Abstract
Hematopoietic stem/progenitor cells (HSPC) in zebrafish emerge from the aortic hemogenic endothelium (HE) and migrate towards the caudal hematopoietic tissue (CHT), where they expand and differentiate during definitive hematopoiesis. Phospholipase C gamma 1 (Plcγ1) has been implicated for hematopoiesis in vivo and in vitro and is also required to drive arterial and HSPC formation. Genetic mutation in plcg1−/− (y10 allele) completely disrupts the aortic blood flow, specification of arterial fate, and HSPC formation in zebrafish embryos. We previously demonstrated that ginger treatment promoted definitive hematopoiesis via Bmp signaling. In this paper, we focus on HSPC development in plcg1−/− mutants and show that ginger/10-gingerol (10-G) can rescue the expression of arterial and HSPC markers in the HE and CHT in plcg1−/− mutant embryos. We demonstrate that ginger can induce scl/runx1 expression, and that rescued HE fate is dependent on Bmp and Notch. Bmp and Notch are known to regulate nitric oxide (NO) production and NO can induce hematopoietic stem cell fate. We show that ginger produces a robust up-regulation of NO. Taken together, we suggest in this paper that Bmp, Notch and NO are potential players that mediate the effect of ginger/10-G for rescuing the genetic defects in blood vessel specification and HSPC formation in plcg1−/− mutants. Understanding the molecular mechanisms of HSPC development in vivo is critical for understanding HSPC expansion, which will have a positive impact in regenerative medicine.
Introduction
During vascular development, endothelial progenitors give rise to a network of blood vessels including arteries and veins. Arterial specification, differentiation and morphogenesis are orchestrated by evolutionarily conserved signaling pathways including vascular endothelial growth factor (Vegf), Notch and ephrinB21,2. The establishment of arterial identity is also a prerequisite for the emergence of definitive hematopoietic stem/progenitor cells (HSPC). Therefore, it is imperative to understand the role of critical genes in the differentiation and specification of arteries and the development of definitive HSPCs. Phospholipase C gamma 1 (Plcγ1) function is required downstream of Vegf receptors (Vegfr1 and Vegfr2) to drive arterial specification and HSPC development during vertebrate embryogenesis3,4. Plcγ1 has been implicated for hematopoiesis in vivo and differentiation of embryonic stem cells into erythrocytes and monocytes/macrophages in vitro5 for classical T-cell receptor signaling and T-cell activation6, and for early B-cell development7. In the developing embryo, expression of Vegf-a is induced by sonic hedgehog (Shh) to establish midline-to-lateral and dorsal-ventral gradients from the hypochord and the ventral part of somites; thus, axial angioblasts are patterned to adopt the arterial fate, while lateral angioblasts receiving a smaller amount of Vegf stimulation become endothelial cells (ECs) with a venous identity8. In addition, a strong gradient of Vegf-a binds to Vegf- and neuropilin- coreceptor complexes (Vegfr2-Nrp1) of arterial differentiating ECs, mediated through Plcγ1 to activate the Raf1-Mek-Erk cascade. As a result, Vegf-a activates Notch signaling to promote arterial cell fate via ephrinB2. Notch signaling is required for the leading endothelial tip cells’ migration and the connection to the pre-existing artery to form a functional vascular network9. On the other hand, a lower gradient of Vegf-a promotes venous EC differentiation. The venous signal activates the PI3K-Akt1 pathway which inhibits the Plcγ1-induced Raf1 signaling and promotes expression of venous markers such as ephrinB4, or COUP-TFII, which also inhibits ephrinB2 expression10,11. Arterial-venous identity is specified when hemodynamic force of blood flow is being established; interestingly, manipulation of blood flow could override the genetic programming of arterial-venous specification and interchange arteries and veins12.
Hematopoiesis and vasculogenesis are closely linked to each other. As in mammals, hematopoiesis in zebrafish is characterized by two waves, primitive and definitive hematopoiesis. Definitive HSPCs arise along the ventral wall of the dorsal aorta (DA) from the presumptive hemogenic endothelium (HE), undergoing endothelial-to-hematopoietic transition (EHT). During definitive hematopoiesis, zebrafish HSPCs undergo EHT around 30 to 35 hour-post-fertilization (hpf)13 from the luminal side of the dorsal aorta. These cells directly emerge from aortic endothelium13,14 and migrate through the sub-aortic space13 to the caudal hematopoietic tissue (CHT). Some of these cells could enter the circulation through the axial vein, then extravasate at widely diverse locations and migrate through the mesenchyme to reach the thymus15. Others could migrate from the CHT to kidney marrow, before matured cells go into circulation13. Scl was identified as a marker of the HE during the EHT process16–18. The emerging HSPCs express scl, myb and runx1 and enter the circulation to home transiently to the CHT, where they could multiply and differentiate from 2 to 7 days-post-fertilization (dpf), prior to seeding their permanent hematopoietic organs19,20. Like other stem cell niches, the CHT is associated with a vascular bed, the caudal vascular plexus (CVP), characterized by large sinusoids in which the reduced flow of blood progenitors helps the homing process at the CHT20. The CVP also provides a microenvironment for interaction of the developing HSPCs with secreted factors and cytokines necessary for the HSPCs to be instructed and to differentiate15,21,22. In this hematopoietic microenvironment, HSPCs undergo extensive proliferation and further migrate to seed the definitive hematopoietic organs, the thymus and kidney marrow, giving rise to many blood lineages20,23. Therefore, understanding the molecular mechanisms of HSPC development in vivo is critical for HSPCs expansion, which will have a positive impact in regenerative medicine.
Bmp signaling acts specifically on the definitive hematopoietic program to induce HSPC emergence within the HE of the DA24. Scl is required for the development of the DA16,25 and promotes EHT in the HE downstream of Shh and Notch, and up-stream of Runx18. Yet, Scl and Myb play important roles in EHT and migration of HSPCs to the CHT26,27, and Notch is required for arterial specification28. plcg1−/− zebrafish embryos are defective in both arterial morphogenesis and HE specification3. This mutant provides the opportunity to investigate the relationship of hemogenic endothelium and HSPC development.
Based on the fact that the EHT process is dependent on Notch13,14,29–31, both Bmp and Notch can regulate NO production and play potential roles in enhancing hematopoiesis32. Thus, we ask if the effect of ginger on induced scl/runx1 expression for the rescued HE fate is dependent on Bmp and/or Notch. We also investigate whether NO plays any role in the rescue of the HSPC fate in plcg1−/− zebrafish embryos. Here, we show that ginger treatment can rescue the deficiencies of plcg1−/− zebrafish embryos in HE and HSPC development, and partially restores the blood circulation. We also demonstrate that ginger can dramatically increase the production of NO independent of nitric oxide synthase (Nos) functions. Using a NO scavenger, we show that ginger-induced NO production is required for rescuing the expression of arterial and HSPC markers and blood circulation.
