S.P. The incidence of treated end-stage renal disease in New Zealand Maori and Pacific Island people and in Indigenous Australians. Nephrol Dial Transplant. 2004;19:678–685. doi: 10.1093/ndt/gfg592. [DOI] [PubMed] [Google Scholar]
- 12.Manrai A.K., Funke B.H., Rehm H.L. Genetic misdiagnoses and the potential for health disparities. N Engl J Med. 2016;375:655–665. doi: 10.1056/NEJMsa1507092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox C.S., Hall J.L., Arnett D.K. Future translational applications from the contemporary genomics era: a scientific statement from the American Heart Association. Circulation. 2015;131:1715–1736. doi: 10.1161/CIR.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soderland P., Lovekar S., Weiner D.E., Brooks D.R., Kaufman J.S. Chronic kidney disease associated with environmental toxins and exposures. Adv Chronic Kidney Dis. 2010;17:254–264. doi: 10.1053/j.ackd.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Weaver V.M., Fadrowski J.J., Jaar B.G. Global dimensions of chronic kidney disease of unknown etiology (CKDu): a modern era environmental and/or occupational nephropathy? BMC Nephrol. 2015;16:145. doi: 10.1186/s12882-015-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Cleary C., Ortiz A. CKD hotspots around the world: where, why and what the lessons are. A CKJ review series. Clin Kidney J. 2014;7:519–523. doi: 10.1093/ckj/sfu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham G., Varughese S., Thandavan T. Chronic kidney disease hotspots in developing countries in South Asia. Clin Kidney J. 2016;9:135–141. doi: 10.1093/ckj/sfv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunyera J., Mohottige D., Isenburg M.V. CKD of uncertain etiology: a systematic review. Clin J Am Soc Nephrol. 2016;11:379–385. doi: 10.2215/CJN.07500715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 20.Debelle F.D., Vanherweghem J.L., Nortier J.L. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 2008;74:158–169. doi: 10.1038/ki.2008.129. [DOI] [PubMed] [Google Scholar]
- 21.Kataria A., Trasande L., Trachtman H. The effects of environmental chemicals on renal function. Nat Rev Nephrol. 2015;11:610–625. doi: 10.1038/nrneph.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correa-Rotter R., Wesseling C., Johnson R.J. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser J., Lemery J., Rajagopalan B. Climate change and the emergent epidemic of CKD from heat stress in rural communities: the case for heat stress nephropathy. Clin J Am Soc Nephrol. 2016;11:1472–1483. doi: 10.2215/CJN.13841215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paula Santos U., Zanetta D.M., Terra-Filho M., Burdmann E.A. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87:792–799. doi: 10.1038/ki.2014.306. [DOI] [PubMed] [Google Scholar]
- 25.Roncal Jimenez C.A., Ishimoto T., Lanaspa M.A. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014;86:294–302. doi: 10.1038/ki.2013.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Trabanino R., Jarquin E., Wesseling C. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador–A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res. 2015;142:746–755. doi: 10.1016/j.envres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Roncal-Jimenez C., Garcia-Trabanino R., Barregard L. Heat stress nephropathy from exercise-induced uric acid crystalluria: a perspective on Mesoamerican nephropathy. Am J Kidney Dis. 2016;67:20–30. doi: 10.1053/j.ajkd.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Wesseling C., Aragon A., Gonzalez M. Kidney function in sugarcane cutters in Nicaragua–A longitudinal study of workers at risk of Mesoamerican nephropathy. Environ Res. 2016;147:125–132. doi: 10.1016/j.envres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Arroyo F.E., Cristobal M., Arellano-Buendia A.S. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am J Physiol Regul Integr Comp Physiol. 2016;311:R57–R65. doi: 10.1152/ajpregu.00354.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wimalawansa S.J. Escalating chronic kidney diseases of multi-factorial origin in Sri Lanka: causes, solutions, and recommendations. Environ Health Prev Med. 2014;19:375–394. doi: 10.1007/s12199-014-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayasumana C., Paranagama P., Agampodi S. Drinking well water and occupational exposure to herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ Health. 2015;14:6. doi: 10.1186/1476-069X-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanigasuriya K. Update on uncertain etiology of chronic kidney disease in Sri Lanka's north-central dry zone. MEDICC Rev. 2014;16:61–65. doi: 10.37757/MR2014.V16.N2.10. [DOI] [PubMed] [Google Scholar]
