Abstract
The human diet is characterized by highly energetic molecules, but it also requires non-energetic compounds that are equally useful for cell functioning and for preserving the organism's health status. These “functional” molecules are represented by a wide variety of plant secondary metabolites, such as terpenoids, vitamins and polyphenols with antioxidant power. Widespread commercial crop varieties often contain scarce levels of functional molecules, because they have been mostly selected for productivity, rather than for the content of secondary metabolites. Different scenarios (global economic situation, foreseeable environmental changes) are pushing farmers to review the use of high yield crops and to focus on the valorization of locally-adapted plants. This renewed interest is strengthened by the growing need of consumers for functional foods with beneficial effects on human health and by the willingness to promote sustainable low-input agricultural practices exploiting local climate, soil, water, and (micro)biota. Here, we want to discuss a specific case study concerning locally-adapted crops in Tuscany (Italy). Analyses of nutraceutical molecules in locally-grown crop varieties (namely tomatoes, sweet cherries and onions) have shown that they are characterized by substantially higher functional molecule contents than commercial varieties. Our goal is to promote the high-throughput study of locally-adapted varieties to understand, in a medium-term perspective, whether the cultivation of such plants is a valuable support for the diet and an adequate local economic resource. Such plants can provide a boost to the regional economy, by diversifying the local crop-market landscape. Moreover, the exploitation of locally-grown plants results in the manufacture of fully-traceable products (from the raw bioresource to the finished product) with a “0 km” concept that minimizes the C footprint.
Keywords: bioactive molecules, Tuscany, autochthonous ancient varieties, nutraceutics, functional food
Functional Molecules in the Diet and Their Effects On Human Health
Plant functional molecules (a.k.a as bioactives or health-promoting compounds) are metabolites produced through the plant secondary metabolism that exert biological effects on humans (Azmir et al., 2013; Berni et al., 2018a). Their role in health protection is one of the most important discoveries of the last decade in the biological, medical, and pharmacological fields. The most noteworthy feature of these health-beneficial molecules is their widespread occurrence in fruits and vegetables. This implies the possibility of assimilating them through the daily diet, thereby avoiding any chemical or industrial process to synthesize them.
Plant secondary metabolites are typically produced in response to changes in the environment or pathogen/herbivore attack and are currently in the spotlight of biotechnology because of their applications in different industrial sectors (i.e., healthcare, cosmetics, to name a few; Guerriero et al., 2018). The chemical structures of plant functional molecules are able to counteract reactive oxygen species (ROS) and reactive nitrogen species (RNS) by acting as natural scavengers (Berni et al., 2018b). A strong body of evidence in the literature has indeed drawn attention on plant secondary metabolites in the light of their antioxidant potential, by focusing on specific classes, namely terpenes, and polyphenols (Ghasemzadeh et al., 2010; Thoppil and Bishayee, 2011).
Terpenes consist of over 40,000 different molecules produced by many organisms, spanning from bacteria, yeasts, fungi and, most notably, plants (Goto et al., 2010). They have been studied for their anti-inflammatory effects (Hortelano, 2009) linked to the regulation of cytokine production in human cells (Ku and Lin, 2013). In the plant kingdom, these molecules are synthesized in large quantities by food crops, such as tomatoes: these vegetables produce large amounts of terpenes in the form of carotenoids and, in particular, of lycopene, a pigment that is responsible for the typical red color (Saini et al., 2015). Lycopene is the most efficient quencher of free radicals with the property of protecting the cellular components against oxidative damage (Nasir et al., 2015).
Polyphenols are the largest group of plant secondary metabolites and are classified into different subsets of compounds, depending on their chemistry (Tsao, 2010; Kabera et al., 2014). Their structure is characterized by aromatic ring(s) linked to hydroxyl groups, which confer antioxidant properties to the molecule (Craft et al., 2012). Particular scientific attention is devoted to the class of flavonoids that are present in common plant food products. Vegetables, such as onions, contain high levels of flavonoids, notably quercetin, that is well known for its applications in cancer prevention (Gibellini et al., 2011). Furthermore, evidence in disease treatment is reported for other flavonoids, such as anthocyanins (Norberto et al., 2013). The role of these molecules is directly linked to the decreased susceptibility in developing cardiovascular diseases (Wallace, 2011).
Red fruits like sweet cherries typically contain high levels of anthocyanins and their dietary intake could help in the prevention of cardiovascular diseases (He and Giusti, 2010; Mirto et al., 2018).
Fruits and vegetables are thus natural sources of different bioactives: in the light of their documented benefit on human health, their intake through the daily diet contributes to boost the natural body defenses.
We have here focused on three examples of crops/fruit tree, i.e., tomatoes, onions, and sweet cherry, which are constituents of the Mediterranean diet and are part of the rich biodiversity of Italian regions. In Tuscany, which is the case study here analyzed, 8 different tomato varieties were previously reported (Naziri, 2009) and specific onion varieties are included in the “PAT” (Prodotti alimentari tradizionali-Traditional food products) by the Ministry of Agricultural Politics (Ferioli and D'Antuono, 2016). The fruits/bulbs of these species are not only consumed fresh, but also dried, or processed as soups/juice/jams/marmalades and canned foods. Additionally, tomatoes and onions are produced for >0,6 million tons and >300,000 tons per year in Italy (statistics from 2010; Sardaro et al., 2013; Caruso et al., 2014) and therefore represent economically-important crops.
