Abstract
Molecular-logic based computation (MLBC) has grown by accumulating many examples of combinational logic gates and a few sequential variants. In spite of many inspirations being available in biology, there are virtually no examples of MLBC in chemistry where sequential and combinational operations are integrated. Here we report a simple alcohol-ketone redox interconversion which switches a macrocycle between a large or small cavity, with erect aromatic walls which create a deep hydrophobic space or with collapsed walls respectively. Small aromatic guests can be captured or released in an all or none manner upon chemical command. During capture, the fluorescence of the alcohol macrocycle is quenched via fluorescent photoinduced electron transfer switching, meaning that its occupancy state is self-indicated. This represents a chemically-driven RS Flip-Flop, one of whose outputs is fed into an INHIBIT gate. Processing of outputs from memory stores is seen in the injection of packaged neurotransmitters into synaptic clefts for onward neural signalling. Overall, capture-release phenomena from discrete supermolecules now have a Boolean basis.
While many processes in biological cells can be understood in terms of molecular logic gates that process information sequentially and combinationally, the design and construction of such devices in the laboratory are unknown. Here the authors achieve this by the reversibly-controlled capture and release of guest molecules from host containers.
Introduction
Molecular-logic-based computation1–23 currently largely consists of combinational logic arrays, with history-dependent sequential logic systems being less common and integrations of these two being rarer still19–21. Indeed, a molecular memory carrying a downstream processor is unknown. Nevertheless, inspiration can be found in stored neurotransmitters being released into the synaptic cleft22,23 for further neural processing of the original signal. Although molecular versions of computer memories or RS flip-flops24–32 are known, all-chemical versions where the device, inputs and outputs are chemical in nature are unavailable.
Reversible atom capture/release is well-established33–35. Molecules can also be imprisoned/liberated36–44 but it is harder to achieve in a reversible all-or-none manner without complications of side-reactions40 or incomplete conversion. On the other hand, related intramolecular studies are available as parts of molecular machines5,6,45–49.
We now present a molecular memory carrying a downstream processor. In this case, it allows us to introduce an all-chemical RS flip-flop. This permits logically controlled reversible capture/release of molecules both in aqueous solution and in the solid state. This pushes the relevance of molecular-logic-based computation1–23 to previously unaffected areas of chemistry, e.g. cleanup of pollutants, generation of chemical signals50 and programmed release of functional species from discrete supermolecules. Logical release (but not capture) from materials (but not discrete molecules) is available51. Digitally capturing/releasing a molecular guest from a molecular host due to molecular inputs shows the ability to create sequential logic devices with input–output homogeneity for gate concatenation, which is a way information is processed inside biological cells. In the present instance, the memory component is serially integrated with an INHIBIT gate whose final output is fluorescence emission. It is also remarkable that all the output states of the device are optically self-indicating. The current work also demonstrates a useful supramolecular mechanism for reversible guest capture/release in water by erecting/collapsing phenylene ring walls in a p-cyclophane by chemical redox conversions. Reduction is the set(S) input which gives the output of guest capture. Oxidation is the reset(R) input which produces the output of guest release. The ideas underlying the RS flip-flop are explained in the Discussion section below.
Results
Synthesis
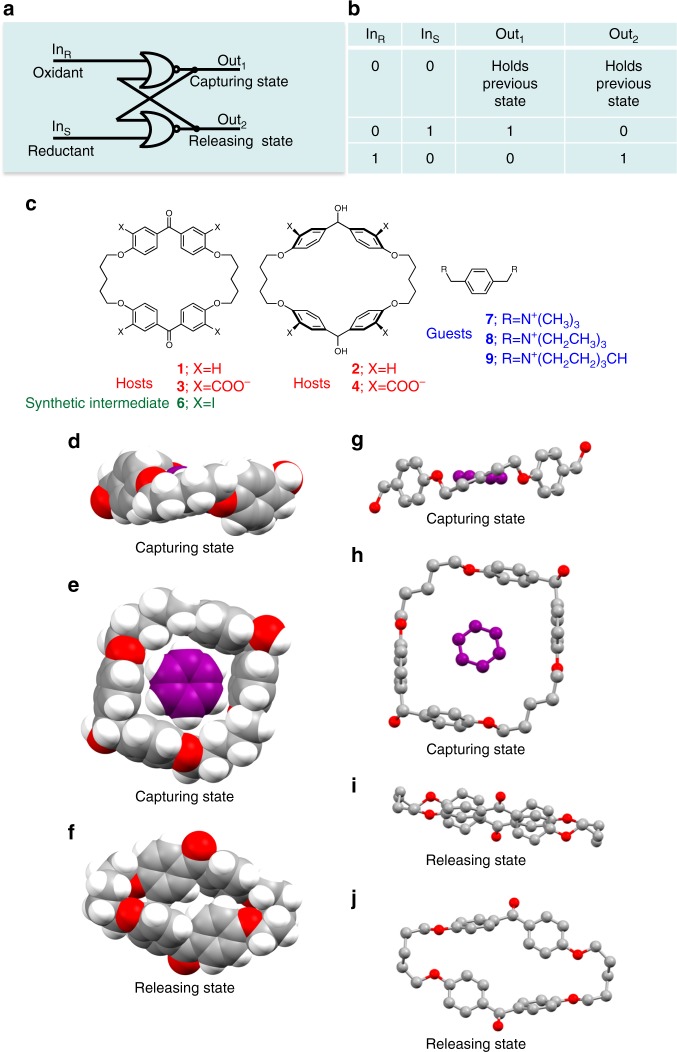
The four compounds required for the systems 1/2 and 3/4 (Fig. 1c) are synthesized as follows. Macrocyclization assisted by Cs2CO3 produces 1 from 4,4′-dihydroxybenzophenone (5) and 1,5-diiodopentane37. NaBH4 reduction52 and work-up of 1 gives 2. Importantly, 1/2 can be derivatized so that biocompatible water-soluble versions 3/4 become available. This begins with selective monoiodination at ortho-positions with respect to the oxygens of all four phenylene rings of 1 to give 6 in good yield53. Each iodo substituent is converted to a carboxylic acid moiety to give 3 in good yield by Pd0-catalyzed carbonylation54. NaBH4 reduction in water to the dialcohol tetracarboxylic acid 4 completes the synthesis (Supplementary Methods). The pair of tetracarboxylic acid derivatives 3/4 is used for aqueous solution-phase experiments at pH 10.0. The oxidation reaction (air, hot DMSO)55 converts dialcohols 2/4 to diketones 1/3. 4 is also oxidized to 3 with KMnO452 in water at 40 °C (Supplementary Methods). 1/3 and 2/4 are stable under ambient conditions (Supplementary Methods).