- 33.Yaxley J., Litfin T. Non-steroidal anti-inflammatories and the development of analgesic nephropathy: a systematic review. Ren Fail. 2016;38:1328–1334. doi: 10.1080/0886022X.2016.1216708. [DOI] [PubMed] [Google Scholar]
- 34.Stanifer J.W., Muiru A., Jafar T.H., Patel U.D. Chronic kidney disease in low- and middle-income countries. Nephrol Dial Transplant. 2016;31:868–874. doi: 10.1093/ndt/gfv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jha V, Rathi M. Natural medicines causing acute kidney injury. Semin Nephrol. 28:416–428. [DOI] [PubMed]
- 36.Jha V., Prasad N. CKD and Infectious diseases in Asia Pacific: challenges and opportunities. Am J Kidney Dis. 2016;68:148–160. doi: 10.1053/j.ajkd.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Yang H.Y., Hung C.C., Liu S.H. Overlooked risk for chronic kidney disease after leptospiral infection: a population-based survey and epidemiological cohort evidence. PLoS Negl Trop Dis. 2015;9:e0004105. doi: 10.1371/journal.pntd.0004105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman B.I. APOL1 and nephropathy progression in populations of African ancestry. Semin Nephrol. 2013;33:425–432. doi: 10.1016/j.semnephrol.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman B.I., Skorecki K. Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol. 2014;9:2006–2013. doi: 10.2215/CJN.01330214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naicker S., Rahmanian S., Kopp J.B. HIV and chronic kidney disease. Clin Nephrol. 2015;83(suppl 1):32–38. doi: 10.5414/CNP83S032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trabanino R.G., Aguilar R., Silva C.R. Nefropatía terminal en pacientes de un hospital de referencia en El Salvador [End-stage renal disease among patients in a referral hospital in El Salvador] Rev Panam Salud Pública. 2002;12:202–206. doi: 10.1590/s1020-49892002000900009. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 42.Almaguer M., Herrera R., Orantes C.M. Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev. 2014;16:9–15. doi: 10.37757/MR2014.V16.N2.3. [DOI] [PubMed] [Google Scholar]
- 43.Weiner D.E., McClean M.D., Kaufman J.S., Brooks D.R. The Central American epidemic of CKD. Clin J Am Soc Nephrol. 2013;8:504–511. doi: 10.2215/CJN.05050512. [DOI] [PubMed] [Google Scholar]
- 44.Laux T.S., Bert P.J., Barreto Ruiz G.M. Nicaragua revisited: evidence of lower prevalence of chronic kidney disease in a high-altitude, coffee-growing village. J Nephrol. 2012;25:533–540. doi: 10.5301/jn.5000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Donnell J.K., Tobey M., Weiner D.E. Prevalence of and risk factors for chronic kidney disease in rural Nicaragua. Nephrol Dial Transplant. 2011;26:2798–2805. doi: 10.1093/ndt/gfq385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peraza S., Wesseling C., Aragon A. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 47.Torres C., Aragon A., Gonzalez M. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Bodin T., Garcia-Trabanino R., Weiss I. Intervention to reduce heat stress and improve efficiency among sugarcane workers in El Salvador: Phase 1. Occup Environ Med. 2016;73:409–416. doi: 10.1136/oemed-2016-103555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanchev I., Evstatiev T.S., Dorosiev D. Study of nephritis in Vrattsa district. Suvr Med (Sofiia) 1956;7:14–29. [PubMed] [Google Scholar]
- 50.Bui-Klimke T., Wu F. Evaluating weight of evidence in the mystery of Balkan endemic nephropathy. Risk Anal. 2014;34:1688–1705. doi: 10.1111/risa.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Batuman V. Fifty years of Balkan endemic nephropathy: daunting questions, elusive answers. Kidney Int. 2006;69:644–646. doi: 10.1038/sj.ki.5000231. [DOI] [PubMed] [Google Scholar]
- 52.Grollman A.P. Aristolochic acid nephropathy: harbinger of a global iatrogenic disease. Environ Mol Mutagen. 2013;54:1–7. doi: 10.1002/em.21756. [DOI] [PubMed] [Google Scholar]
- 53.Ramachandran S. Renal diseases: Sri Lankan and global spectrum. J Ceylon Coll Physicians. 1994;27:27–35. [Google Scholar]
