Abstract
Anemia is a common complication of chronic kidney disease. Use of erythropoiesis-stimulating agents (ESA) has been a mainstay of treatment since 1990. A series of large trials demonstrated that ESAs have serious safety problems, including increasing cardiovascular and thrombotic events, and death. Analyses suggest high pharmacologic doses of ESAs, rather than the highly achieved hemoglobin, may mediate harm. Hypoxia-inducible factor (HIF) activators stimulate endogenous erythropoietin production and enhance iron availability. In early clinical trials, these oral agents appear to be capable of replacing ESA therapy and minimizing the need for i.v. iron therapy for chronic kidney disease–related anemia, while having other potentially advantageous actions. Large phase 3 trials are underway with several HIF activators. This commentary reviews trends in anemia management, the safety issues related to our present therapies, the role of HIF in regulating erythropoiesis, and the diverse actions of HIF activators.
Keywords: epoetin, hepcidin, hypoxia-inducible factor, HIF activators
Anemia is a common complication in patients with chronic kidney disease (CKD), developing gradually and increasing in severity as kidney disease progresses.1 Anemia is associated with poor outcomes, including higher mortality in patients with end-stage renal disease (ESRD)2 and in those with non–dialysis-dependent CKD.3 The major cause of anemia in CKD is a relative deficiency in erythropoietin (EPO) production,4 although the complex clinical picture of most patients with CKD frequently includes additional conditions contributing to the development of anemia, such as inflammation and iron deficiency. Until approximately 1990, anemia of CKD, especially in patients with ESRD, was managed with oral and occasional i.v. iron administration, occasional use of androgens, and blood transfusions for the severely anemic. Transfusion complications included transfusion reactions, sensitization, and iron overload.5 This resulted in lower hemoglobin levels in patients with ESRD until pharmacologic replacement of EPO with Epoetin in 1989 revolutionized the approach to CKD-related anemia.
The prevalence of anemia (hemoglobin ≤12 g/dl) is high (47.7%) in patients with nondialysis CKD and increases as CKD progresses, being present in approximately 42% of patients with stage 3 CKD, increasing to approximately 76% in stage 5 CKD.6 The incidence of more severe anemia (hemoglobin ≤10 g/dl), which is the treatment level described in the package insert of erythropoiesis-stimulating agents (ESAs), is less common: 5.6% prevalence in stage 3 CKD and 27.2% in stage 5 nondialysis CKD.7 Even though anemia is very common in patients with advanced CKD, relatively few of these patients receive treatment for it: among patients with CKD stages 4 and 5, only 20% and 42%, respectively, were on any treatment for anemia defined using gender-specific thresholds (<12 g/dl for female patients and <13 g/dl for male patients).7
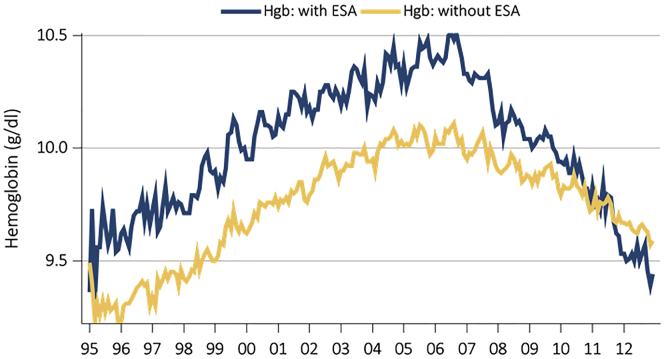
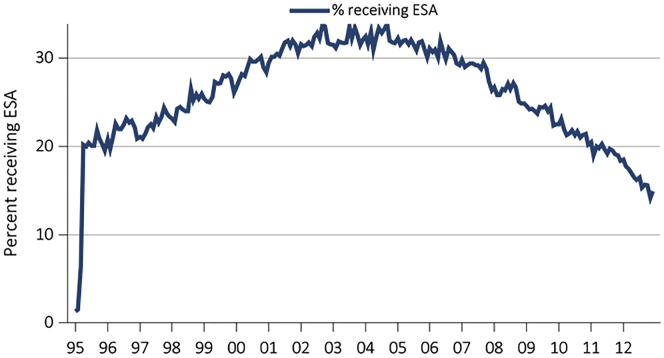
Anemia treatment trends in CKD have shown a secular trend, perhaps as a result of clinical trials changing clinical practices and changes in the regulatory environment. Hemoglobin levels reported in patients initiating chronic dialysis peaked at the end of 2006 in the United States at approximately 10.5 g/dl in ESA-treated patients with CKD, and approximately 10.0 in the remaining patients. Subsequently, mean hemoglobin has fallen steadily in these groups and is approximately 9.5 g/dl in 2013 (Figure 1). This decline comes in the wake of clinical trials showing no benefit or even harm from normalizing hemoglobin with ESA therapy.8 Similar to the trends seen in hemoglobin concentration, ESA use before starting dialysis has also fallen since 2006 and is now below 15% utilization in the United States (Figure 2).8 Use of i.v. iron, in contrast, increased in dialysis patients, with mean ferritin levels steadily increasing. Nondialysis CKD and patients on dialysis have disordered iron metabolism due to increases in hepcidin, the regulator of iron absorption and release from reticuloendothelial cells. Clinically, physicians have compensated for this disordered iron metabolism by administering i.v. iron, but this may have adverse long-term effects due iron increasing oxidative stress.
