Abstract
The objective of this study was to evaluate the hepatoprotective effects of blue honeysuckle (BH) on carbon tetrachloride (CCl4)‐induced acute hepatic damage in mice. The experiment used a total of 60 ICR mice, which were divided into six groups. Except for the intact control groups, all groups received a single intraperitoneal injection of CCl4 after a 7 day pre‐treatment period with distilled water, BH extracts, or silymarin. Twenty‐four hours after the CCl4 injection, the following observations, representative of classical oxidative stress‐mediated centrolobular necrotic acute liver injuries, were observed: decreased body weight; small nodule formation and enlargement on the gross inspections with related liver weight increase; elevation of serum AST and ALT, increases in hepatic lipid peroxidation and related depletion of endogenous antioxidants and antioxidative enzymes; centrolobular necrosis; increases in apoptotic markers, lipid peroxidation markers, and oxidative stress markers. However, liver damage was significantly inhibited by the pre‐treatment with BH extracts. The present study demonstrated that oral administration of BH extracts prior to exposure to CCl4 conferred favorable hepatoprotective effects. These results demonstrated that BHe possessed suitable properties for use as a potent hepatoprotective medicinal food.
Keywords: antioxidant, CCl4, liver, Lonicera caerulea, mice
1. INTRODUCTION
The liver performs various pivotal functions, including protein synthesis, glucose homeostasis, detoxification, and the utilization of various nutrients (Lu et al., 2016; Yang, Zhang, Guan, & Hua, 2015). Generally, when the liver is exposed to high levels of environmental toxins, metabolic dysfunction of the liver may occur, which ranges from the transient elevation of liver enzymes to life‐threatening hepatic fibrosis, liver cirrhosis, and even hepatocellular carcinoma (Sun et al., 2011). Substantial evidence implicates oxidative stress and inflammation in the etiology of liver injury (Berasain et al., 2009). Similar effects are caused by CCl4, an industrial solvent known to induce liver injury and liver diseases, which is widely used in experimental hepatopathy (Yang et al., 2015; Zou, Qi, Ye, & Yao, 2016). CCl4‐induced toxicity depends on the dose and duration of exposure. At a low dose, transient effects occur, including the loss of Ca2+ sequestration, impaired lipid homeostasis, and the release of several cytokines. Longer exposures alter fatty acid metabolism and induce fibrosis, cirrhosis, and cancer (Cui, Yang, Lu, Chen, & Zhao, 2014). CCl4‐induced hepatotoxicity is the result of reductive dehalogenation reactions catalyzed by the hepatic cytochrome P‐450, which forms unstable trichloromethyl and trichloromethyl peroxyl radicals capable of binding to proteins or lipids and initiating lipid peroxidation and liver damage (Cheng et al., 2013). Oxidative stress has been accepted as one of the principal causes of CCl4‐induced hepatic injury, which is mediated by the production of free radical derivatives of CCl4 and is responsible for cell membrane damage and the subsequent release of the marker enzymes of hepatotoxicity (Boll, Weber, Becker, & Stampfl, 2001; Weber, Boll, & Stampfl, 2003). Inflammation is another important pathological mechanism through which CCl4‐induced liver injury is propagated (Ebaid, Bashandy, Alhazza, Rady, & El‐Shehry, 2013; Yang et al., 2015).
Therefore, the hepatoprotective effects of test materials are evaluated on CCl4‐induced acute liver damage through histopathological analyses and the examination of anti‐inflammatory potential and antioxidative activities (Ferreira et al., 2010; Wang et al., 2013).
Although the need for medicines to protect against liver damage has emerged, modern medicine still lacks reliable hepatoprotective drugs; therefore, numerous traditional herbal medicines have been studied for the evaluation of their hepatoprotective efficiency (Lu et al., 2016). Silymarin is a flavonoid found in the herb milk thistle, Silybum marianum. Milk thistle grows wild in a variety of settings, including roadsides. Silymarin is a powerful antioxidant that protects liver cells from toxins (Wellington & Jarvis, 2001). The antioxidant effects of silymarin on CCl4‐induced liver damage have been well documented (Cordero‐Pérez et al., 2013; Vargas‐Mendoza et al., 2014); therefore, it was selected as a reference agent in this study.
Blue honeysuckle (BH) is a shrub traditionally used in folk medicine in northern Russia, China, and Japan, but its fruits are also considered an edible berry in North America, Europe, and Korea (Svarcova, Heinrich, & Valentova, 2007). The berry is a rich source of ascorbic acid and phenolic components, particularly anthocyanins, flavonoids, and low molecular weight phenolic acids (Chaovanalikit, Thompson, & Wrolstad, 2004; Svarcova et al., 2007). These compounds have been reported to exert multiple biological effects, including strong antioxidant activity (Svarcova et al., 2007). Recently, it was reported that orally administered BH protected mice against ionizing radiation (Zhao et al., 2012), ameliorated abnormal lipid and glucose metabolism in rats (Jurgoński, Juśkiewicz, & Zduńczyk, 2013), and exhibited hepatoprotective (Palíková, Valentová, Oborná, & Ulrichová, 2009), anti‐inflammatory (Jin et al., 2006; Zdařilová, Svobodova, Chytilová, Šimánek, & Ulrichová, 2010), and therapeutic effects on hyperthyroidism (Park et al., 2016). In particular, BH extracts showed the strongest antioxidant potential among 12 different colored berries (Chen, Xin, Yuan, Su, & Liu, 2014); the phenolic‐rich extract of BH has been shown to possess anti‐inflammatory and wound‐healing effects in vitro and in vivo (Jin et al., 2006) in addition to protective effects on the skin against ultraviolet‐induced damage (Svobodová, Rambousková, Walterová, & Vostálová, 2008; Vostálová et al., 2013). However, it appears that more detailed studies are needed on the hepatoprotective effects of BH.
In the present study, we aimed to screen the efficacy of three different types of BH extracts in a mouse model of CCl4‐induced acute hepatic damage for their potential as potent hepatoprotective medicinal foods. The effects of the BH extracts were compared with those of silymarin (mixed flavonoids purified from milk thistle, Silybum marianum) (Jain, Lodhi, Jain, Nahata, & Singhai, 2011; Wang, Feng, Zhu, Zhao, & Suo, 2016).
2. MATERIALS AND METHODS
2.1. Preparation of BH extracts
Three different types of BH extract, BHw, BHj, and BHe, were prepared. BHw and BHj were prepared and supplied by Bioport Korea Inc. (Busan, Korea). BHw is the freeze‐dried powder of the hot water extract of BH; briefly, water and dried BH were mixed in a 10:1 (w/w) ratio and then boiled at 90–95°C for 3 h under reflux. The supernatant was condensed (55–65°C) and freeze dried. BHj is the freeze‐dried power of BH squeezed juice and BHe is the freeze‐dried powder of BH solution obtained after enzyme treatment. BH solution was purchased from H&K Bioscience Co. Ltd (Seoul, Korea) and freeze dried by Aribio Co. Ltd (Sungnam, Korea). Briefly, frozen BH fruits were treated by the following: heating (45–55°C for 3 min), pulverization, enzyme treatment (pectinase: Natuzyme DP ultra 0.05% (w/w), Natuzyme olimax 0.05% (w/w), 2–2.5 h, 50 rpm), centrifugation (6,400 g/min), heating (80°C, 15–30 s), addition of chitosan (0.005%, w/w) and guar gum (0.005% w/w), filtration (disc separation, diatomite filtration, filter press), condensation (63 brix, 50°C, 1 min, 0.092 MPa), sterilization (90–95°C, 15–30 s), and then freeze dried; from this process, BHe was obtained at a yield of 10.83%.
In addition, silymarin was purchased in the form of a reddish‐yellow powder from Sigma‐Aldrich Co. LLC. (St. Louis, MO, USA) and used as a reference drug (Jain et al., 2011; Wang et al., 2016).
All three different types of BH extracts were dissolved in distilled water to 20 mg/ml, and orally administered once per day for 7 days, consecutively. The administration volume was 10 ml/kg (equivalent to 200 mg/kg), which was applied through gastric gavage using a zonde attached to a 1 ml syringe. Silymarin was suspended into distilled water at 10 mg/ml, and orally administered in a volume of 10 ml/kg (equivalent 100 mg/kg) once per day for 7 days, consecutively, in accordance with the reference recommendation (Jain et al., 2011; Wang et al., 2016). In the intact vehicle and CCl4‐treated control mice, equal volumes of vehicle (distilled water) were orally administered instead of the test substances.
2.2. Animals and husbandry
A total of sixty, healthy male ICR mice (6 weeks old upon receipt from OrientBio, Seungnam, Korea) were used after acclimatization period of 7 days. Four or five animals were allocated to a polycarbonate cage in a temperature‐ (20–25°C) and humidity‐ (30–35%) controlled room. The light‐dark cycle was 12 h/12 h and the rats were given ad libitum access to feed (Cat. No. 38057; Purinafeed, Seungnam, Korea) and water were accessed.
The animals were divided into six groups based on their body weight prior to test substances administration: Intact control: Distilled water (DW) orally administered and olive oil intraperitoneally (IP) treated mice; CCl4 control: DW orally administered and CCl4 0.5 mg/kg IP treated mice; Silymarin: Silymarin 100 mg/kg orally administered and CCl4 0.5 ml/kg IP treated mice; BHw: BHw 200 mg/kg orally administered and CCl4 0.5 ml/kg IP treated mice; BHj: BHj 200 mg/kg orally administered and CCl4 0.5 ml/kg IP treated mice; and BHe: BHe 200 mg/kg orally administered and CCl4 0.5 ml/kg IP treated mice (Figure 1).
Figure 1.
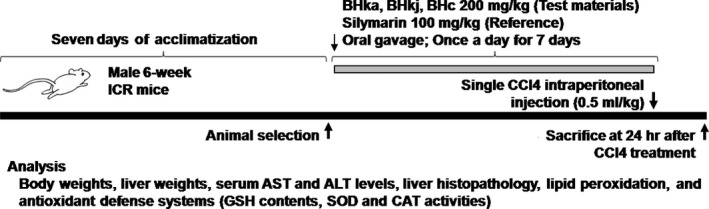
Experimental designs used in this study
2.3. Induction of acute liver damage
Acute liver damage was induced by single IP injection of CCl4 (Sigma‐Aldrich, St Louis, MO, USA), dissolved in olive oil (Sigma‐Aldrich, St Louis, MO, USA) 1:19 (v/v) (5%) in a volume of 10 ml/kg (equivalent to 0.5 ml/kg of CCl4), at 1 h after the seventh administration of the test material in accordance with previously established methods (Al‐Sayed, Abdel‐Daim, Kilany, Karonen, & Sinkkonen, 2015; Al‐Sayed et al., 2014; Fahmy, Al‐Sayed, Abdel‐Daim, Karonen, & Singab, 2016; Ferreira et al., 2010; Wang et al., 2013). Instead of CCl4, equal volumes of olive oils were administered to the intact control mice; further, distilled water was orally administered 1 h after the seventh administration.
2.4. Changes in body weights
Changes in body weight were measured each day, from 1 day before the administration of the initial test, throughout all experimental periods, by using an automatic electronic balance (Precisa Instrument, Zürich, Switzerland). To reduce the individual differences, the body weight gains from the day of initial test substance administration to 24 h after CCl4 injection were calculated as follows: Body weight gains (g) during 7 days of the whole experimental period = Body weight at 24 h after CCl4 treatment – Body weight on the day of initial test substance administration.
