Key Points
In peripheral T-cell lymphomas treated upfront, baseline total metabolic tumor volume and interim 5-point score are prognostic.
Interim PET response (assessed by 5-point score) further risk stratifies patients with low and high baseline clinical risk scores.
Abstract
The prognosis of peripheral T-cell lymphoma (PTCL) is heterogenous. Baseline or interim imaging characteristics may inform risk-adapted treatment paradigms. We identified 112 patients with PTCL who were consecutively treated with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP)/CHOP-like regimens with the intent to consolidate with an autologous transplant. Baseline (n = 93) and interim (after 4 cycles, n = 99) positron emission tomography (PET) images were reevaluated, and we calculated baseline total metabolic tumor volume (TMTV). Interim PET (iPET) responses were graded visually by 5-point score (i5PS) and by percentage change of standardized uptake value. By univariate analysis, predictors of event-free survival (EFS) included Prognostic Index for Peripheral TCL (PIT) higher than 1 (hazard ratio [HR], 1.83; P = .021), International Prognostic Index (IPI) higher than 3 (HR, 2.01; P = .021), high TMTV (>125 cm3; HR, 3.92; P = .003), and positive iPET (HR, 3.57; P < .001). By multivariate analysis, high baseline TMTV predicted worse overall survival (OS; HR, 6.025; P = .022) and EFS (HR, 3.861; P = .005). Patients with i5PS of 1 to 3 had a longer median OS and EFS (104 months, 64 months) than those with i5PS of 4 to 5 (19 months, 11 months; P < .001). Four-year OS and EFS for patients with i5PS of 1 to 3 and PIT of 1 or less were 85% and 62%, respectively. However, 4-year OS and EFS for those with i5PS of 4 to 5 and PIT higher than 1 were both 0% (P < .001). In multivariate analysis, after controlling for IPI and PIT, i5PS was independently prognostic for EFS (HR, 3.400 95% confidence interval, 1.750-6.750; P < .001) and OS (HR, 10.243; 95% confidence interval, 4.052-25.891; P < .001). In conjunction with clinical parameters, iPET helps risk stratify patients with PTCL and could inform risk-adapted treatment strategies. Prospective studies are needed to confirm these findings.
Visual Abstract
Introduction
Peripheral T-cell lymphomas (PTCL) are heterogenous and rare lymphomas comprising approximately 10% of all non-Hodgkin lymphomas.1 This group of diseases is often associated with an aggressive clinical course and relatively poor outcomes compared with their B-cell lymphoma counterparts.1,2 PTCL not otherwise specified (PTCL-NOS), angioimmunoblastic TCL (AITL), and anaplastic lymphoma kinase negative anaplastic large-cell lymphoma (ALK-ALCL) make up 25%, 19%, and 6% of PTCL with 5-year overall survival (OS) estimated at approximately 30% to 40%.1-3 Although there is no universally accepted approach to treatment of these disorders, many centers initially treat fit patients with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP)/CHOP-like regimens, often followed by consolidation with an autologous stem cell transplant (ASCT) in first remission.4-6 With this strategy, progression-free survival at 5 years is approximately 45%.4,7 Depth of remission at the time of transplant has been prognostic in PTCL, as well as other, aggressive lymphomas.7-9 Therefore, it is hypothesized that the degree of responsiveness to upfront chemotherapy is likely clinically important when considering further treatment and consolidation strategies. Although baseline clinical prognostic tools such as the International Prognostic Index (IPI) and Prognostic Index for Peripheral TCL (PIT) have helped risk stratify patients, but are not commonly used to guide therapy.10,11 Interim response assessment may be helpful to guide clinical decisions such as evaluating the benefit or necessity of ASCT as consolidation of first remission compared with consideration of an alternate strategy. Among patients who are not candidates for an allogeneic transplant, the median OS in those with relapsed/refractory disease is 6 months.12 Therefore, earlier identification of patients who are likely to benefit from curative treatment approaches is especially clinically relevant.
The utility of PET has been studied in patients with various TCLs.13-24 The 5-point score (5PS) using interim 18-fluordeoxyglucose (18F-FDG) positron emission tomography (PET) has been adopted in the response evaluation of many forms of aggressive lymphoma, including PTCL as a determinant of response.15,25 The 5PS appears to be a promising parameter for prognostic stratification in multiple lymphomas, including Hodgkin lymphoma, diffuse large B-cell lymphoma, and NK/TCLs.8,9,14,26 Studies of interim PET evaluation in TCL have shown that 70% of cases with PTCL show a favorable response with 5PS 1 to 3 on interim PET during initial chemotherapy. However, those who had early clinical progression would not be represented in these series, as their progression occurred before times for interim evaluation.21,22 More recently, the Response Evaluation Criteria in Lymphoma (RECIL) have emphasized the combined assessment of morphologic and functional changes on imaging studies to characterize response and predict patient outcome.27 RECIL criteria have not previously been applied in TCLs.