Figure 1.
Changes in initial hemoglobin in US incident dialysis patients.
Adapted from the USRDS (US Renal Data System) Annual Report, Volume 2, 2014. ESA, erythropoiesis-stimulating agents; Hgb, hemoglobin.
Figure 2.
Changes in ESA use before initiation of dialysis in US patients.
Adapted from the USRDS (US Renal Data System) Annual Report, Volume 2, 2014. ESA, erythropoiesis-stimulating agents.
In addition to the trends observed in predialysis patients, decreases in hemoglobin levels and ESA dose have also been observed in dialysis patients. The average ESA dose in the United States is now approximately 8000 units per week.9 Due to a more conservative approach to ESA therapy in the past decade, a concomitant rise in blood transfusions from approximately 2.5% to 3.0% has also been observed. Some increase in transfusion rates may reflect appropriate clinical decisions to avoid ESAs in some patients, such as those with cancer or history of strokes, but the concern remains that increased transfusions may have adverse effects, especially among transplant-eligible patients. In summary, disordered iron metabolism and EPO insufficiency contribute to anemia in CKD, whereas treatment of anemia across the CKD stages is less frequent and less aggressive, resulting in lower hemoglobin levels and higher transfusion rates. The growing concerns about the safety of ESA products are a major driver in the shift toward worsening anemia in patients with CKD.
Pathophysiology and epidemiology of anemia of CKD
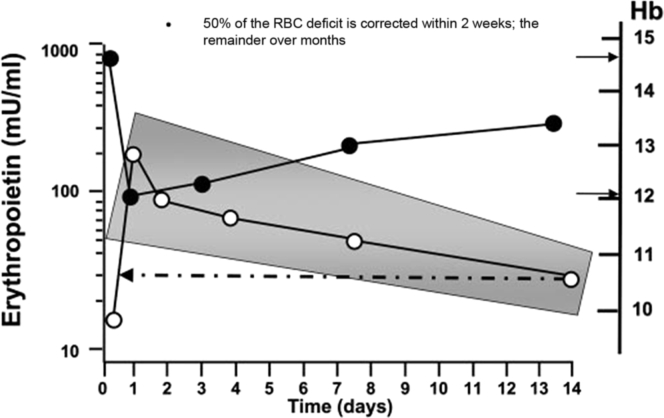
Before the ESA era, patients with CKD and particularly dialysis patients were routinely maintained at a low hematocrit level because interventions available to clinicians were relatively ineffective. During this time, anemia therapy consisted of iron supplements, androgen therapy, and blood transfusions; however, there were predictable complications to these interventions, including iron overload, infections, allosensitization, and cardiovascular complications. The therapy of anemia of CKD was revolutionized with the introduction of Epoetin, the first ESA, in 1989. This led to a major reduction in the burden of illness suffered by patients with CKD. In particular, ESA use led to the virtual elimination of patients with transfusion-dependent anemia. Notwithstanding the major advancement of ESA therapy, its therapeutic application can be considered to be unphysiological regarding the dose, timing, and mode of administration. The early therapeutic paradigm for ESA had been to administer high doses, which were then escalated every 2 to 4 weeks if a desired increase in hemoglobin concentration was not achieved. This strategy ran counter to the human body’s natural response to anemia, which is a short-term rise in endogenous EPO levels (Figure 3), and likely increases the possibility of significant toxicities.10
Figure 3.