Native (Locally-Grown) Plants vs. Commercial Plants
Native crops are wild plants that are consumed for their fruits, leaves, flowers, and seeds and originating in specific geographic areas (Glew et al., 2005). The concept of native crops can be extended to comprise also plants cultivated for several decades in a specific territory and not necessarily originating from the same territory. Native or wild plants have adapted to the territory by establishing a synergy with the local soil and climate. They represent the local germplasm and offer a source of genes and unique phenotypic characters (Berni et al., 2018a). Emblematic is the example of non-commercial apple varieties showing extreme phenotypes, notably russeting (Legay et al., 2015, 2017) and containing molecules with immune-modulatory properties (Andre et al., 2013).
The inexorable industrial development has selected varieties of fruit and vegetables according to the market demand. Varieties that are not productive according to commercial standards are put aside and this leads, indirectly, to a loss of biodiversity (Schmidt and Wei, 2006).
For centuries, wild crops have played a significant role in nutrition, especially during times of weather extremes, thanks to their ability to withstand strong exogenous stresses, thereby representing a key feedstock for human nutrition (Bvenura and Afolayan, 2015). Furthermore, the fruits of such ancient varieties produce a wide range of bioactive molecules that are currently studied for their role in the human diet (Berni et al., 2018c) and as protective molecules against diseases (Francini et al., 2017; Berni et al., 2018b).
The adaptive strategies put in place by these plants to survive in a wild environment (not impacted by any human intervention) translate into an enhanced production of secondary metabolites, such as polyphenols and terpenes. The increased production of such molecules is likely linked to epigenetic changes that have been induced by the interaction with the environment (Baulcombe and Dean, 2014). Genetic modifications confer to ancient crops unique phenotypic and nutritional features, compared to fruits cultivated for commercial purposes (Legay et al., 2017). In this sense, many authors have focused their attention on the functional molecules derived from autochthonous fruits to shed light on their nutritional and nutraceutical potential in the human diet (Stintzing and Carle, 2004). Ancient fruits are considered as natural antioxidant resources and potential scavengers against oxidative stress. Very interesting, in this respect, are the studies that compare the content of these molecules, expressed in terms of antioxidant potential, in ancient and commercial species. Iacopini et al. (2010) report that the ancient apple varieties they studied contain a higher concentration of antioxidant molecules, with respect to commercial counterparts. We have also recently shown that Tuscan tomatoes sampled in 2016 show higher contents of polyphenols and antioxidant molecules with respect to commercial varieties and that this increase is two- and even three-fold (Berni et al., 2018c). As discussed by other authors, the genotype plays a fundamental role on the final content of functional molecules in fruits (Scalzo et al., 2005), since its interaction with the surrounding environment determines the expression of specific traits (King, 2015). Researches on autochthonous fruits intend to valorize and promote the preservation of these territorial species, by exploiting their nutritional value (Lamien-Meda et al., 2008). Considering the huge spectrum of functional molecules produced, ancient crops can, and should be considered as functional foods and could even be used in support of drug therapies.
The Case Study of Native Crops in Tuscany (Italy): Tomatoes, Sweet Cherries and Onions
The region of Tuscany is world renowned as a producer of high-quality products, such as wine and oil. This unrivaled product quality is due to the local production and exclusive use of territorial natural resources (Mangani et al., 2011), as well as to the protection of specific cultivars (Berni et al., 2018a) and to the development of quality labels. The growing interest in these foods is fuelled by their high content in health-beneficial bioactive molecules (Iacopini et al., 2008; Cavallini et al., 2014). The rich composition in bioactives has the potential of making these products key components of a functional diet (Cencic and Chingwaru, 2010) that is able to offer a stronger line of defense against diseases (Pandey and Rizvi, 2009). Thanks to the ability to adapt to the territory, these plants have developed a synergy with the local soil. In this sense, Tuscany has a broad panoply of plant species that constitute the regional germplasm heritage (http://germoplasma.regione.toscana.it/index.php?option=com_content&view=article&id=1&Itemid=127). Most of these plants were commonly used in the past for human consumption or for other uses: fruit harvesting, wood and fodder, to mark the borders and to support other plants and wild animals. Several evidences in the literature indicate that in these woody and herbaceous species the fruits show noteworthy contents of nutritional and functional compounds (Ancillotti et al., 2016). Therefore, analytical studies are necessary to highlight the functional properties of these local species expressed in terms of bioactive compound contents. The case study here reported will shed light on the nutraceutical characteristics of ancient varieties of Tuscan onions, tomatoes and sweet cherries to sensitize the public to a wider use of these varieties that play an important role in the regional biodiversity landscape and that could ultimately improve human nutrition.
Tomatoes are well known for their content in carotenoids, such as lycopene, but also for the occurrence of other molecules, notably flavonoids and hydroxycinnamic acids (García-Valverde et al., 2013). Onions are an excellent source of flavonoids, i.e., myricetin, quercetin and kaempferol. These molecules are typically present in high amount in the fruit flesh; on the contrary, the skins of red onions are rich in anthocyanins (Pérez-Gregorio et al., 2010). Sweet cherries are one of the most abundant red fruits rich in anthocyanins, distributed chiefly in the peel and in the outer layers of the fruit (Kelebek and Selli, 2011). Nevertheless, in some varieties of these fruits, the distribution of anthocyanins is also found in the seeds. Tuscany has classified 8 varieties of tomatoes, 6 of onions and 6 of sweet cherries; these crops are grown in experimental fields (http://www.ivalsa.cnr.it/az-s-paolina.html), which are used to preserve the native genetic resources located in various areas of the region.