Fig. 1.
Memory, chemical compounds and X-ray crystallography. a Physical electronic representation of a computer memory which is conceptually related to the redox-induced reversible guest capture/release by 1/2 in the solid state. b Truth table for redox-induced reversible guest capture/release by 1/2. c Molecular structures 1–4 & 6–9 (no stereochemistry is implied). The structure numbers and group identities of hosts, guests and synthetic intermediate are shown in the arbitrary colours of red, blue and green, respectively. d–j X-ray crystal structures of 2 (d, e, g, h) and 1 (f, i, j) after separate crystallization from benzene. Ball-and-stick representation in elevation view (g, i), space-fill representation (with calculated hydrogen positions included) in plan view (e, f) and ball-and-stick representation in plan view (h, j), are shown in each case, respectively. Case d is a space-fill representation (with calculated hydrogen positions included) of 2 shown in elevation view to emphasize the ‘wall’ aspect of the host
X-ray crystallography
1/2 are switched reversibly between small and large states of their macrocycle cavity by simple chemical redox reactions. Ered for 1 is −2.3 V and Eox for 2 is +1.7 V (vs sce, MeCN, 60 °C and DMF, respectively, glassy carbon working electrode, Bu4NBF4). The large cavity captures aromatic guests, but the small cavity does not, as demonstrated by X-ray crystallography (Fig. 1d–j). Diketone 1 has the small cavity due to flattening of the phenylene rings into the macrocycle plane by π-conjugation with the carbonyl groups. The phenylene rings in dialcohol 2 have no such restrictions and stand orthogonal to the macrocycle plane to create the large cavity to accommodate the benzene guest with edge-to-face interactions.
It is clear from the X-ray crystal structures in Fig. 1d, e, g, h that dialcohol 2 has four erect alkoxyphenylene wall sections as found in classical p-cyclophanes37, where the wall sections are essentially orthogonal to the mean macrocycle plane. One molecule of benzene is symmetrically held within the macrocycle cavity, with the benzene lying in the mean macrocycle plane. The benzene has edge-face contacts56 with the four erect alkoxyphenylene wall sections, which drive the ordered benzene inclusion within 2. The 1:1 host:guest stoichiometry means that, when the host is switched to the releasing state, molecular-scale dosing occurs—a crucial feature for chemical signalling with highly active agents50.
In contrast to those concerning 2, the X-ray crystal structures in Fig. 1f, i, j show that diketone 1 has more-or-less collapsed alkoxyphenylene wall sections that are π-conjugated to the carbonyl groups to some degree. Of each pair, one alkoxyphenylene is aligned much more towards the mean macrocycle plane than the other as a result of the cross-conjugation with the carbonyl. Their orientation with respect to the mean macrocycle plane are 75.85° and 17.67°. The relative orientation of the alkoxyphenylenes and the carbonyl agrees with that of 4,4′-dimethoxybenzophenone57,58 (Supplementary Methods) showing that these angles are the result of cross-conjugation rather than sterics originating from the macrocycle. It is clear that the macrocycle cavity has undergone substantial contraction in 1 (c.f. 2) owing to the wall collapse, and no benzene occupant is found. Smaller molecules, e.g. dimethylformamide, are known to be included in some related macrocycles59,60, though in a disordered fashion59. Examination of the packing diagrams shows that benzene is found only in the internal cavity of 2 and it is completely absent from 1.