Epoetin concentration-time profile following 2-unit phlebotomy in normal male volunteers. The shaded area reflects the 95% confidence interval of the data. The arrows pointing right show the baseline and post-phlebotomy hemoglobin values, while the dotted line and arrow highlights that erythropoietin levels are still above baseline 14 days after phlebotomy.
Adapted with permission from Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2:1274–1282.10 Copyright © American Society of Nephrology. Hb, hemoglobin; RBC, red blood cell.
The risks associated with ESA therapy are highlighted in 3 pivotal clinical trials. The first prospective randomized trial to assess the possibility of benefits in normalizing blood hemoglobin concentration with ESA therapy was the US Normal Hematocrit trial, published in 1998.11, 12 A total of 1265 patients on chronic dialysis were randomized into 2 groups with different target hematocrits (30% vs. 42%), and followed until the development of the composite primary endpoint of death or first nonfatal myocardial infarction. The study was prematurely halted due to a higher proportion of patients in the higher hematocrit target group reaching the primary endpoint, thus obviating the possibility of any benefit in “normalizing” the hematocrit and suggesting that treatment with ESA to a normal hematocrit target may in fact be detrimental. In addition to the primary endpoint, other clinical endpoints, such as vascular access thrombosis, was also significantly increased, suggesting additional harmful effects from the normal hematocrit treatment paradigm.
The 2006 Correction of Hemoglobin and Outcomes in Renal Insufficiency trial was the next large randomized controlled clinical trial designed to assess the effect of different hemoglobin treatment targets (11.3 g/dl vs. 13.5 g/dl) on clinical events in 1432 patients with CKD stages 3 and 4.13 The group assigned to the higher hemoglobin target experienced a significantly higher rate of the composite primary endpoint (congestive heart failure, hospitalizations, stroke, or myocardial infarction) compared with the low-target group, and there was no difference between groups in quality-of-life scores.
The most recent large trial to assess the risks and benefits of ESA therapy was the 2009 Trial to Reduce Cardiovascular Events with Aranesp Therapy trial, which randomized 4038 patients with type 2 diabetes mellitus and CKD stage 3 or 4 to darbepoetin or placebo injections. The hemoglobin target was 13.0 g/dl. The median follow-up was 29.1 months. The darbepoetin-treated arm did not show any reduction in the primary endpoint (death, nonfatal myocardial infarction or stroke, heart failure, or unstable angina), and experienced significantly more strokes (hazard ratio 1.92; 95% confidence interval, 1.68–2.38), venous thromboembolic events, arterial thromboembolic events, and deaths from recurrent cancer compared with the placebo arm.14 The only benefits observed from darbepoetin therapy were fewer transfusions and a modest improvement in patient-reported fatigue.
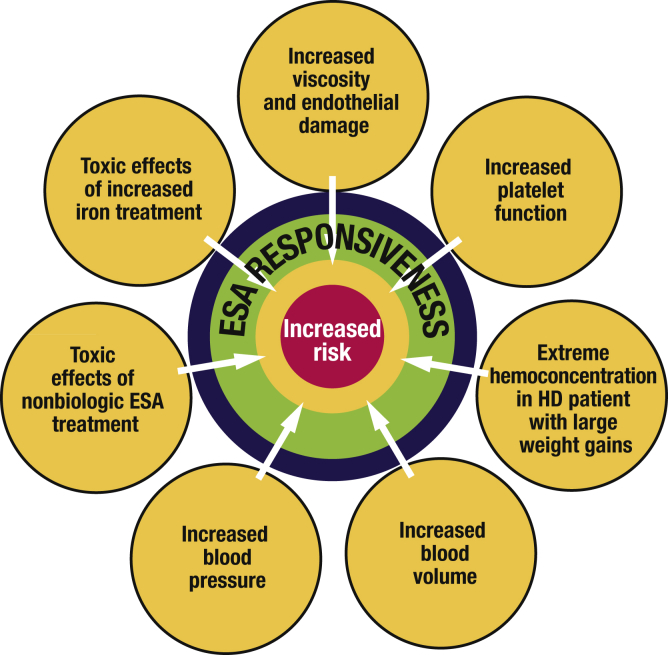
For each of these studies, the hypothesis was that giving ESAs and achieving a higher hematocrit or hemoglobin would bring significant clinical benefits, although potential complications associated with the use of ESAs were already known or suspected. Analyses of the major trial results have suggested that the high ESA doses administered, rather than the specific hemoglobin target, may be a major mediator of harm, although a higher target leads to higher overall ESA doses. During the early days, the use of ESAs in dialysis was associated with hypertension, seizures, and vascular access thrombosis. Subsequently, usage of ESAs was also linked to hemoglobin “overshooting,” ESA-resistance, hemoglobin cycling, strokes, and associations with cancer. There are several proposed mechanisms of ESA toxicity (Figure 4), which are to some extent masked by the difficulty in detecting hypertension due to ESAs and the high underlying risk of death due to cardiovascular events and possibly even neoplasia in dialysis patients.