2.5. Measurements of liver weights
All animals were sacrificed 24 h after the CCl4 injection, gross inspection was conducted under anesthesia induced with 2–3% isoflurane (Hana Pharm. Co. Ltd, Hwasung, Korea) in a mixture of 70% N2O and 28.5% O2 using by using a rodent inhalation anesthesia apparatus (Surgivet, Waukesha, WI, USA) and rodent ventilator (Model 687, Harvard Apparatus, Cambridge, UK) and the weight of liver was measured (absolute wet‐weights). To reduce the differences from individual body weights, the relative liver weights (as a percentage of body weights) were also calculated from the following formula: relative liver weights (% of body weight) = (absolute liver wet‐weights/body weight at sacrifice) × 100.
2.6. Measurement of serum AST and ALT levels
At sacrifice, approximately 1 ml of venous blood was collected from the vena cava. All collected blood samples were centrifuged at 12,600 g for 10 min under cool temperatures (4°C) by using clotting activated serum tubes for serum separations and stored in an ultradeep freezer (Model MDF‐1156, Sanyo, Tokyo, Japan) below −150°C until analysis. Serum AST and ALT levels were detected by using an automated blood analyzer (Model Dri‐Chem NX500i, Fuji Medical System Co., Ltd, Tokyo, Japan).
2.7. Measurement of liver lipid peroxidation
The separated hepatic tissues were weighed and homogenized in ice‐cold 0.01 M Tris‐HCl buffer (pH 7.4) and centrifuged at 12,000 g for 15 min, as described by Kavutcu et al. (1996). Tissue homogenates were stored in an ultradeep freezer below −150°C until analysis. The concentration of liver lipid peroxidation was determined through the estimation of MDA by using the thiobarbituric acid test and a UV/Vis spectrophotometer (Model OPTIZEN POP, Mecasys, Daejeon, Korea) to measure the absorbance of the solution at 525 nm, and determined as nM of MDA/mg protein (Jamall & Smith, 1985). The total protein content was measured by a previously described method (Lowry, Rosenbrough, Farr, & Randall, 1951) using bovine serum albumin (Invitrogen, Carlsbad, CA, USA) as internal standard.
2.8. Measurement of hepatic antioxidant defense systems
The prepared hepatic homogenates were mixed with 0.1 ml 25% trichloroacetic acid (Merck, West Point, CA, USA) and centrifuged (1,627 g, 40 min, 4°C). The GSH content was spectrophotometrically determined through the measurement of absorbance at 412 nm by using 2‐nitrobenzoic acid (Sigma‐Aldrich, St. Louis, MO, USA) (Sedlak & Lindsay, 1968). The decomposition of H2O2 in the presence of CAT was followed at 240 nm by using a spectrophotometer (Aebi, 1974). CAT activity was defined as the amount of enzyme required to decompose 1 nM of H2O2 per minute at 25°C and pH 7.8. The results were expressed as U/mg protein. The measurement of SOD activity was performed in accordance with the method of Sun, Larry, and Ying (1988). SOD estimation was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which react with nitrotetrazolium blue to produce formazan dye. SOD activity, which was related to the degree of inhibition of this reaction, was then spectrophotometrically measured at 560 nm and expressed as U/mg protein. One unit of SOD enzymatic activity is equal to the amount of enzyme that diminishes the initial absorbance of nitroblue tetrazolium by 50% over 1 min.
2.9. Histopathology
The left lateral lobes of the liver were separated and fixed in 10% neutral buffered formalin (NBF), embedded in paraffin, sectioned (3–4 μm), and stained with hematoxylin and eosin (H&E) for general histopathological analysis (Ki et al., 2013; Lee et al., 2014). The histopathological profiles of each sample were generated by observation under a light microscope (Eclipse 80i, Nikon, Tokyo, Japan). To observe more detailed changes, hepatic damage was evaluated by the modified HAI (histological activity index) grading scores based on a previous well‐established semiquantitative histopathological scoring system (Ishak et al., 1995), which includes assessment of confluent necrosis, focal lytic necrosis, apoptosis, and focal and portal inflammation (Table 1). In addition, the percentage of degenerative regions (%/mm2) in the liver that exhibited centrolobular necrosis, congestion, and inflammatory cell infiltrations on hepatic lobules was computed by using a software‐based automated image analyzer (iSolution FL ver 9.1, IMT i‐solution Inc., Vancouver, Quebec, Canada). The numbers of hepatocytes that showed degenerative changes, including necrosis, acute cellular swelling (ballooning), and severe fatty acid changes, and inflammatory cells infiltrated were also calculated by using an automated image analyzer and expressed as cells/1000 hepatocytes and cells/mm2 of hepatic parenchyma in accordance with previously described methods (Ki et al., 2013; Lee et al., 2014). The histopathologist was blinded to the group distribution when the analysis was performed.
Table 1.
Modified HAI grading: inflammatory scores used in the present study
| A. Confluent necrosis | ||
| Absent | 0 | |
| Focal confluent necrosis | 1 | |
| Zone 3 necrosis in some areas | 2 | |
| Zone 3 necrosis in most areas | 3 | |
| Zone 3 necrosis + occasional portal‐central bridging | 4 | |
| Zone 3 necrosis + multiple portal‐central bridging | 5 | |
| Panacinar or multiacinar necrosis | 6 | |
| B. Focal (spotty) lytic necrosis, apoptosis and focal inflammation | ||
| Absent | 0 | |
| One focus or less per 10× objective | 1 | |
| Two to four foci per 10× objective | 2 | |
| Five to ten foci per 10× objective | 3 | |
| More than ten foci per 10× objective | 4 | |
HAI: Histological Activity Index; HAI grading scores = A + B; Possible maximum total scores = 10. Modified from the method described by Ishak et al. (1995).
2.10. Immunohistochemistry
The changes in apoptotic markers (cleaved caspase‐3 and PARP) (Jiang et al., 2012; Talwar et al., 2013; Yu et al., 2014), a marker of lipid peroxidation (4‐HNE) (Lee et al., 2014; Smathers, Galligan, Stewart, & Petersen, 2011), and an NO related oxidative stress marker (NT) (Lee et al., 2014; Pacher, Beckman, & Liaudet, 2007) were observed by the use of immunohistochemical staining methods by using purified primary antibodies (Table 2) with an ABC and peroxidase substrate kit (Vector Labs, Burlingame, CA, USA). Briefly, endogenous peroxidase activity was blocked by incubation in methanol and 0.3% H2O2 for 30 min and non‐specific binding of immunoglobulin was blocked through incubation with normal horse serum blocking solution for 1 h in a humidity chamber after heating (95–100°C); epitope retrievals were conducted in 10 mM citrate buffers (pH 6.0) (Ki et al., 2013; Lee et al., 2014; Yu et al., 2014). The primary antisera were treated overnight at 4°C in a humidity chamber and incubated with biotinylated universal secondary antibody and ABC reagents for 1 h at room temperature in humidity chamber. Finally, the sections were reacted with a peroxidase substrate kit for 3 min at room temperature. All sections were rinsed three times in 0.01 M PBS between each step. Cells that contained over 20% of immunoreactive staining by density, of cleaved caspase‐3, cleaved PARP, NT, and 4‐HNE, were regarded as positive in this study, and the numbers of cleaved caspase‐3, cleaved PARP, NT and 4‐HNE‐immunolabeled cells located within a restricted view field of hepatic parenchyma around centrolobular regions, around central veins as cells/1000 hepatocytes were measured by using a computer‐based automated image analyzer as previously described (Ki et al., 2013; Lee et al., 2014; Yu et al., 2014), respectively. The histopathologist was blinded to the group distribution when this analysis was performed.
Table 2.
Primary antisera and detection kits for immunohistochemistry
| Antisera or detection kits | Code | Source | Dilution |
|---|---|---|---|
| Primary antisera | |||
| Anti‐cleaved caspase‐3 (Asp175) antibody | 9661 | Cell Signaling Technology Inc, Beverly, MA, USA | 1:400 |
| Anti‐cleaved PARP (h215) antibody | sc‐23461 | Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA | 1:100 |
| Anti‐Nitrotyrosine polyclonal antibody | 06‐284 | Millipore Corporation, Temecula, CA, USA | 1:200 |
| Anti‐4‐Hydroxynonenal polyclonal antibody | Ab46545 | Abcam, Cambridge, UK | 1:100 |
| Detection kits | |||
| Vectastain Elite ABC kit | PK‐6200 | Vector Lab., Burlingame, CA, USA | 1:50 |
| Peroxidae substrate kit | SK‐4100 | Vector Lab., Burlingame, CA, USA | 1:50 |
All antiserum were diluted using 0.01 M phosphate buffered saline (pH 7.2). PARP: Poly(ADP‐ribose) polymerase.
2.11. Statistical analyses
All numerical data were expressed as the mean ± SD of 10 mice. Multiple comparison tests for different dose groups were conducted. Variance homogeneity was examined by using the Levene test (Levene, 1981). If the Levene test indicated no significant deviations from variance homogeneity, the obtain data were analyzed by one‐way ANOVA followed by a least‐significant differences (LSD) multi‐comparison test to determine which pairs of groups were significantly different. In the case that significant deviations from variance homogeneity were observed in the Levene test, a non‐parametric comparison test, the Kruskal‐Wallis H test, was conducted. When a significant difference was observed in the Kruskal‐Wallis H test, the Mann‐Whitney U (MW) test was conducted to determine the specific pairs of groups that are significantly different (Ludbrook, 1997). Differences were considered significant at p < 0.05. Statistical analyses were computed by using SPSS for Windows (Release 14.0K, SPSS Inc., Chicago, IL, USA).
In addition, the percentage change between intact control mice and CCl4 control mice was calculated to evaluate the severity of hepatic damages, including the induction of centrolobular necrosis, and the percentage changes compared with the CCl4 control and BH‐ or silymarin‐treated mice were also calculated to help elucidate the efficacy of the test substances. These calculations were performed by Equations (1) and (2), respectively, in accordance with our previously established method (Kang et al., 2014).
| (1) |
| (2) |
3. RESULTS
3.1. Changes in body weight
A significant (p < 0.01) decrease in body weight was detected on the day of sacrifice in the treated mice compared with the intact control; the body weight gain over the 7 day experimental period was also significantly (p < 0.01) decreased in the CCl4 control mice compared with that of the intact control mice. Significant (p < 0.01 or p < 0.05) increases in body weight at sacrifice and body weight gain during the 7 day experimental period were demonstrated in all mice administered each test substance in comparison with those administered the CCl4‐treated control. The order of change was as follows (largest to smallest): BHe, silymarin, BHj, and BHw (Table 3, Figure 2).
Table 3.
Body weight gains in CCl4‐treated mice
| Periods Groups | Body weights at | Body weight gains [B‐A] | |||
|---|---|---|---|---|---|
| One day before test substance administration | First test substance administration [A]* | Last 7th test substance administration | 24 h after CCl4 treatment [B]* | ||
| Controls | |||||
| Intact | 33.35 ± 1.14 | 30.24 ± 1.25 | 36.14 ± 1.14 | 33.72 ± 1.46 | 3.48 ± 0.73 |
| CCl4 | 33.32 ± 1.33 | 30.33 ± 1.49 | 36.05 ± 1.34 | 31.04 ± 1.57a | 0.71 ± 1.14a |
| Silymarin | 33.37 ± 1.43 | 30.13 ± 1.48 | 36.18 ± 1.27 | 32.79 ± 1.62d | 2.66 ± 0.91c |
| BH extracts | |||||
| BHw | 33.40 ± 1.66 | 30.26 ± 1.77 | 36.19 ± 1.30 | 32.60 ± 1.28d | 2.34 ± 0.95ac |
| BHj | 33.40 ± 1.42 | 30.28 ± 1.53 | 36.15 ± 1.45 | 32.64 ± 1.37d | 2.36 ± 1.27bc |
| BHe | 33.34 ± 1.70 | 30.40 ± 1.81 | 36.18 ± 1.67 | 33.12 ± 1.88c | 2.72 ± 0.41c |
Values are expressed mean ± SD of 10 mice, g. BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract.