Spectroscopy in solution
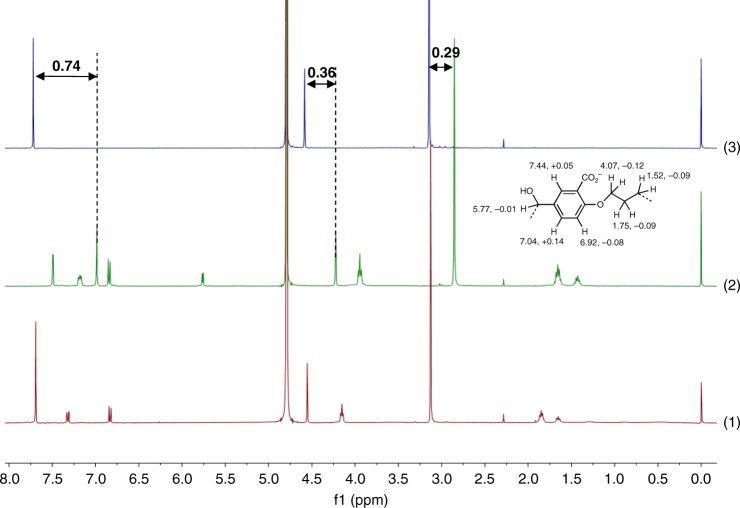
Since the X-ray crystallography studies clearly showed that the walled 2 contained one benzene occupant whereas the wall-collapsed 1 lost its occupant, we next examine aqueous solution-phase data of solubilized counterparts 4 and 3, respectively. No suitable crystals of 3 and 4 could be grown from solvents in which they were soluble. 4 and 3 are employed at levels which are below their critical aggregation concentrations (Table 1, footnote a). 7–9 (in the form of their dibromide salts) are evaluated as potential occupants. If the geometric fit is suitable, the dications of these p-xylyldiammonium salts (9 being a bicyclo[2,2,2]octane derivative) should be electrostatically attracted to the tetraanionic macrocycle cavity, besides some favourable hydrophobic attractions between the central parts of both the hosts and guests. The p-xylyldiammonium unit is stable under our redox conditions. The 1H NMR spectra of 7 with/without 3 or 4 are shown in Fig. 2. Complexation-induced chemical shift differences (Δδ) are observed for 7 in the presence of dialcohol 4, but not in the presence of diketone 3. The corresponding Δδ values for dialcohol 4 in the presence of 7 are shown in the inset in Fig. 2. The pattern of these Δδ values, which is evidence for an axial inclusion complex in the solution state, are similar to those seen for classical examples of cyclophane inclusion complexes37,61,62, but with some differences. No significant chemical shift differences are found for diketone 3 in the presence of 7. Somewhat analogous to the X-ray crystallography studies (which employs the smaller and neutral guest benzene), inclusive complexation is clear between 4 and 7, and no binding is observed for 3 and 7 in spite of ion-pairing in both cases. Although p-cyclophane hosts are known to be shape-dependent binders37, reversible all-or-none switching between binding and releasing states in water has not been demonstrated until now. The complexation between 4 and 7–9 is quantified by measurement of concentration-dependent Δδ values to obtain binding constants (log β) which have values around 4 (Table 1).
Table 1.
1H NMR and fluorescence emission spectroscopic data for complexation of 4 and 7–9a
| Datum | 7 | 8 | 9 | |||
|---|---|---|---|---|---|---|
| δArH, −Δδ | 7.72 | 0.74 | 7.66 | 0.58 | 7.62 | 0.52 |
| δBzH, −Δδ | 4.58 | 0.36 | 4.50 | 0.26 | 4.40 | 0.31 |
| δα, −Δδ | 3.14 | 0.29 | 3.27 | 0.22 | 3.46 | 0.42 |
| δβ, −Δδ | — | — | 1.42 | 0.11 | 2.19 | 0.16 |
| δγ, −Δδ | — | — | — | — | 1.97 | 0.26 |
| logβb | 4.2 | 3.7 | 4.6 | |||
| logβc | 4.5 | 4.5 | 4.6 | |||
| QFd | 3.1 | 2.4 | 1.7 | |||
aCompound numbers 4 and 7–9 are given in bold formatting. D2O, pD 10.0 for all experiments except fluorescence spectroscopy. δ values for 7–9 are given. Δδ values of −0.02 + 0.02 for all protons are seen when 3 is used instead of 4. The uncertainty in this case is the sample-to-sample variability. For details, see Supplementary Tables 1–3. The critical aggregation concentrations (CAC) of 4 and 3 are 1.6 × 10−3 and 2.0 × 10−3 M, respectively
bDetermined by 1H NMR spectroscopy from analysis of Δδ values for δArH, according to the equation (Δδ / Δδmax)/[1 − (Δδ / Δδmax)]2 = βa73, where a is the concentration of 7–9. 1:1 molar ratios of 4:7–9 are maintained in the concentration range 10−5–10−3 M
cH2O, pH 10.0. Determined by fluorescence emission spectroscopy from analysis of integrated fluorescence intensity (IF) (excited at 290 nm), according to the equation log[(IFmax − IF) / (IF − IFmin)] = logβ − pa73,74. 2.5 × 10−5 M 4, 0–10−3 M of 7–9
dQuenching factor (QF) for integrated fluorescence intensity (IFmax / IFmin)
Fig. 2.
1H NMR spectral evidence for guest binding/non-binding. 1H NMR spectra in D2O, pD 10.0. (1) 7 + 3, (2) 7 + 4 and (3) 7 (all 10−3 M). Δδ values for 7 caused by 4 are shown. Inset. δ values in the 1H NMR spectra of 4 (a part structure is shown) and Δδ values caused by 7
Fluorescence spectra also illuminate the interaction between 4 and 7–9. 7–9 quench the fluorescence of 4 (Supplementary Methods)63. Thus, 4 optically self-indicates its guest occupancy status. 4 is excited without significant absorption by 7–9 except at rather high concentrations of the latter. The addition of self-indication to host 4’s redox switchability immediately extends its logic integration64 (Fig. 3a). Since a high level of the guests 7–9 quenches the fluorescence, 7–9 serve as the inhibiting input (In4) to the INHIBIT logic gate (Fig. 3a), which is now integrated to the Out1 line of the RS Flip-Flop in the form of In3 (Fig. 3a, b). This is the bridge between the sequential logic component and the combinatorial component. Such fluorescence quenching is likely caused by photoinduced electron transfer (PET)65,66 from the alkoxyphenylene units of 4 to the electron-poor phenylene group of 7–9 within the pseudo-intramolecular system. PET from 4 to 7 is thermodynamically allowed, with ΔGPET = −0.2 eV (Supplementary Methods). No exciplex emissions are seen67. Analysis of the variation of fluorescence intensity with the concentration of 7–9 gives logβ results essentially in agreement with the corresponding values obtained via 1H NMR spectra (Table 1). The releasing state has a high ε of 31,000 M−1 cm−1 at 304 nm for the ππ* band owing to the well-delocalized aromatic ketone units and has a low ΦFlu of 0.00368 (λFlu = 458 nm) due to energetically low-lying nπ* excited states69. The capturing states have low ε values of 3900 M−1 cm−1 at 290 nm owing to the smaller π-delocalization of the benzhydrol moieties, while the ΦFlu value is low (0.004) when the guest 7 is bound and high (0.013, λFlu = 356, 416 nm) when guest-free. All three output states, i.e. vacant capturing state, occupied capturing state and releasing state, are therefore distinguishable by optical spectroscopy.