Although the role of total metabolic tumor volume (TMTV) analysis has been elucidated in Hodgkin lymphoma and diffuse large B-cell lymphoma, there is little known about the prognostic utility of TMTV in PTCL.8,28-30
In this retrospective study of consecutive and uniformly treated patients, we characterized treatment response based on interim 5PS and measured TMTV to better understand the potential clinical utility of these imaging parameters in PTCL. In particular, we sought to risk-stratify patients according to their baseline PET characteristics and interim response, thus setting the stage for future risk-adapted protocols in this disease.
Methods
Patients
The institutional review board approved this retrospective study and waived the informed consent requirement in accordance with the Declaration of Helsinki; the study was compliant with Health Insurance Portability and Accountability Act requirements. Through the Memorial Sloan Kettering Cancer Center lymphoma database, we identified patients who either were consecutively treated at our center at the time of initial diagnosis or were seen at our center during their initial therapy from 2001 to 2015 and met the following criteria: age, 21 years or older; a confirmed histological diagnosis of PTCL-NOS, AITL, or ALK-ALCL based on the Revised European American Lymphoma Classification, World Health Organization 2003, or World Health Organization 2008 classifications (according to the schema in use at the time of diagnosis); initial treatment with CHOP or CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone) with intent to consolidate with autologous transplantation; and at least 6 months follow-up or an event defined as relapse or death from any cause. Patients with ALK+ ALCL were excluded. In all cases, the diagnosis was confirmed by hematopathology review at Memorial Sloan Kettering Cancer Center. Clinical data collected included age, sex, histologic subtype of TCL, stage, number of extranodal sites, sites of involvement including bone marrow, level of lactate dehydrogenase, IPI score, PIT score, death, and relapse.
18F-FDG PET/computed tomography protocol
PET/computed tomography (CT) images were collected at baseline, at interim evaluation (after 4 cycles of CHOP-based therapy), and at end of treatment (EOT) before clinical decisions for ASCT, according to our institutional practice. Before injection of 18F-FDG, patients fasted for at least 6 hours. If plasma glucose levels were lower than 200 mg/dL, patients were injected IV with approximately 12 mCi radiotracer. After a standard 60- to 90-minute uptake time, 18F-FDG PET/CT was performed on various scanners (GE Discovery Series: VCT, ST, STE, and 690). The subjects were scanned while in the supine position. Cross-calibration between the dose-calibrator and the PET scanners was routinely performed. Phantom studies were used to ensure that the quantitative measure was exact and minimized standard reuptake value (SUV) differences between scanners, generally within 10%. CT images of the PET/CT were used for attenuation correction of the PET scan. The attenuation-corrected data were reconstructed using an ordered-subset expectation maximization algorithm and displayed for review using standard manufacture-supplied equipment (Advantage Workstation, GE Healthcare).
Response assessments using FDG PET and CT
All PET/CT scans were reviewed by 2 board-certified nuclear medicine physicians, and interim and EOT PET images were scored using the 5PS.31 Controversial cases and all score 3 and 4 cases were discussed by these 2 physicians, and consensus was reached. The reviewers finalized their scan interpretation based on CT and PET imaging features and known normal distribution of FDG to account for non-tumor-related FDG uptake. The PET scores were incorporated into RECIL for treatment response classification.
Quantitative PET parameters were computed by a board-certified nuclear medicine physician. Using PET-VCAR software ver.3.2 (Advantage Workstation, GE Healthcare) on dedicated workstations, the reviewer delineated the volume of interests in each region so as to obtain the SUVmax. Lesional MTV was defined as the region enclosed by a 41% isocontour around maximum PET voxel of a lesion. Contours of conglomerated masses or a series of connected lymph nodes were delineated using a semiautomatic contouring system on the software. This method used the 41% SUVmax threshold, similar to previous studies.24,25,32,33 A volume of interest was set around each lesion (node or organ involvement). The spleen was considered to be involved if there was focal uptake or diffuse uptake higher than 150% of the liver background.20 Bone marrow involvement was considered in volume measurement only if there was clear focal uptake higher than background. The TMTV for a given patient represents the sum of all individual lesional MTVs. Occasional adjustments of the 41% threshold were performed if the PET volume clearly extended beyond the lesion as seen on CT (a blooming artifact).
We also analyzed quantitative changes between baseline and follow-up PET by calculating percentage decrease in SUVmax between baseline PET and interim PET (interim ΔSUV) in 80 patients. The cutoff value of interim ΔSUVmax was set at −73% (after 4 cycles), derived from previous studies.21 In addition to metabolic parameters, we also measured the sum of the longest diameter (SLD) in target lesions. This was done using the CT images (at most 3.75 mm thickness) from the PET/CT or from a separately acquired CT scan covering the same field of view (from neck to upper thigh) and performed with or without IV contrast. Up to 3 target lesions were selected according to RECIL 2017.27 ΔSLD (baseline − interim) and ΔSLD (baseline − EOT) were calculated as percentage change. Response to chemotherapy was characterized based on ΔSLD in combination with PET response as complete response (CR), partial response (PR), stable disease (SD) including minor responses, or progressive disease.