*All animals were overnight fasted (about 18 h; water was not restrict). a p < 0.01 and b p < 0.05 as compared with intact control by LSD test. c p < 0.01 and d p < 0.05 as compared with CCl4 control by LSD test.
Figure 2.
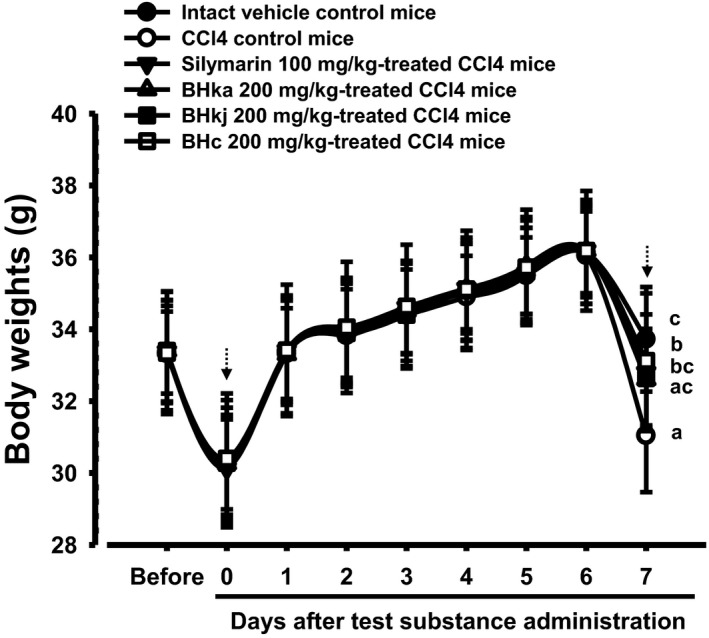
Body weights changes in intact or CCl4‐treated mice. Values are expressed mean ± SD of 10 mice. BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract. D‐1 means 1 day before first test substance administration. Day 7 means the day of sacrifice, 24 h after CCl4 treatment. All animals were overnight fasted before initial test substance administration and sacrifice (dot arrows). a p < 0.01 as compared with intact control by LSD test. b p < 0.01 and c p < 0.05 as compared with CCl4 control by LSD test
The body weight gains during the 7 day whole experimental period in the CCl4 control were found to be −79.60% compared with intact control, but the values in silymarin 100 mg/kg and BHw, BHj, and BHe 200 mg/kg treated mice were 274.65%, 229.58%, 232.39%, and 283.10%, respectively, compared with the CCl4 control.
3.2. Changes on gross appearance and liver weights
Marked small nodule formation and the enlargement of the liver were demonstrated in the CCl4 control compared with the intact control upon gross inspection, with related significant (p < 0.01) increases in the absolute and relative weights of the liver. However, noticeable decreases in small nodule formations and hepatic enlargements, and related significant (p < 0.01) decreases of liver weights were observed in all mice administered the test substance compared with the CCl4 control. The induced changes occurred in the following order (largest to smallest): BHe, silymarin, BHj, and BHw (Figures 3 and 4).
Figure 3.
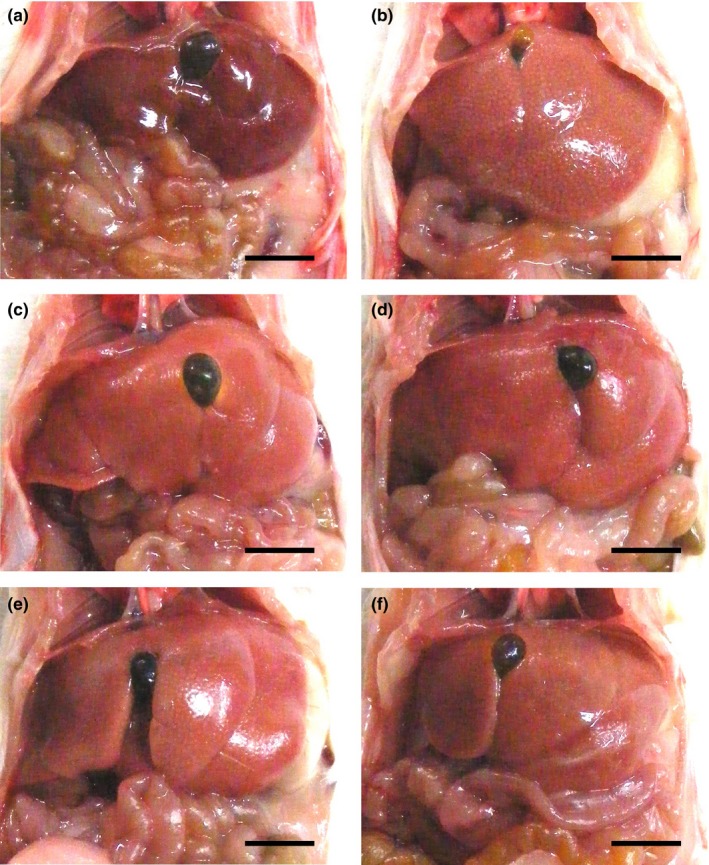
Representative gross liver images, taken from intact or CCl4‐treated mice. (a) Intact control (Distilled water and olive oil treated mice). (b) CCl4 control (Distilled water and CCl4 0.5 ml/kg treated mice). (c) Silymarin control (Silymarin 100 mg/kg and CCl4 0.5 ml/kg treated mice). (d) BHw (BHw 200 mg/kg and CCl4 0.5 ml/kg treated mice). (e) BHj (BHj 200 mg/kg and CCl4 0.5 ml/kg treated mice). (f) BHe (BHe 200 mg/kg and CCl4 0.5 ml/kg treated mice). BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract, BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract; CCl4: Carbone tetrachloride. Scale bars: 6.5 mm
Figure 4.
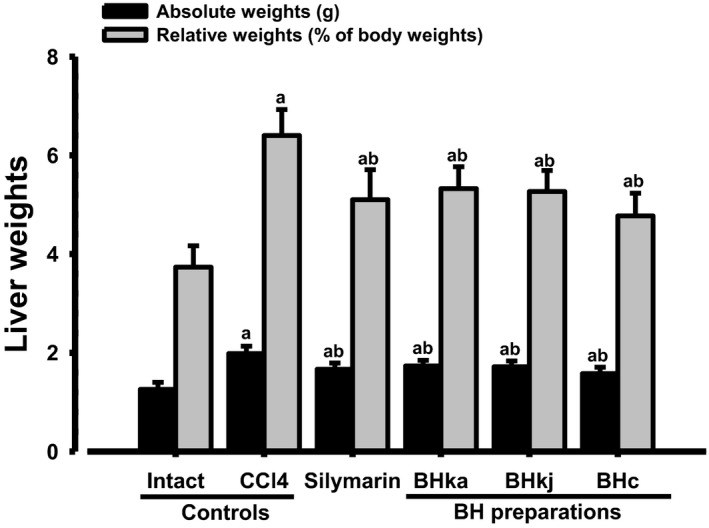
Liver weights in the CCl4‐treated mice. Values are expressed mean ± SD of 10 mice. BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract. a p < 0.01 as compared with intact control by LSD test. b p < 0.01 as compared with CCl4 control by LSD test
The absolute liver weight in the CCl4 control was changed by 57.75% compared with the intact control, but this was ameliorated by the administration of silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −16.09%, −12.67%, −13.49%, and −20.56%, respectively, compared with the CCl4 control. The relative liver weights in the CCl4 control were 71.50% of the intact control, but were altered by the treatment of silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −20.31%, −16.84%, −17.74%, and −25.48%, respectively, compared with the CCl4 control.
3.3. Changes in the serum AST and ALT levels
Significant (p < 0.01) elevations of serum AST and ALT levels (intracellular enzymes indicative of hepatic damages) were observed in the CCl4 control compared with the intact control, but significant (p < 0.01) decreases were induced by the treatment of all BH extracts at 200 mg/kg, and by silymarin 100 mg/kg, compared with the CCl4 control. The changes were largest in BHe, followed by silymarin, BHj, and BHw (Figure 5).
Figure 5.
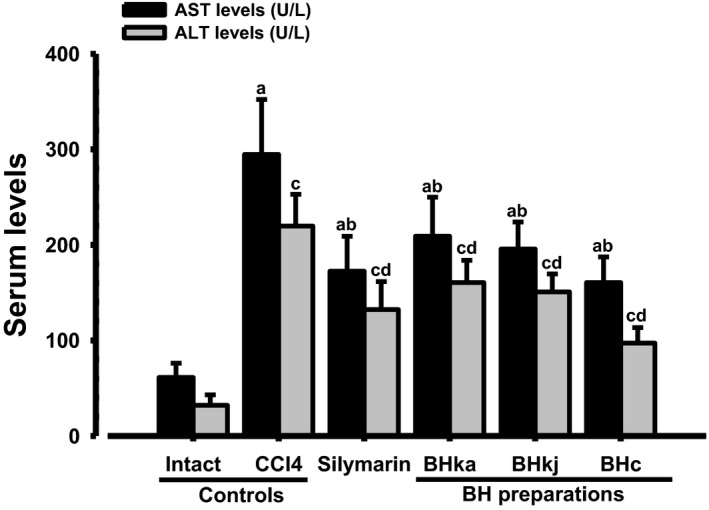
Serum AST and ALT levels in the CCl4‐induced liver damaged mice. Values are expressed mean ± SD of 10 mice. BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase. a p < 0.01 as compared with intact control by LSD test. b p < 0.01 as compared with CCl4 control by LSD test. c p < 0.01 as compared with intact control by MW test. d p < 0.01 as compared with CCl4 control by MW test
The serum AST levels in the CCl4 control were increased to 380.59% compared with the intact control, but were found to be decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −41.48%, −29.06%, −33.60%, and −45.52%, respectively, compared with the CCl4 control. The serum ALT levels in the CCl4 control were increased to 584.42% compared with the intact control, but they were decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −39.78%, −26.95%, −31.36%, and −55.76%, respectively, compared with the CCl4 control.
3.4. Effects on the hepatic lipid peroxidation
Significant (p < 0.01) increases in hepatic lipid peroxidation and MDA content were found in the CCl4 control compared with the intact control. However, significant (p < 0.01) decreases in MDA content were induced by all BH extracts at 200 mg/kg and silymarin 100 mg/kg compared with the CCl4 control; the largest changes were induced by BHe, followed by silymarin, BHj, and BHw (Table 4).
Table 4.