Fig. 3.
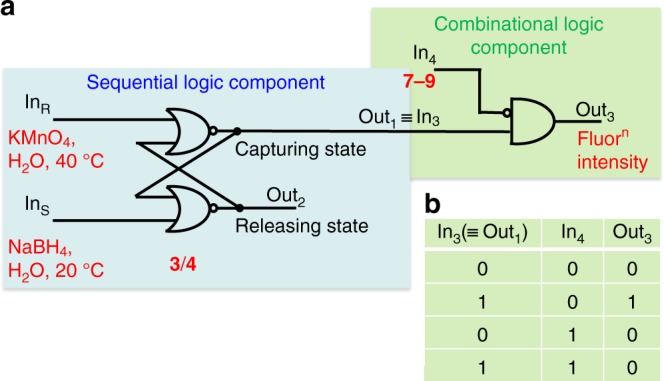
Integrated sequential-combinational logic. a Physical electronic representation of redox-induced reversible guest release/capture by 3/4 in water followed in the latter case by fluorescence signalling according to INHIBIT logic. Output1 is the status of the capturing state, which is characterized by the UV spectroscopic parameter ε = 3,900 M−1 cm−1 at the absorption maximum wavelength (λmax) 290 nm. Output2 is the status of the releasing state, which is characterized by ε = 31,000 M−1 cm−1 at λmax 304 nm. b Truth table for guest (7)-induced quenching of fluorescence of device 3/4 in water corresponding to an INHIBIT logic gate. Guest-free fluorescence quantum yields (ΦFlu) are 0.013 and 0.003 for 4 and 3, respectively, at pH 10.0, determined by comparison with 2-methoxybenzoate in water at pH 8.0 (ΦFlu = 0.011) as secondary standard68. 7 quenches the fluorescence of 4 by a factor of 3.1. 7 does not measurably affect the fluorescence of 3. The output is digitized by applying a threshold value of 0.008 to the ΦFlu values
Discussion
Having been inspired by the roles played by cyclophanes in supramolecular chemistry37,42,45,48, we felt that we could apply our experience of molecular switching4,10–12 to make a contribution to this area. Excellent examples of switchable cyclophanes and related macrocycles already exist37–44, but binary all-or-none switching of cyclophanes in water still remains a worthwhile goal. Since cyclophanes are well-known to bind shape-complementary organic guests in water37, a method of switching the cavity size/shape should enable the guest’s release. Such guest capture/release would have immediate consequences for dosing of drugs and other bio-signalling agents, as well as environmental beneficiation by toxin removal. Notably, each of these interventions could remain under the user’s external control or could be triggered by an existing (e.g. intracellular) condition. The capabilities of such a switchable system could be made more intelligent if it could be endowed with self-indicating properties. Self-indication is key for nanometric molecules to communicate their status to their far-larger human handlers, i.e. to say when a task has been accomplished successfully18.
Such relatively complex molecular-logic behaviour can benefit from the insights of computer science4. Computer scientists and electrical engineers have dealt with such situations in larger-sized systems. This relationship between the disciplines is clear to us since we had the pleasure to introduce the field of molecular-logic-based computation10.
The inclusive complexation of a guest by a cyclophane can be seen, from some viewpoints, to be a case of a guest being corralled by a surrounding wall, with the corral itself being specified by the macrocycle and with the wall height being determined by the width of the aromatic rings. Notably, the walls are perpendicular to the corral area, i.e. the aromatic ring planes are orthogonal to the mean macrocycle plane. Human experience70 suggests that a guest cannot be corralled if the walls have fallen down for some reason. The present work shows that these human situations are mirrored in the molecular world.
Although cyclophanes of this type are well-known37, a prominent feature of our particular cyclophanes is the presence of a pair of ketones or secondary alcohols with flanking phenylene moieties. The benzhydrol version behaves as a more-or-less normal cyclophane host by orienting its phenylene ring planes orthogonal to the macrocycle plane, thereby creating a rather deep and hydrophobic cavity which can include an aromatic guest37,61. On the other hand, the benzophenone version has additional cross-conjugation due to the ketones flanked by the phenylene units. This flattens one phenylene of the pair beside each ketone so that the ketone and the phenylene form a plane which is nearly in the macrocycle plane. Such phenylene ring rotation closes off a large part of the macrocycle cavity. Thus a suitable aromatic guest which is inclusively complexed by the benzhydrol version is rejected by the corresponding benzophenone.
It is well-appreciated that ketones and secondary alcohols can be interconverted by appropriate reduction and oxidation52, each of which are efficient unidirectional chemical reactions. When the ketone is treated with a reducing agent, the corresponding alcohol is formed and it stays as an alcohol whether excess reductant is present or whether the reductant is removed entirely. Similarly, when the alcohol is treated with an oxidant, the corresponding ketone is formed which persists whether the oxidant is absent or present in excess.
