Abstract
Objectives
To assess neurodevelopment of breastfed HIV-exposed uninfected (HEU) and breastfed HIV-unexposed (HU) children in the context of universal maternal antiretroviral therapy (ART).
Design
Prospective study with antenatal enrolment and follow-up of breastfeeding HEU and HU mother-infant pairs through 12–18 months postpartum.
Setting
Peri-urban community, Cape Town, South Africa.
Subjects
HEU (n=215) and HU (n=306) children.
Main outcome measures
Cognitive, motor and language development at median 13 (IQR 12–14) months of age: continuous and dichotomous BSID-III scores (Bayley Scales of Infant and Toddler Development 3rd edition; delay defined as composite score <85)
Results
Incidence of preterm delivery (PTD, <37 weeks) was similar among HEU and HU children (11% vs. 9%, p=0.31; median gestation 39 weeks); 48% were boys. Median breastfeeding duration was shorter among HEU vs. HU children (6 vs. 10 months). All HIV-infected mothers initiated lifelong ART (TDF/FTC/EFV) antenatally. HEU (vs. HU) children had higher odds of cognitive delay [OR 2.28 (95%CI 1.13–4.60)] and motor delay [OR 2.10 (95% CI 1.03–4.28)], but not language delay, in crude and adjusted analysis. PTD modified this relationship for motor development: compared to term HU, term HEU children had similar odds of delay, preterm HU children had 5-fold increased odds of delay (aOR 4.73, 95% CI 1.32; 16.91) and preterm HEU children, 16-fold (aOR 16.35, 95% CI 5.19; 51.54).
Conclusions
Young HEU children may be at increased risk for cognitive and motor delay despite universal maternal ART and breastfeeding; those born preterm may be particularly vulnerable.
Keywords: HIV, Prevention of mother to child transmission, HIV-exposed uninfected children, Preterm birth, Child developmen, Africa
INTRODUCTION
With the rapid expansion of lifelong, triple-drug antiretroviral therapy (ART) across sub-Saharan Africa, the incidence of pediatric HIV infection is declining while a large and growing proportion of the region’s children are born perinatally HIV-exposed but uninfected (HEU). In some areas, HEU newborns constitute 20–30% of births annually, and there is growing concern regarding the potential adverse health outcomes in this specific group of children.[1, 2]
HEU children may be at higher risk of neurodevelopmental delays than their HIV-unexposed (HU) counterparts.[3] While findings have been inconsistent, [4–9] neurodevelopmental delays across cognitive, motor and/or language domains have been documented among preschool HEU children, [3, 10–12] with grade repetition,[13] poor school grades,[14] reduced working memory profiles[15] and lower IQ scores[16] reported among school-age children. However, data come predominantly from non-breastfeeding populations in high income countries, and/or predate the widespread availability of universal ART (treatment for all, irrespective of disease stage) in resource-limited settings.[17] In addition, inferences have been limited by the scarcity of appropriately sampled HU control groups from the same communities, inadequate consideration of psycho-social and environmental confounders including alcohol and drug use in pregnancy, as well as inconsistent use of standardized, validated assessment tools.[3]
As a result, there is a clear need for comparison of early development in HEU and HU infants and young children under conditions of breastfeeding with universal maternal ART, particularly from settings with high HIV prevalence. To address this gap, we compared cognitive, motor and language development in a well-characterized, prospective cohort of young, breastfed HU, and HEU children born to women who initiated universal ART in pregnancy, in Cape Town, South Africa.
METHODS
Study design and population
HIV-infected women and HEU children were participants of the Maternal and Child Health Antiretroviral Therapy study (MCH-ART; 2013–2016), a prospective study of strategies to improve postpartum adherence and retention in ART care.[18] HIV-uninfected women and HU children were participants of the HIV-unexposed-uninfected mother and child health study (HU2; 2014–2017), a prospective cohort study specifically designed to complement MCH-ART, using the same study structure, design, staff and measures.[18] HIV-uninfected women, and HIV-infected women initiating ART (tenofovir-emtricitabine-efavirenz, TDF/FTC/EFV) in pregnancy, were followed from first antenatal clinic visit, through pregnancy to delivery, and with their breastfed children, until 12–18 months postpartum. Study methodology has been described elsewhere.[18] Briefly, after enrolment in pregnancy, women attended 1–3 antenatal study visits and were asked to return within 7 days postpartum. Breastfeeding mother-infant pairs were eligible for continued postnatal follow-up, with visits scheduled at 6 weeks; 3, 6, 9 and 12 months. MCH-ART participants returned for a final visit at 18 months. At the final or near-final study visit, eligible children (11–18 months old, HIV-uninfected, gestational age at birth ≥ 28 weeks, without known congenital abnormalities or severe cerebral palsy) of consenting mothers from both studies received a single developmental assessment.
Study setting
Research was based at a primary health care center in Gugulethu, a peri-urban township in Cape Town, South Africa. The facility serves a population of about 350 000, with an estimated 30% antenatal HIV seroprevalence.[19] The Gugulethu Midwife Obstetric Unit (MOU) provides antenatal and obstetric care, and universal ART to all HIV-infected pregnant women since 2013.[19, 20] Study visits, including developmental assessments, were conducted at the research unit adjacent to, but separate from, routine care.
Measurements
Trained interviewers administered questionnaires to both groups of women. Study-specific questionnaires, identical except for HIV-related items, asked about pregnancy intentions, maternal demographic and health information, and psycho-social measures including alcohol/drug use (AUDIT, alcohol use disorders identification test; DUDIT, drug use disorders identification test)[21, 22], depression (Edinburgh postnatal depression scale, EPDS)[23] and experiences of intimate partner violence (IPV; WHO Violence against women questionnaire)[24]. After delivery, additional questionnaires assessed infant feeding practices, maternal-infant health and demographics. Obstetric, child health and laboratory data were abstracted from medical records. In addition to HIV-related phlebotomy and developmental assessments, clinical measurements included antenatal ultrasound at enrolment, repeated at 20–22 weeks for fetal anomalies where possible, and during the third trimester. Maternal-infant anthropometry was measured at all postnatal visits, with gestation-adjusted Z-scores generated using the Intergrowth-21st growth reference standards.[25]
Routine PMTCT services conducted antenatal HIV counseling and testing (HCT) using a rapid finger-prick test (Alere Determine®). Positive women provided serum for CD4 cell count and HIV viral load, and all initiated ART (TDF/FTC/EFV) at the MOU.[18] HIV-exposed children received HIV-PCR testing to exclude MTCT at 6 weeks and 12 months.[18] HU2 mothers received repeat HCT via routine health services during and after pregnancy. At final study visit, all HU2 mothers had repeat HCT at the study site.