Statistical analysis
Sensitivity, specificity, and diagnostic accuracy of FDG PET and CT parameters were calculated for diagnostic values. Median and receiver operating characteristic (ROC) curves were used to analyze imaging quantitative value to determine the cutoff point that yielded the highest combined sensitivity and specificity. To compare FDG PET and CT for prediction of outcome, patients were dichotomized into a good-response group and a poor-response group by conventional criteria.32 Important prognostic factors found to be significant by univariate analysis (P < .05) were entered simultaneously into a Cox multivariate regression analysis model. Event-free survival (EFS) was defined as time from diagnosis until progression, relapse, death from any cause, or last follow-up visit. OS was defined as time from diagnosis until death from any cause or last follow-up visit. EFS and OS were estimated by Kaplan-Meier method and compared by the log-rank test. Surviving patients were censored at last follow-up. Characteristics of the population were compared between groups, using Fisher’s exact or χ2 tests, as appropriate. Φ coefficient was used to estimate covariant of association between nominal data. P < .05 was considered statistically significant. All analyses were performed using SPSS 24.0 (SPSS, Chicago, IL).
Results
Patient characteristics
One hundred twelve patients with PTCL who were treated with the intent to pursue autologous transplant after CHOP-like chemotherapy in the upfront setting were identified. Their median age was 60 years (range, 22-79 years). Of these 112 patients, 99 had sufficient-quality interim PET/CT images for independent review. Their histologies were PTCL-NOS (n = 38), AITL (n = 40), and ALK- ALCL (n = 21) (Table 1). In these 99 patients, the median age of the cohort was 60 years, and 66 patients (67%) were male. Of the 99 patients with available interim PET, 53 patients (54%) had a PIT score of 0 to 1 and 46 patients (47%) had a PIT score of 2 to 4. In addition, 20 (20%), 61 (62%), and 18 (18%) had an IPI score of 0 to 1, 2 to 3, and 4 to 5, respectively. Other clinical parameters are summarized in Table 1. There were no significant differences between the entire cohort (n = 112) and the group of patients undergoing interim PET (n = 99; supplemental Table 1).
Table 1.
Patient characteristics of the interim PET cohort (n = 99)
| Characteristics | No. (%) | Characteristics | No. (%) |
|---|---|---|---|
| Age, y | Treatment | ||
| ≤60 | 51 (52) | CHOP/ICE | 39 (39) |
| >60 | 48 (48) | CHOEP | 22 (22) |
| Sex | EPOCH | 1 (1) | |
| Male | 66 (67) | CHOP | 21 (21) |
| Female | 33 (33) | Other | 16 (16) |
| Histology | Transplant | ||
| PTCL-NOS | 38 (38) | Autologous | 61 (62) |
| AITL | 40 (40) | Allogeneic | 6 (6) |
| ALCL, ALK− | 21 (21) | None | 32 (32) |
| Ann Arbor stage | Interim PET by 5PS | ||
| I-II | 13 (13) | 1 and 2 | 78 (79) |
| III-IV | 86 (87) | 3 | 5 (5) |
| Performance status (ECOG) | 4 and 5 | 16 (16) | |
| 0-1 | 71 (72) | EOT PET by 5PS (n=90) | |
| 2-3 | 27 (27) | 1 and 2 | 68 (76) |
| NA | 1 | 3 | 2 (2) |
| Bone marrow involvement | 4 and 5 | 20 (22) | |
| No | 72 (73) | ||
| Yes | 27 (27) | ||
| LDH | |||
| Normal | 50 (51) | ||
| High | 45 (45) | ||
| NA | 4 (4) | ||
| IPI | |||
| 0-1 | 20 (20) | ||
| 2-3 | 61 (62) | ||
| 4-5 | 18 (18) | ||
| PIT | |||
| 0 | 17 (17) | ||
| 1 | 36 (36) | ||
| 2 | 25 (25) | ||
| 3 | 16 (16) | ||
| 4 | 5 (5) |
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; NA, not available; NS, not significant.
Characterization of imaging findings
Baseline imaging
At baseline, all patients had FDG avid lesions. The median baseline SUVmax was 13.52 (range, 1.75-51.02). Baseline TMTV was calculated for 68 patients whose baseline PET/CT scans were of sufficient quality (supplemental Figure 1). The median TMTV was 188.6 cm3 (range, 2.49-1839.42 cm3). All patients had at least 1 measurable target lesion on baseline CT. The median SLD was 32.68 cm (range, 1.57-171.67 cm). There was no significant difference in survival based on the presence of extranodal involvement with regard to EFS (P = .368) or OS (P = .353). There were no differences in OS (P = .286) or EFS (P = .245) by histology. In addition, the TMTVs of 3 histological subtypes were similar.