Hepatic lipid peroxidation, GSH contents, and CAT and SOD activities in CCl4‐treated mice
| Items (Unit) Groups | Lipid Peroxidation (nM of MDA/mg protein) | GSH Contents (nM/mg protein) | Enzyme activity | |
|---|---|---|---|---|
| SOD (U/mg protein) | CAT (U/mg protein) | |||
| Controls | ||||
| Intact | 1.49 ± 0.86 | 38.48 ± 10.33 | 418.83 ± 135.08 | 242.68 ± 102.48 |
| CCl4 | 8.31 ± 1.44a | 3.78 ± 1.21c | 58.76 ± 27.74c | 45.60 ± 18.65c |
| Silymarin | 5.00 ± 1.13ab | 17.59 ± 3.71cd | 184.74 ± 72.99cd | 121.85 ± 22.91cd |
| BH extracts | ||||
| BHw | 6.05 ± 1.10ab | 10.74 ± 2.89cd | 126.68 ± 35.63cd | 88.15 ± 21.04cd |
| BHj | 5.54 ± 1.12ab | 12.51 ± 2.50cd | 145.29 ± 39.18cd | 104.39 ± 20.66cd |
| BHe | 4.45 ± 1.45ab | 21.41 ± 5.08cd | 223.66 ± 55.75cd | 133.21 ± 20.74cd |
Values are expressed mean ± SD of 10 mice. BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract, BHj: lyophilized powder of BH squeezed juice, BHe: lyophilized powder of BH enzyme extract; MDA: Malondialdehyde; CAT: Catalase; SOD: Superoxide dismutase.
a p < 0.01 as compared with intact control by LSD test. b p < 0.01 as compared with CCl4 control by LSD test. c p < 0.01 as compared with intact control by MW test. d p < 0.01 as compared with CCl4 control by MW test.
The hepatic MDA contents in the CCl4 control were increased to 457.65% compared with the intact control, but were decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −39.78%, −27.19%, −33.34%, and −46.48%, respectively, compared with the CCl4 control.
3.5. Effects on hepatic GSH, an endogenous antioxidant
A significant (p < 0.01) decrease of hepatic GSH was observed in the CCl4 control mice compared with the intact control, but these changes were significantly (p < 0.01) ameliorated by oral pre‐administration for 7 days for all BH extracts at 200 mg/kg and by silymarin 100 mg/kg compared with the CCl4 control. BHe exerted the strongest effect, followed by silymarin, BHj, and BHw (Table 4).
The hepatic GSH contents in the CCl4 control were changed to −90.17% compared with the intact control, but they were increased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to 364.72%, 183.85%, 230.58%, and 465.70%, respectively, compared with the CCl4 control.
3.6. Changes in activity of CAT, an endogenous antioxidative enzyme
A significant (p < 0.01) decrease in hepatic CAT activity was observed in the CCl4 control compared with the intact control, but the decrease induced by CCl4 was significantly (p < 0.01) ameliorated by the 7 day pre‐treatment of silymarin 100 mg/kg and all BH extracts at 200 mg/kg; BHe exerted the strongest effect, followed by silymarin, BHj, and BHw (Table 4).
The hepatic CAT activity in the CCl4 control was decreased to −81.21% compared with the intact control, but was increased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to 167.20%, 93.31%, 128.92%, and 192.13% compared with the CCl4 control, respectively.
3.7. Effects on activity of SOD, another endogenous antioxidative enzyme
A significant (p < 0.01) decrease in hepatic SOD activity was detected in the CCl4 control compared with the intact control. However, significant (p < 0.01) increases in SOD activities occurred in mice that received pre‐treatment with all BH extracts at 200 mg/kg and silymarin 100 mg/kg compared with that of the CCl4 control. BHe exerted the strongest effect, followed by BHe, silymarin, BHj, and BHw (Table 4).
The hepatic SOD activity in the CCl4 control was decreased to −85.97% compared with the intact control, but was increased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to 214.39%, 115.59%, 147.26%, and 280.62%, respectively, compared with the CCl4 control.
3.8. Histopathological inspection
Classic centrolobular necrosis (hepatocyte vacuolation and ballooning, deposition of lipid droplets in hepatocytes, and infiltration of inflammatory cells) was observed after a single IP treatment of CCl4 0.5 ml/kg. However, this microscopic centrolobular necrosis was markedly inhibited by 7 days of continuous oral pre‐treatment of silymarin 100 mg/kg and by all BH extracts at 200 mg/kg compared with the CCl4 control; BHe was the most effective, followed by silymarin, BHj, and BHw. The histomorphometrical and semi‐quantitative analysis indicated significant (p < 0.01) increases in the percentage area of degenerative regions, the numbers of degenerative hepatocytes, and the numbers of inflammatory cells infiltrated in hepatic parenchyma; therefore, a related increase of modified HAI grading scores was observed in the CCl4 control compared with the intact control. These changes were significantly (p < 0.01) ameliorated by treatment with all BH extracts at 200 mg/kg and by silymarin 100 mg/kg compared with the CCl4 control; BHe exerted the strongest effect, followed by silymarin, BHj, and BHw (Table 5, Figure 6).
Table 5.
General histomorphometrical analysis of hepatic tissues from CCl4‐treated mice
| Items (Unit) Groups | General histomorphometry | |||
|---|---|---|---|---|
| Histological Activity Index (Scores; Max = 10) | Percentages of degenerative regions (%/mm2) | Numbers of degenerative hepatocytes (cells/1000 hepatocytes) | Numbers of inflammatory cells infiltrated (cells/mm2) | |
| Controls | ||||
| Intact | 0.40 ± 0.52 | 2.53 ± 1.95 | 29.60 ± 19.41 | 43.40 ± 16.60 |
| CCl4 | 8.40 ± 1.07a | 79.82 ± 10.06c | 809.50 ± 100.54c | 269.10 ± 74.04c |
| Silymarin | 4.40 ± 0.84ab | 44.22 ± 11.44cd | 446.90 ± 108.91cd | 73.50 ± 15.86cd |
| BH extracts | ||||
| BHw | 5.00 ± 1.33ab | 54.92 ± 12.47cd | 563.30 ± 136.44cd | 102.20 ± 25.42cd |
| BHj | 4.80 ± 1.14ab | 50.21 ± 11.52cd | 496.10 ± 122.23cd | 89.40 ± 21.54cd |
| BHe | 3.50 ± 1.27ab | 38.32 ± 11.02cd | 407.40 ± 121.06cd | 62.40 ± 23.80d |
Values are expressed mean ± SD of 10 mice. BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract.
a p < 0.01 as compared with intact vehicle control by LSD test. b p < 0.01 as compared with CCl4 control by LSD test. c p < 0.01 as compared with intact vehicle control by MW test. d p < 0.01 as compared with CCl4 control by MW test.
Figure 6.
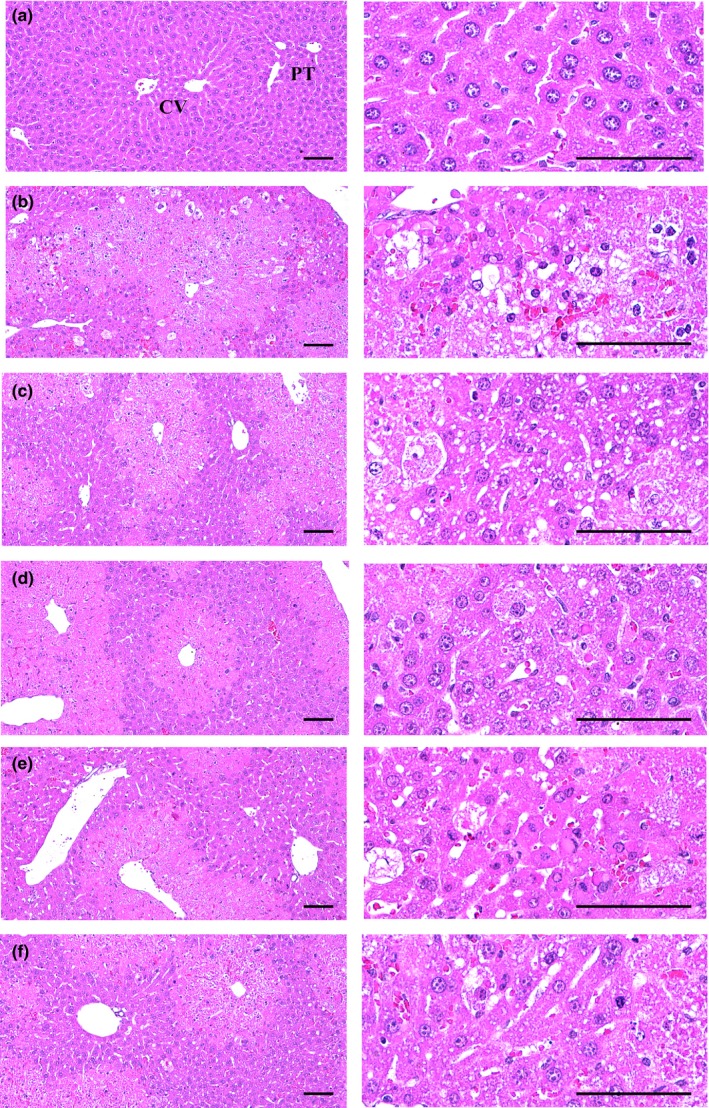
Histopathogical profiles of the CCl4 damaged liver. (a) Intact control (Distilled water and olive oil treated mice). (b) CCl4 control (Distilled water and CCl4 0.5 ml/kg treated mice). (c) Silymarin control (Silymarin 100 mg/kg and CCl4 0.5 ml/kg treated mice). (d) BHw (BHw 200 mg/kg and CCl4 0.5 ml/kg treated mice). (e) BHj (BHj 200 mg/kg and CCl4 0.5 ml/kg treated mice). (f) BHe (BHe 200 mg/kg and CCl4 0.5 ml/kg treated mice). BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract; CV: Central vein; PT: Portal triad regions. All Hematoxylin‐eosin stain. Scale bars = 120 μm
The percentage of degenerative regions in the CCl4 control was increased to 3053.58% compared with the intact control, but was decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −44.59%, −31.20%, −37.09%, and −51.99%, respectively, compared with the CCl4 control. The mean number of degenerated hepatocytes in the CCl4 control was increased to 2634.80% compared with the intact control, but decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −44.79%, −30.41%, −38.72%, and −49.67%, respectively, compared with the CCl4 control. The mean number of inflammatory cells infiltrated in the hepatic parenchyma of the CCl4 control was increased to 520.05% compared with the intact control, but was decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −72.69%, −62.39%, −66.78%, and −76.81%, respectively, compared with the CCl4 control. The mean modified HAI grading score in the CCl4 control was increased to 2000.00% compared with the intact control, but decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −47.62%, −40.48%, −42.86%, and −58.33%, respectively, compared with the CCl4 control.
3.9. Immunohistochemical analysis
Significant (p < 0.01) increases in the number immunoreactive hepatocytes to apoptotic markers (cleaved caspase‐3 and PARP), a marker of lipid peroxidation (4‐HNE) and an NO‐related oxidative stress marker (NT) were observed in the CCl4 control compared with the intact control by using ABC‐based immunohistochemistry. However, significant (p < 0.01) decreases of the mean numbers of cleaved caspase‐3, PARP, NT, and 4‐HNE immunoreactive hepatocytes were induced by all three types of BH extracts at 200 mg/kg and by silymarin 100 mg/kg compared with the CCl4 control; BHe exerted the strongest effect, followed by silymarin, BHj, and BHw (Table 6, Figures 7 and 8).
Table 6.