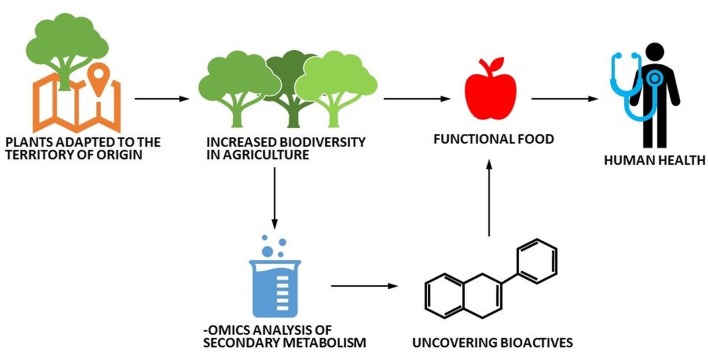
The plant material used in the present study was provided by the “Trees and Timber Institute—CNR-IVALSA” (Follonica, Italy). The institute is a germplasm regional bank for the propagation and enhancement of regional genetic resources. Our analysis focused on functional molecules in native varieties collected in 2017, with the aim of comparing their content to that found in the most widespread commercial Italian varieties. All the analyses performed follow the standards used for the determination of functional and nutraceutical molecules, i.e., Ferric Reducing Antioxidant Power (FRAP), Folin–Ciocalteu, aluminum chloride assay, pH differential method (Aramwit et al., 2010; Dai and Mumper, 2010; Jagtap et al., 2010) and confirmed with HPLC analysis (see Supplementary Material for a description of the methods used). The results are reported in Table 1. Regional varieties showed interesting results, especially when compared with commercial counterparts. Autochthonous fruits contained indeed large quantities of functional molecules (polyphenols, flavonoids, and terpenes) that, in many cases, were higher than those found in commercial fruits (Berni et al., 2018c). Notably, a higher functional molecule content has been demonstrated in other crop landraces (Renna et al., 2014, 2018). These results show that local fruits produce higher amounts of molecules that can be exploited as health-beneficial bioactive compounds (Williamson, 2017; Figure 1).
Table 1.
Total content of antioxidants as mmol Fe2+ per 100g FW, polyphenols as mg of GAE (gallic acid equivalents) per 100 g of FW, flavonoids as mg of QeE (quercetin equivalents) per 100 g of FW, carotenoids as TCC (total carotenoids content) per 100 g of FW and anthocyanins as CyE (cyanidin-3-glucoside equivalents) per 100g of FW.
| Variety names | Antioxidants (mmol Fe2+/100 g FW) ± S.D | Polyphenols (mg GAE/100g FW) ± S.D | Flavonoids (mg QeE/100g FW) ± S.D | Carotenoids (mg TCC/100g FW) ± S.D | Anthocyanins (mg CyE/100g FW) ± S.D | |
|---|---|---|---|---|---|---|
| Tomatoes (Solanum lycopersicum L.) | ||||||
| Liscio da Serbo Toscano | 0.84 ± 0.04a | 74.24 ± 1.7a | 12.96 ± 0.2ae | 0.80 ± 0.02a | / | |
| Rosso Pitigliano | 1.19 ± 0.01b | 75.67 ± 1.7a | 7.25 ± 0.1b | 0.86 ± 0.06ab | / | |
| Quarantino ec Valdarno | 1.03 ± 0.02c | 90.35 ± 1.1b | 20.31 ± 0.5c | 0.90 ± 0.04b | / | |
| Fragola | 0.64 ± 0.03d | 63.68 ± 1.2c | 8.83 ± 0.1d | 0.87 ± 0.03ab | / | |
| Canestrino di Lucca | 0.52 ± 0.04e | 53.81 ± 2.5d | 7.88 ± 0.1b | 0.89 ± 0.01ab | / | |
| Costoluto Fiorentino | 1.14 ± 0.03b | 76.78 ± 2.4ae | 13.42 ± 0.2e | 0.75 ± 0.05c | / | |
| Giallo di Pitigliano | 0.93 ± 0.02a | 79.24 ± 0.7e | 21.49 ± 0.4c | 0.71 ± 0.07c | / | |
| Pisanello | 0.76 ± 0.02f | 66.52 ± 0.4c | 11.95 ± 0.2a | 0.74 ± 0.01c | / | |
| Cuore di Bue (Commercial) | 0.48 ± 0.07e | 51.35 ± 1.5d | 6.58 ± 0.1f | 0.69 ± 0.07c | / | |
| Onions (Allium cepa L.) | ||||||
| Maremma | 1.84 ± 0.10a | 69.25 ± 2.6a | 63.2 ± 1.3a | / | 7.1 ± 0.8a | |
| Rossa Massese | 3.08 ± 0.14b | 302.83 ± 3.9b | 280.4 ± 3.2b | / | 40.2 ± 2.1b | |
| Treschietto | 1.18 ± 0.08cde | 150.42 ± 4.9c | 69.3 ± 1.4c | / | 6.8 ± 0.6a | |
| Rossa della Valtiberina | 1.15 ± 0.11cd | 98.58 ± 3.1d | 68.3 ± 3.5c | / | 55.5 ± 2.8c | |
| Rossa di Lucca | 1.23 ± 0.12d | 117.91 ± 4.2e | 80.2 ± 2.1d | / | 23.3 ± 1.8d | |
| Rossa Fiorentina | 0.97 ± 0.09e | 89.52 ± 2.9f | 60.3 ± 2.9a | / | 47.3 ± 2.3b | |