In summary, ginger/10-G treatment can induce NO and potential players in Bmp, Notch and NO pathways, which are required for the rescue of the genetic defects in blood vessel specification and HSPC formation in plcg1−/− zebrafish embryos, thus providing a foundation for future study of signaling crosstalk for promoting hematopoiesis.
Results
Ginger rescues the DA and HSPC in plcg1−/− embryos
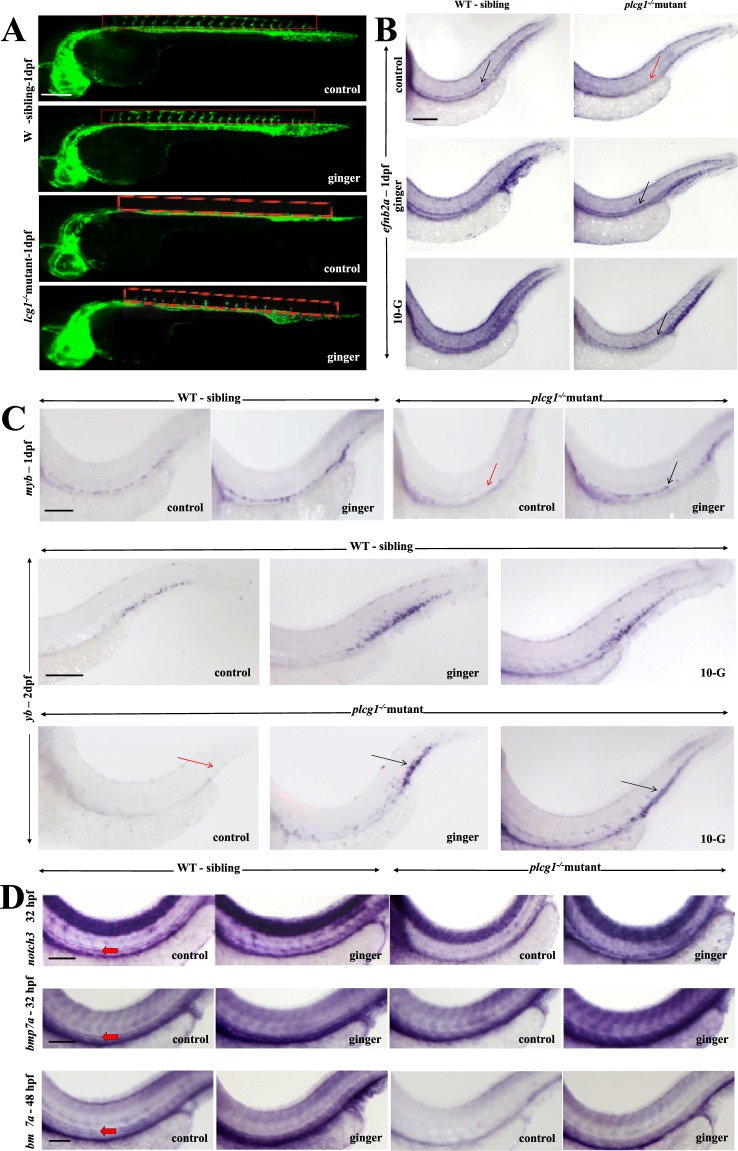
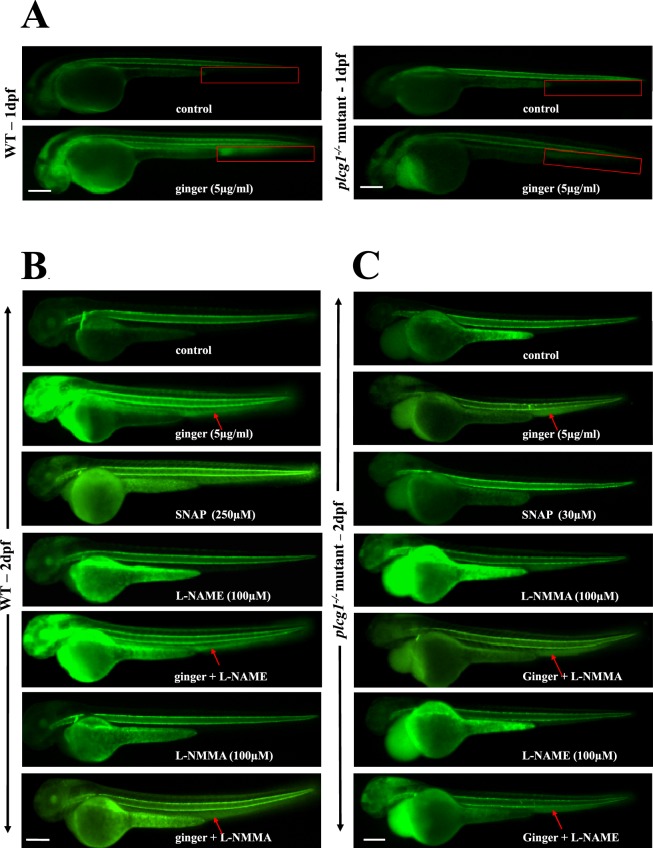
Zebrafish with the plcg1−/− recessive mutation showed impaired arterial-venous vasculogenesis3 and cardiac ventricular contractility33. Similar to dead beat33 (another lethal allele of plcg1), here, the y10 allele3 homozygous mutation completely abolished Plcγ1 function, resulting in the absence of arteries, HSPCs and blood circulation3,34. No arterial-venous specification is found in plcg1−/− embryos, with these embryos harboring only a single axial cord with no lumen at 1dpf (Fig. 1A). In vertebrates, Plcγ1 controls arterial morphogenesis downstream of Vegf signaling3. We use the transgenic and mutant zebrafish line tg(fli1:EGFP);plcg1−/− (y10 allele)3 to visualize the developing vasculature, sort homozygous mutants from their wildtype (WT) siblings (Fig. 1A), and study the effect of ginger/10-G on their compromised definitive hematopoiesis. Surprisingly, real-time observation of the fluorescent vessels reveals a partial rescue (intersegmental vessel, ISV formation in 17.5% embryos) of the vasculature in arterial-venous morphogenesis at 1dpf by ginger/10-G treatment (Fig. 1A). This is done by exposure of plcg1−/− embryos to ginger (or its active compound 10-G) from the late gastrulation (9–10 hpf) stage onward35. This also reveals an increase of vasculature in the CHT region in ginger-treated WT sibling embryos. Ginger (83%) and 10-G (89.5%) rescue the formation of the DA at 32 hpf, as shown by the restored expression of the arterial marker efnb2a, which is not expressed in plcg1−/− embryos (Fig. 1B). Circulation is also partially rescued in some of the plcg1−/− mutants (17.5%) at 2 dpf (data not shown). This also rescues the expression of the HSPC marker myb along the aortic HE, and later in the CHT at 2 dpf stage (Fig. 1C) of mutants, suggesting the rescue of definitive hematopoiesis. We choose two different timings using the myb marker because around 1 dpf, the myb + cells originally emerge along the DA, and around 2 dpf, they migrate to the CHT region. Notch signaling is necessary for arterial cell-fate specification during vasculogenesis as part of the Vegf-Notch-ephrinB2a signaling cascade and for HSPC specification in the HE36. In situ hybridization analysis confirms the rescue of arterial identity in plcg1−/− embryos treated with ginger by using the arterial marker notch3 which is absent in the mutants (Fig. 1D). Further supporting the above finding, we demonstrate that bmp7a is also expressed in the DA of WT siblings at 1–2 dpf stage (but not in plcg1−/− embryos); bmp7a expression is also rescued in the restored DA of ginger-treated plcg1−/−embryos (Fig. 1D).