Figure 4.
Potential mechanism of increased cardiovascular risk with higher hemoglobin targets in ESA studies.
Adapted with permission from Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2:1274–1282.10 Copyright © American Society of Nephrology. ESA, erythropoiesis-stimulating agents; HD, hemodialysis.
These toxicities may be mediated by one or more of the several nonerythropoietic actions of high levels of EPO. The appearance of hypertension could be related to increases in renin-angiotensin-aldosterone system activation, asymmetric dimethylarginine, thromboxane, and vascular smooth muscle cell calcium concentration, and a decrease in prostacyclin, leading to reductions in nitric oxide. The risk of thrombosis is likely mediated through an increase in platelet production and activity along with increases in Von Willebrand factor and plasminogen activator inhibitor-1, whereas tumor growth and vascular remodeling could be a result of vascular smooth muscle cell and EC proliferation and angiogenesis. Overall, targeting higher hemoglobin concentrations in patients with CKD or ESRD is associated with increased risk of all-cause mortality (risk ratio 1.17; 95% confidence interval, 1.01–1.35; P = 0·031), risk of arteriovenous access thrombosis (1.34; 1.16–1.54; P = 0.0001), and higher risk of poorly controlled blood pressure (1.27; 1.08–1.50; P = 0.004).15
The current pattern of anemia management in CKD is characterized by fewer patients being treated with ESA and more patients receiving oral and i.v. iron. However, this approach is still unphysiological and suboptimal, as there are significant concerns about the long-term benefits of an anemia management strategy that centers on iron administration in often supraphysiologic doses. Potential complications include allergic reactions, infectious risks, and long-term complications related to the induction of oxidative stress, such as kidney injury leading to worsened renal outcomes in patients with CKD.16 The lack of long-term clinical trials assessing clinical outcomes associated with various iron administration strategies makes it difficult to determine whether or not the current therapeutic paradigms of i.v. iron administration in dialysis patients are safe, and leaves many unanswered questions about what the best approach should be for the optimization of iron homeostasis in CKD. The more desirable approach moving forward is the use of a more physiological strategy that has a gentle, configurable correction and maintenance of hematocrit.
Anemia in CKD: changing treatment paradigms
As discussed previously, the currently available treatment options for anemia of CKD and ESRD have significant limitations. The circulating levels of EPO required to stimulate erythropoiesis is 7 to 30 mU/ml, with higher levels required in patients with CKD and ESRD due to shortened red cell survival. The unphysiologic administration of high-dose ESA for anemia of CKD and ESRD can result in EPO levels as high as 700 mU/ml, which is suspected to account for at least some of the harm observed in the randomized trials. One treatment strategy that may improve outcomes is to design interventions that stimulate intermittent increases in endogenous EPO levels, instead of use of high pharmacologic doses of ESAs.