Cognitive, motor and language development was assessed using the Bayley Scales of Infant and Toddler Development®, Third Edition (BSID-III), which has been validated in South Africa.[26, 27] Developmental assessments were conducted by either a paediatric occupational therapist or a child health physician; all assessors received systematic supervised training in the use of BSID-III and were assisted by a trained, isi-Xhosa-speaking counsellor. Composite cognitive, motor and language scores were generated from cognitive, fine and gross motor, and expressive language subscale scores using BSID-III normative and conversion tables, which account for gestation at delivery.[26] Receptive language testing using standardized BSID-III tools proved contextually challenging; throughout, results represent expressive language scores only. For interrater reliability, video-graphed assessment scores were compared between assessors, generating estimates for interrater variability (correlation coefficients and percent agreement) per developmental domain. Correlation coefficients for cognitive and motor scores were above 0.9; language ranged from 0.7 to 0.98. There was perfect agreement between the binary classifications of some vs. no delay in all three domains.
Several known risk factors for maternal HIV acquisition may also be independent determinants of development in early childhood.[28, 29] Potential confounders identified a priori for this analysis included maternal age, education, relationship status, pregnancy intentions and socio-economic status (employment and housing). Psychosocial measures included alcohol use (risky drinking at enrolment and/or in late pregnancy, AUDIT-C score ≥ 3), postpartum depression (EPDS score ≥ 13 at enrolment and/or 6 weeks), and IPV (any violence reported at enrolment). We also assessed infant sex, gestational age and anthropometry at birth, giving special consideration to the role of preterm delivery (PTD, <37 weeks’ gestation) given its potential mediating role in the HIV-exposure-development relationship. Postnatal factors included breastfeeding duration and at 12 months, maternal smoking and child attendance at a nursery.
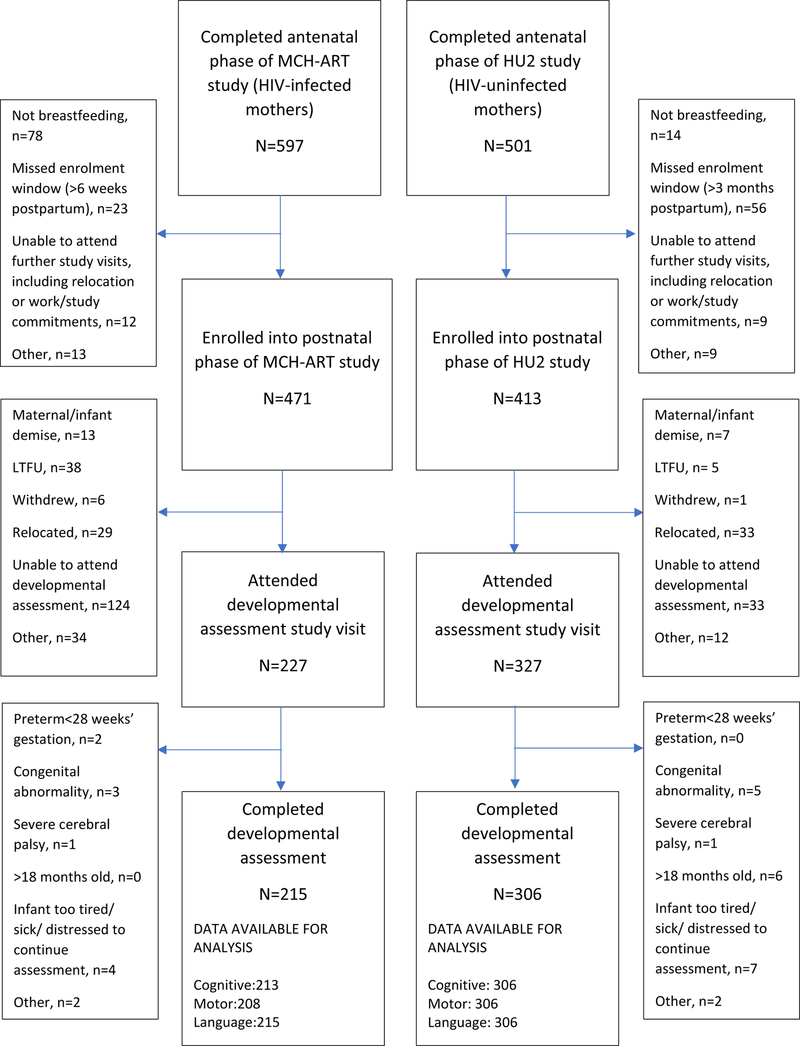
Loss to follow-up was minimized through use of telephonic contact and household tracing. Systematic differences between those with and without developmental assessments within strata of maternal HIV status were explored and findings are interpreted accordingly (figure 1; Supplemental digital content 3, table).
FIGURE 1. Study flow diagram.
Statistical methodology
BSID-III composite scores generally have an expected mean (standard deviation, SD) of 100 (15).[26] While these expected values are based on the US-based reference population, similar expected values have been reported in low-resourced settings including South Africa.[27] In clinical practice, a BSID-III score below 1 SD from the mean (<85) typically indicates some delay and below 2SD, severe delay.[30] We estimated that an overall sample size of 500, including 200–250 HEU children, would achieve >90% power to detect a mean difference of ≥ 5 points (0.33 of SD).
Data were analyzed using Stata 14.0 (Statacorp, College Station TX). Composite scores were analyzed in continuous and binary form (score <85 indicating “any” delay).[30] For between-group comparisons of severity, delay was further categorized into [(i) no delay (composite score ≥85); (ii) mild/moderate delay (≥70, <85); and (iii) severe delay (<70)].[30] Exposure-outcome relationships were explored graphically and tested using correlation coefficients, Kruskal-Wallis or chi2 tests as appropriate. Categorization of continuous variables followed published boundaries where available, or locally weighted regression plots. Absolute differences in mean composite scores and relative odds of delay were obtained from linear and logistic regression, respectively. Multivariable model selection was based on improvements in Akaike’s information criterion (AIC) building on a null model that included variables chosen a priori (maternal education, alcohol use and IPV; infant gestational age at birth, birth-size and duration of breastfeeding; directed acyclic graph, supplemental digital content 1). Based on a priori hypotheses, effect modification was assessed between HIV-exposure and (i) gestation at birth, (ii) infant sex and (iii) duration of breastfeeding; effect modification by other variables was tested as exploratory analysis. In sensitivity analyses, we examined the HIV-exposure-development relationship among relatively “healthy” children (term, appropriate-for-gestational age (AGA); no maternal IPV, risky drinking or substance use; breastfed for at least 6 months; HIV-infected maternal pre-ART CD4 ≥ 200 cells/mm3).
Ethical considerations
Both MCH-ART and HU2 are approved by the University of Cape Town’s Faculty of Health Sciences Research Ethics Committee (UCT-HREC, 567/2014; 451/2012).