Interim imaging
At interim, after 4 cycles of chemotherapy, 84% (83/99) of patients had a 5PS of 1 to 3, and 16% (16/99) had a 5PS of 4 or 5. (Table 1) Median SUVmax on interim PET had declined to 1.15, corresponding to median percentage decline (ΔSUV) by −92.6% (range, −100% to +10.8%).
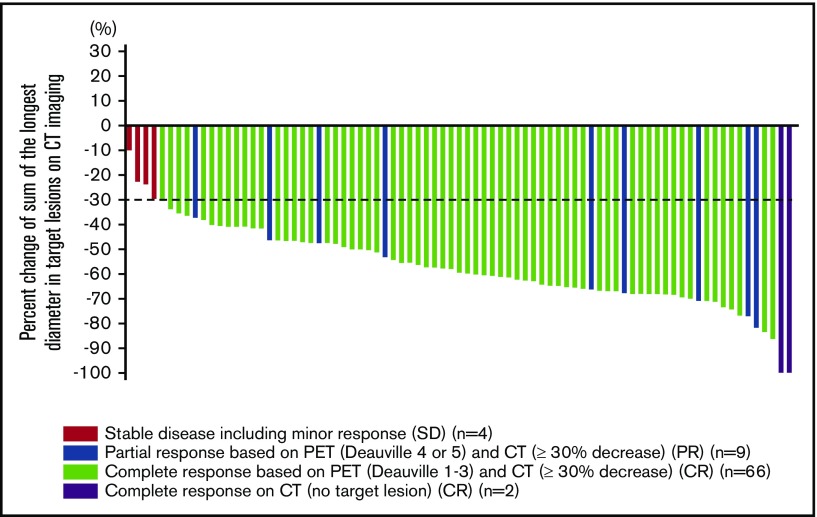
RECIL response at the interim could be calculated for 81 patients (Figure 1). The median percentage decrease of the SLD (ΔSLD) at interim was −59.7% (range, −100% to −9.9%).
Figure 1.
Waterfall plot according to RECIL in interim PET/CT (n = 81).
EOT imaging
At the EOT, 78% (70/90) of patients had a 5PS of 1 to 3, and 22% (20/90) had a 5PS of 4 or 5. According to RECIL criteria, 52 of the 70 patients with 5PS of 1 to 3 were classified as CR. In contrast, 19 of the 20 patients with 5PS of 4 or 5 were classified as SD (n = 6) or PR (n = 13); no patient had progressive disease. Among those who had CR at the EOT by REICL, 13 did not ultimately undergo an autologous transplant because of comorbidities or patient choice. Of note, 9 patients with an interim PET did not have an EOT evaluation, 7 had a PET with insufficient imaging quality for review, and 2 progressed before their EOT evaluation.
Patient outcome and identification of prognostic groups
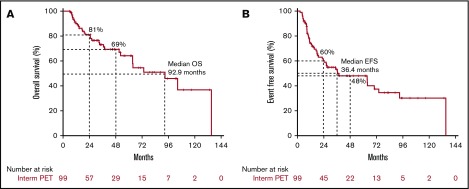
The median OS and EFS of the entire cohort were 93 and 36 months, respectively. The survival curves for the entire cohort and patients undergoing interim PET showed no significant difference (data not shown). Figure 2 shows the OS and EFS for the interim PET cohort. At 4 years, OS and EFS of this cohort were 69% and 48%.
Figure 2.
OS and EFS for the cohort (n = 99). OS (A) and EFS (B) curves in interim PET cohort.
In univariate analysis, IPI higher than 3 was predictive of OS (hazard ratio [HR], 2.13; 95% confidence interval [CI], 1.023-4.513; P = .043) and EFS (HR, 2.012; 95% CI, 1.112-3.641; P = .021). PIT higher than 1 was also predictive of EFS (HR, 1.825; 95% CI, 1.094-3.043; P = .021) and showed a trend toward being predictive of OS (HR, 1.663; 95% CI, 0.877-3.155; P = .12)
Baseline TMTV and prognosis
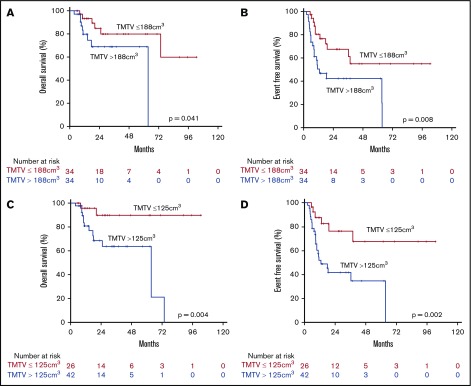
To identify subsets of patients with different outcomes as early as possible, we first assessed the prognostic value of baseline TMTV by dichotomizing patients by the median TMTV, which was 188.6 cm3. In this analysis, median TMTV proved prognostic for both OS and EFS. We applied ROC analysis to derive the optimal cutoff point to separate patients with better and poor prognosis.