| Tropea (Commercial 1) | 0.91 ± 0.02e | 55.98 ± 0.9g | 24.4 ± 0.9e | / | 39.5 ± 2.4b | |
| Giarratana (Commercial 2) | 0.83 ± 0.01e | 58.76 ± 4.7g | 27.9 ± 0.8e | / | / | |
| Sweet cherries (Prunus avium L.) | ||||||
| Carlotta | 1.49 ± 0.06a | 200.49 ± 15.8a | 47.21 ± 2.4a | / | 30.80 ± 1.8a | |
| Benedetta | 1.51 ± 0.04a | 250.91 ± 11.3b | 65.33 ± 2.3b | / | 27.52 ± 1.8a | |
| Morellona | 2.51 ± 0.03b | 300.87 ± 13.1c | 63.01 ± 3.7b | / | 59.41 ± 2.5b | |
| Maggiola | 1.92 ± 0.12c | 276.71 ± 7.5d | 46.41 ± 2.5a | / | 33.17 ± 1.7a | |
| Moscatella | 1.51 ± 0.05a | 215.15 ± 11.9a | 44.82 ± 4.8a | / | 27.04 ± 2.4ca | |
| Crognola | 3.17 ± 0.01d | 368.18 ± 5.7e | 81.62 ± 3.3c | / | 67.75 ± 2.7c | |
| Durone (Commercial) | 1.13 ± 0.04e | 146.05 ± 2.7f | 32.21 ± 2.8d | / | 31.65 ± 2.3a | |
| Tomatoes (Solanum lycopersicum L.) | Caffeic Acid (μg/g FW) ± S.D | Ferulic Acid (μg/g FW) ± S.D | Chlorogenic Acid (μg/g FW) ± S.D | Naringenin (μg/g FW) ± S.D | Quercetin (μg/g FW) ± S.D | Lycopene (μg/g FW) ± S.D |
| Liscio da Serbo Toscano | 18.75 ± 0.9a | 3.89 ± 0.5a | 41.23 ± 2.4a | 5.48 ± 0.9ad | 14.08 ± 2.6a | 4.65 ± 0.3a |
| Rosso Pitigliano | 17.21 ± 1.4a | 8.25 ± 1.2b | 28.26 ± 1.8b | 14.24 ± 2.1b | 27.45 ± 3.4b | 4.23 ± 0.1a |
| Quarantino ec Valdarno | 9.50 ± 0.8b | 8.56 ± 1.4b | 39.56 ± 2.9a | 4.01 ± 0.7a | 20.14 ± 2.9c | 6.12 ± 0.5b |
| Fragola | 7.45 ± 2.1c | 7.81 ± 0.9b | 20.14 ± 1.7c | 19.12 ± 1.4c | 18.01 ± 1.7c | 5.06 ± 0.3a |
| Canestrino di Lucca | 8.14 ± 0.8b | 3.01 ± 1.2a | 19.84 ± 1.9c | 6.57 ± 1.2ad | 17.12 ± 2.7c | 5.89 ± 0.6ab |
| Costoluto Fiorentino | 14.56 ± 1.7d | 8.12 ± 1.7b | 30.21 ± 2.1b | 6.14 ± 0.9ad | 9.87 ± 1.5d | 4.15 ± 0.2a |
| Giallo di Pitigliano | 8.23 ± 1.2b | 9.16 ± 1.9b | 18.54 ± 0.7c | 8.14 ± 1.9d | 10.48 ± 2.7d | 4.58 ± 0.5a |
| Pisanello | 19.54 ± 1.4a | 2.28 ± 0.9a | 16.02 ± 2.4c | 3.89 ± 0.4a | 10.47 ± 2.8d | 6.21 ± 0.7b |
| Cuore di Bue (Commercial) | 5.14 ± 1.2e | 0.98 ± 0.8c | 13.24 ± 1.8d | 2.01 ± 0.2e | 6.48 ± 0.7e | 4.02 ± 0.1a |
| Onions (Allium cepa L.) | Quercetin (μg/g FW) ± S.D | Myricetin (μg/g FW) ± S.D | Kaempferol (μg/g FW) ± S.D | Peonidin-3-glucoside (μg/g FW) ± S.D | Petunidin-3-glucoside (μg/g FW) ± S.D | |
| Maremma | 29.59 ± 0.6a | 30.59 ± 7.6a | 216.58 ± 8.4a | 9.12 ± 0.4a | 5.12 ± 1.3a | |
| Rossa Massese | 583.77 ± 3.1b | 583.77 ± 10.2b | 849.14 ± 10.4b | 21.64 ± 2.3b | 15.24 ± 1.4b | |
| Treschietto | 23.54 ± 0.7c | 23.54 ± 8.1a | 305.68 ± 7.8c | 9.57 ± 0.8a | 4.91 ± 1.2a | |
| Rossa della Valtiberina | 24.26 ± 0.9c | 24.26 ± 9.6a | 304.45 ± 8.7c | 36.47 ± 1.9c | 21.45 ± 1.3c | |
| Rossa di Lucca | 183.12 ± 2.1d | 183.12 ± 8.2c | 251.06 ± 8.1d | 10.57 ± 1.1a | 7.15 ± 1.9ad | |
| Rossa Fiorentina | 22.67 ± 1.2c | 22.67 ± 6.4a | 221.08 ± 8.9a | 31.18 ± 0.7d | 20.14 ± 2.1c | |
| Tropea (Commercial 1) | 19.86 ± 2.3c | 19.86 ± 8.1a | 66.68 ± 2.4e | 10.96 ± 0.5a | 10.24 ± 2.7d | |
| Giarratana (Commercial 2) | 20.45 ± 2.5c | 20.14 ± 7.4a | 87.78 ± 5.6f | / | / | |
| Sweet cherries (Prunus avium L.) | Chlorogenic Acid (μg/g FW) ± SD | p-coumaric acid (μg/g FW) ± SD | (+)-Catechin (μg/g FW) ± SD | Rutin (μg/g FW) ± SD | Cyanidin-3-glucoside (μg/g FW) ± SD | |
| Carlotta | 121.91 ± 0.6a | 19.41 ± 02a | 25.93 ± 1.9a | 30.83 ± 1.3a | 51.78 ± 1.8a | |
| Benedetta | 81.27 ± 0.4b | 16.61 ± 0.3b | 201.54 ± 1.4b | 53.96 ± 3.4b | 33.88 ± 1.5b | |
| Morellona | 324.59 ± 0.4c | 30.05 ± 0.2c | 72.54 ± 2.3c | 38.54 ± 1.4c | 74.82 ± 1.1c | |
| Maggiola | 94.32 ± 0.2d | 12.43 ± 0.2d | 45.99 ± 1.9d | 34.46 ± 1.8c | 35.28 ± 1.3b | |