RESULTS
Overall, 521 mother-infant pairs contributed to this analysis (HEU, n=215; HU, n=306; figure 1, table 1). HIV-infected women (median nadir CD4 cell count 346 cells/mm3; 75% with HIV viral suppression < 50 copies/mL at delivery) were significantly less likely to have completed high school (27% vs. 46%, p<0.0001), and more likely to report risky drinking (29% vs. 8%, p<0.0001) and IPV (20% vs. 8%, p<0.0001) at first antenatal visit, than HIV-uninfected women. One HIV-infected mother reported drug use in pregnancy. Comparing HEU to HU children, there were no significant differences in gestation at delivery [median 39 (IQR 39–40) weeks in both groups], the incidence (13% vs. 9%, p=0.31) or relative odds of PTD [OR 1.49 (95%CI 0.85; 2.59)]. Similar proportions of HEU and HU children were born small-for-gestational age (SGA, <10th percentile). Median duration of breastfeeding was shorter among HEU than HU children (6 vs. 10 months, p=0.0004). Differences in maternal and infant characteristics by preterm delivery-HIV exposure status reflected the overall differences between HEU and HU children (table, Supplemental digital content 2). HU children contributing to these analyses were largely representative of the larger HU cohort (table, supplemental digital content 3). HEU children contributing to analyses had somewhat older mothers and better living conditions than those not included in the analysis (table, supplemental digital content 3). In both HEU and HU groups, children included in the analyses had longer median duration of breastfeeding than those not included, partly due to breastfeeding censoring at last attended study visit.
TABLE 1.
Maternal and infant characteristics of HIV-exposed uninfected (HEU) and HIV-unexposed (HU) children with completed neurodevelopmental assessments
| Total (N=521) |
HIV-infected women and HEU children (n=215) |
HIV-uninfected women and HU children (n=306) |
p-value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age in years | 28 (24 – 33) | 29 (25–33) | 28 (24–32) | 0.07 |
| Married/cohabiting | 227 (44%) | 89 (41%) | 138 (45%) | 0.40 |
| Completed secondary education | 202 (39%) | 59 (27%) | 143 (46%) | <0.0001 |
| Employed | 225 (43%) | 81 (38%) | 144 (47%) | 0.03 |
| Formal housing | 281 (54%) | 119 (55%) | 162 (53%) | 0.59 |
| Primigravida | 110 (21%) | 30 (14%) | 80 (26%) | 0.001 |
| Planned pregnancy | 163 (31%) | 54 (25%) | 109 (36%) | 0.01 |
| Risky drinking, enrolment1 | 85 (16%) | 61 (29%) | 24 (8%) | <0.0001 |
| Risky drinking, 3rd trimester1 | 27 (5%) | 25 (12%) | 2 (<1%) | <0.0001 |
| Any drug use, 3rd trimester1 | 1 (<1%) | 1 (<1%) | 0 | - |
| Intimate partner violence2 | 66 (13%) | 43 (20%) | 23 (8%) | <0.0001 |
| Depression, enrolment3 | 39 (7%) | 19 (9%) | 20 (7%) | 0.31 |
| Depression, 6 weeks postpartum3 | 17 (3%) | 9 (4%) | 8 (2%) | 0.33 |
| Log10 HIV viral load at ART initiation (copies/mL) | - | 4.1 (3.6–4.6) | - | - |
| CD4 cell count at ART initiation (cells/mm3) | - | 346 (235–522) | - | - |
| Birth and infant characteristics | ||||
| Gestational age at delivery (weeks) | 39 (38 – 40) | 39 (38 – 40) | 39 (38 – 40) | 0.72 |
| Term (≥37 weeks) | 465 (89%) | 187 (87%) | 278 (91%) | 0.31 |
| Late preterm (≥34 to <37) | 32 (6%) | 17 (8%) | 15 (5%) | |
| Preterm (≥28 to <34) | 24 (5%) | 11 (5%) | 13 (5%) | |
| Caesarian section delivery | 183 (35%) | 63 (29%) | 120 (39%) | 0.02 |
| Male | 252 (48%) | 114 (53%) | 138 (45%) | 0.08 |
| Birth weight for age, Z-score4 | −0.1 (−0.8 to 0.5) | −0.2 (−0.9 to 0.4) | 0 (−0.8 to 0.6) | 0.02 |
| Small for gestational age | 64 (12%) | 29 (14%) | 35 (11%) | 0.48 |
| Birth head circumference for age, Z-score4 | 0.7 (−0.3 to 1.5) | 0.4 (−0.6 to 1.4) | 0.8 (−0.1 to 1.7) | 0.004 |
| Duration of any breastfeeding (months) | 9 (3–12) | 6 (1–12) | 10 (3–12) | 0.0004 |
| Age at assessment (months) | 13 (12–14) | 13 (13–14) | 13 (12–14) | 0.30 |
| Attending nursery/creche at time of assessment5 | 71 (14%) | 23 (11%) | 48 (16%) | 0.11 |
Values are median (interquartile range) or n (column %); p-values are based on Kruskal-Wallis or chi2 and are not corrected for multiple testing
Hazardous drinking, defined as Alcohol use disorders identification test (AUDIT-C) score ≥3 as reported at first antenatal visit and at approximately 34 weeks’ gestation (missing data, n=2); Drug use defined as any Drug use disorders identification test (DUDIT) score > 0, at approximately 34 weeks’ gestation (missing data, n=12)
Any physical, sexual or psychological violence as measured with World Health Organization violence against women questionnaire at first antenatal visit (missing data, n=3)
Maternal depression, EPDS (Edinburgh postnatal depression scale) score of ≥ 13 at first antenatal visit and at 6 weeks’ postpartum (missing data, n=16)
Corrected for gestational age at birth, calculated using Intergrowth-21st reference standards (missing data for birth length, n=9; birth head circumference, n=11)
Maternal self-report; missing data, n=1
There were no significant differences between HEU compared to HU children in median cognitive scores [100 (IQR 95–110) vs. 100 (IQR 95–110)], motor scores [97 (IQR 89–107) vs. 97 (IQR 91–103)] or language scores [94 (IQR 89–112) vs. 100 (IQR 94–106)]. Average scores were comparable to the BSID reference standards (table, supplemental digital content 4). A larger proportion of HEU than HU children demonstrated any delay (composite score <85) in cognitive and motor domains [HEU vs. HU: 10% vs. 5%, relative risk (RR) 2.15 (95% CI 1.12;4.14); and 9% vs. 5% (RR 2.00, 95% CI 1.02;3.89), respectively]. Risk of language delay was similar between HEU and HU children (RR 1.23, 95% CI 0.83; 1.83). Among children with scores <85, a very small number had severe delay (score <70), with no substantial differences noted between HEU and HU children (tables, supplemental digital content 5–7).
Cognitive development
Overall, the average cognitive scores of HEU and HU children were similar (table 2). However, in both crude and adjusted logistic regression models, HEU children were twice as likely to be diagnosed with any cognitive delay compared to HU children [adjusted odds ratio, aOR 2.56 (95% CI 1.22; 5.40), table 3]. Increasing gestational age at birth was protective in both linear and logistic regression (tables 2 and 3). There was some evidence for interaction between HIV exposure and preterm delivery on the odds of cognitive delay (figure 2a).