The area under the curve for 4-year OS was 0.671 (95% CI, 0.53-0.81; P = .04), and for 4-year EFS it was 0.671 (95% CI, 0.54-0.80; P = .014). For both endpoints, the optimal cutoff value was 125 cm3, yielding a combined sensitivity and specificity of 88% and 46% for survival at 4 years, and 81% and 56% for EFS at 4 years, respectively (Figure 3). Low and high TMVT were defined by ROC cutpoints as less than 125 cm3 vs at least 125 cm3. TMTV higher than 125 cm3 was associated with worse OS (HR, 6.813; 95% CI, 1.511-30.725; P = .013) and EFS (HR, 3.921; 95% CI, 1.579-97.36; P = .003).
Figure 3.
OS and EFS by TMTV. OS (A) and EFS (B) curves by median (188 cm3) TMTV. OS (C) and EFS (D) curves using a ROC cutoff (125 cm3) for TMTV.
Treatment responses on interim PET and prognosis
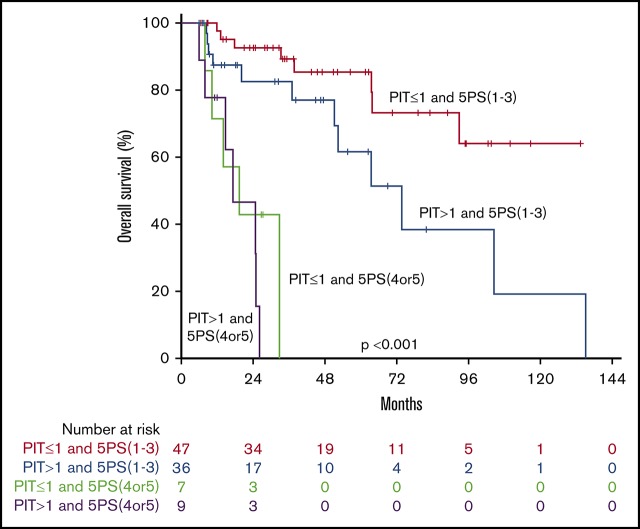
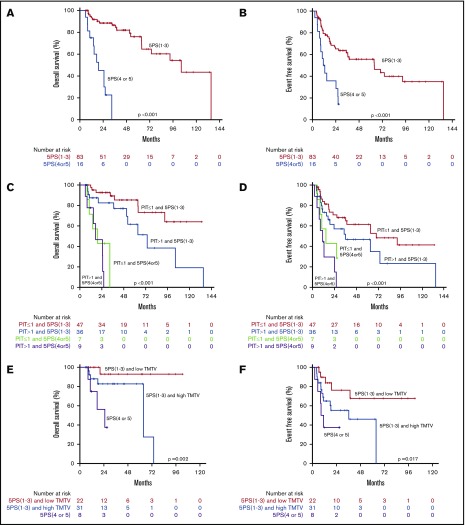
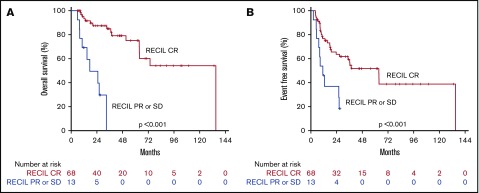
We then evaluated the prognostic value of interim scan findings. In univariate analysis, interim 5PS of 4 or 5 was predictive of worse OS (HR, 11.025; 95% CI, 4.409-27.571; P < .001) and worse EFS (HR, 3.573; 95% CI, 1.824-6.997; P < .001; Table 2). Patients with 5PS of 1 to 3 had a median OS and EFS of 104 and 64 months, respectively (Figure 4). In contrast, those with 5PS of 4 or 5 had a median OS and EFS of only 19 and 11 months, respectively. Further analyses were performed evaluating the combined prognostic significance of interim 5PS and PIT (Figure 4). By RECIL, patients with complete remission at interim evaluation had superior OS (HR, 9.699; 95% CI, 3.539-26.578; P < .001) and EFS (HR, 3.187; 95% CI, 1.508-6.736; P = .002) (Table 2; Figure 5).
Table 2.