Immunohistochemistrical‐histomorphometrical analysis of hepatic tissues from CCl4‐treated mice
| Items (Unit) Groups | Positive cells by immunohistochemistry (cells/1000 hepatocytes) | |||
|---|---|---|---|---|
| Cleaved caspase‐3 | Cleaved Poly(ADP‐ribose) polymerase | Nitrotyrosine | 4‐Hydroxynonenal | |
| Controls | ||||
| Intact | 2.00 ± 1.41 | 4.10 ± 2.88 | 17.20 ± 10.12 | 15.80 ± 8.38 |
| CCl4 | 718.20 ± 108.68a | 714.40 ± 111.79c | 707.10 ± 101.38c | 757.10 ± 120.07c |
| Silymarin | 383.00 ± 104.97ab | 288.80 ± 64.00cd | 331.50 ± 115.94cd | 440.20 ± 135.73cd |
| BH extracts | ||||
| BHw | 488.90 ± 119.27ab | 470.40 ± 126.21cd | 486.90 ± 108.44cd | 535.70 ± 107.30cd |
| BHj | 433.20 ± 107.51ab | 454.80 ± 96.07cd | 442.10 ± 66.79cd | 480.90 ± 112.66cd |
| BHe | 310.10 ± 111.16ab | 196.90 ± 62.99cd | 291.60 ± 69.14cd | 264.10 ± 70.92cd |
Values are expressed mean ± SD of 10 mice. BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract.
a p < 0.01 as compared with intact vehicle control by LSD test. b p < 0.01 as compared with CCl4 control by LSD test. c p < 0.01 as compared with intact vehicle control by MW test. d p < 0.01 as compared with CCl4 control by MW test.
Figure 7.
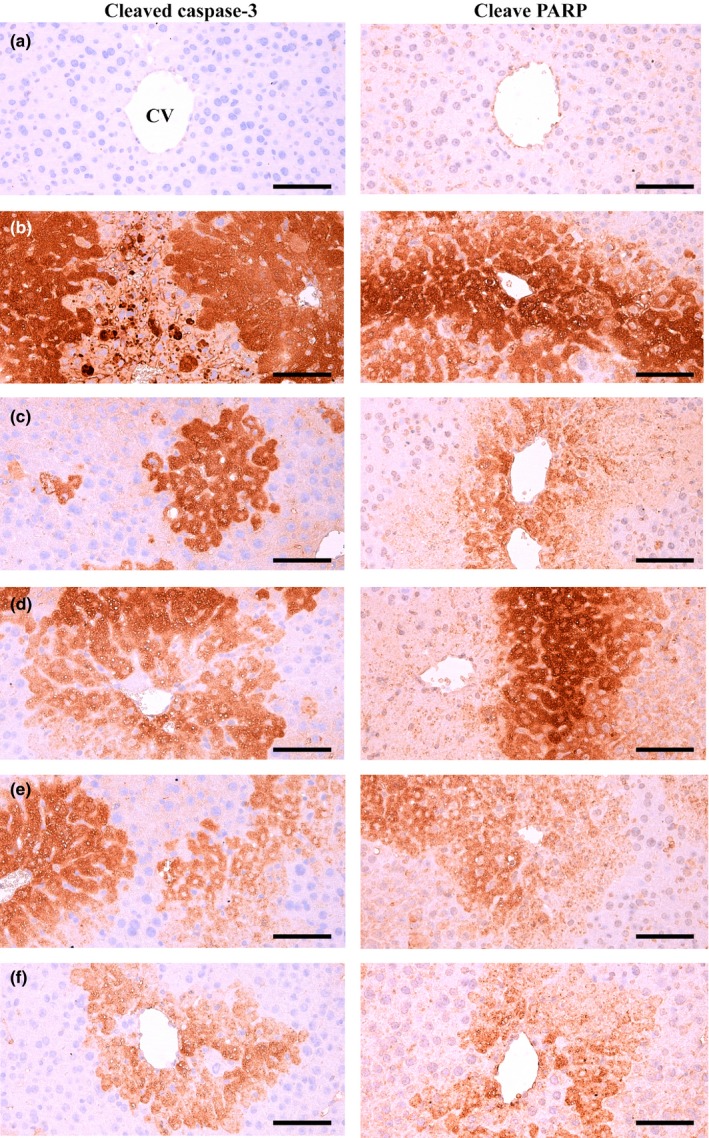
Cleaved caspase‐3 and PARP immunoreactivities in the CCl4 damaged liver. (a) Intact control (Distilled water and olive oil treated mice). (b) CCl4 control (Distilled water and CCl4 0.5 ml/kg treated mice). (c) Silymarin control (Silymarin 100 mg/kg and CCl4 0.5 ml/kg treated mice). (d) BHw (BHw 200 mg/kg and CCl4 0.5 ml/kg treated mice). (e) BHj (BHj 200 mg/kg and CCl4 0.5 ml/kg treated mice). (f) BHe (BHe 200 mg/kg and CCl4 0.5 ml/kg treated mice). BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract; BHj: lyophilized powder of BH squeezed juice; BHe: lyophilized powder of BH enzyme extract; CV: Central vein; PARP: Poly(ADP‐ribose) polymerase. Immunoreactive cells were stained by avidin‐biotin‐peroxidase methods. Scale bars = 120 μm
Figure 8.
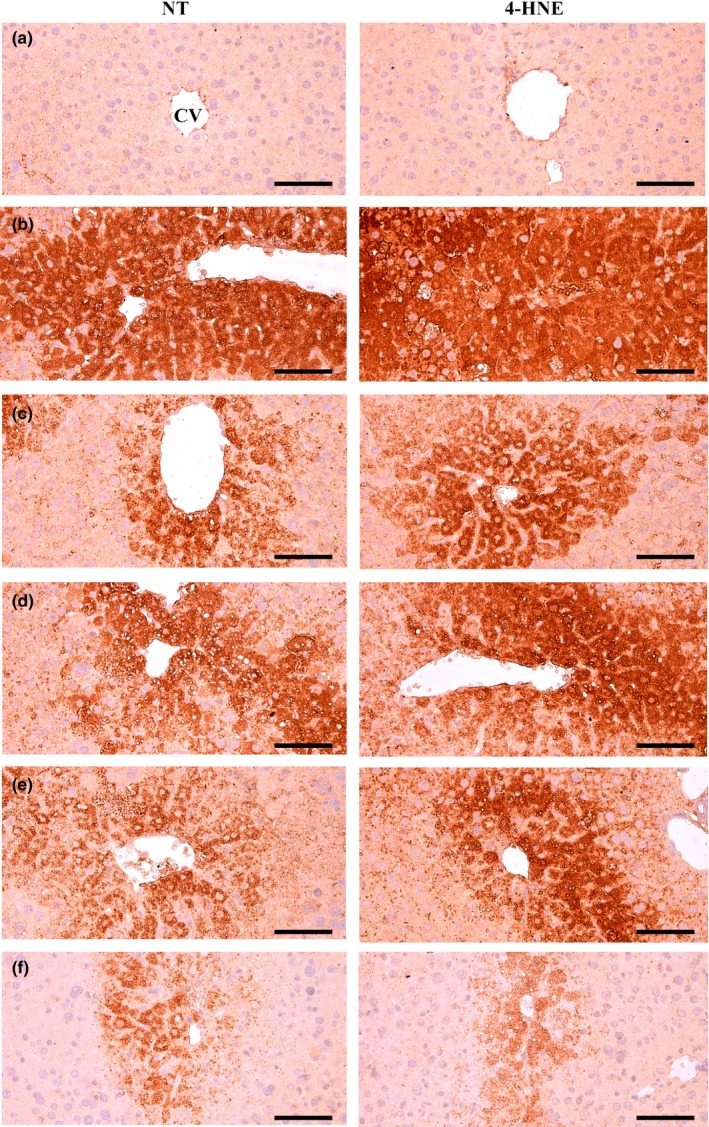
NT and 4‐HNE Immunoreactivities in the CCl4 induced damaged liver. (a) Intact control (Distilled water and olive oil treated mice). (b) CCl4 control (Distilled water and CCl4 0.5 ml/kg treated mice). (c) Silymarin control (Silymarin 100 mg/kg and CCl4 0.5 ml/kg treated mice). (d) BHw (BHw 200 mg/kg and CCl4 0.5 ml/kg treated mice). (e) BHj (BHj 200 mg/kg and CCl4 0.5 ml/kg treated mice). (f) BHe (BHe 200 mg/kg and CCl4 0.5 ml/kg treated mice). BH: Blue honeysuckle (Berries of Lonicera caerulea var. edulis L., Caprifoliaceae); BHw: lyophilized powder of BH aqueous extract, BHj: lyophilized powder of BH squeezed juice, BHe: lyophilized powder of BH enzyme extract; CV: Central vein; NT: Nitrotyrosine; 4‐HNE: 4‐Hydroxynonenal. Immunoreactive cells were stained by avidin‐biotin‐peroxidase methods. Scale bars = 120 μm
The mean number of cleaved caspase‐3 immunoreactive hepatocytes in the CCl4 control was increased to 35810.00% compared with the intact control, but was decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −46.67%, −31.93%, −39.68%, and −56.82%, respectively, compared with the CCl4 control. The mean number of PARP immunopositive hepatocytes in the CCl4 control was increased to 17324.39% compared with the intact control, but was decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −59.57%, −34.15%, −36.34%, and −72.44%, respectively, compared with the CCl4 control. The mean number of NT immunolabeled hepatocytes in the CCl4 control was increased to 4011.05% compared with the intact control, but was decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −53.12%, −31.14%, −37.48%, and −58.76% compared with the CCl4 control, respectively. The mean number of 4‐HNE immunostained hepatocytes in the CCl4 control was increased to 4691.77% compared with the intact control, but was decreased by silymarin 100 mg/kg, BHw, BHj, and BHe 200 mg/kg to −41.86%, −29.24%, −36.48%, and −65.12%, respectively, compared with the CCl4 control.
4. DISCUSSION
The common industrial solvent, CCl4, is one of the most potent inducers of acute liver injury; it is often used in animal studies to model human liver injury (Yang et al., 2015; Zou et al., 2016). As the liver is a vital organ with known importance in several physiological processes (Lu et al., 2016; Yang et al., 2015), the need for hepatoprotective medicines has gradually emerged. Modern medicine is still hampered by a lack of reliable hepatoprotective drugs; therefore, numerous traditional herbal medicines have been studied for their hepatoprotective efficiency (Lu et al., 2016). BH is a rich source of ascorbic acid and phenolic components, particularly anthocyanins, flavonoids, and low molecular weight phenolic acids, which exert multiple biological activities, including strong antioxidant activity (Chaovanalikit et al., 2004; Svarcova et al., 2007). BH extracts were shown to have the strongest antioxidant potential among 12 types of colored berries (Chen et al., 2014) and possess hepatoprotective effects (Palíková et al., 2009). However, as more detailed studies on these hepatoprotective effects are lacking, we aimed to evaluated the effect of BH extracts against CCl4‐induced acute hepatic damage in mice (Ferreira et al., 2010; Wang et al., 2013); we examined three different extract types, BHw, BHj, and BHe, for their suitability as potent hepatoprotective medicinal foods.
The decreased body weight after the administration of CCl4 is considered to result from the direct toxicity of CCl4 and/or indirect toxicity related to the liver damage; hence, the change in body weight after CCl4 treatment has been used as a valuable index in the efficacy test of CCl4‐related organ damage (Pradeep, Mohan, Anand, & Karthikeyan, 2005; Yang, Li, Wang, & Wu, 2010). All mice in the intact control group in this study experienced normal increases in body weight within the range of normal age‐matched mice of the same strain (Fox, Cohen, & Loew, 1984; Tajima, 1989). In the current study, a significant decrease in body weight was detected up to the day of sacrifice day (24 h after CCl4 treatment) compared with those of the intact control; accordingly, the body weight gains during the 7 day experimental period were also significantly decreased in the CCl4 control compared with those of the intact control. However, significant increases in the body weight at sacrifice and the body weight gain during the 7 day experimental period were demonstrated in all mice treated with the test substances compared with the CCl4 control. The strongest effects were exerted by BHe, followed by silymarin, BHj, and BHw. These findings were considered reliable evidence that all three BH extracts exerted favorable inhibitory effects on the CCl4‐induced body weight changes; the strongest effects were exerted by BHe, followed by BHj and BHw. In particular, BHe 200 mg/kg showed greater inhibition of the CCl4‐induced changes in body weights than that caused by silymarin 100 mg/kg.