TABLE 2.
Linear regression analysis of BSID-III composite scores comparing HIV-exposed uninfected (HEU) to HIV-unexposed (HU) children on cognitive, motor and language* development.
| Variable | Cognitive | Motor | Language*1 | |||
|---|---|---|---|---|---|---|
| β (95% CI) | aβ (95% CI) | β (95% CI) | aβ (95% CI) | β (95% CI) | aβ (95% CI) | |
| Maternal HIV (HEU vs. HU)2 | 0.31 (−2.07; 2.69) | 0.64 (−1.89; 3.16) | 0.55 (−1.84; 2.95) | 1.23 (−1.28; 3.74) | 1.97 (−0.6; 4.54) | 2.68 (−0.08; 5.43) |
| Gestation at delivery (weeks) | 1.19 (0.64; 1.75) | 1.20 (0.63; 1.76) | 1.35 (0.79; 1.91) | 1.35 (0.78; 1.92) | 0.50 (−0.10; 1.11) | 0.51 (−0.11; 1.12) |
| Sex: male vs. female2 | 1.09 (−1.26; 3.43) | - | 1.33 (−1.02; 3.68) | - | −0.12 (−2.65; 2.42) | - |
| Weight-for-age Z-score at birth3 | 0.63 (−0.58; 1.84) | - | −0.38 (−1.60; 0.83) | - | −0.43 (−1.74; 0.88) | - |
| Small-for-gestational age4 | −2.83 (−6.39; 0.72) | −2.78 (−6.36; 0.81) | −0.92 (−4.53; 2.70) | −0.20 (−3.80; 3.40) | −0.86 (−4.72; 3.00) | −1.16 (−5.09; 2.77) |
| Maternal age ≥ 30 years5 | 1.59 (−0.81; 3.99) | - | 1.76 (−0.65; 4.17) | - | −0.12 (−2.72; 2.48) | - |
| Maternal education6 | 0.43 (−1.98; 2.83) | 0.65 (−1.82; 3.13) | −0.61 (−3.02; 1.81) | −0.73 (−3.20; 1.73) | 1.78 (−0.81; 4.38) | 2.50 (−0.18; 5.18) |
| Maternal employment7 | 0.16 (−2.21; 2.52) | - | 0.07 (−2.30; 2.44) | - | −0.28 (−2.84; 2.28) | - |
| Informal housing8 | −1.41 (−3.76; 0.94) | −1.69 (−4.06; 0.67) | −1.81 (−4.16; 0.55) | −2.48 (−4.83; −0.13) | −1.33 (−3.87; 1.21) | - |
| Planned pregnancy9 | 0.34 (−2.19; 2.87) | - | −1.15 (−3.68; 1.38) | - | −1.43 (−4.16; 1.30) | - |
| Intimate partner violence10 | −0.65 (−4.20; 2.90) | −1.19 (−4.86; 2.47) | −2.45 (−5.99; 1.09) | −2.77 (−6.39; 0.86) | 0.49 (−3.33; 4.30) | 0.36 (−3.61; 4.34) |
| Risky drinking11 | 0.14 (−3.04; 3.31) | 0.88 (−2.52; 4.28) | −2.17 (−5.39; 1.04) | −1.30 (−4.71; 2.11) | −0.50 (−3.93; 2.94) | −1.40 (−5.11; 2.31) |
| Postpartum depression12 | 3.08 (−3.54; 9.70) | - | −3.07 (−9.73; 3.59) | - | −1.94 (−9.15; 5.27) | - |
| Breastfeeding duration (months)13 | 0.11 (−0.11; 0.34) | 0.09 (−0.13; 0.31) | 0.19 (−0.04; 0.41) | 0.15 (−0.08; 0.37) | 0.02 (−0.22; 0.26) | 0.01 (−0.24; 0.25) |
Based on expressive language only
Test for HIV-exposure/infant sex interaction in multivariable model: cognitive, p=0.79; motor, p=0.96; language, p=0.77
Intergrowth-21 reference standards
<10th vs. ≥ 10th percentile
vs. <30 years of age
completed vs. did not complete secondary schooling
any employment vs. none
vs. brick housing
vs. unplanned pregnancy
any physical, sexual or psychological violence reported at first antenatal visit vs. none;
Alcohol use disorders identification test (AUDIT-C) score ≥3 vs. <3, first antenatal visit
EPDS (Edinburgh postnatal depression scale) score >=13 vs. <13 at 6 weeks’ postpartum study visit
Based on maternal self-report, last study visit date at which any breastfeeding was reported used as date of breastfeeding cessation
TABLE 3.
Logistic regression analysis of odds of developmental delay (Bayley scales of infant development composite score<85), comparing HIV-exposed uninfected (HEU) to HIV-unexposed (HU) children on cognitive, motor and language* development
| Variable | Cognitive | Motor | Language*1 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | |
| Maternal HIV (HEU vs. HU) | 2.28 (1.13; 4.60) | 2.56 (1.22; 5.40) | 2.10 (1.03; 4.28) | 1.59 (0.70; 3.64) | 1.28 (0.80; 2.05) | 0.95 (0.56; 1.60) |
| Gestation at delivery (weeks) | 0.82 (0.72; 0.93) | 0.82 (0.72; 0.93) | 0.68 (0.60; 0.78) | 0.66 (0.57; 0.76) | 0.99 (0.89; 1.11) | 1.00 (0.89; 1.12) |
| Maternal-HIV/preterm categories | ||||||
| Term, HIV-unexposed (ref) | Reference | - | Reference | - | Reference | - |
| Term, HIV-exposed | 2.14 (0.96; 4.77) | - | 1.56 (0.64; 3.82) | - | 1.55 (0.93; 2.57) | - |
| Preterm, HIV-unexposed | 2.91 (0.76; 11.13) | - | 4.47 (1.30; 15.32) | - | 2.69 (1.10; 6.56) | - |
| Preterm, HIV-exposed | 6.62 (2.24; 19.6) | - | 14.19 (5.09; 39.56) | - | 0.81 (0.23; 2.81) | - |
| Sex: male vs. female2 | 1.15 (0.58; 2.28) | - | 0.90 (0.44; 1.83) | - | 1.08 (0.67; 1.73) | - |
| Weight-for-age Z-score at birth3 | 0.91 (0.64; 1.30) | - | 1.01 (0.70; 1.46) | - | 0.95 (0.74; 1.21) | - |
| Small-for-gestational age4 | 0.91 (0.31; 2.67) | 0.92 (0.30; 2.78) | 1.33 (0.49; 3.58) | 1.38 (0.47; 4.05) | 1.60 (0.84; 3.06) | 1.92 (0.97; 3.80) |
| Maternal age ≥ 30 years5 | 1.73 (0.87; 3.43) | -x | 0.90 (0.43; 1.87) | - | 1.61 (1.00; 2.59) | 1.78 (1.09; 2.90) |
| Maternal education6 | 0.93 (0.46; 1.89) | 1.08 (0.51; 2.29) | 0.66 (0.31; 1.43) | 0.74 (0.31; 1.77) | 0.46 (0.26; 0.78) | 0.40 (0.23; 0.70) |
| Maternal employment7 | 0.76 (0.37; 1.54) | - | 0.96 (0.47; 1.96) | - | 0.97 (0.60; 1.57) | - |
| Informal housing8 | 1.43 (0.71; 2.84) | - | 1.62 (0.80; 3.31) | 2.39 (1.06; 5.40) | 1.14 (0.71; 1.82) | - |
| Planned pregnancy9 | 1.01 (0.48; 2.12) | - | 0.94 (0.44; 2.03) | - | 0.72 (0.42; 1.23) | - |
| Intimate partner violence10 | 1.17 (0.44; 3.13) | 1.20 (0.42; 3.43) | 3.34 (1.51; 7.40) | 3.63 (1.43; 9.23) | 1.21 (0.62; 2.38) | 1.06 (0.51; 2.17) |
| Risky drinking11 | 0.64 (0.22; 1.86) | 0.44 (0.14; 1.38) | 2.09 (0.93; 4.67) | 1.17 (0.45; 3.04) | 1.55 (0.87; 2.78) | 1.43 (0.74; 2.74) |
| Postpartum depression12 | n/a | - | 1.94 (0.42; 8.85) | - | 1.14 (0.32; 4.07) | - |
| Breastfeeding duration (months)13 | 1.01 (0.95; 1.08) | 1.02 (0.96; 1.09) | 0.95 (0.89; 1.01) | 0.97 (0.90; 1.04) | 0.97(0.93; 1.02) | 0.97 (0.93; 1.02) |
Based on expressive language only
HIV exposure/infant sex interaction terms (multivariable models): cognitive, p=0.39; motor, p=0.23; language, p=0.85
Intergrowth-21 reference standards
<10th vs. ≥ 10th percentile
vs. <30 years of age
completed vs. did not complete secondary schooling
any employment vs. none
vs. brick housing
vs. unplanned pregnancy
any physical, sexual or psychological violence reported at first antenatal visit vs. none
Alcohol use disorders identification test (AUDIT-C) score ≥3 vs. <3, first antenatal visit
EPDS (Edinburgh postnatal depression scale) score >=13 vs. <13 at 6 weeks’ postpartum study visit; odds ratio for cognitive delay not calculable due to null cell
Based on maternal self-report, last study visit date at which any breastfeeding was reported used as date of breastfeeding cessation
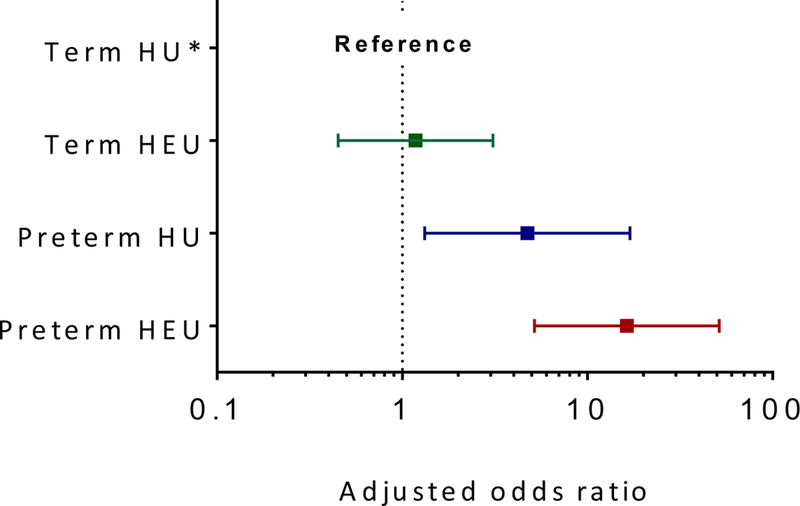
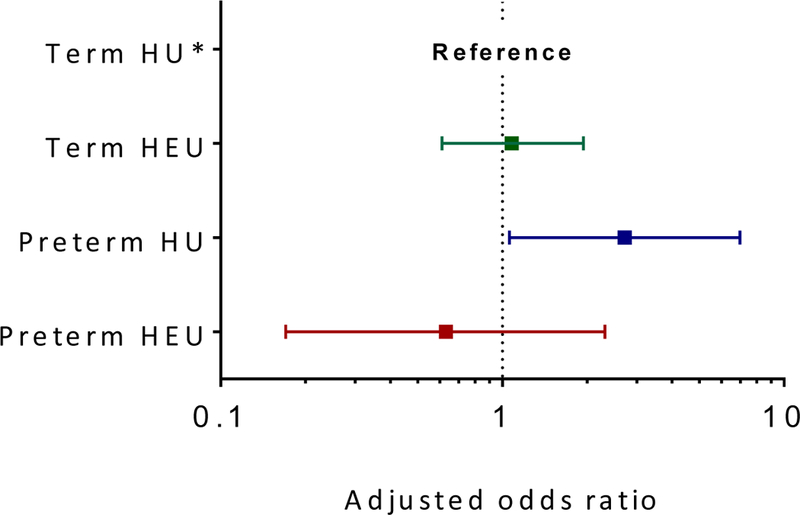
FIGURE 2. Forest plots of adjusted odds ratios for developmental delay (BSID-III composite scores <85) by maternal HIV status and preterm delivery with term HIV-unexposed children as reference category across (a) cognitive, (b) motor and (c) language domains.