EPO is a proliferation and maturation factor produced in response to tissue hypoxia, as a result of complex regulatory mechanisms,17, 18, 19, 20 which were not yet fully understood at the time when EPO was first used therapeutically. Ninety percent of all EPO produced in the body originates from the kidneys and approximately 10% is produced by the liver.21 Kidney-derived EPO is produced by cortical peritubular fibroblasts located near the proximal tubular cells in the outer medulla and inner cortex in the kidney. This production is expanded into the outer cortex in response to hypoxia and anemia, a region that is especially susceptible to hypoxia. Indeed, tissue hypoxia is the pivotal factor that increases EPO production as a final step in a signal transduction pathway involving several proteins, among which hypoxia-inducible factors (HIFs) play a central role (vide infra).18
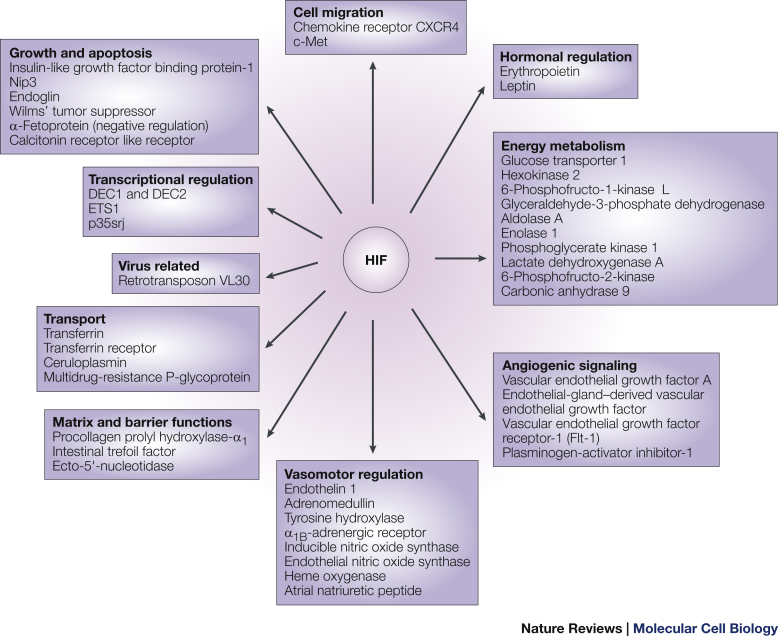
HIFs are proteins that play a pivotal role in the signal transduction leading to EPO production.22 The HIF complex consists of either HIF-1α or an oxygen-sensitive HIF-2α subunit that associates with a constitutively expressed HIF-1β subunit.22 Human renal fibroblasts, under hypoxic conditions, activate only HIF-2α and not HIF-1α. HIF-2α, in the absence of hypoxia, is hydroxylated at 2-proline residues by prolyl-hydroxylases, which mark the HIF-2α for binding by von Hippel Lindau protein and subsequently for ubiquitination and proteasomal degradation.23 Prolyl-hydroxylase 2 has been identified as the key enzyme that regulates HIF-2α stability; prolyl-hydroxylase 2 has also been identified in mutations leading to erythrocytosis.24 HIF-2α, in hypoxic states, is not hydroxylated due to inhibition of prolyl-hydroxylase and hence forms a heterodimer with HIF-1β that translocates to the nucleus and binds to the hypoxia response element of EPO and induces EPO transcription. Prolyl-hydroxylase 2 enzymes have a wide range of intracellular functions and targets, which include various genes involved in erythropoiesis, including those for EPO, EPO receptor, and proteins promoting iron absorption, iron transport, and heme synthesis (Figure 5).
Figure 5.
Hypoxia-inducible factor (HIF) gene targets.
Reprinted from Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354, by permission from Macmillan Publishers Ltd.
HIF-prolyl-hydroxylase 2 inhibitors or HIF stabilizers represent a novel therapeutic intervention in the future management of anemia.25 HIF stabilizers inhibit the prolyl-hydroxylase enzyme leading to increased levels of HIF and thus increased production of endogenous EPO. In the first study to show the proof of concept of this therapeutic intervention, patients with normal kidneys and patients on hemodialysis (including anephric patients who were analyzed separately) received an HIF stabilizer with subsequent measurement of plasma EPO.26 EPO levels increased after administration of the HIF stabilizing agent, with an exaggerated response in plasma EPO in the nephric hemodialysis patients. The anephric hemodialysis patients still had an increase in EPO but not as exaggerated as the nephric patients, possibly a result of hepatic EPO production.26
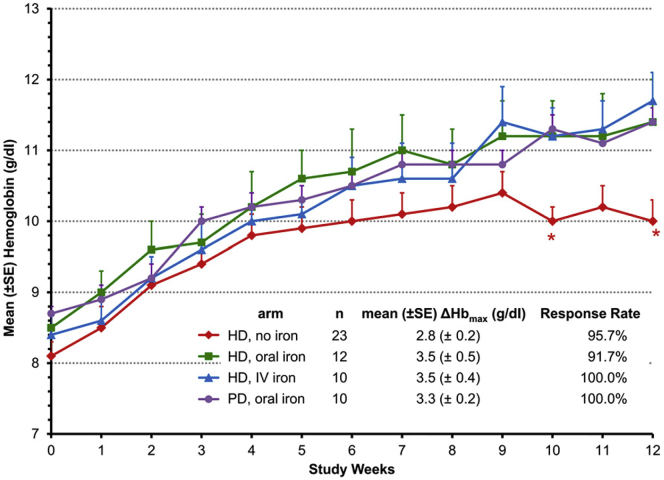
There are currently 4 HIF stabilizers that are in phase 2/3 clinical trials (roxadustat, daprodustat, vadadustat, and molidustat), and all are orally administered agents (Table 1). In a phase 2 clinical trial of roxadustat, 4 different groups of patients were evaluated: hemodialysis receiving no iron, hemodialysis receiving oral iron, hemodialysis receiving i.v. iron, and peritoneal dialysis receiving oral iron (see Figure 6).27 Oral iron in hemodialysis patients receiving conventional ESA therapy has been shown to be no better than no iron due to blockade of iron absorption by high hepcidin levels. Hemoglobin levels increased in all the groups, including the patients who did not receive iron; however, the response in this group was less than in the patients who were receiving i.v. and oral iron, suggesting HIF stabilizers enhance iron availability. In another phase 2 dose-finding trial of daprodustat examining nondialysis patients