| Moscatella | 239.25 ± 01e | 10.13 ± 0.3e | 34.86 ± 2.7e | 27.93 ± 1.6a | 33.54 ± 1.6b | |
| Crognola | 387.73 ± 07f | 28.11 ± 0.3f | 163.51 ± 1.4f | 95.33 ± 2.1d | 151.2 ± 1.2d | |
| Durone (Commercial) | 78.25 ± 0.4b | 10.85 ± 0.1e | 15.85 ± 1.1g | 25.65 ± 2.1a | 36.27 ± 1.7b | |
The table also reports the HPLC quantifications of specific secondary metabolites in tomatoes, onions and sweet cherries expressed as μg of component per gram of FW. Values are indicated with the relative standard deviation; different letters refer to statistically significant differences (p < 0.05).
Figure 1.
Schematic cartoon depicting the potential valorization route of local ancient plant varieties.
The use of these fruits could represent the next step towards the diversification of the current staple food crop market and a functional diet valorizing the local agrobiodiversity (Cantini et al., 2018). Additionally, such varieties could be the object of high-throughput studies based on—omics to uncover the bioactive molecules produced and to test their nutraceutical and health-promoting effects (Figure 1).
Is the Cultivation of Local Plants An Economic Resource?
Assuming the importance to take functional molecules with the diet, there are contrasting aspects that sometimes make vain the scientific interest on these molecules. One example is the input for the local population to consume local products in opposition to mass-merchandised products, which is often more a cultural than an economic issue. The consumption of products from the local agriculture might be perceived as a return to “old-fashioned” agriculture that is considered less productive and less profitable. Therefore, the consumption of local products is often seen by consumers as the return to an “ancient” agriculture. This may be more a problem for younger generations than older ones. The new generations have had different cultural backgrounds in which nutrition was not seen as a way to improve health, but essentially as a way to simply take on calories (Popkin et al., 2012). Not going too far into the basic rhetoric about fast-food, this different perception has greatly contributed to affect the trends in agriculture. The consumption of products from the local agriculture also necessarily requires the cultivation of local plants on a large scale. The problem then shifts from the consumer to the farmer and the question is: how ready are farmers to grow local plants instead of more traditional and therefore more commercial ones? This is a very serious question because it implies rethinking on the production yield of these plants. Obviously, no farmer prefers to earn less and it is therefore clear that locally adapted plants must be as productive in economic terms as more traditional ones (Reganold and Wachter, 2016). So far, few researches have been focused on the differences in biodiversity and on the economic benefits between unconventional and conventional crops.
Tuscany has been particularly active in recent years by promoting research and transfer activities in the agro-food sector (Berni et al., 2018a). Through a series of financially-supported projects, Tuscany has actively promoted and supported the research on and the cultivation of autochthonous species rich in functional molecules. An emblematic output of these projects is the manufacture of dark chocolate bars (Toscolata®) functionalized with Tuscan autochthonous food products (Cantini et al., 2018).