From a computer science perspective, the ketone/alcohol system can exist in either of two stable states, depending on the input condition. If a high concentration of reductant is applied as the input, the resulting output state is the alcohol because of the reduction of any starting ketone. This input is called the set(S) input because it sets the alcohol as the output state (Out1 = 1, Out2 = 0 in Fig. 1a, b). If a high concentration of oxidant is applied as the input, the resulting output state is the ketone (Out1 = 0, Out2 = 1 in Fig. 1a, b). This input is called the reset(R) input because it returns the output state to the ketone with which we started. If no input is applied, the system maintains its previous state.
The above behaviour pattern is well-known to computer scientists as a RS flip-flop, which is the memory element in modern computers4. Indeed, we have here an all-chemical RS flip-flop, where the inputs, outputs and the device itself are all chemical species. Molecular RS flip-flops based on photochromic27, enzyme29, and DNA28 systems are known. We have pointed out that chemical oxidation and reduction of molecules can also lead to RS flip-flop action4. The truth table of a RS flip-flop annotated for our situation is shown in Fig. 1b. However, it is important to note some key differences between the electronic and chemical RS flip-flops arising from the diversity present within chemistry. The electronic version is prevented from receiving two high inputs (hence the truth table having only three rows) since it leads to an unstable output situation. The chemical version naturally avoids receiving two high inputs because it is recognized that mixing equivalent amounts of reductants and oxidants would not have a net effect on a substrate. Secondly, the two outputs of an electronic RS flip-flop are always opposite whereas the chemical case is more nuanced. Opposition is available in terms of the guest-capturing and -releasing states. Opposition is also available in terms of the ketones and the alcohols being redox-interconvertible. On the other hand, there is no natural opposition between ketones and alcohols per se or in terms of their different chemistries.
In conclusion, X-ray crystallography shows that, when π-conjugation opportunities are depleted in benzhydrol derivative 2, the phenylene units in a p-cyclophane orient essentially orthogonal to the mean macrocycle plane37. This macrocyclic cavity encapsulates benzene. When extra π-conjugation is arranged via ketone groups in benzophenone derivative 1, the phenylene groups flatten significantly into the mean macrocycle plane so that no encapsulation is possible. The aqueous solution-phase spectroscopy experiments on 4 and 3 confirm that inclusive macrocycle occupancy (by 7–9) is only permitted for the benzhydrol 4 with its erected phenylene walls. An all-chemical RS flip-flop feeding an INHIBIT gate is demonstrated since chemical redox inputs applied to the p-cyclophane devices 1/2 or 3/4 permit reversible all-or-none capture or release of the guests where guest occupancy is self-indicated by the fluorescence output being quenched. This augurs well for the small-scale serial integration of sequential and combinational logic devices in the context of guest capture/release applications.
Methods
Synthesis and characterization of the molecular-logic gate arrays and their precursors
All the procedures are given in the Supplementary Methods.
X-ray crystallography of the guest-binding and guest-releasing states
Single crystals of 1 and 2 were obtained by evaporation of concentrated solutions of the compounds in benzene. Suitable crystals were selected and measured on a Rigaku Saturn724 + (2 × 2 bin mode) diffractometer. The crystals were kept at 100 K during data collection. Using Olex271, the structures were solved with the ShelXS72 structure solution programme using direct methods and refined with the ShelXL72 refinement package using least squares minimization. X-ray crystallographic data for 1 and 2, as well those for control compounds are detailed in the Supplementary Methods.
Guest-dependent fluorescence spectroscopy of the dialcohol cyclophane
Fluorescence spectroscopic data obtained on a Perkin-Elmer LS55 spectrometer running FLWinlab software for 4 as a function of added guest are detailed in the Supplementary Methods.
Supplementary information
Acknowledgements
We thank the Department of Education and Learning of Northern Ireland, Engineering and Physical Sciences Research Council of UK, China Scholarship Council, Queen’s University Belfast, Dr. A.P. Doherty, Dr. W.D.J.A. Norbert, C. Kelly, Z.Q. Chen and L.F. Gui for support and help. In Memoriam Professor John McGarvey, Professor Ken Seddon and Dr. Sergiy Shekhtman.
Author contributions
H.Q.N.G. designed the synthesis. B.D., T.S.M., A.J.M.H., C.Y.Y., B.S. and H.Q.N.G. contributed to the synthesis, optimization and characterization. B.D., A.A.-A., J.F.M. and P.N. carried out the X-ray crystallography. B.D., T.S.M. and A.J.M.H. conducted binding studies and analyzed data. C.Y.Y. assisted during the revision. A.P.deS. planned the research, analyzed data and wrote the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information files. The X-ray crystallographic coordinates for structures 1 and 2 reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1875556–1875557. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-07902-7.