- 54.Redmon J.H., Elledge M.F., Womack D.S. Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka–lessons learned from the WHO CKDu population prevalence study. BMC Nephrol. 2014;15:125. doi: 10.1186/1471-2369-15-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayatilake N., Mendis S., Maheepala P., Mehta F.R. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nanayakkara S., Senevirathna S.T., Abeysekera T. An integrative study of the genetic, social and environmental determinants of chronic kidney disease characterized by tubulointerstitial damages in the North Central Region of Sri Lanka. J Occup Health. 2014;56:28–38. doi: 10.1539/joh.13-0172-oa. [DOI] [PubMed] [Google Scholar]
- 57.Reddy D.V., Gunasekar A. Chronic kidney disease in two coastal districts of Andhra Pradesh, India: role of drinking water. Environ Geochem Health. 2013;35:439–454. doi: 10.1007/s10653-012-9506-7. [DOI] [PubMed] [Google Scholar]
- 58.State of the Nation. 2016 Kidney Health Week. Chronic Kidney Disease Hotspots. Mapping the Impact of Chronic Kidney Disease in Australia. Kidney Health Australia. 2016. Available at: www.kidney.org.au. Accessed July 4, 2016.
- 59.Hoy W.E. Kidney disease in Aboriginal Australians: a perspective from the Northern Territory. Clin Kidney J. 2014;7:524–530. doi: 10.1093/ckj/sfu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collins J.F. Kidney disease in Maori and Pacific people in New Zealand. Clin Nephrol. 2010;74(suppl 1):S61–S65. [PubMed] [Google Scholar]
- 61.Wesseling C., Crowe J., Hogstedt C. Resolving the enigma of the mesoamerican nephropathy: a research workshop summary. Am J Kidney Dis. 2014;63:396–404. doi: 10.1053/j.ajkd.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Wesseling C., Crowe J., Hogstedt C., editors. Mesoamerican nephropathy: report from the first international research workshop on MeN. SALTRA/IRET-UNA; Heredia, Costa Rica: 2013. http://www.regionalnephropathy.org/wp-content/uploads/2013/04/Technical-Report-for-Website-Final.pdf Available at: Accessed July 2, 2016. [Google Scholar]
- 63.Ordunez P., Saenz C., Martinez R. The epidemic of chronic kidney disease in Central America. Lancet Glob Health. 2014;2:e440–e441. doi: 10.1016/S2214-109X(14)70217-7. [DOI] [PubMed] [Google Scholar]
- 64.Tzur S., Rosset S., Shemer R. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amendola L.M., Dorschner M.O., Robertson P.D. Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res. 2015;25:305–315. doi: 10.1101/gr.183483.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacArthur D.G., Manolio T.A., Dimmock D.P. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lek M., Karczewski K.J., Minikel E.V. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rehm H.L., Berg J.S., Brooks L.D. ClinGen—the clinical genome resource. N Engl J Med. 2015;372:2235–2242. doi: 10.1056/NEJMsr1406261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Green R.C., Goddard K.A., Jarvik G.P. Clinical sequencing exploratory research consortium: accelerating evidence-based practice of genomic medicine. Am J Hum Genet. 2016;99:246. doi: 10.1016/j.ajhg.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen R., Shi L., Hakenberg J. Analysis of 589,306 genomes identifies individuals resilient to severe Mendelian childhood diseases. Nat Biotechnol. 2016;34:531–538. doi: 10.1038/nbt.3514. [DOI] [PubMed] [Google Scholar]
- 71.Genomes Project C. Auton A., Brooks L.D. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jarvik G.P., Amendola L.M., Berg J.S. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet. 2014;94:818–826. doi: 10.1016/j.ajhg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tekola-Ayele F., Rotimi C.N. Translational genomics in low- and middle-income countries: opportunities and challenges. Public Health Genomics. 2015;18:242–247. doi: 10.1159/000433518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Green R.C., Goddard K.A., Jarvik G.P. Clinical sequencing exploratory research consortium: accelerating evidence-based practice of genomic medicine. Am J Hum Genet. 2016;98:1051–1066. doi: 10.1016/j.ajhg.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kottgen A., Pattaro C., Boger C.A. New loci associated with kidney function and chronic kidney disease. Nat Genet. 2010;42:376–384. doi: 10.1038/ng.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boger C.A., Chen M.H., Tin A. CUBN is a gene locus for albuminuria. J Am Soc Nephrol. 