In particular, we hereby wish to mention two projects to which we have recently participated: the first concerns the genetic, qualitative and sustainability characterization of products and derivatives from autochthonous Tuscan horticultural crops (BASIQ project: http://www.valdimersegreen.com/basiq/). The second project is about the use of different cultivation practices of wheat to see how it might affect the content of bioactive molecules (INNOVACEREALI project: http://innovacereali.maidicolasovicille.it/).
The Cultivation of Native Plants Preserves the Local Agrobiodiversity
This Perspective paper on the importance of cultivating native species, albeit limited to a specific region of Central Italy, can be enriched by one further final consideration: the cultivation of native plants cannot only preserve the local agrobiodiversity (Berni et al., 2018a), but also contribute to the restoration of original regional habitats. Nowadays, the loss of habitat diversification is a major threat linked to the ever-growing industrialization, climatic changes and land-use. Future forecasts highlight the dramatic scenarios of habitat loss with the increasing use of wild lands for the massive cultivation of man-selected plants (Pereira et al., 2010). Landscape and plant biodiversity are directly linked: the loss of one involves the loss of the other. The cultivation of native crops can play an important role in the preservation of both, while more traditional agricultural practices, especially if intense, can have negative consequences because of the massive use of land resources to promote large-scale products. Therefore, a well-conceived agricultural management, based on the regional valorization of autochthonous species, has been shown to preserve biodiversity and local ecosystems (Tscharntke et al., 2005). Native plants are also useful to maintain the local soil microbiota and composition, thereby preserving an optimal interaction between plants and microorganisms. Lange and colleagues reported the importance of plant diversity in the microbial soil composition with indirect effects also on C storage of the whole ecosystem (Lange et al., 2015).
Based on these evidences, the use of native crops should not aim at massive agricultural production, but rather at maintaining and restoring habitats in regional “niches.” From this perspective, the cultivation of local plants has both an economically- and ecologically-relevant impact.
Author Contributions
RB, GG, and MR wrote the manuscript and prepared the figures. J-FH, CC, and GC revised the text.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the Tuscany Region and the National Research Council (CNR-Italy) for support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01983/full#supplementary-material
References
- Ancillotti C., Ciofi L., Pucci D., Sagona E., Giordani E., Biricolti S., et al. (2016). Polyphenolic profiles and antioxidant and antiradical activity of Italian berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. subsp. gaultherioides (Bigelow) SB Young. Food Chem. 204, 176–184. 10.1016/j.foodchem.2016.02.106 [DOI] [PubMed] [Google Scholar]
- Andre C. M., Larsen L., Burgess E. J., Jensen D. J., Cooney J. M., Evers D., et al. (2013). Unusual immuno-modulatory triterpene-caffeates in the skins of russeted varieties of apples and pears. J. Agric. Food Chem. 61, 2773–2779. 10.1021/jf305190e [DOI] [PubMed] [Google Scholar]
- Aramwit P., Bang N., Srichana T. (2010). The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 43, 1093–1097. 10.1016/j.foodres.2010.01.022 [DOI] [Google Scholar]
- Azmir J., Zaidul I. S. M., Rahman M. M., Sharif K. M., Mohamed A., Sahena F., et al. (2013). Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Eng. 117, 426–436. 10.1016/j.jfoodeng.2013.01.014 [DOI] [Google Scholar]
- Baulcombe D. C., Dean C. (2014). Epigenetic regulation in plant responses to the environment. Cold Spring Harb. Perspect. Biol. 6:a019471. 10.1101/cshperspect.a019471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni R., Cantini C., Romi M., Hausman J. F., Guerriero G., Cai G. (2018a). Agrobiotechnology goes wild: ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 19:2248 10.3390/ijms19082248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni R., Luyckx M., Xu X., Legay S., Sergeant K., Hausman J. F., et al. (2018b). Reactive Oxygen Species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 10.1016/j.envexpbot.2018.10.017. [Epub ahead of print]. [DOI] [Google Scholar]
- Berni R., Romi M., Parrotta L., Cai G., Cantini C. (2018c). Ancient Tomato (Solanum lycopersicum L.) varieties of tuscany have high contents of bioactive compounds. Horticulturae 4:51 10.3390/horticulturae4040051 [DOI] [Google Scholar]
- Bvenura C., Afolayan A. J. (2015). The role of wild vegetables in household food security in South Africa: a review. Food Res. Int. 76, 1001–1011. 10.1016/j.foodres.2015.06.013 [DOI] [Google Scholar]