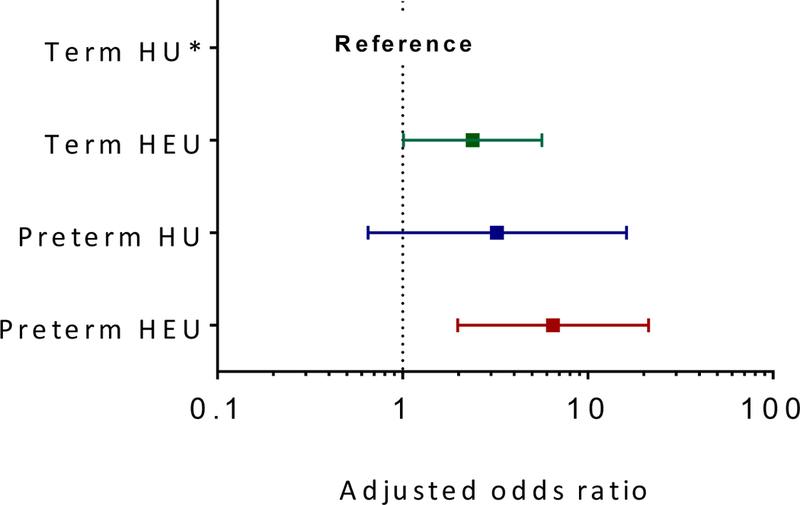
(a) Adjusted OR (95% CI) for cognitive delay in term HIV-exposed uninfected children, 2.52 (1.09; 5.83); preterm HIV-unexposed children, 3.30 (0.85; 12.78); and preterm HIV-exposed uninfected children, 8.25 (2.69; 25.28) [Reference group, term HIV-unexposed children; model adjusted for maternal education, intimate partner violence, risky drinking, infant size (small-for-gestational-age) and duration of breastfeeding; p-value for interaction = 0.15]
(b). Adjusted OR (95% CI) for motor delay in term HIV-exposed uninfected children, 1.17 (0.45; 3.07); preterm HIV-unexposed children, 4.73 (1.32; 16.91); and preterm HIV-exposed uninfected children, 16.35 (5.19; 51.54) [Reference group, term HIV-unexposed children; model adjusted for maternal education, housing, intimate partner violence, risky drinking, infant size (small-for-gestational-age) and duration of breastfeeding; p-value for interaction = 0.07]
(c). Adjusted OR (95% CI) for language delay in term HIV-exposed uninfected children, 1.14 (0.65; 1.98); preterm HIV-unexposed children, 2.49 (1.00; 6.29); and preterm HIV-exposed uninfected children, 0.65 (0.18; 2.37). [Reference group, term HIV-unexposed children; model adjusted for maternal education, maternal age, intimate partner violence, risky drinking, infant size (small-for-gestational-age) and duration of breastfeeding; p-value for interaction = 0.04]
Motor development
HEU and HU children had similar mean motor scores [β 0.55 (95% CI −1.84; 2.95), table 2], but HEU children were at higher odds for any motor delay [(OR 2.10 (95% CI 1.03; 4.28), table 3]. The latter association was attenuated (aOR 1.59, 95% CI 0.70; 3.64) after adjusting for several other significant predictors of motor development including gestational age, informal housing and IPV (table 3). There was evidence for interaction between HIV-exposure and gestational age (figure 2b). While term HEU children had similar odds of motor delay compared to the reference group of term HU children (aOR 1.17, 95% CI 0.45; 3.07), preterm delivery increased the odds of motor delay almost 5-fold among HU children (preterm HU vs. term HU: aOR 4.73, 95% CI 1.32; 16.91) while the combination of both HIV exposure and preterm delivery increased the odds 16-fold (preterm HEU vs. term HU: aOR 16.35, 95% CI 5.19; 51.54; figure 2b).
Language development
Overall, HEU children had an average 2.8 point higher composite language score than their HU counterparts (aβ 2.8; 95% CI 0.08; 5.59; table 2). Compared to term HU, preterm HU were at higher odds of any language delay (aOR 2.49, 95% CI 1.00; 6.29) but the odds of delay were similar comparing either term HEU or preterm HEU to term HU (figure 2c).
Sensitivity analyses
Point estimates for relative odds of any delay comparing “healthy” HEU (n=48) to similar HU (n=160) children approximated that of the full cohort for all three domains (table, supplemental digital content 8): OR (95% CI) for cognitive delay, 2.21 (0.69;7.10); motor delay, 2.28 (0.37;14.03); and language delay, 1.40 (0.57;3.42). In exploratory subgroup analysis, the effects of HIV-exposure on child development varied somewhat within strata of various maternal-child characteristics (tables and figures, supplemental digital content 9–12).
DISCUSSION
Compared to HIV-unexposed community controls, we observed increased odds of cognitive and motor, but not language, delay among young, breastfed HEU children born to women who initiated universal ART in pregnancy. Overall, median developmental scores of HEU children approximated those of HU children. That is, although the average scores of HEU and HU children were similar at a group level, there was an excess of minor deficits detectable among HEU children. Severe delays were scarce, and equally distributed between the groups.
Our findings are in keeping with results from several other studies, including a recent meta-analysis of developmental outcomes in young children with HIV-infected mothers.[3, 10–12, 14, 15] Notably, this analysis included only one African study where mothers received ART during pregnancy.[3] Our findings contrast with some other recent African studies. A large cohort study in Botswana found no substantial differences between HEU and HU children at 24 months of age.[6] Maternal ART in pregnancy was restricted to women with low CD4 cell count (36% of all HIV-infected women); only 10% of HEU children were breastfed. However, living conditions were significantly better than in our cohort, and only 6% of HIV-infected women reported any prior alcohol use compared to almost 30% in ours. It may be that despite better access to ART and prolonged breastfeeding in our cohort, differences in socio-economic conditions and alcohol exposure disproportionately predisposed our HEU children towards developmental vulnerability. In a South African cohort (all mothers receiving ART; 40% of HEU children breastfeeding by 2 weeks; similar living conditions and antenatal use of alcohol) no differences in mean BSID-III composite cognitive, motor or language scores were seen comparing HEU to HU children at 12 months of age.[9] However, a larger proportion of HEU than HU children had some evidence of developmental delay (composite score <85) in cognitive (HEU vs. HU, 9% vs. 0%) and language (HEU vs. HU, 28% vs. 18%) domains; precision was limited due to relatively small sample size.
We found no differences in language delay between HEU and HU children. However, language assessment in a multicultural setting is difficult, and the use of US-designed BSID-III language tests may not be optimal for language assessment in this setting. Reassuringly, average language scores in our cohort approximated those of the US reference group.[26] Nevertheless, assessments were conducted at a young age, when much reliance is on sounds rather than words or grammar, particularly in expressive language testing. As recently demonstrated among Kenyan HEU children, subtle differences in language development may only become detectable at an older age, underscoring the importance of repeated developmental assessments throughout childhood and adolescence.[31]
Taken together, these data indicate that breastfed HEU children born to women initiating universal ART in pregnancy may be at increased risk for some developmental delay, which is identifiable at a young age. However, delays appear to be in the mild-moderate range and associated with similar risk factors as neurodevelopmental delays in HU children.[32]
We observed a strong positive relationship between gestation at birth and neurodevelopment, reflecting findings from HIV-uninfected populations globally. [33] In our cohort, children born both preterm and HEU had the highest relative odds of motor and cognitive delay. Similar synergistic effects have been described among very preterm HIV-uninfected infants, with the highest risks of delay observed among those who were also SGA and had evidence of systemic inflammation.[34] These interaction effects can be explained by the so-called “two-hit” hypothesis, wherein intrauterine insult(s) increase vulnerability to later perinatal insults.[34, 35] Our findings are particularly concerning given the known association between maternal HIV infection and preterm delivery, potentially compounded by maternal use of ART.[36, 37]
There is biological plausibility for a relationship between maternal HIV infection and neurodevelopmental delay in HEU children. The immune system plays a critical role in brain development and homeostasis.[38] Neuroinflammation, including pathological microglial activation, may disrupt early brain development.[39, 40] A growing body of evidence from HIV-unrelated epidemiological, preclinical and clinical studies points to antenatal maternal immune activation (mIA) as an important risk factor for offspring neurodevelopmental disorders.[39, 40] Immune activation and inflammation are hallmarks of HIV infection itself; chronic inflammation can persist despite suppressive ART, particularly among those with microbial translocation and microbiome dysbiosis.[41, 42] Additionally, maternal viral co-infections such as CMV typically exacerbate immune activation in both mothers and infants, while congenital CMV infection has direct effects on the developing brain. [43, 44] In utero exposure to mIA may partly explain the pro-inflammatory immunological changes typically observed among HEU infants.[45] In animal models, perinatal neuroinflammation has consistently been associated with white matter damage.[46] Concordantly, two recent studies using diffusion tensor imaging described alterations in white matter when comparing otherwise healthy HEU and HU children.[47, 48] White matter changes are also typical of perinatal brain injury in preterm infants, with the worst injuries described among those who also had in utero exposure to mIA.[46, 49] Thus in HEU children, particularly those born to women with viral co-infections and/or altered microbiota, neuroinflammation may be a mechanism of developmental delay, and further research is required to better understand these and other related causal pathways.