References
- 1.Katz E, editor. Molecular and Supramolecular Information Processing. Weinheim: Wiley-VCH; 2012. [Google Scholar]
- 2.Katz E, editor. Biomolecular Information Processing. Weinheim: Wiley-VCH; 2012. [Google Scholar]
- 3.Szacilowski K. Infochemistry. Chichester: Wiley-VCH; 2012. [Google Scholar]
- 4.de Silva AP. Molecular Logic-based Computation. Cambridge: Royal Society of Chemistry; 2013. [Google Scholar]
- 5.Balzani V, Venturi M, Credi A. Molecular Devices and Machines. 2nd ed. Weinheim: Wiley-VCH; 2008. [Google Scholar]
- 6.Feringa BL, Browne WR, editors. Molecular Switches. 2nd ed. Weinheim: Wiley-VCH; 2011. [Google Scholar]
- 7.Baroncini M, Semeraro M, Credi A. Processing chemical and photonic signals by artificial multicomponent molecular systems. Isr. J. Chem. 2011;51:23–35. doi: 10.1002/ijch.201000065. [DOI] [Google Scholar]
- 8.Andréasson J, Pischel U. Molecules with a sense of logic: a progress report. Chem. Soc. Rev. 2015;44:1053–1069. doi: 10.1039/C4CS00342J. [DOI] [PubMed] [Google Scholar]
- 9.Erbas-Cakmak S, et al. Molecular logic gates: the past, present and future. Chem. Soc. Rev. 2018;47:2228–2248. doi: 10.1039/C7CS00491E. [DOI] [PubMed] [Google Scholar]
- 10.de Silva AP, Gunaratne HQN, McCoy CP. A molecular photoionic and gate based on fluorescent signaling. Nature. 1993;364:42–44. doi: 10.1038/364042a0. [DOI] [Google Scholar]
- 11.de Silva AP, Uchiyama S. Molecular logic and computing. Nat. Nanotechnol. 2007;2:399–410. doi: 10.1038/nnano.2007.188. [DOI] [PubMed] [Google Scholar]
- 12.Ling J, Naren GW, Kelly J, Moody TS, de Silva AP. Building pH sensors into paper-based small-molecular logic systems for very simple detection of edges of objects. J. Am. Chem. Soc. 2015;137:3763–3766. doi: 10.1021/jacs.5b00665. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar T, Selvakumar K, Motiei L, Margulies D. Message in a molecule. Nat. Commun. 2016;7:11374. doi: 10.1038/ncomms11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rout B, Bigliardi PL. Pattern-generating unimolecular sensors: for future differential sensing and molecular computing. Synlett. 2017;28:1005–1010. doi: 10.1055/s-0036-1588721. [DOI] [Google Scholar]
- 15.Margulies D, Felder CE, Melman G, Shanzer A. A molecular keypad lock: a photochemical device capable of authorizing password entries. J. Am. Chem. Soc. 2007;129:347–354. doi: 10.1021/ja065317z. [DOI] [PubMed] [Google Scholar]
- 16.Yu X, Li ML, Li TR, Zhou S, Liu HY. A resettable and reprogrammable biomolecular keypad lock with dual outputs based on glucose oxidase-Au nanoclusters-Prussian blue nanocomposite films on an electrode surface. Nanoscale. 2016;8:20027–20036. doi: 10.1039/C6NR07344A. [DOI] [PubMed] [Google Scholar]
- 17.Erbas-Cakmak S, Cakmak FP, Topel SD, Uyar TB, Akkaya EU. Selective photosensitization through an AND logic response: optimization of the pH and glutathione response of activatable photosensitizers. Chem. Commun. 2015;51:12258–12261. doi: 10.1039/C5CC01261A. [DOI] [PubMed] [Google Scholar]
- 18.Turan IS, Gunaydin G, Ayan S, Akkaya EU. Molecular demultiplexer as a terminator automaton. Nat. Commun. 2018;9:805. doi: 10.1038/s41467-018-03259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remon P, Balter M, Li SM, Andreasson J, Pischel U. An all-photonic molecule-based D flip-flop. J. Am. Chem. Soc. 2011;133:20742–20745. doi: 10.1021/ja2100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreasson J, et al. All-photonic multifunctional molecular logic device. J. Am. Chem. Soc. 2011;133:11641–11648. doi: 10.1021/ja203456h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puntoriero F, et al. Molecular logics: a mixed bodipy-bipyridine dye behaving as a concealable molecular switch. New J. Chem. 2011;35:948–952. doi: 10.1039/c0nj00770f. [DOI] [Google Scholar]
- 22.Yin CX, et al. A two-input fluorescent logic gate for glutamate and zinc. ACS Neurosci. 2017;8:1159–1162. doi: 10.1021/acschemneuro.6b00420. [DOI] [PubMed] [Google Scholar]
- 23.Hettie KS, Klockow JL, Glass TE. Three-input logic gates with potential applications for neuronal imaging. J. Am. Chem. Soc. 2014;136:4877–4880. doi: 10.1021/ja501211v. [DOI] [PubMed] [Google Scholar]
- 24.de Ruiter G, van der Boom M. Surface-confined assemblies and polymers for molecular logic. Acc. Chem. Res. 2011;44:563–573. doi: 10.1021/ar200002v. [DOI] [PubMed] [Google Scholar]
- 25.de Ruiter G, Motiei L, Choudhury J, Oded N, van der Boom M. Electrically addressable multistate volatile memory with flip-flop and flip-flap-flop logic circuits on a solid support. Angew. Chem. Int. Ed. 2010;49:4780–4783. doi: 10.1002/anie.201000785. [DOI] [PubMed] [Google Scholar]
- 26.Baron, R. et al. An electrochemical/photochemical information processing system using a monolayer-functionalized electrode. Chem. Commun. 10.1039/B518378B (2006). [DOI] [PubMed]