with CKD and hemodialysis patients, 5 mg of daprodustat resulted in an increase in hemoglobin concentration in nondialysis patients with CKD, and was maintained at the level of control group (which received ESA) in hemodialysis patients.28 Vadadustat was studied in a 20-week double-blind, randomized, placebo-controlled phase 2b study in nondialysis-dependent patients with CKD.29 Similar to the other agents, there was a significant increase in hemoglobin concentration compared with placebo. Significant increases in both reticulocytes and total iron-binding capacity and significant decreases in both serum hepcidin and ferritin levels were observed in patients on vadadustat compared with placebo.29 Finally, molidustat was studied in a phase 2 clinical trial, also showing a dose-dependent increase in hemoglobin levels.30
Table 1.
Hypoxia-inducible factor stabilizers under development for treatment of anemia in chronic kidney disease
| Company | Molecule | Drug name | Phase of development |
|---|---|---|---|
| FibroGen Astellas AstraZeneca |
FG-4592 | Roxadustat | Phase 3 |
| GlaxoSmithKline | GSK 1278863 | Daprodustat | Phase 3 |
| Akebia | AKB-6548 | Vadadustat | Phase 3 |
| Bayer | BAY 85–3934 | Molidustat | Phase 2/3 |
| Japan Tobacco Inc | JTZ-951 | Phase 1 |
Figure 6.
Mean change in hemoglobin with the HIF stabilizer, roxadustat, in dialysis patients. Patients on hemodialysis and/or peritoneal dialysis were given oral roxadustat, with oral iron, i.v. iron, or no iron supplementation. The change in mean hemoglobin ± SE is shown by study week. ∗P < 0.05 in comparisons between the no-iron cohort to the pooled-iron cohorts. Hb, hemoglobin; HD, hemodialysis; PD, peritoneal dialysis.
Reprinted with permission from Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. Copyright © American Society of Nephrology.
There are several important differences between HIF stabilizers and ESAs in their effects on circulating EPO levels. HIF stabilizers increase hemoglobin with much lower levels of circulating EPO,31 thus eliminating the risk of patient exposure to such high levels. In addition, the roxadustat study indicated that patients supplemented with oral and i.v. iron had the exact same response rates to these treatments, in contradistinction of what has been previously described in ESA studies whereby patients receiving i.v. iron typically had better responses.27 One potential explanation for this observation is that HIF stabilizing agents appear to lower hepcidin levels, which results in increased iron availability and increased reticulocyte hemoglobin content, as demonstrated in patients treated with roxadustat when compared with patients receiving EPO. The effects of HIF stabilizers on hepcidin levels may also explain why the hemoglobin response seen with this new class of drugs is independent of background inflammation. In contrast, patients with inflammation typically require higher ESA doses and are less likely to achieve target hemoglobin.
In addition to the fundamentally different mechanisms of action that mediate the effect of HIF activators on hemoglobin production, there are additional effects of these agents that are independent of EPO production and the regulation of iron metabolism, some of which may be beneficial (e.g., alleviation of ischemic injury32 and beneficial effects in diabetic nephropathy33), but others that may be harmful (such as its effects on angiogenesis34 and glucose metabolism35). Currently there are no early signs of concerns regarding adverse effects in the available clinical trials. Furthermore, living at high altitudes, which results in physiologic changes similar to the effects of HIF activators (e.g., reduction in EPO requirements and resistance), is associated with lower all-cause mortality, lower cancer rates, and fewer cardiac events in dialysis patients.36, 37, 38, 39, 40 Nevertheless, more extensive evaluations are warranted to ensure the safety of HIF activators. There are several phase 3 trials of HIF activator agents. They uniformly include thousands of patients and hard safety endpoints, which should inform us about the benefits and risks of these agents versus ESA therapy and placebo.