It was observed that marked small nodulation and enlargement occurred in CCl4‐treated livers with related increases in liver weights (Han et al., 2016; Pinto, Duque, Rodríguez‐Galdón, Cestero, & Macías, 2012), but was also present in the CCl4 control. However, a noticeable decrease in small nodule formations and hepatic enlargements, with a related significant decrease in liver weights were observed in all mice administered test substances compared with those of the CCl4 control; BHe exerted the most favorable effect, followed by silymarin, BHj, and BHw. These findings were also considered to present clear evidence that all three BH extracts tested in this study induced favorable hepatoprotective effects on CCl4‐induced acute liver injury in mice. In particular, BHe 200 mg/kg showed more favorable inhibitory effects on the CCl4‐induced nodulation and enlargement of the liver with related increases of liver weights in mice compared with those of silymarin 100 mg/kg.
Generally, AST and ALT (formerly known as SGOT and SGPT) have been used as serum markers to represent liver damage (Sodikoff, 1995); these enzymes are markedly elevated in CCl4‐induced hepatic damage (Lu et al., 2016; Yang et al., 2015) and were elevated in the CCl4 control in the present study. Therefore, the results of our study provided further evidence that all three BH extracts tested in this study exerted favorable hepatoprotective effects against CCl4‐induced liver injuries; the strongest effect was induced by BHe, followed by BHj and BHw, as demonstrated by the marked and significant inhibitions on the CCl4‐induced serum AST and ALT elevations in these groups compared with the CCl4 control. Better inhibition of the CCl4‐induced elevation of serum AST and ALT were observed in mice treated with BHe 200 mg/kg compared with those treated with silymarin 100 mg/kg.
Considerable experimental and clinical evidence supports the prominent role of oxidative stress in the pathophysiological processes of liver injury related to CCl4 exposure (Yang et al., 2015; Zou et al., 2016). Lipid peroxidation is an autocatalytic mechanism that leads to the oxidative destruction of cellular membranes (Subudhi, Das, Paital, Bhanja, & Chainy, 2008; Videla, 2000). Such destruction can lead to cell death and to the production of toxic and reactive aldehyde metabolites called free radicals, of which MDA is one of the most important (Messarah et al., 2010; Venditti & Di Meo, 2006). It is known that reactive oxygen species (ROS) lead to the oxidative damage of biological macromolecules, including lipids, proteins, and DNA (Das & Chainy, 2001; Messarah et al., 2010), and that oxidative stress also influences adipocytes, causing decreases in body fat mass and, subsequently, body weight decrease (Voldstedlund, Tranum‐Jensen, Handberg, & Vinten, 1995). As MDA is a terminal product of lipid peroxidation, the content of MDA can be used to estimate the extent of lipid peroxidation (Messarah et al., 2010). Marked increases of liver MDA content have been observed in CCl4‐treated animals (Yang et al., 2015; Zou et al., 2016); in this study, MDA was increased at 24 h after single IP treatment of CCl4 0.5 ml/kg. GSH is a representative endogenous antioxidant, which prevents tissue damage by suppression of ROS levels; furthermore, at certain cellular concentrations, it is accepted to be a protective antioxidant factor in tissues (Odabasoglu et al., 2006). SOD is another antioxidant enzyme that contributes to the enzymatic defense mechanisms and the enzyme CAT is responsible for the conversion of H2O2 to H2O (Cheeseman & Slater, 1993). Decreases in antioxidant enzyme activities, such as SOD and CAT, and decreases in the content of GSH may be indicative of the failure of cells to respond to the oxidative stress induced by CCl4 (Yang et al., 2015; Zou et al., 2016). Trichloromethyl radicals also react with the sulfhydryl groups of GSH leading to its deactivation (Al‐Sayed et al., 2014; Srivastava & Shivanandappa, 2010). In this experiment, the hepatic antioxidant defense system was clearly enhanced by treatment of all BH extracts; the effects of BHe were the strongest, followed by BHj and BHw, compared with the CCl4 control. Our results suggested that all BH extracts reduced the effect of CCl4‐induced liver injury through the augmentation of the hepatic antioxidant defense system. In particular, mice treated with BHe 200 mg/kg showed more favorable inhibitory effects on CCl4‐induced hepatic lipid peroxidation, with depletions of the endogenous antioxidant (GSH) and enzymes (SOD and CAT) observed, compared with silymarin 100 mg/kg‐treated mice, which supported the aforementioned hepatoprotective effects.
As previously reported (Ki et al., 2013; Lee et al., 2014), vacuolation (the deposition of lipid droplets), the ballooning of hepatocytes, and inflammatory cell infiltration were detected after single IP injection of CCl4 0.5 ml/kg, which are indications of classic centrolobular necrosis. Damaged hepatocytes were mainly located around the central veins and the fatty changed cells were marginally located. CCl4 treatment‐related acute hepatic damages were confirmed by using HAI grading scores, based on the assessment of confluent necrosis, focal lytic necrosis, apoptosis, and focal and portal inflammation, which provides a well‐established semi‐quantitative histopathological scoring system in which higher grade scores indicate severe hepatitis (Ishak et al., 1995). The percentage of degenerative regions, the number of degenerative hepatocytes, and the numbers of inflammatory cells infiltrated in hepatic parenchyma were significantly increased in the CCl4 control compared with the intact control. However, the CCl4 treatment‐related centrolobular acute hepatic damage was significantly inhibited by the treatment of all BH extracts (in the order BHe, BHj, and BHw) compared with those of the CCl4 control. These histopathological findings were considered to provide direct and consistent evidence that all BH extracts tested in this study exerted favorable hepatoprotective effects on acute hepatic damage. In particular, mice treated with BHe 200 mg/kg showed more favorable hepatoprotective effects at the histopathological level on the CCl4‐induced centrolobular necrosis‐related acute liver damage compared with mice treated with silymarin 100 mg/kg.
Apoptosis occurs through two pathways, an extrinsic pathway that involves the interaction of death ligands with their respective cell surface receptors and an intrinsic pathway that is initiated by insults that damage the DNA, such as ultraviolet light and chemotherapeutic agents. Both pathways eventually result in mitochondrial damage, the release of cytochrome c, and the downstream activation of caspases, such as caspase‐3. The activation of other downstream caspases results in the cleavage of cellular proteins, such as PARP, cytokeratin 18, and other caspases, which lead to the morphologic and biochemical changes of apoptosis (Barrett, Willingham, Garvin, & Willingham, 2001; Nunez, Benedict, Hu, & Inohara, 1998). PARP is a nuclear DNA‐binding protein that functions in DNA base excision repair (Trucco, Oliver, de Murcia, & Menissier‐de Murcia, 1998). PARP cleavage results in decrease enzymatic repair functions and contributes to the progression of apoptosis, although it is not strictly necessary for apoptosis to proceed (Smulson et al., 1998). Caspase 3, a downstream effector caspase, is responsible for the cleavage of several critical nuclear targets in the apoptotic cascade, including the inhibitor of caspase‐activated deoxynuclease, which results in nuclear fragmentation, and PARP, which results in a defective DNA repair function (Smyth, Berman, & Bursztajn, 2002). It has been reported that severe apoptosis of liver hepatocytes has been observed in CCl4‐induced acute liver injury (Sun et al., 2011), and the inhibition of cleaved caspase‐3 and PARP have been regarded as hepatoprotective indicators (Jiang et al., 2012; Talwar et al., 2013; Yu et al., 2014). In our study, increased cleaved caspase‐3 and PARP immunoreactive hepatocytes were also demonstrated in the CCl4 control, mainly in the centrolobular regions, compared with the intact control. Noticeably, all BH extracts significantly reduced the CCl4‐induced increases in the number of cleaved caspase‐3 and PARP immunolabeled hepatocytes; at 200 mg/kg, BHe exerted the strongest effects, followed by BHj and BHw. These immunohistochemical results on the hepatic cleaved caspase‐3 and PARP immunostained cells provided direct evidence that the hepatoprotective effects on CCl4‐induced acute hepatic damage exerted by all BH extracts tested in this study may occur through anti‐apoptotic activity. BHe exerted the strongest effect, followed by BHj and BHw; in particularly, mice treated with BHe 200 mg/kg consistently demonstrated more favorable anti‐apoptotic effects against CCl4 treatment compared with those induced by silymarin.
NT is a product of tyrosine nitration mediated by reactive nitrogen species, such as the peroxynitrite anion and nitrogen dioxide. It is detectable in many pathological conditions, including CCl4‐induced acute and acute hepatic damages, and is considered to be marker of NO‐dependent, reactive nitrogen species‐induced nitrative stress (Lee et al., 2014; Pacher et al., 2007). 4‐HNE is an α, β‐unsaturated hydroxyalkenal produced by lipid peroxidation in cells; both these compounds are considered as possible causative agents of numerous diseases, including chronic inflammation, neurodegenerative diseases, adult respiratory distress syndrome, atherogenesis, diabetes, and different types of cancer (Lee et al., 2014; Smathers et al., 2011). The metabolism of CCl4 also initiates the peroxidation of polyunsaturated fatty acids that produce α, β‐unsaturated aldehydes, including 4‐HNE and malondialdehyde (Hartley, Kolaja, Reichard, & Petersen, 1999; Sigala et al., 2006). In the present study, marked and significant increases of NT and 4‐HNE immunostained hepatocytes were observed in the CCl4 control compared with the intact control, but were significantly reduced by the 7 day continuous pretreatment of all BH extracts at 200 mg/kg and by silymarin 100 mg/kg. In particular, mice treated with BHe 200 mg/kg showed better inhibitory effects on the CCl4‐induced increases of NT and 4‐HNE immunopositive hepatocytes in mice compared with silymarin 100 mg/kg‐treated mice. These immunohistochemical findings on the hepatic NT and 4‐HNE immunostained cells provided direct evidence that the hepatoprotective effects on the CCl4‐induced acute hepatic damages of all three different BH extracts detected in this study may occur through antioxidant effects.
5. CONCLUSION
Through the assessment of the key parameters of the hepatoprotective effects on CCl4‐induced acute liver injury in mice, the present work demonstrated that the oral pre‐administration of BHe, BHw, and BHj exerted favorable hepatoprotective effects through the activation of hepatic antioxidant defense systems; the strongest effects occurred in BHe, followed by BHw and BHj. In particular, BHe 200 mg/kg showed more favorable hepatoprotective effects compared with those of silymarin 100 mg/kg on CCl4‐induced acute liver damage in mice in the current study. Therefore, BHe is a suitable candidate BH extract for the development of potent hepatoprotective medicinal foods.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICAL APPROVAL
This experiment was conducted in accordance with the international regulations of the usage and welfare of laboratory animals, and approved by the Institutional Animal Care and Use Committee of Daegu Haany University (Gyeongsan, Korea) (Approval No. DHU2016‐090).
ACKNOWLEDGEMENT
This study work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value‐added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA; 116019‐3) and partly supported by Cooperative Research Program for Agriculture Science and Technology Development funded by Rural Development Administration (Project No. PJ01227802), Republic of Korea.