- 27.Pischel U, Andreasson J. A simplicity-guided approach toward molecular set-reset memories. New J. Chem. 2010;34:2701–2703. doi: 10.1039/c0nj00498g. [DOI] [Google Scholar]
- 28.Elbaz J, Moshe M, Willner I. Coherent activation of DNA tweezers: a “set-reset” logic system. Angew. Chem. Int. Ed. 2009;48:3834–3837. doi: 10.1002/anie.200805819. [DOI] [PubMed] [Google Scholar]
- 29.Pita M, Strack G, MacVittie K, Zhou J, Katz E. Set-reset flip-flop memory based on enzyme reactions: toward memory systems controlled by biochemical pathways. J. Phys. Chem. B. 2009;113:16071–16076. doi: 10.1021/jp908291f. [DOI] [PubMed] [Google Scholar]
- 30.Periyasamy G, Collin JP, Sauvage JndashP, Levine RD, Remacle F. Electrochemically driven sequential machines: an implementation of copper rotaxanes. Chem. Eur. J. 2009;15:1310–1313. doi: 10.1002/chem.200802249. [DOI] [PubMed] [Google Scholar]
- 31.Green JE, et al. A 160-kilobit molecular electronic memory patterned at 1011 bits per square centimeter. Nature. 2007;445:414–417. doi: 10.1038/nature05462. [DOI] [PubMed] [Google Scholar]
- 32.Irie M, Fukaminato T, Sasaki T, Tamai N, Kawai T. A digital fluorescent molecular photoswitch. Nature. 2002;420:759–760. doi: 10.1038/420759a. [DOI] [PubMed] [Google Scholar]
- 33.Shinkai S, Yoshioka A, Nakayama H, Manabe O. A photochemically activated cyclophane. J. Chem. Soc. Perkin Trans. 1990;2:1905–1909. doi: 10.1039/p29900001905. [DOI] [Google Scholar]
- 34.Hashim PK, Basheer MC, Tamaoki N. Chirality induction by E-Z photoisomerization in [2,2]paracyclophane-bridged azobenzene dimer. Tetrahedron Lett. 2013;54:176–178. doi: 10.1016/j.tetlet.2012.10.121. [DOI] [Google Scholar]
- 35.Li ZY, et al. Switchable azo-macrocycles: from molecules to functionalisation. Supramol. Chem. 2014;26:54–65. doi: 10.1080/10610278.2013.822970. [DOI] [Google Scholar]
- 36.Wise DL, editor. Handbook of Pharmaceutical Controlled Release Technology. New York: Dekker; 2000. [Google Scholar]
- 37.Diederich F. Cyclophanes. Cambridge: Royal Society of Chemistry; 1991. [Google Scholar]
- 38.Bernardo AR, Stoddart JF, Kaifer AE. Cyclobis(paraquat-p-phenylene) as a synthetic receptor for electron-rich aromatic-compounds - electrochemical and spectroscopic studies of neurotransmitter binding. J. Am. Chem. Soc. 1992;114:10624–10631. doi: 10.1021/ja00052a069. [DOI] [Google Scholar]
- 39.Kanazawa H, Higuchi M, Yamamoto K. An electric cyclophane: cavity control based on the rotation of a paraphenylene by redox switching. J. Am. Chem. Soc. 2005;127:16404–16405. doi: 10.1021/ja055681i. [DOI] [PubMed] [Google Scholar]
- 40.Seward EM, Hopkins RB, Sauerer W, Tam SW, Diederich F. Redox-dependent binding ability of a flavin cyclophane in aqueous-solution - hydrophobic stacking versus cavity-inclusion complexation. J. Am. Chem. Soc. 1990;112:1783–1790. doi: 10.1021/ja00161a020. [DOI] [Google Scholar]
- 41.Pochorovski I, Diederich F. Development of redox-switchable resorcin[4]arene cavitands. Acc. Chem. Res. 2014;47:2096–2105. doi: 10.1021/ar500104k. [DOI] [PubMed] [Google Scholar]
- 42.Zhu ZX, et al. Controlling switching in bistable [2]catenanes by combining donor-acceptor and radical-radical interactions. J. Am. Chem. Soc. 2012;134:11709–11720. doi: 10.1021/ja3037355. [DOI] [PubMed] [Google Scholar]
- 43.Schlosser F, Moos M, Lambert C, Wuerthner F. Redox-switchable intramolecular pi-pi-stacking of perylene bisimide dyes in a cyclophane. Adv. Mater. 2013;25:410–414. doi: 10.1002/adma.201201266. [DOI] [PubMed] [Google Scholar]
- 44.Kurihara K, Yazaki K, Akita M, Yoshizawa M. A switchable open/closed polyaromatic macrocycle that shows reversible binding of long hydrophilic molecules. Angew. Chem. Int. Ed. 2017;56:11360–11364. doi: 10.1002/anie.201703357. [DOI] [PubMed] [Google Scholar]
- 45.Asakawa M, et al. Photoactive azobenzene-containing supramolecular complexes and related interlocked molecular compounds. Chem. Eur. J. 1999;5:860–875. doi: 10.1002/(SICI)1521-3765(19990301)5:3<860::AID-CHEM860>3.0.CO;2-K. [DOI] [Google Scholar]
- 46.Lane AS, Leigh DA, Murphy A. Peptide-based molecular shuttles. J. Am. Chem. Soc. 1997;119:11092–11093. doi: 10.1021/ja971224t. [DOI] [Google Scholar]
- 47.Brouwer AM, et al. Photoinduction of fast, reversible translational motion in a hydrogen-bonded molecular shuttle. Science. 2001;291:2124–2128. doi: 10.1126/science.1057886. [DOI] [PubMed] [Google Scholar]
- 48.Bissell RA, Córdova E, Kaifer AE, Stoddart JF. A chemically and electrochemically switchable molecular shuttle. Nature. 1994;369:133–137. doi: 10.1038/369133a0. [DOI] [Google Scholar]
- 49.Sauvage, J.-P., Stoddart, J. F. & Feringa, B. L. The Nobel Prize in Chemistry 2016. The Nobel Prize, www.nobelprize.org (2016).