Figure 1.
Ginger rescues the dorsal aorta (DA) and the formation of hematopoietic stem/progenitor cells (HSPCs) in plcg1−/− embryos. (A) Real-time imaging of the vasculature in tg(fli1:GFP) embryos at 30 hpf. Red rectangle shows the location of ISV. (B) In situ hybridization of the DA marker ephrin-B2a (efnb2a) at 1dpf (32 hpf). Black arrow indicates the artery, red arrow shows absence of artery in mutant fish. (C) In situ hybridization of the HSPC marker myb at 1 (32 hpf) vs 2dpf (54hpf). Black arrow points to myb expression in hemogenic endothelium (1 dpf) and CHT region (2 dpf), red arrow indicates absence of expression in mutant fish. (D) In situ hybridization of notch3 (normally expressed in the DA at 1 dpf (32 hpf)) and bmp7a (shown here for the first time to be expressed in the DA from 1(32 hpf) to 2 dpf (48 hpf)). These marker expressions are lost in plcg1−/− embryos and rescued following exposure to ginger. Red arrow points to DA. Scale bars = 250 μm.
These data indicate a robust effect of ginger in rescuing the plcg1−/− embryos in specification of arterial fate and HSPC formation; however, ginger treatment is not sufficient to rescue the mutant embryos from a severe heart dysfunction. On the other hand, there is no significant change in the expression of the vein marker fms-related tyrosine kinase 4 (flt4) in ginger-treated plcg1−/− embryos; however, a rescue of lumenization of the cardinal vein is observed (Supplemental Fig. S1).
Bmp and Notch signaling in ginger-induced arteriogenesis and HSPC formation
We previously demonstrated that ginger promoted definitive hematopoiesis via Bmp signaling in zebrafish embryos35. We showed that treatment with a highly selective Bmp inhibitor DMH-1, which had no effect on arterial morphogenesis, inhibited myb expression in the CHT of WT embryos. Our finding was supported by Wilkinson and colleagues’ demonstration that the arterial program was unaffected by loss of Bmp signaling, but the latter was required for HSPC emergence within the aortic HE24. Here, we show ginger can rescue both arteriogenesis (Fig. 2A,B) and hematopoiesis (Fig. 3A,B) in plcg1−/− embryos, which are dependent on an intact and functional Bmp signaling.
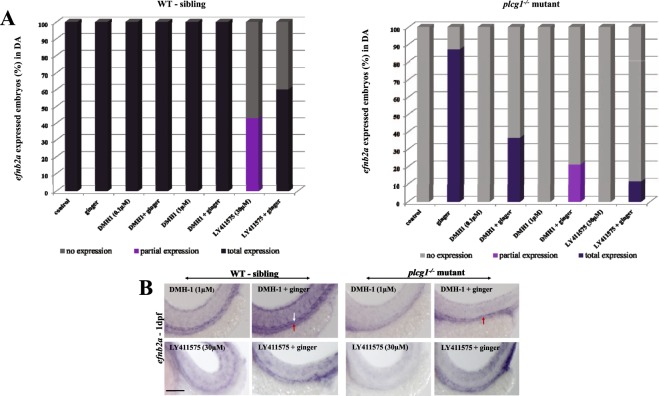
Figure 2.
Signaling pathways involved in the rescue of arteriogenesis by ginger in plcg1−/− embryos. (A) Pharmacological inhibition of Bmp (DMH-1) and Notch (LY411575) signaling pathways affect the rescue of dorsal aorta, analyzed by efnb2a in situ at 1 dpf. (B) Representative images of different treatment. White arrow indicates the dorsal aorta, underneath is the vein indicated by red arrow. Scale bars = 250 μm.
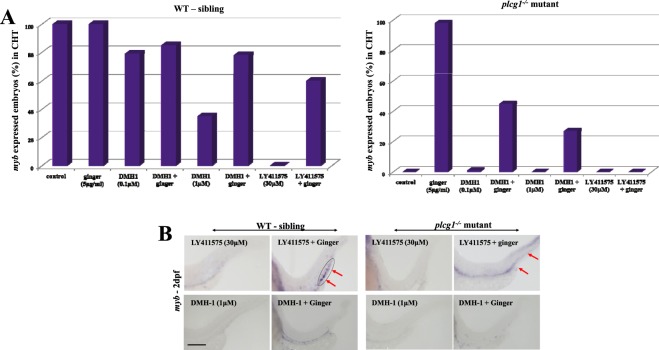
Figure 3.
Signaling pathways involved in the rescue of definitive hematopoiesis by ginger in plcg1−/− embryos. (A) Pharmacological inhibition of Bmp (DMH-1) and Notch (LY411575) signaling pathways affect the rescued HSPCs, analyzed by myb in situ at 2 dpf. (B) Representative images of different treatment. Encircled area indicates myb expression in the CHT region, whereas red arrows indicate the CHT region only. Scale bars = 250 μm.