- Cantini C., Salusti P., Romi M., Francini A., Sebastiani L. (2018). Sensory profiling and consumer acceptability of new dark cocoa bars containing Tuscan autochthonous food products. Food Sci. Nutr. 6, 245–252. 10.1002/fsn3.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso G., Conti S., Villari G., Borrelli C., Melchionna G., Minutolo M., et al. (2014). Effects of transplanting time and plant density on yield, quality and antioxidant content of onion (Allium cepa L.) in southern Italy. Sci. Hortic. 166, 111–120. 10.1016/j.scienta.2013.12.019 [DOI] [Google Scholar]
- Cavallini G., Dachà M., Potenza L., Ranieri A., Scattino C., Castagna A., et al. (2014). Use of red blood cell membranes to evaluate the antioxidant potential of plant extracts. Plant Foods Hum. Nutr. 69, 108–114. 10.1007/s11130-014-0414-0 [DOI] [PubMed] [Google Scholar]
- Cencic A., Chingwaru W. (2010). The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2, 611–625. 10.3390/nu2060611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft B. D., Kerrihard A. L., Amarowicz R., Pegg R. B. (2012). Phenol-based antioxidants and the in vitro methods used for their assessment. Compr. Rev. Food Sci. Food Saf. 11, 148–173. 10.1111/j.1541-4337.2011.00173.x [DOI] [Google Scholar]
- Dai J., Mumper R. J. (2010). Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352. 10.3390/molecules15107313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferioli F., D'Antuono L. F. (2016). Evaluation of phenolics and cysteine sulfoxides in local onion and shallot germplasm from Italy and Ukraine. Genet. Resour. Crop Evol. 63, 601–614. [Google Scholar]
- Francini A., Romeo S., Cifelli M., Gori D., Domenici V., Sebastiani L. (2017). 1H NMR and PCA-based analysis revealed variety dependent changes in phenolic contents of apple fruit after drying. Food Chem. 221, 1206–1213. 10.1016/j.foodchem.2016.11.038 [DOI] [PubMed] [Google Scholar]
- García-Valverde V., Navarro-González I., García-Alonso J., Periago M. J. (2013). Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food Bioprocess Technol. 6, 391–402. 10.1007/s11947-011-0687-3 [DOI] [Google Scholar]
- Ghasemzadeh A., Jaafar H. Z., Rahmat A. (2010). Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 15, 4324–4333. 10.3390/molecules15064324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini L., Pinti M., Nasi M., Montagna J. P., De Biasi S., Roat E., et al. (2011). Quercetin and cancer chemoprevention. Evid. Based Complement. Alternat. Med. 2011. 10.1093/ecam/neq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. S., Vanderjagt D. J., Chuang L. T., Huang Y. S., Millson M., Glew R. H. (2005). Nutrient content of four edible wild plants from West Africa. Plant Foods Hum. Nutr. 60, 187–193. 10.1007/s11130-005-8616-0 [DOI] [PubMed] [Google Scholar]
- Goto T., Takahashi N., Hirai S., Kawada T. (2010). Various terpenoids derived from herbal and dietary plants function as PPAR modulators and regulate carbohydrate and lipid metabolism. PPAR Res. 2010:483958 10.1155/2010/483958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero G., Berni R., Muñoz-Sanchez J., Apone F., Abdel-Salam E., Qahtan A., et al. (2018). Production of plant secondary metabolites: examples, tips and suggestions for biotechnologists. Genes 9:309. 10.3390/genes9060309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Giusti M. M. (2010). Anthocyanins: natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 1, 163–187. 10.1146/annurev.food.080708.100754 [DOI] [PubMed] [Google Scholar]
- Hortelano S. (2009). Molecular basis of the anti-inflammatory effects of terpenoids. Inflamm. Allergy-Drug Targets Former. Curr. Drug Targets-Inflamm. Allergy 8, 28–39. 10.2174/187152809787582534 [DOI] [PubMed] [Google Scholar]
- Iacopini P., Baldi M., Storchi P., Sebastiani L. (2008). Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 21, 589–598. 10.1016/j.jfca.2008.03.011 [DOI] [Google Scholar]
- Iacopini P., Camangi F., Stefani A., Sebastiani L. (2010). Antiradical potential of ancient Italian apple varieties of Malus x domestica Borkh. in a peroxynitrite-induced oxidative process. J. Food Compos. Anal. 23, 518–524. 10.1016/j.jfca.2009.05.004 [DOI] [Google Scholar]
- Jagtap U. B., Panaskar S. N., Bapat V. A. (2010). Evaluation of antioxidant capacity and phenol content in jackfruit (Artocarpus heterophyllus Lam.) fruit pulp. Plant Foods Hum. Nutr. 65, 99–104. 10.1007/s11130-010-0155-7 [DOI] [PubMed] [Google Scholar]
- Kabera J. N., Semana E., Mussa A. R., He X. (2014). Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2, 377–392. [Google Scholar]
- Kelebek H., Selli S. (2011). Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars. Int. J. Food Sci. Technol. 46, 2530–2537. 10.1111/j.1365-2621.2011.02777.x [DOI] [Google Scholar]
- King G. J. (2015). Crop epigenetics and the molecular hardware of genotype x environment interactions. Front. Plant Sci. 6:968. 10.3389/fpls.2015.00968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku C. M., Lin J. Y. (2013). Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 141, 1104–1113. 10.1016/j.foodchem.2013.04.044 [DOI] [PubMed] [Google Scholar]
- Lamien-Meda A., Lamien C. E., Compaoré M. M., Meda R. N., Kiendrebeogo M., Zeba B., et al. (2008). Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 13, 581–594. 10.3390/molecules13030581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M., Eisenhauer N., Sierra C. A., Bessler H., Engels C., Griffiths R. I., et al. (2015). Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 6, 6707. 10.1038/ncomms7707 [DOI] [PubMed] [Google Scholar]