Univariate and multivariate analysis of factors predictive of survival in patients
| Variable | OS | EFS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Univariate analysis | ||||||
| IPI | ||||||
| >3 | 2.148 | 1.023-4.513 | .043 | 2.012 | 1.112-3.641 | .021 |
| ≤3 | 1 | (reference) | 1 | (reference) | ||
| PIT | ||||||
| >1 | 1.663 | 0.877-3.155 | .12 | 1.825 | 1.094-3.043 | .021 |
| ≤1 | 1 | (reference) | 1 | (reference) | ||
| TMTV, cm3 | ||||||
| >125 | 6.813 | 1.511-30.725 | .013 | 3.921 | 1.579-9.736 | .003 |
| ≤125 | 1 | (reference) | 1 | (reference) | ||
| Interim 5PS | ||||||
| 4 or 5 | 11.025 | 4.409-27.571 | <.001 | 3.573 | 1.824-6.997 | <.001 |
| 1-3 | 1 | (reference) | 1 | (reference) | ||
| Interim ΔSUV, % | ||||||
| >−73 | 6.253 | 2.320-16.857 | <.001 | 7.076 | 3.283-15.252 | <.001 |
| ≤−73 | 1 | (reference) | 1 | (reference) | ||
| Interim RECIL | ||||||
| PR or SD | 9.699 | 3.539-26.578 | <.001 | 3.187 | 1.508-6.736 | .002 |
| CR | 1 | (reference) | 1 | (reference) | ||
| EOT 5PS | ||||||
| 4 or 5 | 3.199 | 1.502-6.814 | .003 | 3.315 | 1.808-6.076 | <.001 |
| 1-3 | 1 | (reference) | 1 | (reference) | ||
| EOT RECIL | ||||||
| PR or SD | 2.054 | 0.866-4.870 | .102 | 3.101 | 1.603-6.000 | .001 |
| CR | 1 | (reference) | 1 | (reference) | ||
| Multivariate analyses | ||||||
| TMTV, cm3 | ||||||
| >125 | 6.025 | 1.288-28.177 | .022 | 3.861 | 1.499-9.943 | .005 |
| ≤125 | 1 | (reference) | 1 | (reference) | ||
| Interim 5PS | ||||||
| 4 or 5 | 6.506 | 1.715-24.687 | .006 | 3.407 | 1.212-9.579 | .020 |
| 1-3 | 1 | (reference) | 1 | (reference) | ||
Figure 4.
Combining interim PET with clinical risk factors. OS (A) and EFS (B) curves by interim 5PS alone. OS (C) and EFS (D) curves of combined 5PS with PIT. OS (E) and EFS (F) curves of combined 5PS with metabolic tumor volume (TMTV).
Figure 5.
Survival by interim RECIL. OS (A) and EFS (B) curves by interim RECIL.
Patients with interim ΔSUVmax higher than 73% had a significantly better EFS (HR, 7.076; 95% CI, 3.283-15.282; P < .001) and OS (HR, 6.253; 95% CI, 2.320-16.857; P < .001) compared with those with a ΔSUV of 73% or less (Table 2; supplemental Figure 3). Regarding changes of nodal size at interim, the optimal cutoff point for ΔSLD (as derived from ROC analysis) to predict EFS was −53.7%; log-rank; P = .019).
Combining imaging and clinical factors to evaluate prognosis
In multivariate analysis, patients with favorable interim PET (5PS, 1-3) and PIT of 1 or less had a 4-year OS and EFS of 85% and 62% compared with those with unfavorable interim PET (5PS, 4 or 5) and PIT higher than 1 (4-year OS and EFS both 0%). In a subanalysis by Kaplan-Meier log-ranked evaluation, we confirmed that patients with a 5PS of 1 to 3 and PIT of 1 or less had significantly better OS than patients with a 5PS of 1 to 3 and PIT higher than 1 (P = .035; Figure 4; supplemental Figure 2). When controlling for PIT, interim 5PS PET was independently prognostic of EFS (HR, 3.441 95% CI, 1.730-6.684; P < .001) and OS (HR, 10.277; 95% CI, 4.073-25.931; P < .001). When controlling for IPI, interim 5PS PET was independently prognostic of EFS (HR, 3.437 95% CI, 1.750-6.750; P < .001) and OS (HR, 10.616; 95% CI, 4.216-26.733; P < 001). Similarly, in a multivariate analysis, when controlling for IPI and PIT, interim 5PS PET was independently prognostic of EFS (HR, 3.400 95% CI, 1.750-6.750; P < .001) and OS (HR, 10.243; 95% CI, 4.052-25.891; P < .001; Table 2).
Under the hypothesis that larger initial tumor burden and poor response on interim PET probably identifies a patient group with worst prognosis, we conducted further analysis looking at both TMTV and interim PET. As shown in Figure 4, patients with low baseline TMTV and interim PET 5PS of 1 to 3 had superior outcome. In particular, patients with low TMTV and interim PET 5PS of 1 to 3 had significantly better EFS and OS than patients with high TMTV and interim PET 5PS of 1 to 3 (P = .018 and P = .023). It was confirmed on multivariate analysis that TMTV higher than 125 cm3 and interim 5PS of 4 to 5 remained independently predictive of OS and EFS (Table 2).