Since the process of definitive hematopoiesis occurs later during development, we cannot use a knockdown approach such as antisense morpholino to inhibit genetic pathways in which morpholinos are normally delivered by microinjection into the 1-cell stage embryos, or a knockout approach, because both will interfere with the signaling pathways during gastrulation. Therefore, we choose a chemical-genetic approach using chemical inhibitors to modulate signaling pathways after the gastrulation stage. Inhibitors can be applied to the embryo medium at the end of gastrulation (9–10hpf) to study the signaling of ginger-induced arteriogenesis and hematopoiesis. Since Plcγ1 is already a downstream signal of the Vegf pathway for arterial and HSPCs development during vertebrate embryogenesis3, here, we intend to study other downstream players of potential interacting pathways during the rescue of HSPC formation in the plcg1−/− embryos.
Notch signaling was downstream of Shh and Vegf pathways and was essential, but not sufficient for inducing arterial identity (efnb2a)37,38, although it was crucial for HSPC generation from the HE39. Figure 2A,B shows Notch signaling is required for rescuing specification of the DA in plcg1−/− mutants after ginger treatment. For HSPCs, treating WT siblings with the γ-secretase inhibitor LY411575 (Notch inhibitor) abolishes myb expression in the CHT, whereas 60% of embryos co-exposed to LY411575 and ginger show a rescue of myb-expressing cells in their CHT. In plcg1−/− embryos, Notch inhibition abolishes the effect of ginger on the rescue of HSPC formation in the CHT (myb) (Fig. 3A,B). Taken together, Notch signal is required for the rescue effect of ginger on the DA specification and HSPC formation in the plcg1−/− mutant. These findings provide preliminary steps for future study of signaling crosstalk between ginger-induced Bmp and Notch pathways in hematopoiesis.
Ginger expands the CVP at the perivascular niche
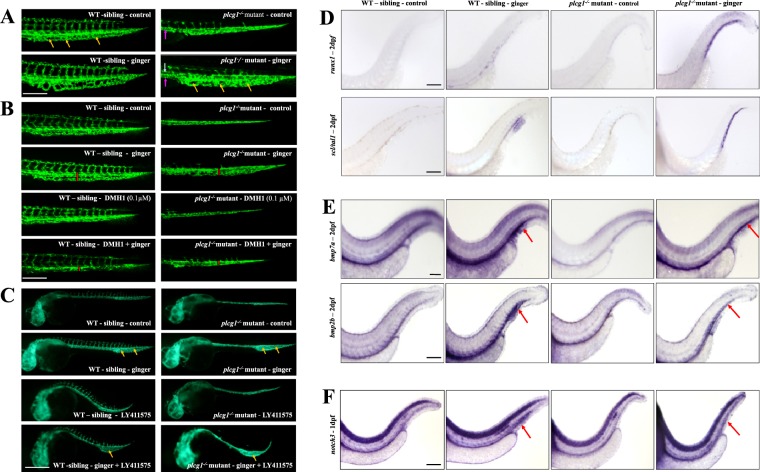
The formation of the CVP involved active sprouting, ventral migration of endothelial cells and anastomosis at 1 dpf, then maturation and lumenization of the primordial CVP to allow blood circulation at 2 dpf20,40. Treating WT siblings with ginger results in enlarged CVP sinusoids (Fig. 4A). This might help HSPC homing and docking to the CHT associated with such vascular beds. Ginger-treated plcg1−/− embryos harbor a rescued axial aorta from the trunk to the CVP region and rescued sinusoids within the CVP (Fig. 4A). These rescue effects are dependent on Bmp, when we incubate these embryos with the Bmp inhibitor DMH-1 (0.1 µM), the rescue effect of ginger is diminished (Fig. 4B).
Figure 4.
Ginger expands the Caudal Vein Plexus (CVP). (A) Ginger increases or rescues the CVP sinusoids in tg(fli1:GFP) WT siblings or plcg1−/− mutant embryos respectively (2 dpf). Yellow arrows show enlarged CVP with big sinusoid. White arrow indicates dorsal aorta and pink arrow cardinal vein. (B) Bmp is necessary for the effect of ginger on the CVP in tg(fli1:GFP) WT siblings and plcg1−/− mutant embryos (2 dpf). Vertical red bar represents the width of CVP. (C) Inhibition of Notch compromised the effect of ginger on the developing CVP at the sinusoidal level (1 dpf). Yellow arrows show enlarged CVP with big sinusoid. (D) runx1 and scl are ectopically expressed in the CVP/CHT area following ginger treatment. (E,F) Bmp7a/bmp2b expression (E) and notch3 expression (F), are highly promoted by ginger in the CVP/CHT of WT and plcg1−/−mutant embryos. Red arrow indicates high expression in CVP/CHT (E,F). Pictures are the representative images of ≥30 embryos per condition. Red box indicates the CHT region. Scale bars = 250 μm.
Notch is also an important signal for the development of the perivascular niche and the regulation of HSC emergence from the hemogenic endothelium41. Embryos exposed to the Notch inhibitor LY411575 do not develop a CVP; co-exposure to ginger reverses the effect and results in an expansion of CVP in WT siblings and mutant embryos (Fig. 4C). Since HSC fate is established by the Notch–Runx pathway39, and Notch and Scl promote the embryonic endothelial-to-hematopoietic transition17, we want to investigate the effect of ginger on scl and runx1 in the rescue of the plcg1−/− embryos. It is known that scl plays a role in the specification of the HE18, and runx1 specifically orchestrates HSPC trans-differentiation from the HE via EHT13. scl is then required for HSPC maintenance18, and myb is required for the migration of these HSPCs towards the CHT26. Here, we show there is no expression of scl and runx1 at 2 dpf in the CHT of WT siblings and plcg1−/− embryos (Fig. 4D). Interestingly, ginger treatment to WT siblings can induce a weak expression of runx1 and an over-expression of scl in the CHT (and tail fin region), while plcg1−/− mutants treated with ginger show strong ectopic expression of both scl and runx1 in the ventral tail portion, along the vascular structure of the CHT. These results suggest that ginger might have the potential to induce transcription factors like scl and runx1 to possibly activate the HE and EHT activities in the developing CHT of plcg1−/− embryos. This raises an interesting question: do the rescued embryos have the capability to utilize the CHT as a novel site for HE and HSPC development in response to ginger treatment?
We cannot directly confirm if the expanded CHT becomes a novel site for HE and HSPC development, but we do observe that ginger induces expression of bmp7a/bmp2b and notch3 in the CHT of WT siblings and mutant embryos (Fig. 4E,F). The arterial marker efnb2a and HSPC marker myb are also over-expressed in the CHT/CVP of ginger-treated embryos (Fig. 1B,C). To further confirm that ginger induces more HSPCs in the CHT, blood lineage markers are evaluated by whole-mount in situ. Indeed, erythroid (gata1), early myeloid (pu.1), neutrophil (mpo), myeloid (lcp1), and lymphoid (pan-leukocyte cd45) cell fates are all promoted by ginger (Supplemental Fig. S2).