Lee Y‐S, Cho IJ, Kim JW, et al. Hepatoprotective effects of blue honeysuckle on CCl4‐induced acute liver damaged mice. Food Sci Nutr. 2019;7:322–338. 10.1002/fsn3.893
This study “Hepatoprotective effects of blue honeysuckle on CCl4 induced acute liver damaged mice” has not been published or submitted for publication elsewhere, and all the listed authors have read and approved the submitted manuscript. All authors have contributed significantly and that all authors are in agreement with the content of the manuscript and agree to the conditions outlined in the copyright assignment form. In addition, this manuscript revised by an English‐speaking consultant.
Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value‐added Food Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (grant 116019‐3) and Cooperative Research Program for Agriculture Science and Technology Development funded by Rural Development Administration (Project No. PJ01227802), Republic of Korea.
REFERENCES
- Aebi, H. (1974). Catalase In Bergmeyer H. U. (Ed.), Methods in enzymatic analysis (pp. 673–686). New York, NY: Academic Press; 10.1016/B978-0-12-091302-2.50032-3 [DOI] [Google Scholar]
- Al‐Sayed, E. , Abdel‐Daim, M. M. , Kilany, O. E. , Karonen, M. , & Sinkkonen, J. (2015). Protective role of polyphenols from Bauhinia hookeri against carbon tetrachloride‐induced hepato‐ and nephrotoxicity in mice. Renal Failure, 37, 1198–1207. 10.3109/0886022X.2015.1061886 [DOI] [PubMed] [Google Scholar]
- Al‐Sayed, E. , Martiskainen, O. , el‐Din, S. H. S. , Sabra, A. N. A. , Hammam, O. A. , & Abdel‐Daim, M. M. (2014). Hepatoprotective and antioxidant effect of Bauhinia hookeri extract against carbon tetrachloride‐induced hepatotoxicity in mice and characterization of its bioactive compounds by HPLC‐PDA‐ESI‐MS/MS. BioMed Research International, 2014, 245171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, K. L. , Willingham, J. M. , Garvin, J. A. , & Willingham, M. C. (2001). Advances in cytochemical methods for detection of apoptosis. Journal of Histochemistry and Cytochemistry, 49, 821–832. 10.1177/002215540104900703 [DOI] [PubMed] [Google Scholar]
- Berasain, C. , Castillo, J. , Perugorria, M. J. , Latasa, M. U. , Prieto, J. , & Avila, M. A. (2009). Inflammation and liver cancer: New molecular links. Annals of the New York Academy of Sciences, 1155, 206–221. 10.1111/j.1749-6632.2009.03704.x [DOI] [PubMed] [Google Scholar]
- Boll, M. , Weber, L. W. , Becker, E. , & Stampfl, A. (2001). Mechanism of carbon tetrachloride‐induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Zeitschrift für Naturforschung C, 56(7–8), 649–659. 10.1515/znc-2001-7-826 [DOI] [PubMed] [Google Scholar]
- Chaovanalikit, A. , Thompson, M. M. , & Wrolstad, R. E. (2004). Characterization and quantification of anthocyanins and polyphenolics in blue honeysuckle (Lonicera caerulea L.). Journal of Agricultural and Food Chemistry, 52, 848–852. 10.1021/jf030509o [DOI] [PubMed] [Google Scholar]
- Cheeseman, K. H. , & Slater, T. F. (1993). An introduction to free radical biochemistry. British Medical Bulletin, 49, 481–493. 10.1093/oxfordjournals.bmb.a072625 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Xin, X. , Yuan, Q. , Su, D. , & Liu, W. (2014). Phytochemical properties and antioxidant capacities of various colored berries. Journal of the Science of Food and Agriculture, 94, 180–188. 10.1002/jsfa.6216 [DOI] [PubMed] [Google Scholar]
- Cheng, N. , Ren, N. , Gao, H. , Lei, X. , Zheng, J. , & Cao, W. (2013). Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4‐induced acute liver damage in mice. Food and Chemical Toxicology, 55, 234–240. 10.1016/j.fct.2012.11.022 [DOI] [PubMed] [Google Scholar]
- Cordero‐Pérez, P. , Torres‐González, L. , Aguirre‐Garza, M. , Camara‐Lemarroy, C. , Guzmán‐de la Garza, F. , Alarcón‐Galván, G. , … Muñoz‐Espinosa, L. E. (2013). Hepatoprotective effect of commercial herbal extracts on carbon tetrachloride‐induced liver damage in Wistar rats. Pharmacognosy Research, 5, 150–156. 10.4103/0974-8490.112417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Yang, X. , Lu, X. , Chen, J. , & Zhao, Y. (2014). Protective effects of polyphenols‐enriched extract from Huangshan Maofeng green tea against CCl4‐induced liver injury in mice. Chemico‐Biological Interactions, 220(C), 75–83. 10.1016/j.cbi.2014.06.018 [DOI] [PubMed] [Google Scholar]
- Das, K. , & Chainy, G. B. (2001). Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochimica et Biophysica Acta, 1537, 1–13. [DOI] [PubMed] [Google Scholar]
- Ebaid, H. , Bashandy, S. A. , Alhazza, I. M. , Rady, A. , & El‐Shehry, S. (2013). Folic acid and melatonin ameliorate carbon tetrachloride‐induced hepatic injury, oxidative stress and inflammation in rats. Nutrition and Metabolism, 10, Article No. 20 10.1186/1743-7075-10-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy, N. M. , Al‐Sayed, E. , Abdel‐Daim, M. M. , Karonen, M. , & Singab, A. N. (2016). Protective effect of Terminalia muelleri against carbon tetrachloride‐induced hepato and nephro‐toxicity in mice and characterization of its bioactive constituents. Pharmaceutical Biology, 54, 303–313. 10.3109/13880209.2015.1035794 [DOI] [PubMed] [Google Scholar]
- Ferreira, E. A. , Gris, E. F. , Felipe, K. B. , Correia, J. F. , Cargnin‐Ferreira, E. , Wilhelm Filho, D. , & Pedrosa, R. C. (2010). Potent hepatoprotective effect in CCl4‐induced hepatic injury in mice of phloroacetophenone from Myrcia multiflora . Libyan Journal of Medicine, 5, Article No. 4891 10.3402/ljm.v5i0.4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, J. G. , Cohen, B. J. , & Loew, F. M. (1984). Laboratory animal medicine. Orlando, FL: Academic Press Inc.. [Google Scholar]
- Han, B. , Gao, Y. , Wang, Y. , Wang, L. , Shang, Z. , Wang, S. , & Pei, J. (2016). Protective effect of a polysaccharide from Rhizoma Atractylodis Macrocephalae on acute liver injury in mice. International Journal of Biological Macromolecules, 87, 85–91. 10.1016/j.ijbiomac.2016.01.086 [DOI] [PubMed] [Google Scholar]
- Hartley, D. P. , Kolaja, K. L. , Reichard, J. , & Petersen, D. R. (1999). 4‐Hydroxynonenal and malondialdehyde hepatic protein adducts in rats treated with carbon tetrachloride: Immunochemical detection and lobular localization. Toxicology and Applied Pharmacology, 161, 23–33. 10.1006/taap.1999.8788 [DOI] [PubMed] [Google Scholar]
- Ishak, K. , Baptista, A. , Bianchi, L. , Callea, F. , De Groote, J. , Gudat, F. , … Thaler, H. (1995). Histological grading and staging of chronic hepatitis. Journal of Hepatology, 22, 696–699. 10.1016/0168-8278(95)80226-6 [DOI] [PubMed] [Google Scholar]
- Jain, N. K. , Lodhi, S. , Jain, A. , Nahata, A. , & Singhai, A. K. (2011). Effects of Phyllanthus acidus (L.) Skeels fruit on carbon tetrachloride‐induced acute oxidative damage in livers of rats and mice. Zhong Xi Yi Jie He Xue Bao, 9, 49–56. 10.3736/jcim [DOI] [PubMed] [Google Scholar]
- Jamall, I. S. , & Smith, J. C. (1985). Effects of cadmium on glutathione peroxidase, superoxidase dismutase and lipid peroxidation in the rat heart: A possible mechanism of cadmium cardiotoxicity. Toxicology and Applied Pharmacology, 80, 33–42. 10.1016/0041-008X(85)90098-5 [DOI] [PubMed] [Google Scholar]
- Jiang, W. , Gao, M. , Sun, S. , Bi, A. , Xin, Y. , Han, X. , … Luo, L. (2012). Protective effect of L‐theanine on carbon tetrachloride‐induced acute liver injury in mice. Biochemical and Biophysical Research Communications, 422, 344–350. 10.1016/j.bbrc.2012.05.022 [DOI] [PubMed] [Google Scholar]
- Jin, X. H. , Ohgami, K. , Shiratori, K. , Suzuki, Y. , Koyama, Y. , Yoshida, K. , … Ohno, S. (2006). Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide‐induced inflammation in vitro and in vivo. Experimental Eye Research, 82, 860–867. 10.1016/j.exer.2005.10.024 [DOI] [PubMed] [Google Scholar]
- Jurgoński, A. , Juśkiewicz, J. , & Zduńczyk, Z. (2013). An anthocyanin‐rich extract from Kamchatka honeysuckle increases enzymatic activity within the gut and ameliorates abnormal lipid and glucose metabolism in rats. Nutrition, 29, 898–902. 10.1016/j.nut.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Kang, S. J. , Lee, J. E. , Lee, E. K. , Jung, D. H. , Song, C. H. , Park, S. J. , … Lee, Y. J. (2014). Fermentation with Aquilariae Lignum enhances the anti‐diabetic activity of green tea in type II diabetic db/db mouse. Nutrients, 6, 3536–3571. 10.3390/nu6093536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavutcu, M. , Canbolat, O. , Oztürk, S. , Olcay, E. , Ulutepe, S. , Ekinci, C. , … Durak, I. (1996). Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin‐treated guinea pigs: Effects of vitamins E and C. Nephron, 72, 269–274. 10.1159/000188853 [DOI] [PubMed] [Google Scholar]
- Ki, S. H. , Yang, J. H. , Ku, S. K. , Kim, S. C. , Kim, Y. W. , & Cho, I. J. (2013). Red ginseng extract protects against carbon tetrachloride‐induced liver fibrosis. Journal of Ginseng Research, 37, 45–53. 10.5142/jgr.2013.37.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Jang, E. J. , Seo, H. L. , Ku, S. K. , Lee, J. R. , Shin, S. S. , … Kim, Y. W. (2014). Sauchinone attenuates liver fibrosis and hepatic stellate cell activation through TGF‐β/Smad signaling pathway. Chemico‐Biological Interactions, 224C, 58–67. 10.1016/j.cbi.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Levene, A. (1981). Pathological factors influencing excision of tumours in the head and neck. Part I”. Clinical Otolaryngology and Allied Sciences, 6, 145–151. 10.1111/j.1365-2273.1981.tb01800.x [DOI] [PubMed] [Google Scholar]
- Lowry, O. H. , Rosenbrough, N. J. , Farr, A. L. , & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275. [PubMed] [Google Scholar]
- Lu, Y. , Hu, D. , Ma, S. , Zhao, X. , Wang, S. , Wei, G. , … Wang, J. (2016). Protective effect of wedelolactone against CCl4‐induced acute liver injury in mice. International Immunopharmacology, 34, 44–52. 10.1016/j.intimp.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Ludbrook, J. (1997). Update: Microcomputer statistics packages. A personal view. Clinical and Experimental Pharmacology and Physiology, 24, 294–296. 10.1111/j.1440-1681.1997.tb01823.x [DOI] [PubMed] [Google Scholar]