There was direct correlation (r = 0.7; P < .001) between the groups stratified by ΔSUVmax (decline of >73% vs decline ≤73%) and groups stratified by interim 5PS (1-3 vs 4 or 5). Of note, use of quantitative ΔSUV in combination with baseline TMTV was not superior to using 5PS combined with TMTV in a multivariate analysis. In a subanalysis by Kaplan Meier log rank analysis, we confirmed that patients with low TMTV and ΔSUV (decline of ≥73%) had significantly better EFS and OS than patients with high TMTV and ΔSUV (decline of <73%; P = .012 and P = .014; supplemental Figure 3).
Similarly, using RECIL response at interim did not add prognostic value when compared with interim 5PS alone in a multivariate analysis. This occurred because interim RECIL classification (CR vs PR+SD) showed near complete correlation with classification by interim 5PS (1-3 vs 4 or 5) alone.
In summary, univariate analysis showed interim treatment response parameters (5PS, ΔSUV, and RECIL) as factors significantly associated with EFS and OS (Table 2). In a multivariate analysis, both interim 5PS and MTV were statistically significant factors associated with EFS and OS. Interim 5PS was also a significant predictor in the other models combining PIT or IPI (supplemental Table 2).
Treatment responses at EOT
EOT PET using the 5PS predicted both EFS (P < .001) and OS (P = .001) (supplemental Figure 4). Considering PIT or IPI in addition to EOT PET did not improve the prediction of patient outcome on multivariate analysis.
Discussion
We present the results of a retrospective study of consecutive, uniformly treated patients with the more common forms of PTCL (excluding ALK+ ALCL) to evaluate the prognostic value of interim PET by 5PS, as well as TMTV and ΔSUV. We sought to examine whether these functional imaging parameters added to commonly used clinical risk stratification scores such as PIT and IPI.
This retrospective study generated several important findings: First, we showed that the PET parameter baseline TMTV has prognostic value in PTCL, alone or in combination with clinical indices. We further demonstrated that interim PET using the 5PS can further discriminate groups of patients with variable prognosis independent of clinical prognostic indices such as PIT and IPI. Moreover, TMTV in combination with interim imaging data (using 5PS, ΔSUV, or interim RECIL response) was able to stratify the patients and identify a group of patients with PTCL with much better outcome.
Previous studies have evaluated the role of interim PET/CT in TCLs before the adoption of the 5PS system. Major studies performed before the use of the 5PS system showed that patients with negative interim PET achieved an approximately 70% PFS at 3 to 4 years compared with only 20% to 30% among patients with positive interim scan.16,21 In comparison, in our series using the 5PS, the 4-year EFS in patients with negative interim PET was 58%, and patients with positive interim PET had a 4-year EFS of 0%. Furthermore, we demonstrated that the interim PET helped to better stratify patients with low or high PIT.
Accordingly, overall, the 5PS helped to risk-stratify patients with PTCL who were treated with a uniform approach of induction chemotherapy followed by an intent to consolidate with ASCT. Of course, our findings do not pertain to patients with progressive disease before their interim evaluation, which may explain some of the differences in outcomes for our cohort compared with published registry studies. In a previously published study from our group that evaluated patients (2001-2011) treated with the intent to transplant, we identified that 12% of patients had disease progression by the time of their interim scan. This study does not address whether interim PET should be performed at an earlier time. We know of at least 1 prospective study that will provide data regarding interim PET at an earlier time (NCT 02561273).
We further showed that baseline TMTV carries prognostic information among patients with PTCL. In this series, TMTV of 125 cm3 was identified as the best threshold to separate patients with good from those with poor prognosis. Although several studies have shown that patients with high initial metabolic tumor burden have generally worse outcomes, the optimal method to derive TMTV and the reproducibility of the method are still the subject of ongoing investigations. The methods currently being tested vary in their way to calculate volumes.33 In addition, the optimal cutoff value to segregate patients with good from those with poor prognosis depends on the characteristics of the study population. In a similar retrospective study, Cottereau et al recently published their analysis of interim PET and TMTV in 140 patients with PTCLs, including those who were not treated with the intent to transplant in first remission and those with ALK+ ALCL.20,21 The researchers demonstrated that baseline TMTV of 230 cm3 or less risk-stratified patients with PTCL.21 Although their results also demonstrate that interim PET and TMTV can help in stratifying patients with PTCL, 16% of patients in their series had ALK+ ALCL, which is a more chemosensitive disease with favorable prognosis.20,21 In the previous studies of PTCL, TMTV classified patients with unfavorable prognosis defined as combined with 5PS or PIT into subgroups with very poor prognosis.20,21 Conversely, TMTV in our study was able to further classify patients with favorable prognosis defined as combination of interim 5PS (or interim ΔSUV) into subgroups with excellent and poor prognostic groups. These differences of stratifications might depend on the difference of the optimal TMTV cutoff and study population. Nevertheless, both studies demonstrate that patients with PTCL with high tumor burden are at higher risk for treatment failure and have generally shorter survival than those with low tumor burden. Therefore, we believe that baseline TMTV, as an independent prognostic factor, has a potential ability to extract a patient population with excellent outcome. Whether these most favorable patients could fare just as well with a less intensive approach than that given here could be the subject of a future study. However, we would point out that no constellation of favorable baseline characteristics (low TMTV and low PIT) could identify a group with a 5-year EFS higher than 70%. Furthermore, the added value of 5PS in patients with high or low TMTV could not be addressed in our analysis.