Ginger induces nitric oxide (NO) production throughout the embryo
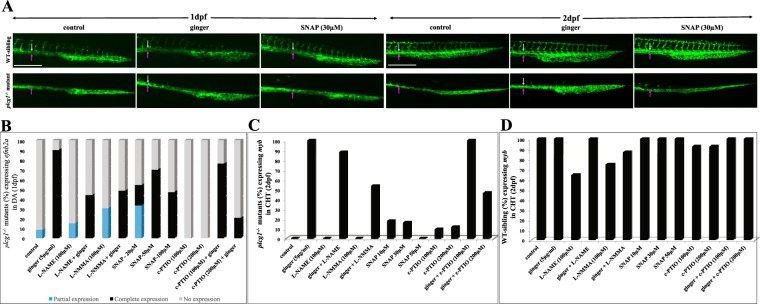
Vegf signaling can regulate NO via its downstream effectors Plcγ1 and Nos in endothelial cells. NO is a short-lived gaseous intercellular signal that regulates many important functions such as arteriogenesis, cardiovascular homeostasis, vascular tone and micro-vascular permeability. NO was required for blood flow and definitive hematopoiesis32, and was the most consistent mediator of collateral arteriogenesis in mammals42 and in zebrafish43. For NO detection in vivo, we use the diaminofluorescein-FM diacetate (DAF-FM-DA) probe, a sensitive fluorescent dye, for detecting the physiological range of NO concentrations. WT control embryos produce NO in the notochord from 1 dpf onwards (Fig. 5A) and in the cleithrum from 2 dpf (Fig. 5B), which is consistent with Lepiller et al.44. Upon exposure to ginger (from late gastrulation onwards, or following a 30-minute incubation at 2 dpf), embryos harbor a tremendous, ectopic increase of the NO levels in the heart, head, notochord, vessels/blood, and CHT, which can be completely inhibited by co-incubation with the NO scavenger c-PTIO (400 μM, 30 mins incubation) (Supplemental Fig. S3A). On the other hand, inhibition of nitric oxide synthases by L-NMMA or L-NAME shows no effect on the NO-promoting action of ginger in WT embryos (Fig. 5B). Using the NO donor SNAP as a positive control, we observe increased levels of NO in the notochord and caudal fin, but not in the head and heart. Most interestingly, ginger treatment has a greater effect on NO production than SNAP (30 uM) in the zebrafish embryos. SNAP and c-PTIO data support the finding that ginger induces NO production (Fig. 5B,C, Supplemental Fig. S3A). This effect of ginger on NO production is also observed in plcg1−/− embryos, especially in the CVP/CHT and the circulation. The Nos inhibitors have no dramatic effect on the ginger-induced increase of NO production in the CHT region (Fig. 5B,C). As an additional control, sodium nitrite (NaNO2; 1–2 mM) (Supplemental Fig. S3B) is able to induce NO levels with the same pattern as observed in WT and plcg1−/− SNAP-treated embryos (Fig. 5B,C). However, the tg(fli1:EGFP);plcg1−/− embryos in the same experimental conditions show no effect of NaNO2 on the CVP (Supplemental Fig. S3C), which look like the CVP of control plcg1−/− mutants (with no sinusoids), in contrast to the effect of ginger on these mutant embryos (Fig. 4A).
Figure 5.
Ginger increases the level of nitric oxide (NO) in non-transgenic WT and plcg1−/− mutant embryos. (A) Effect of ginger on NO staining at 1 dpf WT and plcg1−/− embryos. (B,C) Effect of NO donor (SNAP) and 2 Nos inhibitors (L-NAME, L-NMMA) on 2 dpf WT (B) and plcg1−/− embryos (C). Red arrows show ginger effect in CVP region. The effect of the NO donor SNAP is shown for comparison. Non-transgenic WT and mutant embryos are used to see the NO fluorescent dye. Pericardial edema, a typical phenotype occurred in plcg1−/− mutants at 2 dpf (C). Pictures are the representative images of ≥30 embryos per condition. Scale bars = 250 μm.
Ginger-induced NO production is essential for arteriogenesis and hematopoiesis
Co-incubation with ginger and the Nos inhibitors L-NAME or L-NMMA lead to a 50%-decrease in the number of rescued plcg1−/− mutant embryos showing expression of efnb2a in the DA; exposures to SNAP at various concentrations rescue efnb2a expression in the DA in 70% of plcg1−/− embryos (compare with 90% rescued by ginger); c-PTIO can abolish the ginger-induced rescue of DA specification (Fig. 6A,B). The rescue of defective arterial ECs in SNAP-treated plcg1−/− embryos is observed at 1 dpf stage (Fig. 6A,B). In contrast to ginger, SNAP does not rescue the NO signal in the CVP region, the morphogenesis of the CVP nor definitive HSPC proliferation at 2 dpf (Figs 5C and 6C). In the same experimental series, L-NAME slightly inhibits ginger-induced rescue of HSPCs in plcg1−/− mutants, whereas L-NMMA and c-PTIO inhibit ginger effect by about 50% (Fig. 6C). Figure 6D shows the effects of the same treatments on hematopoiesis in WT siblings: the Nos inhibitor L-NAME inhibits about 30% of the number of WT siblings with HSPCs in their CHT. This partial inhibition is reversed when co-treated with ginger, whereas L-NMMA inhibits about 25% of the number of embryos with definitive hematopoiesis (a 13.6% is reversed when co-incubated with ginger). In contrast to what is observed in plcg1−/− mutants, c-PTIO combined with ginger does not affect hematopoiesis in WT embryos (Fig. 6C,D).
Figure 6.
Effects of nitric oxide on arterial and hematopoietic gene marker expression. (A) Vasculature visualized by tg(fli1:GFP) at 1 dpf and 2 dpf embryos. (B–D) The graphical results are shown in 1 dpf (B) and 2 dpf (C,D) embryos. NO synthase inhibitors L-NAME and L-NMMA, NO donor SNAP, and NO scavenger c-PTIO. White arrow indicates dorsal aorta and pink arrow indicates cardinal vein. Scale bars = 250 μm.