To our knowledge, this is the first large study of neurodevelopment among young, breastfed HEU children who were all born to relatively healthy women initiating universal ART in pregnancy. Unlike many of the large, US-based studies, our cohort was homogenous in the use of a single WHO first-line ART regimen.[50] In addition, we were able to obtain detailed longitudinal measures of several major determinants of developmental outcomes in early childhood, with a large group of community-control HU comparators sampled and followed using the same methodology. We used a comprehensive, robust and validated measuring tool, supported by demonstration of reliability in quality assurance. Nonetheless, our findings need to be interpreted in the light of several limitations. Without measures of maternal-infant inflammation and viral co-infections we were unable to assess underlying causal mechanisms. Our inferences on language development are limited by the lack of receptive language measures. We assessed development cross-sectionally, among a subgroup of HEU children who were still in follow-up a year after birth, and whose mothers were willing to return for the assessment. All women received good perinatal care including ART for those with HIV infection, the majority of whom achieved viral suppression before delivery. Furthermore, all children in our cohort were breastfed; breastfeeding promotes neurodevelopment [51]. As such, our findings may underestimate differences between HEU and HU children in less fortunate settings. Simultaneously, our findings may not extend to populations with lower levels of antenatal alcohol use and IPV.
HEU children are vulnerable, at least partly due to social determinants of disease that cluster with maternal HIV infection, but possibly also via exposure to maternal HIV. Although our data adds significantly to the knowledge base of HEU child development at a young age, little is known about the long-term effects of in utero exposure to maternal HIV in the context of universal ART and breastfeeding. As such, continued follow-up and assessment throughout childhood and adolescence will be critical. Finally, our data highlight challenging environments for many families in settings such as ours, including those of HIV-uninfected women and their children. Without effectively addressing the broader social determinants of health, efforts to improve childhood developmental trajectories in resource-limited settings are unlikely to succeed.
CONCLUSIONS
Despite universal ART during pregnancy and breastfeeding, HEU children may be at increased risk of cognitive and motor delays. Early developmental screening and intervention programs are clearly warranted for this growing group of vulnerable children, prioritizing those born preterm. Data are required on neurodevelopment of HEU children born to women who initiated suppressive ART prior to conception.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by PEPFAR through NICHD under Cooperative Agreement 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council, the Fogarty Foundation (NIH Fogarty International Center Grant #5R25TW009340) and the Office of AIDS Research.
SLR was responsible for implementation and management of the HU2 study, assisted with collection of data, conducted the analyses and wrote the first draft of the manuscript. KD provided training and supervision of developmental assessments; KD and MK provided supervision for all child health aspects of the study. KB and TKP were responsible for data management and oversight. TKP and KN were the study coordinators. AZ was the senior study manager and provided oversight of all study administration processes. AS conducted developmental assessments and assisted with training and data management. LM and EJA conceived the MCH-ART study, and were responsible for study design, funding, implementation and overall leadership. All authors contributed to and approved the final manuscript.
source of funding: This research was supported by PEPFAR through NICHD under Cooperative Agreement 1R01HD074558. Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council, the Fogarty Foundation (NIH Fogarty International Center Grant #5R25TW009340) and the Office of AIDS Research.
Footnotes
Conflicts of interest: The authors have no conflict of interest to declare.
REFERENCES
- 1.UNICEF. State of the World’s children: A fair chance for every child 2016.
- 2.UNAIDS. Global AIDS update. Ending AIDS: Progress towards the 90-90-90 targets 2017.
- 3.McHenry MS, McAteer CI, Oyungu E, McDonald BC, Bosma CB, Mpofu PB, et al. Neurodevelopment in Young Children Born to HIV-Infected Mothers: A Meta-analysis. Pediatrics 2018. [DOI] [PMC free article] [PubMed]
- 4.Rochat TJ, Houle B, Stein A, Coovadia H, Coutsoudis A, Desmond C, et al. Exclusive Breastfeeding and Cognition, Executive Function, and Behavioural Disorders in Primary School-Aged Children in Rural South Africa: A Cohort Analysis. PLoS Med 2016,13:e1002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajan R, Seth A, Mukherjee SB, Chandra J. Development assessment of HIV exposed children aged 6–18 months: a cohort study from North India. AIDS Care 2017,29:1404–1409. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhury S, Williams PL, Mayondi GK, Leidner J, Holding P, Tepper V, et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Pediatrics 2017,140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngoma MS, Hunter JA, Harper JA, Church PT, Mumba S, Chandwe M, et al. Cognitive and language outcomes in HIV-uninfected infants exposed to combined antiretroviral therapy in utero and through extended breast-feeding. AIDS 2014,28:S323–S330. [DOI] [PubMed] [Google Scholar]
- 8.Boivin MJ, Barlow-Mosha L, Chernoff MC, Laughton B, Zimmer B, Joyce C, et al. Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS 2018,32:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Springer PE, Slogrove AL, Laughton B, Bettinger JA, Saunders HH, Molteno CD, et al. Neurodevelopmental outcome of HIV-exposed but uninfected infants in the Mother and Infants Health Study, Cape Town, South Africa. Trop Med Int Health 2018,23:69–78. [DOI] [PubMed] [Google Scholar]
- 10.Smith ML, Puka K, Sehra R, Read SE, Bitnun A. Longitudinal development of cognitive, visuomotor and adaptive behavior skills in HIV uninfected children, aged 3–5 years of age, exposed pre- and perinatally to anti-retroviral medications. AIDS Care 2017,29:1302–1308. [DOI] [PubMed] [Google Scholar]
- 11.Boivin M, Maliwichi-Senganimalunje L, Nyakoto M, Sikorskii A, Ogwang LW, Kawalazira R, et al. Neurodevelopment of Ugandan and Malawian PROMISE exposed and unexposed uninfected children at 12 and 24 months of age. In: International AIDS Society Meeting Durban, South Africa. [Google Scholar]
- 12.Van Rie A, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr 2009,52:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell JM, Rochat TJ, Houle B, Stein A, Newell ML, Bland RM. The effect of maternal and child early life factors on grade repetition among HIV exposed and unexposed children in rural KwaZulu-Natal, South Africa. J Dev Orig Health Dis 2016,7:185–196. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson L, Chisenga M, Siame J, Kasonka L, Filteau S. Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC Pediatr 2015,15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milligan R, Cockcroft K. Working Memory Profiles in HIV-Exposed, Uninfected and HIV-Infected Children: A Comparison with Neurotypical Controls. Front Hum Neurosci 2017,11:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr SJ, Puthanakit T, Vibol U, Aurpibul L, Vonthanak S, Kosalaraksa P, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care 2014,26:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach Second edition.; 2016http://apps.who.int/iris/bitstream/10665/246200/1/9789241511124-eng.pdf (accessed 28 July 2016). [PubMed]
- 18.Myer L, Phillips TK, Zerbe A, Ronan A, Hsiao NY, Mellins CA, et al. Optimizing Antiretroviral Therapy (ART) for Maternal and Child Health (MCH): Rationale and Design of the MCH-ART Study. JAIDS 2016,72 Suppl 2:S189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myer L, Phillips T, Manuelli V, McIntyre J, Bekker LG, Abrams EJ. Evolution of antiretroviral therapy services for HIV-infected pregnant women in Cape Town, South Africa. J Acquir Immune Defic Syndr 2015. [DOI] [PMC free article] [PubMed]
- 20.Department of Health RoSA. The South African Antiretroviral Treatment Guidelines 2013 (PMTCT guidelines revised March 2013) In; 2013.