- 50.Reddy UG, et al. Co-registered molecular logic gate with a CO-releasing molecule triggered by light and peroxide. J. Am. Chem. Soc. 2017;139:4991–4994. doi: 10.1021/jacs.7b00867. [DOI] [PubMed] [Google Scholar]
- 51.Badeau BA, Comerford MP, Arakawa CK, Shadish JA, DeForest CA. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem. 2018;10:251–258. doi: 10.1038/nchem.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donohoe TJ. Oxidation and Reduction in Organic Chemistry. Oxford: Oxford University Press; 2000. [Google Scholar]
- 53.Racys DT, Sharif SAI, Pimlott SL, Sutherland A. Silver(I)-catalyzed iodination of arenes: tuning the Lewis acidity of N-iodosuccinimide activation. J. Org. Chem. 2016;81:772–780. doi: 10.1021/acs.joc.5b02761. [DOI] [PubMed] [Google Scholar]
- 54.Bumagin NA, Nikitin KV, Beletskaya IP. Palladium-catalyzed carbonylation of aryl iodides in aqueous-media. J. Organo. Chem. 1988;358:563–565. doi: 10.1016/0022-328X(88)87103-1. [DOI] [Google Scholar]
- 55.Zhang WW, Liu MC, Wu HY, Ding JC, Cheng J. Cesium hydroxide-promoted aerobic oxidation of sec-aromatic alcohols. Tetrahedron Lett. 2008;49:5336–5338. doi: 10.1016/j.tetlet.2008.06.061. [DOI] [Google Scholar]
- 56.Jennings WB, Farrell BM, Malone JF. Attractive intramolecular edge-to-face aromatic interactions in flexible organic molecules. Acc. Chem. Res. 2001;34:885–894. doi: 10.1021/ar0100475. [DOI] [PubMed] [Google Scholar]
- 57.Fun HK, Franklin S, Jebas SR, Balasubramanian T. 4,4 ‘-dimethoxybenzophenone: a triclinic polymorph. Acta Cryst. 2008;E64:o1256. doi: 10.1107/S1600536808017315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norment HG, Karle IL. The crystal structures of deoxyanisoin and p,p’-dimethoxybenzophenone. Acta Cryst. 1962;15:873–878. doi: 10.1107/S0365110X62002303. [DOI] [Google Scholar]
- 59.Weber E, Pollex R, Czugler M. A new channel-forming host macroring - x-ray crystal-structure of its inclusion compound with DMF. J. Org. Chem. 1992;57:4068–4070. doi: 10.1021/jo00041a005. [DOI] [Google Scholar]
- 60.Kuś P, Jones PJ. Synthesis of new tetraoxacyclophanes containing benzophenone units. Pol. J. Chem. 2000;74:965–979. [Google Scholar]
- 61.Odashima K, Itai A, Iitaka Y, Koga K. Host-guest complex-formation between a water-soluble polyparacyclophane and a hydrophobic guest molecule. J. Am. Chem. Soc. 1980;102:2504–2505. doi: 10.1021/ja00527a083. [DOI] [Google Scholar]
- 62.Cameron KS, et al. Anionic cyclophanes as potential reversal agents of muscle relaxants by chemical chelation. Bioorg. Med. Chem. Lett. 2002;12:753–755. doi: 10.1016/S0960-894X(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 63.Spenst P, Wurthner F. A cyclophane with perylenebisimides in chloroform: A perylene bisimide cyclophane as a ‘turn-on’ and ‘turn-off’ fluorescence probe. Angew. Chem. Int. Ed. 2015;54:10165–10168. doi: 10.1002/anie.201503542. [DOI] [PubMed] [Google Scholar]
- 64.de Silva AP, et al. Integration of logic functions and sequential operation of gates at the molecular-scale. J. Am. Chem. Soc. 1999;121:1393–1394. doi: 10.1021/ja982909b. [DOI] [Google Scholar]
- 65.de Silva AP, et al. Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 1997;97:1515–1566. doi: 10.1021/cr960386p. [DOI] [PubMed] [Google Scholar]
- 66.Balzani V, editor. Electron Transfer in Chemistry. Weinheim: Wiley-VCH; 2001. [Google Scholar]
- 67.Benson DR, Fu J, Johnson CK, Williamson DA. Exciplex formation in complexes between cyclophane hosts and aromatic guests: evidence that the ground-state complexes exist in more than one distinct geometry. J. Org. Chem. 1998;63:9935–9945. doi: 10.1021/jo981755f. [DOI] [Google Scholar]
- 68.Pozdnyakov IP, et al. The photophysics of salicylic acid derivatives in aqueous solution. J. Phys. Org. Chem. 2009;22:449–454. doi: 10.1002/poc.1489. [DOI] [Google Scholar]
- 69.Jornet D, Tormos R, Miranda MA. Photobehavior of mixed n pi*/pi pi* triplets: simultaneous detection of the two transients, solvent-dependent hydrogen abstraction, and reequilibration upon protein binding. J. Phys. Chem. B. 2011;115:10768–10774. doi: 10.1021/jp2051432. [DOI] [PubMed] [Google Scholar]
- 70.Taylor F. The Berlin Wall. New York: Harper; 2008. [Google Scholar]
- 71.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009;42:339–341. doi: 10.1107/S0021889808042726. [DOI] [Google Scholar]
- 72.Sheldrick GM. A short history of SHELX. Acta Cryst. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 73.Connors KA. Binding Constants. New York: Wiley; 1987. [Google Scholar]
- 74.de Silva AP, Gunaratne HQN, Lynch PLM, Patty AJ, Spence GL. Luminescence and charge-transfer 3. The use of chromophores with ICT (internal charge-transfer) excited-states in the construction of fluorescent PET (photoinduced electron-transfer) pH sensors and related absorption pH sensors with aminoalkyl side-chains. J. Chem. Soc. Perkin Trans. 1993;2:1611–1616. doi: 10.1039/p29930001611. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information files. The X-ray crystallographic coordinates for structures 1 and 2 reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1875556–1875557. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.