Summary
In summary, the field of anemia therapy in CKD and ESRD has progressed from one with very limited options in the pre-ESA era to one in which novel agents are developed to induce therapeutic effects that more closely mimic the body’s own responses to hypoxia, raising hope that the complications caused by the prevailing ESA-based paradigms can be eliminated. The new class of orally administered HIF stabilizers that is under clinical development mimics hypoxia, resulting in the upregulation of EPO gene expression, and in an increase in both hemoglobin concentration and iron transport proteins. Because these agents may upregulate other hypoxia-sensitive genes that are involved in angiogenesis and tumor growth, their long-term safety will need to be proven.
Disclosure
Publication of this article was supported by AstraZeneca and FibroGen. DWC has received research support from FibroGen, GlaxoSmithKline, Celgene, Eli Lilly, and Janssen; and speaking and consultant honoraria or paid advisory boards from AMAG, Eli Lilly, GlaxoSmithKline, and AstraZeneca. DG has received speaking and consultant honoraria or paid advisory boards from Roche, Amgen, Sandoz, Abbott, AstraZeneca, Bristol-Myers Squibb, Akebia, Astellas, Vifor, Bayer, Fresenius Medical Care, Genzyme, Keryx, and Sanofi-Aventis; and research support from Shire. IM has received research support from Akebia, Astellas, Bayer, and GlaxoSmithKline; speaking and consultant honoraria or paid advisory boards from Akebia, AMAG, Vifor, Astellas, Bayer, FibroGen, and GlaxoSmithKline; and lecture fees from Vifor, FibroGen, and Akebia.
Acknowledgments
Publication of this article was supported by AstraZeneca and FibroGen. This supplement summarizes the two continuing medical education meetings held during the International Society of Nephrology/World Congress of Nephrology conference. The two educational meetings were supported by an educational grant from FibroGen and AstraZeneca. FibroGen and AstraZeneca had no involvement in the development of the two continuing medical education meetings or this supplement. The Med Ed Group, Inc, the meeting organizers, provided editorial support for this supplement. SynAptiv accredited the two continuing medical education meetings and has reviewed this supplement.
References
- 1.Fehr T., Ammann P., Garzoni D. Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int. 2004;66:1206–1211. doi: 10.1111/j.1523-1755.2004.00880.x. [DOI] [PubMed] [Google Scholar]
- 2.Regidor D.L., Kopple J.D., Kovesdy C.P. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy C.P., Trivedi B.K., Kalantar-Zadeh K. Association of anemia with outcomes in men with moderate and severe chronic kidney disease. Kidney Int. 2006;69:560–564. doi: 10.1038/sj.ki.5000105. [DOI] [PubMed] [Google Scholar]
- 4.Artunc F., Risler T. Serum erythropoietin concentrations and responses to anaemia in patients with or without chronic kidney disease. Nephrol Dial Transplant. 2007;22:2900–2908. doi: 10.1093/ndt/gfm316. [DOI] [PubMed] [Google Scholar]
- 5.Macdougall I.C., Obrador G.T. How important is transfusion avoidance in 2013? Nephrol Dial Transplant. 2013;28:1092–1099. doi: 10.1093/ndt/gfs575. [DOI] [PubMed] [Google Scholar]
- 6.McClellan W., Aronoff S.L., Bolton W.K. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510. doi: 10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer M.E., Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9:e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R., Li Y., Robinson B. US Renal Data System 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67 doi: 10.1053/j.ajkd.2015.12.014. Svii, S1–Svii, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaboyas A., Zee J., Morgenstern H. Understanding the recent increase in ferritin levels in United States dialysis patients: potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. Clin J Am Soc Nephrol. 2015;10:1814–1821. doi: 10.2215/CJN.02600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishbane S., Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007;2:1274–1282. doi: 10.2215/CJN.02380607. [DOI] [PubMed] [Google Scholar]
- 11.Besarab A., Bolton W.K., Browne J.K. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 12.Coyne D.W. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney Int. 2012;82:235–241. doi: 10.1038/ki.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh A.K., Szczech L., Tang K.L. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 14.Pfeffer M.A., Burdmann E.A., Chen C.Y. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 15.Phrommintikul A., Haas S.J., Elsik M. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet. 2007;369:381–388. doi: 10.1016/S0140-6736(07)60194-9. [DOI] [PubMed] [Google Scholar]