- 21.WHO. AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care. 2nd Edition. World Health Organization; Department of Mental Health and Substance Dependence; 2001http://apps.who.int/iris/bitstream/10665/67205/1/WHO_MSD_MSB_01.6a.pdf (accessed 23 June 2016). [Google Scholar]
- 22.Berman AH, Bergman H, Palmstierna T, Schlyter F. Evaluation of the Drug Use Disorders Identification Test (DUDIT) in criminal justice and detoxification settings and in a Swedish population sample. Eur Addict Res 2005,11:22–31. [DOI] [PubMed] [Google Scholar]
- 23.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987,150:782–786. [DOI] [PubMed] [Google Scholar]
- 24.García-Moreno C, Jansen HAFM, Ellsberg M, Heise L, Watts C. WHO Multi-country Study on Women’s Health and Domestic Violence against Women: Initial results on prevalence, health outcomes and women’s responses. WHO 2005.
- 25.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014,384:857–868. [DOI] [PubMed] [Google Scholar]
- 26.Bayley N Bayley Scales of Infant and Toddler Development - third edition. Pearson clinical assessments, San Antonio, TX: The Psychological Corporation 2006. [Google Scholar]
- 27.Rademeyer V, Jacklin L. A study to evaluate the performance of black South African urban infants on the Bayley Scales of Infant Development III. South AFrican Journal of Child Health 2013,7. [Google Scholar]
- 28.McKinnon LR, Karim QA. Factors Driving the HIV Epidemic in Southern Africa. Curr HIV/AIDS Rep 2016,13:158–169. [DOI] [PubMed] [Google Scholar]
- 29.Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, et al. Nurturing care: promoting early childhood development. Lancet 2017,389:91–102. [DOI] [PubMed] [Google Scholar]
- 30.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014,75:670–674. [DOI] [PubMed] [Google Scholar]
- 31.Abubakar A, Holding P, Van Baar A, Newton CR, Van de Vijver FJ, Espy KA. The performance of children prenatally exposed to HIV on the A-not-B task in Kilifi, Kenya: a preliminary study. Int J Environ Res Public Health 2013,10:4132–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter LM, Daelmans B, Lombardi J, Heymann J, Boo FL, Behrman JR, et al. Investing in the foundation of sustainable development: pathways to scale up for early childhood development. Lancet 2017,389:103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res 2013,74 Suppl 1:17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leviton A, Fichorova RN, O’Shea TM, Kuban K, Paneth N, Dammann O, et al. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr Res 2013,73:362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O. Cerebral Palsy-Trends in Epidemiology and Recent Development in Prenatal Mechanisms of Disease, Treatment, and Prevention. Front Pediatr 2017,5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med 2016,375:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zash R, Jacobson DL, Diseko M, Mayondi G, Mmalane M, Essex M, et al. Comparative Safety of Antiretroviral Treatment Regimens in Pregnancy. JAMA Pediatr 2017,171:e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilbo SD, Schwarz JM. The immune system and developmental programming of brain and behavior. Front Neuroendocrinol 2012,33:267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 2014,10:643–660. [DOI] [PubMed] [Google Scholar]
- 40.Mottahedin A, Ardalan M, Chumak T, Riebe I, Ek J, Mallard C. Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front Cell Neurosci 2017,11:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koay WLA, Siems LV, Persaud D. The microbiome and HIV persistence: implications for viral remission and cure. Curr Opin HIV AIDS 2018,13:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hileman CO, Funderburg NT. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Curr HIV/AIDS Rep 2017,14:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filteau S, Rowland-Jones S. Cytomegalovirus Infection May Contribute to the Reduced Immune Function, Growth, Development, and Health of HIV-Exposed, Uninfected African Children. Front Immunol 2016,7:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianella S, Letendre S. Cytomegalovirus and HIV: A Dangerous Pas de Deux. J Infect Dis 2016,214 Suppl 2:S67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Altfeld M, Bunders MJ. Impact of HIV-1 infection on the feto-maternal crosstalk and consequences for pregnancy outcome and infant health. Semin Immunopathol 2016,38:727–738. [DOI] [PubMed] [Google Scholar]
- 46.Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, et al. The role of inflammation in perinatal brain injury. Nat Rev Neurol 2015,11:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jankiewicz M, Holmes MJ, Taylor PA, Cotton MF, Laughton B, van der Kouwe AJW, et al. White Matter Abnormalities in Children with HIV Infection and Exposure. Front Neuroanat 2017,11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tran LT, Roos A, Fouche JP, Koen N, Woods RP, Zar HJ, et al. White Matter Microstructural Integrity and Neurobehavioral Outcome of HIV-Exposed Uninfected Neonates. Medicine (Baltimore) 2016,95:e2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis 2014,14:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams PL, Marino M, Malee K, Brogly S, Hughes MD, Mofenson LM. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics 2010,125:e250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Horta BL, de Mola CL, Victora CG. Breastfeeding and intelligence: systematic review and meta-analysis. Acta Paediatr 2015. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.