2011;22:555–570. doi: 10.1681/ASN.2010060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boger C.A., Gorski M., Li M. Association of eGFR-related loci identified by GWAS with incident CKD and ESRD. PLoS Genet. 2011;7:e1002292. doi: 10.1371/journal.pgen.1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pattaro C., Teumer A., Gorski M. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun. 2016;7:10023. doi: 10.1038/ncomms10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilkinson M.D., Dumontier M., Aalbersberg I.J. The FAIR guiding principles for scientific data management and stewardship. Sci Data. 2016;3:160018. doi: 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Go forth and replicate! Nature. 2016;536:373. doi: 10.1038/536373a. [DOI] [PubMed] [Google Scholar]
- 81.Welter D., MacArthur J., Morales J. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42:D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Margolis R., Derr L., Dunn M. The National Institutes of Health's Big Data to Knowledge (BD2K) initiative: capitalizing on biomedical big data. J Am Med Inform Assoc. 2014;21:957–958. doi: 10.1136/amiajnl-2014-002974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flannick J., Florez J.C. Type 2 diabetes: genetic data sharing to advance complex disease research. Nat Rev Genet. 2016;17:535–549. doi: 10.1038/nrg.2016.56. [DOI] [PubMed] [Google Scholar]
- 84.Roadmap Epigenomics C., Kundaje A., Meuleman W. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suhre K., Shin S.Y., Petersen A.K. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477:54–60. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rhee E.P., Ho J.E., Chen M.H. A genome-wide association study of the human metabolome in a community-based cohort. Cell Metab. 2013;18:130–143. doi: 10.1016/j.cmet.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nelson M.R., Tipney H., Painter J.L. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47:856–860. doi: 10.1038/ng.3314. [DOI] [PubMed] [Google Scholar]
- 88.Ferreiro A., Álvarez-Estévez G., Cerdas-Calderón M. Confirmed clinical case of chronic kidney disease of nontraditional causes in agricultural communities in Central America: a case definition for surveillance. Rev Panam Salud Pública. 2016;40:301–308. [PubMed] [Google Scholar]
- 89.Kidney Health for Life (KH4L). Chronic Kidney Disease. Multinational Inventory. Available at: KH4L_-_CKD_Multinational_Inventory.pdf. Accessed March 31, 2014.
- 90.Said S., Hernandez G.T. Environmental exposures, socioeconomics, disparities, and the kidneys. Adv Chronic Kidney Dis. 2015;22:39–45. doi: 10.1053/j.ackd.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 91.Luyckx V.A., Brenner B.M. Birth weight, malnutrition and kidney-associated outcomes[mdash]a global concern. Nat Rev Nephrol. 2015;11:135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 92.Gutierrez O.M. Contextual poverty, nutrition, and chronic kidney disease. Adv Chronic Kidney Dis. 2015;22:31–38. doi: 10.1053/j.ackd.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wesseling C., Crowe J., Hogstedt C. The epidemic of chronic kidney disease of unknown etiology in Mesoamerica: a call for interdisciplinary research and action. Am J Public Health. 2013;103:1927–1930. doi: 10.2105/AJPH.2013.301594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiryluk K., Li Y., Scolari F. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomson R., Genovese G., Canon C. Evolution of the primate trypanolytic factor APOL1. Proc Natl Acad Sci U S A. 2014;111:E2130–E2139. doi: 10.1073/pnas.1400699111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKnight A.J., McKay G.J., Maxwell A.P. Genetic and epigenetic risk factors for diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:287–296. doi: 10.1053/j.ackd.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 97.Harjutsalo V., Groop P.H. Epidemiology and risk factors for diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21:260–266. doi: 10.1053/j.ackd.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 98.Sekula P., Li Y., Stanescu H.C. Genetic risk variants for membranous nephropathy: extension of and association with other chronic kidney disease aetiologies. Nephrol Dial Transplant. 2017;32:325–332. doi: 10.1093/ndt/gfw001. [DOI] [PMC free article] [PubMed] [Google Scholar]