- 16.Charytan D.M., Pai A.B., Chan C.T. Considerations and challenges in defining optimal iron utilization in hemodialysis. J Am Soc Nephrol. 2015;26:1238–1247. doi: 10.1681/ASN.2014090922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumitriu B., Patrick M.R., Petschek J.P. Sox6 cell-autonomously stimulates erythroid cell survival, proliferation, and terminal maturation and is thereby an important enhancer of definitive erythropoiesis during mouse development. Blood. 2006;108:1198–1207. doi: 10.1182/blood-2006-02-004184. [DOI] [PubMed] [Google Scholar]
- 18.Haase V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299:F1–F13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La F.K., Reimann C., Jelkmann W. Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-kappaB. FASEB J. 2002;16:1811–1813. doi: 10.1096/fj.02-0168fje. [DOI] [PubMed] [Google Scholar]
- 20.Scortegagna M., Morris M.A., Oktay Y. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102:1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- 21.Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 22.Semenza G.L., Wang G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberger C., Mandriota S., Jurgensen J.S. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13:1721–1732. doi: 10.1097/01.asn.0000017223.49823.2a. [DOI] [PubMed] [Google Scholar]
- 24.Siddiq A., Aminova L.R., Ratan R.R. Hypoxia inducible factor prolyl 4-hydroxylase enzymes: center stage in the battle against hypoxia, metabolic compromise and oxidative stress. Neurochem Res. 2007;32:931–946. doi: 10.1007/s11064-006-9268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagel S., Talbot N.P., Mecinovic J. Therapeutic manipulation of the HIF hydroxylases. Antioxid Redox Signal. 2010;12:481–501. doi: 10.1089/ars.2009.2711. [DOI] [PubMed] [Google Scholar]
- 26.Bernhardt W.M., Wiesener M.S., Scigalla P. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besarab A., Chernyavskaya E., Motylev I. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016;27:1225–1233. doi: 10.1681/ASN.2015030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holdstock L., Meadowcroft A.M., Maier R. Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016;27:1234–1244. doi: 10.1681/ASN.2014111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pergola P.E., Spinowitz B.S., Hartman C.S. Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016;90:1115–1122. doi: 10.1016/j.kint.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Macdougal IC, Lentini S, Schmidt A, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral HIF stabilizer molidustat in pre-dialysis patients with renal anemia. Abstract presented at: European Renal Association-European Dialysis and Transplant Association Congress. May 2015. Vienna.
- 31.Maxwell P.H., Eckardt K.U. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol. 2016;12:157–168. doi: 10.1038/nrneph.2015.193. [DOI] [PubMed] [Google Scholar]
- 32.Song Y.R., You S.J., Lee Y.M. Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant. 2010;25:77–85. doi: 10.1093/ndt/gfp454. [DOI] [PubMed] [Google Scholar]
- 33.Nordquist L., Friederich-Persson M., Fasching A. Activation of hypoxia-inducible factors prevents diabetic nephropathy. J Am Soc Nephrol. 2015;26:328–338. doi: 10.1681/ASN.2013090990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pugh C.W., Ratcliffe P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 35.Denko N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro B.B., Streja E., Rhee C.M. Revisiting the association between altitude and mortality in dialysis patients. Hemodial Int. 2014;18:374–383. doi: 10.1111/hdi.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winkelmayer W.C., Hurley M.P., Liu J. Altitude and the risk of cardiovascular events in incident US dialysis patients. Nephrol Dial Transplant. 2012;27:2411–2417. doi: 10.1093/ndt/gfr681. [DOI] [PubMed] [Google Scholar]
- 38.Brookhart M.A., Bradbury B.D., Avorn J. The effect of altitude change on anemia treatment response in hemodialysis patients. Am J Epidemiol. 2011;173:768–777. doi: 10.1093/aje/kwq423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkelmayer W.C., Liu J., Brookhart M.A. Altitude and all-cause mortality in incident dialysis patients. JAMA. 2009;301:508–512. doi: 10.1001/jama.2009.84. [DOI] [PubMed] [Google Scholar]
- 40.Brookhart M.A., Schneeweiss S., Avorn J. The effect of altitude on dosing and response to erythropoietin in ESRD. J Am Soc Nephrol. 2008;19:1389–1395. doi: 10.1681/ASN.2007111181. [DOI] [PMC free article] [PubMed] [Google Scholar]