- Messarah, M. , Boumendjel, A. , Chouabia, A. , Klibet, F. , Abdennour, C. , Boulakoud, M. S. , & Feki, A. E. (2010). Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Experimental and Toxicologic Pathology, 62, 301–310. 10.1016/j.etp.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Nunez, G. , Benedict, M. A. , Hu, Y. , & Inohara, N. (1998). Caspases: The proteases of the apoptotic pathway. Oncogene, 17, 3237–3245. 10.1038/sj.onc.1202581 [DOI] [PubMed] [Google Scholar]
- Odabasoglu, F. , Cakir, A. , Suleyman, H. , Aslan, A. , Bayir, Y. , Halici, M. , & Kazaz, C. (2006). Gastroprotective and antioxidant effects of usnic acid on indomethacin‐induced gastric ulcer in rats. Journal of Ethnopharmacology, 103, 59–65. 10.1016/j.jep.2005.06.043 [DOI] [PubMed] [Google Scholar]
- Pacher, P. , Beckman, J. S. , & Liaudet, L. (2007). Nitric oxide and peroxynitrite in health and disease. Physiological Reviews, 87, 315–424. 10.1152/physrev.00029.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palíková, I. , Valentová, K. , Oborná, I. , & Ulrichová, J. (2009). Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. Journal of Agricultural and Food Chemistry, 57, 6584–6589. 10.1021/jf9003994 [DOI] [PubMed] [Google Scholar]
- Park, S. I. , Lee, Y. J. , Choi, S. H. , Park, S. J. , Song, C. H. , & Ku, S. K. (2016). Therapeutic effects of blue honeysuckle on lesions of hyperthyroidism in rats. American Journal of Chinese Medicine, 44, 1441–1456. 10.1142/S0192415X16500804 [DOI] [PubMed] [Google Scholar]
- Pinto, C. , Duque, A. L. , Rodríguez‐Galdón, B. , Cestero, J. J. , & Macías, P. (2012). Xanthohumol prevents carbon tetrachloride‐induced acute liver injury in rats. Food and Chemical Toxicology, 50, 3405–3412. 10.1016/j.fct.2012.07.035 [DOI] [PubMed] [Google Scholar]
- Pradeep, K. , Mohan, C. V. , Anand, K. G. , & Karthikeyan, S. (2005). Effect of pretreatment of Cassia fistula Linn. leaf extract against subacute CCl4 induced hepatotoxicity in rats. Indian Journal of Experimental Biology, 43, 526–530. [PubMed] [Google Scholar]
- Sedlak, J. , & Lindsay, R. H. (1968). Estimation of total, protein‐bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical Biochemistry, 25, 192–205. 10.1016/0003-2697(68)90092-4 [DOI] [PubMed] [Google Scholar]
- Sigala, F. , Theocharis, S. , Sigalas, K. , Markantonis‐Kyroudis, S. , Papalabros, E. , Triantafyllou, A. , … Andreadou, I. (2006). Therapeutic value of melatonin in an experimental model of liver injury and regeneration. Journal of Pineal Research, 40, 270–279. 10.1111/j.1600-079X.2005.00310.x [DOI] [PubMed] [Google Scholar]
- Smathers, R. L. , Galligan, J. J. , Stewart, B. J. , & Petersen, D. R. (2011). Overview of lipid peroxidation products and hepatic protein modification in alcoholic liver disease. Chemico‐Biological Interactions, 192C, 107–112. 10.1016/j.cbi.2011.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulson, M. E. , Pang, D. , Jung, M. , Dimtchev, A. , Chasovskikh, S. , Spoonde, A. , … Dritschilo, A. (1998). Irreversible binding of poly‐(ADP) ribose polymerase cleavage product to DNA ends revealed by atomic force microscopy: Possible role in apoptosis. Cancer Research, 58, 3495–3498. [PubMed] [Google Scholar]
- Smyth, P. G. , Berman, S. A. , & Bursztajn, S. (2002). Markers of apoptosis: Methods for elucidating the mechanism of apoptotic cell death from the nervous system. BioTechniques, 32, 648–665. 10.2144/02323dd02 [DOI] [PubMed] [Google Scholar]
- Sodikoff, C. H. (1995). Laboratory profiles of small animal diseases: A guide to laboratory diagnosis (pp. 1–36). St. Louise, MO: Mosby. [Google Scholar]
- Srivastava, A. , & Shivanandappa, T. (2010). Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride‐induced oxidative stress in rats. Food Chemistry, 118, 411–417. 10.1016/j.foodchem.2009.05.014 [DOI] [Google Scholar]
- Subudhi, U. , Das, K. , Paital, B. , Bhanja, S. , & Chainy, G. B. (2008). Alleviation of enhanced oxidative stress and oxygen consumption of L‐thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chemico‐Biological Interactions, 173, 105–114. 10.1016/j.cbi.2008.02.005 [DOI] [PubMed] [Google Scholar]
- Sun, H. , Chen, L. , Zhou, W. , Hu, L. , Li, L. , Tu, Q. , … Wang, H. (2011). The protective role of hydrogen‐rich saline in experimental liver injury in mice. Journal of Hepatology, 54, 471–480. 10.1016/j.jhep.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Larry, W. O. , & Ying, L. (1988). A simple method for clinical assay of superoxide dismutase. Clinical Chemistry, 34, 497–500. [PubMed] [Google Scholar]
- Svarcova, I. , Heinrich, J. , & Valentova, K. (2007). Berry fruits as a source of biologically active compounds: The case of Lonicera caerulea . Biomedical Papers of the Medical Faculty of the University Palacky, Olomouc, Czechoslovakia, 151, 163–174. 10.5507/bp.2007.031 [DOI] [PubMed] [Google Scholar]
- Svobodová, A. , Rambousková, J. , Walterová, D. , & Vostálová, J. (2008). Protective effects of phenolic fraction of blue honeysuckle fruits against UV A‐induced damage to human keratinocytes. Archives of Dermatological Research, 300, 225–233. 10.1007/s00403-008-0850-5 [DOI] [PubMed] [Google Scholar]
- Tajima, Y. (1989). Biological reference data book on experimental animals. Tokyo, Japan: Soft Science Inc.. [Google Scholar]
- Talwar, S. , Jagani, H. V. , Nayak, P. G. , Kumar, N. , Kishore, A. , Bansal, P. , … Nandakumar, K. (2013). Toxicological evaluation of Terminalia paniculata bark extract and its protective effect against CCl4‐induced liver injury in rodents. BMC Complementary and Alternative Medicine, 13, Article no. 127 10.1186/1472-6882-13-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco, C. , Oliver, F. J. , de Murcia, G. , & Menissier‐de Murcia, J. (1998). DNA repair defect in poly (ADP‐ribose) polymerase‐deficient cell lines. Nucleic Acids Research, 26, 2644–2649. 10.1093/nar/26.11.2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas‐Mendoza, N. , Madrigal‐Santillán, E. , Morales‐González, A. , Esquivel‐Soto, J. , Esquivel‐Chirino, C. , García‐Luna, Y. , … Morales‐González, J. A. (2014). Hepatoprotective effect of silymarin. World Journal of Hepatology, 6, 144–149. 10.4254/wjh.v6.i3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti, P. , & Di Meo, S. (2006). Thyroid hormone‐induced oxidative stress. Cellular and Molecular Life Sciences, 63, 414–434. 10.1007/s00018-005-5457-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videla, L. A. (2000). Energy metabolism, thyroid calorigenesis, and oxidative stress: Functional and cytotoxic consequences. Redox Report, 5, 265–275. 10.1179/135100000101535807 [DOI] [PubMed] [Google Scholar]
- Voldstedlund, M. , Tranum‐Jensen, J. , Handberg, A. , & Vinten, J. (1995). Quantity of Na/K‐ATPase and glucose transporters in the plasma membrane of rat adipocytes is reduced by in vivo triiodothyronine. European Journal of Endocrinology, 133, 626–634. 10.1530/eje.0.1330626 [DOI] [PubMed] [Google Scholar]
- Vostálová, J. , Galandáková, A. , Palíková, I. , Ulrichová, J. , Doležal, D. , Lichnovská, R. , … Rajnochová Svobodová, A. (2013). Lonicera caerulea fruits reduce UVA‐induced damage in hairless mice. Journal of Photochemistry and Photobiology B, 128, 1–11. 10.1016/j.jphotobiol.2013.07.024 [DOI] [PubMed] [Google Scholar]
- Wang, R. , Feng, X. , Zhu, K. , Zhao, X. , & Suo, H. (2016). Preventive activity of banana peel polyphenols on CCl4‐induced experimental hepatic injury in Kunming mice. Experimental and Therapeutic Medicine, 11, 1947–1954. 10.3892/etm.2016.3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. H. , Wang, Y. N. , Ge, J. Y. , Liu, H. Y. , Zhang, H. J. , Qi, Y. , … Cui, X. L. (2013). Role of activin A in carbon tetrachloride‐induced acute liver injury. World Journal of Gastroenterology, 19, 3802–3809. 10.3748/wjg.v19.i24.3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, L. W. , Boll, M. , & Stampfl, A. (2003). Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Critical Reviews in Toxicology, 33, 105–136. 10.1080/713611034 [DOI] [PubMed] [Google Scholar]
- Wellington, K. , & Jarvis, B. (2001). Silymarin: A review of its clinical properties in the management of hepatic disorders. BioDrugs: Clinical Immunotherapeutics, Biopharmaceuticals and Gene Therapy, 15, 465–489. 10.2165/00063030-200115070-00005 [DOI] [PubMed] [Google Scholar]
- Yang, J. , Li, Y. , Wang, F. , & Wu, C. (2010). Hepatoprotective effects of apple polyphenols on CCl4‐induced acute liver damage in mice. Journal of Agricultural and Food Chemistry, 58, 6525–6531. 10.1021/jf903070a [DOI] [PubMed] [Google Scholar]
- Yang, B. Y. , Zhang, X. Y. , Guan, S. W. , & Hua, Z. C. (2015). Protective effect of procyanidin B2 against CCl4‐induced acute liver injury in mice. Molecules, 20, 12250–12265. 10.3390/molecules200712250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H. , Zheng, L. , Yin, L. , Xu, L. , Qi, Y. , Han, X. , … Peng, J. (2014). Protective effects of the total saponins from Dioscorea nipponica Makino against carbon tetrachloride‐induced liver injury in mice through suppression of apoptosis and inflammation. International Immunopharmacology, 19, 233–244. 10.1016/j.intimp.2014.01.019 [DOI] [PubMed] [Google Scholar]
- Zdařilová, A. , Svobodova, A. R. , Chytilová, K. , Šimánek, V. , & Ulrichová, J. (2010). Polyphenolic fraction of Lonicera caerulea L. fruits reduces oxidative stress and inflammatory markers induced by lipopolysaccharide in gingival fibroblasts. Food and Chemical Toxicology, 48, 1555–1561. [DOI] [PubMed] [Google Scholar]
- Zhao, H. , Wang, Z. , Ma, F. , Yang, X. , Cheng, C. , & Yao, L. (2012). Protective effect of anthocyanin from Lonicera caerulea var. edulis on radiation‐induced damage in mice. International Journal of Molecular Sciences, 13, 11773–11782. 10.3390/ijms130911773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, J. , Qi, F. , Ye, L. , & Yao, S. (2016). Protective Role of grape seed proanthocyanidins against CCl4 induced acute liver injury in mice. Medical Science Monitor, 22, 880–889. 10.12659/MSM.895552 [DOI] [PMC free article] [PubMed] [Google Scholar]