In addition, Notch inhibitor (LY411575) does not reverse the increase in NO observed in the ventral tail region following ginger treatment, but it does promote the ginger-induced NO production in the CVP/CHT region (Supplemental Fig. S4A). The Bmp inhibitor DMH-1 decreases the control levels of NO production in vivo at 2 dpf, but only partially reverses the effect induced by ginger; in both cases, the slight decrease in NO is observed only in the notochord (Supplemental Fig. S4B). Consistently, Bmp inhibitor DMH-1 decreases NO production in the notochord and caudal fin, and slightly reverses ginger-induced increase of NO in the notochord and in all regions of the 3 dpf embryos (Supplemental Fig. S4C). Thus, the NO stimulating effect of ginger, which is sustained and widely distributed throughout the embryos (compared to SNAP), might be at least partially required for rescuing the defects in arteriogenesis and definitive hematopoiesis of plcg1−/− mutants.
Discussion
Development of the endothelial and hematopoietic lineages has long been considered to be (EHT) closely related processes. Back in 1917, Sabin suggested that some hematopoietic cells were derived from hematogenous yolk sac endothelial cells in the developing chick embryo45. Murray then named these multipotent mesenchyme-derived cells, common to endothelium and blood, as “hemangioblasts”46. Murray was the first to suggest that there was also a specific endothelium retaining the capacity to give rise to new blood cells. “Hemogenic endothelium” (HE) was observed in real-time in vivo using the transparent zebrafish embryo; Bertrand et al., showed the emergence of flk1+/myb+ cells from the ventral wall of the DA, as well as the presence of these markers in adult differentiated blood cells14. At the same time, Kissa and Herbomel also described the trans-differentiation of endothelial-like cells into hematopoietic precursors through a maturation process, called the “endothelial hematopoietic transition” (EHT)13. In zebrafish, some of these HE-derived definitive HSPCs could migrate to the bilateral thymi, while others transiently reside at the posterior CHT to expand before seeding the kidney marrows47. We show that ginger, a natural product, can rescue both defects of the vasculogenesis of the DA and the formation of HSPCs in the CHT in plcg1−/− mutants. These effects are dependent on the pathways involved in the control of aortic specification and definitive hematopoiesis such as Bmp/Notch/Scl/Myb. In addition, ginger treatment dramatically increases the level of NO overall, particularly in the axial vasculature, CHT, heart and brain. Blocking the activity of the Nos by inhibitors does not dramatically reduce the ginger-induced NO production (Fig. 5B,C); however, it does reduce about 50% of ginger-induced rescue of DA (Fig. 6B) and HSPCs (Fig. 6C) in plcg1−/− embryos. In contrast to the Nos inhibitors, the NO scavenger c-PTIO is able to completely abolish the effect of ginger on the induced-production of NO (Supplemental Fig. S3A). Consistent with these results, c-PTIO is also more efficient than Nos inhibitors for blocking the effect of ginger for rescuing arteriogenesis and hematopoiesis in plcg1−/− embryos (Fig. 6B,C). NO is a small lipophilic, uncharged, and short-lived molecule that can cross the plasma membranes48. Endogenous NO is formed by both enzymatic (Nos) and non-enzymatic reactions in the cardiovascular system. Nitrate and especially nitrite in the blood and extra vascular tissues could be reduced to form NO49. In normal conditions, beside Nos, other enzymes and compounds can generate NO via nitrite reductase activity. These enzymes and compounds include xanthine oxidoreductase, aldehyde oxidase, hemoglobin, deoxy-hemoglobin, myoglobin, carbonic anhydrase, mitochondrial enzymes of the transport chain, glutathione-S-transferases, cytochrome P450, carbonic anhydrase, vitamin C, antioxidants, and polyphenols50,51. Thus, the ginger-induced production of NO might be related to the ginger-induced increase in erythrocytes35, as well as to its antioxidant pathways52–54. These data are consistent with our observations and the well-known properties of ginger. Moreover, North et al., also demonstrated that NO was a conserved regulator of HSPC development and functioned downstream of Notch for arterial identity and HSPC induction32. This agrees with our data, suggesting that the ginger-induced increase in NO production could compensate the Notch signaling pathway as it pertains to arteriogenesis. Interestingly, NO is required for the ginger-induced rescue of arteriogenesis and hematopoiesis in plcg1−/− embryos, and the NO staining pattern in the notochord (immediately above the developing DA) and surrounding area around the CHT, suggesting that it could provide direct inductive signals for DA formation.
This study provides a foundation that intervention such as ginger treatment could rescue the hematopoiesis defect in plcg1−/− mutant embryos. It raises an unanswered but interesting question: can interventions such as induction by ginger/Bmp be used to manipulate a highly vascular region like CVP to become a favorable microenvironment to support the nurture or proliferation of HSPC in zebrafish, as well as the bone marrow niche in human? Future study is needed to evaluate if it is possible that the zebrafish CVP can be induced to become a secondary site for EHT for the emergence of HSPC from the endothelium, or if in vitro culture of human bone marrow cells can be manipulated to support HSPC expansion.
Our study of the rescue of genetic defects in HSPC formation may provide insight to understanding the genetic basis induced by interventions such as with ginger. It may also indicate a future path of a new experimental paradigm to regulate hematopoiesis, such as our previous study on stress erythropoiesis in hemolytic anemia model35, or in this study of a genetic model (plcg1−/−) to overcome the defect in HSPC formation. Taken together, our results provide a foundation to dissect potential novel mechanisms induced by ginger, and the future study of signaling interaction of Bmp-Notch-NO pathways to promote hematopoiesis.
Methods
Zebrafish husbandry
Zebrafish AB wildtype and transgenic lines tg(flk1:GFP); tg(fli1:EGFP) y1 allele and plcg1 y10 allele have been described previously3,36,55,56. Identified tg(fli1:EGFP) adult fish carriers of the heterozygous plcg1+/− mutation are used and embryos are staged and maintained according to standard protocols57,58 and NCCU IACUC guidelines. “plcg1−/− mutant” refers to homozygous (−/−) embryos, whereas “WT siblings” are phenotypically wildtype siblings that are heterozygous (+/−) or homozygous (+/+) embryos. WT refers to phenotypically wildtype non-transgenic embryos of AB strain. Zebrafish embryos are incubated in 0.3X Danieau’s solution (19.3 mM NaCl, 0.23 mM KCl, 0.13 mM MgSO4, 0.2 mM Ca(NO3)2, 1.7 mM HEPES, pH 7.4) at 28.5 °C; phenylthiourea (PTU, 30 µg/ml) is added around 8 hpf (75% epiboly stage) to inhibit pigment formation. Following exposure to ginger and/or pharmacological inhibitors as described, embryos are washed, dechorionated, and anaesthetized before imaging or fixing in 4% paraformaldehyde (PFA).