- Legay S., Cocco E., André C. M., Guignard C., Hausman J. F., Guerriero G. (2017). Differential lipid composition and gene expression in the semi-russeted “Cox Orange Pippin” apple variety. Front. Plant Sci. 8:1656 10.3389/fpls.2017.01656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay S., Guerriero G., Deleruelle A., Lateur M., Evers D., André C. M., et al. (2015). Apple russeting as seen through the RNA-seq lens: strong alterations in the exocarp cell wall. Plant Mol. Biol. 88, 21–40. 10.1007/s11103-015-0303-4. [DOI] [PubMed] [Google Scholar]
- Mangani S., Buscioni G., Collina L., Bocci E., Vincenzini M. (2011). Effects of microbial populations on anthocyanin profile of Sangiovese wines produced in Tuscany, Italy. Am. J. Enol. Vitic. 10.5344/ajev.2011.11047 [DOI] [Google Scholar]
- Mirto A., Iannuzzi F., Carillo P., Ciarmiello L. F., Woodrow P., Fuggi A. (2018). Metabolic characterization and antioxidant activity in sweet cherry (Prunus avium L.) Campania accessions: metabolic characterization of sweet cherry accessions. Food Chem. 240, 559–566. 10.1016/j.foodchem.2017.07.162 [DOI] [PubMed] [Google Scholar]
- Nasir M. U., Hussain S., Jabbar S. (2015). Tomato processing, lycopene and health benefits: a review. Sci. Lett. 3, 1–5. [Google Scholar]
- Naziri D. (2009). Direct sale as a means for promoting the sustainable use of plant genetic resources: the case of the tuscany region. J. Agric. Environ. Int. Dev. 103, 65–80. 10.12895/jaeid.20091/2.25 [DOI] [Google Scholar]
- Norberto S., Silva S., Meireles M., Faria A., Pintado M., Calhau C. (2013). Blueberry anthocyanins in health promotion: a metabolic overview. J. Funct. Foods 5, 1518–1528. 10.1016/j.jff.2013.08.015 [DOI] [Google Scholar]
- Pandey K. B., Rizvi S. I. (2009). Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2, 270–278. 10.4161/oxim.2.5.9498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H. M., Leadley P. W., Proença V., Alkemade R., Scharlemann J. P., Fernandez-Manjarrés J. F., et al. (2010). Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501. 10.1126/science.1196624 [DOI] [PubMed] [Google Scholar]
- Pérez-Gregorio R. M., García-Falcón M. S., Simal-Gándara J., Rodrigues A. S., Almeida D. P. (2010). Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. J. Food Compos. Anal. 23, 592–598. 10.1016/j.jfca.2009.08.013 [DOI] [Google Scholar]
- Popkin B. M., Adair L. S., Ng S. W. (2012). Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 70, 3–21. 10.1111/j.1753-4887.2011.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reganold J. P., Wachter J. M. (2016). Organic agriculture in the twenty-first century. Nat. Plants 2 :15221. 10.1038/nplants.2015.221 [DOI] [PubMed] [Google Scholar]
- Renna M., Durante M., Gonnella M., Buttaro D., D'Imperio M., Mita G., et al. (2018). Quality and nutritional evaluation of regina tomato, a traditional long-storage landrace of puglia (Southern Italy). Agriculture 8:83 10.3390/agriculture8060083 [DOI] [Google Scholar]
- Renna M., Serio F., Signore A., Santamaria P. (2014). The yellow–purple Polignano carrot (Daucus carota L.): a multicoloured landrace from the Puglia region (Southern Italy) at risk of genetic erosion. Genet. Resour. Crop Evol. 61, 1611–1619. 10.1007/s10722-014-0155-9 [DOI] [Google Scholar]
- Saini R. K., Nile S. H., Park S. W. (2015). Carotenoids from fruits and vegetables: chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 76, 735–750. 10.1016/j.foodres.2015.07.047 [DOI] [PubMed] [Google Scholar]
- Sardaro M. L., Marmiroli M., Maestri E., Marmiroli N. (2013). Genetic characterization of Italian tomato varieties and their traceability in tomato food products. Food Sci. Nutr. 1, 54–62. 10.1002/fsn3.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo J., Politi A., Pellegrini N., Mezzetti B., Battino M. (2005). Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 21, 207–213. 10.1016/j.nut.2004.03.025 [DOI] [PubMed] [Google Scholar]
- Schmidt M. R., Wei W. (2006). Loss of agro-biodiversity, uncertainty, and perceived control: a comparative risk perception study in Austria and China. Risk Anal. Int. J. 26, 455–470. 10.1111/j.1539-6924.2006.00744.x [DOI] [PubMed] [Google Scholar]
- Stintzing F. C., Carle R. (2004). Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 15, 19–38. 10.1016/j.tifs.2003.07.004 [DOI] [Google Scholar]
- Thoppil R. J., Bishayee A. (2011). Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 3:228. 10.4254/wjh.v3.i9.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao R. (2010). Chemistry and biochemistry of dietary polyphenols. Nutrients 2, 1231–1246. 10.3390/nu2121231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharntke T., Klein A. M., Kruess A., Steffan-Dewenter I., Thies C. (2005). Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol. Lett. 8, 857–874. 10.1111/j.1461-0248.2005.00782.x [DOI] [Google Scholar]
- Wallace T. C. (2011). Anthocyanins in cardiovascular disease. Adv. Nutr. 2, 1–7. 10.3945/an.110.000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson G. (2017). The role of polyphenols in modern nutrition. Nutr. Bull. 42, 226–235. 10.1111/nbu.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.