Treatment of embryos with ginger and small-molecule inhibitors
Ginger and 10-gingerol preparation were described previously35. Ginger extract (5 µg/ml) and 10-gingerol (2 µg/ml) are diluted in 0.3X Danieau/PTU. All inhibitors are tested in a broad range of concentrations in 0.3X Danieau/PTU. LY411575 (γ-secretase inhibitor that blocks Notch activation), DMH1 (dorsomorphin analogue; highly selective Bmp inhibitor), SB505124 (inhibitor of TGF-β type I receptors ALK4/5/7), and L-NAME (Nos inhibitor) were obtained from Sigma-Aldrich; L-NMMA (Nos inhibitor) is obtained from Calbiochem. c-PTIO (NO scavenger) and SNAP (NO donor) are obtained from Molecular Probes.
Whole-mount in situ hybridization
For experiments using plcg1 mutants, embryos are sorted under a fluorescent stereomicroscope at 1dpf and distributed into two dishes: homozygous mutants (−/−) versus wildtype (WT) siblings (+/−; +/+), according to phenotype. Paraformaldehyde-fixed embryos at indicated stages are processed for in situ hybridization as described in Leung et al.59. The following antisense RNA probes are used: scl60, runx114, myb14, efnb2a3, notch361, flt461, bmp2b62, bmp7a63, pu.114, gata164, mpo14, lcp165, and cd4565. A minimum of 30 plcg1−/− mutants embryos and 100 WT siblings per condition are used and 2–3 independent experiments are conducted per analysis. Pictures of zebrafish embryos are acquired using a Nikon SMZ1500 stereomicroscope with digital color camera DXM1200c (Nikon) and associated NIS-Element AR software.
NO labeling in vivo
Control and treated embryos are incubated for 2 hours at 28.5 °C in the dark in their culture medium containing 5 µM DAF-FM-DA (Diaminofluorescein-FM diacetate, Molecular-Probes), then washed thoroughly and imaged using a fluorescence microscope. For NO scavenger c-PTIO, treatments are performed for only 30 minutes prior to NO labeling. For positive control, NO donor SNAP (10–50 µM) are applied to bud stage (end of gastrulation around 10 hpf) onward until analysis, whereas SNAP treatment at 250 µM, 2dpf embryos are incubated for 1 hr, which are previously loaded with DAF-FM-DA.
Fluorescence microscopy
Real time imaging of embryos is performed using a MVX10 Macro-View Fluorescence Microscope (Olympus) with C9300-221 high-speed digital CCD camera (Hamamatsu). Picture acquisition parameters are kept constant to allow direct comparisons. Raw data are analyzed using MetaMorph Basic software (Olympus). Green fluorescence vasculature of tg(fli1:GFP) and NO staining are pseudo color images using MetaMorph.
Ethical approval
All experimental protocols and procedures are approved by and conformed to the guidelines of the Animal Care and Use Committee of North Carolina Central University (Durham, NC). (NCCU IACUC Protocol # TCL-07-14-2008).
Electronic supplementary material
Acknowledgements
We thank Drs. Brant Weinstein (National Institutes of Health, Bethesda, MD) and Nathan Lawson (UMass Medical School) for the transgenic tg(fli1:EGFP) and mutant plcg1 zebrafish lines; Suk-Won Jin (Yale Medical School) for the transgenic tg(flk1:GFP) zebrafish; Drs. David Traver (University of California at San Diego) and Matthias Hammerschmidt (University of Cologne, Germany) for in situ probes. We thank Ms. Xiaoyan Huang and Ms. Chunyu Xu for maintaining the zebrafish facility and Drs. Greg Cole, Faye Calhoun, and Deepti Diwan for critical comments on the manuscript. This study is supported by funding from the University of North Carolina System. T. Leung is partially supported by grants U54MD012392 from NIH/NIMHD and U54CA156735 from NIH/NCI.
Author Contributions
K.F.F.L. and T.C.L. design the zebrafish methodology, analyze and interpret the results; T.C.L., K.F.F.L. and S.S. design and interpret the ginger related experiments; K.F.F.L. and J.H. perform experiments and analyze results; K.F.F.L. prepares the draft manuscript, and J.H. and T.C.L. revise the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and supplementary figures.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36338-8.
References
- 1.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 2.Quillien A, et al. Distinct Notch signaling outputs pattern the developing arterial system. Development. 2014;141:1544–1552. doi: 10.1242/dev.099986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson ND, Mugford JW, Diamond BA, Weinstein B. M. phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–1351. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao HJ, et al. Absence of erythrogenesis and vasculogenesis in Plcg1-deficient mice. The Journal of biological chemistry. 2002;277:9335–9341. doi: 10.1074/jbc.M109955200. [DOI] [PubMed] [Google Scholar]
- 5.Shirane M, et al. Deficiency of phospholipase C-gamma1 impairs renal development and hematopoiesis. Development. 2001;128:5173–5180. doi: 10.1242/dev.128.24.5173. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, et al. 1,25(OH)2D3 induces regulatory T cell differentiation by influencing the VDR/PLC-gamma1/TGF-beta1/pathway. Molecular immunology. 2017;91:156–164. doi: 10.1016/j.molimm.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Wen R, et al. An important role of phospholipase Cgamma1 in pre-B-cell development and allelic exclusion. The EMBO journal. 2004;23:4007–4017. doi: 10.1038/sj.emboj.7600405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohli V, Schumacher JA, Desai SP, Rehn K, Sumanas S. Arterial and venous progenitors of the major axial vessels originate at distinct locations. Dev Cell. 2013;25:196–206. doi: 10.1016/j.devcel.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasan, S. S. et al. Endothelial Notch signalling limits angiogenesis via control of artery formation. Nature cell biology, 10.1038/ncb3574 (2017). [DOI] [PMC free article] [PubMed]
- 10.Ren B, et al. ERK1/2-Akt1 crosstalk regulates arteriogenesis in mice and zebrafish. J Clin Invest. 2010;120:1217–1228. doi: 10.1172/JCI39837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Y, et al. Endothelial RAF1/ERK activation regulates arterial morphogenesis. Blood. 2013;121(3988–3996):S3981–3989. doi: 10.1182/blood-2012-12-474601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.le Noble F, et al. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 13.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissa K, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111:1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 16.Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–3511. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- 17.Kim PG, et al. Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition. Proc Natl Acad Sci USA. 2013;110:E141–150. doi: 10.1073/pnas.1214361110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhen F, Lan Y, Yan B, Zhang W, Wen Z. Hemogenic endothelium specification and hematopoietic stem cell maintenance employ distinct Scl isoforms. Development. 2013;140:3977–3985. doi: 10.1242/dev.097071. [DOI] [PubMed] [Google Scholar]
- 19.Jin H, Xu J, Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109:5208–5214. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- 20.Murayama E, et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Stachura DL, et al. Clonal analysis of hematopoietic progenitor cells in the zebrafish. Blood. 2011;118:1274–1282. doi: 10.1182/blood-2011-01-331199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamplin OJ, et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160:241–252. doi: 10.1016/j.cell.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, et al. TopBP1 Governs Hematopoietic Stem/Progenitor Cells Survival in Zebrafish Definitive Hematopoiesis. PLoS Genet. 2015;11:e1005346. doi: 10.1371/journal.pgen.1005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson RN, et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bussmann J, Bakkers J, Schulte-Merker S. Early endocardial morphogenesis requires Scl/Tal1. PLoS Genet. 2007;3:e140. doi: 10.1371/journal.pgen.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Jin H, Li L, Qin FX, Wen Z. cMyb regulates hematopoietic stem/progenitor cell mobilization during zebrafish hematopoiesis. Blood. 2011;118:4093–4101. doi: 10.1182/blood-2011-03-342501. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson RN, et al. Hedgehog signaling via a calcitonin receptor-like receptor can induce arterial differentiation independently of VEGF signaling in zebrafish. Blood. 2012;120:477–488. doi: 10.1182/blood-2011-10-383729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawson ND, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 29.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 30.Rybtsov S, et al. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem cell reports. 2014;3:489–501. doi: 10.1016/j.stemcr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ditadi A, et al. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nature cell biology. 2015;17:580–591. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rottbauer W, et al. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 2005;19:1624–1634. doi: 10.1101/gad.1319405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Ferri-Lagneau KF, et al. Ginger stimulates hematopoiesis via Bmp pathway in zebrafish. PLoS One. 2012;7:e39327. doi: 10.1371/journal.pone.0039327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawson ND, Vogel AM, Weinstein B. M. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/S1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 37.Yamamizu K, Yamashita JK. Roles of cyclic adenosine monophosphate signaling in endothelial cell differentiation and arterial-venous specification during vascular development. Circ J. 2011;75:253–260. doi: 10.1253/circj.CJ-10-0915. [DOI] [PubMed] [Google Scholar]
- 38.Sacilotto N, et al. Analysis of Dll4 regulation reveals a combinatorial role for Sox and Notch in arterial development. Proc Natl Acad Sci USA. 2013;110:11893–11898. doi: 10.1073/pnas.1300805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi J, et al. Aplexone targets the HMG-CoA reductase pathway and differentially regulates arteriovenous angiogenesis. Development. 2011;138:1173–1181. doi: 10.1242/dev.054049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butko E, Pouget C, Traver D. Complex regulation of HSC emergence by the Notch signaling pathway. Dev Biol. 2016;409:129–138. doi: 10.1016/j.ydbio.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prior BM, et al. Arteriogenesis: role of nitric oxide. Endothelium. 2003;10:207–216. doi: 10.1080/10623320390246388. [DOI] [PubMed] [Google Scholar]
- 43.Gray C, et al. Ischemia is not required for arteriogenesis in zebrafish embryos. Arterioscler Thromb Vasc Biol. 2007;27:2135–2141. doi: 10.1161/ATVBAHA.107.143990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lepiller S, et al. Imaging of nitric oxide in a living vertebrate using a diamino-fluorescein probe. Free Radic Biol Med. 2007;43:619–627. doi: 10.1016/j.freeradbiomed.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 45.Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. 1917. Journal of hematotherapy & stem cell research. 2002;11:5–7. doi: 10.1089/152581602753448496. [DOI] [PubMed] [Google Scholar]
- 46.Murray P. The development in vitro of the blood of the early chick embryo. Proc R Soc Biol Sci. 1932;3:497–519. doi: 10.1098/rspb.1932.0070. [DOI] [Google Scholar]
- 47.Avagyan S, Zon LI. Fish to Learn: Insights into Blood Development and Blood Disorders from Zebrafish Hematopoiesis. Human gene therapy. 2016;27:287–294. doi: 10.1089/hum.2016.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, et al. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem. 2006;281:12546–12554. doi: 10.1074/jbc.M511803200. [DOI] [PubMed] [Google Scholar]
- 50.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 51.Rocha BS, Nunes C, Pereira C, Barbosa RM, Laranjinha J. A shortcut to wide-ranging biological actions of dietary polyphenols: modulation of the nitrate-nitrite-nitric oxide pathway in the gut. Food Funct. 2014;5:1646–1652. doi: 10.1039/c4fo00124a. [DOI] [PubMed] [Google Scholar]
- 52.Wang S, Penchala S, Prabhu S, Wang J, Huang Y. Molecular basis of traditional Chinese medicine in cancer chemoprevention. Curr Drug Discov Technol. 2010;7:67–75. doi: 10.2174/157016310791162794. [DOI] [PubMed] [Google Scholar]
- 53.Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem Biophys Res Commun. 2003;307:973–979. doi: 10.1016/S0006-291X(03)01308-1. [DOI] [PubMed] [Google Scholar]
- 54.Liu XM, et al. Nitric oxide stimulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival. Cardiovasc Res. 2007;75:381–389. doi: 10.1016/j.cardiores.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langenau DM, et al. In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc Natl Acad Sci USA. 2004;101:7369–7374. doi: 10.1073/pnas.0402248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beis D, et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132:4193–4204. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- 57.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 58.Westerfield, M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). (1993).
- 59.Leung T, et al. Zebrafish G protein gamma2 is required for VEGF signaling during angiogenesis. Blood. 2006;108:160–166. doi: 10.1182/blood-2005-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dooley KA, Davidson AJ, Zon LI. Zebrafish scl functions independently in hematopoietic and endothelial development. Dev Biol. 2005;277:522–536. doi: 10.1016/j.ydbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Herpers R, van de Kamp E, Duckers HJ, Schulte-Merker S. Redundant roles for sox7 and sox18 in arteriovenous specification in zebrafish. Circ Res. 2008;102:12–15. doi: 10.1161/CIRCRESAHA.107.166066. [DOI] [PubMed] [Google Scholar]
- 62.Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- 63.Dick A, et al. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development. 2000;127:343–354. doi: 10.1242/dev.127.2.343. [DOI] [PubMed] [Google Scholar]
- 64.Long Q, et al. GATA-1 expression pattern can be recapitulated in living transgenic zebrafish using GFP reporter gene. Development. 1997;124:4105–4111. doi: 10.1242/dev.124.20.4105. [DOI] [PubMed] [Google Scholar]
- 65.Bertrand JY, et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and supplementary figures.