Of note, although some prognostic information can already be obtained from baseline PET and clinical parameters before initiation of therapy, more accurate stratification can happen at the time of interim assessment, using the interim 5PS alone or in combination with clinical parameters (Figure 5). Although interim ΔSUV also stratified patients with good vs poor outcome (supplemental Figure 3), this more quantitative approach did not provide any additional information beyond that available from the widely established 5PS alone. As calculation of ΔSUV is technically more challenging, we favor the use of 5PS for interim prognostic evaluation. In addition to metabolic response, morphological response on CT images is incorporated into response criteria such as Lugano classification and RECIL. The objective of RECIL is to simplify response assessment and to characterize suboptimal response in greater detail. In the current study, RECIL could be applied, but it did not provide better risk stratification than PET alone, largely as a result of complete concordance with the 5PS. Favorable RECIL response corresponded with the presence of complete remission on interim PET by 5PS, and both are markers of responsiveness to chemotherapy.
In an era of precision medicine biomarker-driven treatment algorithms, there has been an emphasis on response assessment using modern imaging studies in lymphoma. Current prognostic indices primarily rely on clinical factors. However, our study demonstrates that PET response to therapy is a key component of prognostic stratification. Patients with interim 5PS of 4 or 5 had uniformly poor outcome; no patient survived beyond 4 years. In contrast, patients with favorable interim 5PS had significantly better outcomes, even when considered to be at high risk based on baseline PIT. These data are in concordance with prior studies demonstrating generally poor outcomes for patients not achieving response to upfront chemotherapy.1,3,7 Accordingly, because outcomes are generally poor for patients with interim 5PS of 4 or 5, it would seem reasonable to change their chemotherapy regimen and consider consolidation with allogeneic transplant if remission is achieved. Identifying this especially high-risk subgroup serves as evidence that this population warrants further study; this population could be targeted for novel therapeutic approaches in clinical trials. Most clinical trials in relapsed/refractory TCLs have not had predefined subgroup analysis. For instance, one may consider further studies to evaluate a PET adaptive approach to considering consolidation with ASCT in CR1. Alternatively, interim PET evaluation could allow earlier identification of patients in whom this strategy is destined to fail, directing them to other approaches such as clinical trials of nonchemotherapeutic agents and/or allogeneic stem cell transplantation. It is important to note that all subjects included in our analysis were treated with the intent to proceed to ASCT in CR1. Therefore, our study does not address the need for, or added benefit of, consolidation. However, our data also could serve as background to a study seeking to identify subjects with favorable prognosis without consolidation.
The usual limitations of retrospective analysis apply to our study. In our study, only 16 patients had an interim 5PS of 4 to 5. In other studies, the rate of interim PET positivity was higher. However those studies included a more heterogeneous patient population (including ALK+ ALCL) and were not treated uniformly.21,22 All patients in this series were treated uniformly with the intent to transplant in first remission, and the overall response rate to CHOP-based therapy was similar to other prospective series.4 Our results may not be able to be extrapolated to patients who are not treated with that approach. Only 5 patients had an interim 5PS of 3, leading to uncertainty of whether patients with 5PS 3 can indeed by lumped together with those with 5PS 1 or 2 in PTCL. As our series had a long observation period of 15 years, technological developments of PET scanners and software may have affected SUV and TMTV measurements. However, this change in technology would not be expected to influence 5PS, which is a relative score, based on comparison of tumor FDG uptake with normal activity in reference regions within the same scan. Similarly, although modern PET scanners often yield higher SUV numbers for a given lesion (related to improved scanner performance and image reconstruction), calculation of ΔSUV is likely not affected by this, as baseline and follow-up scans are performed within a few months, and thus on the same generation of equipment.
In conclusion, baseline TMTV and at interim 5PS are both independent prognostic factors in patients with PTCL. In this series, the use of interim 5PS in PTCL was independently predictive of outcome when controlling for clinical risk scores such as PIT and IPI. Interim PET evaluation also identified a particularly high-risk group that may benefit from alternative treatment strategies earlier in the treatment course. This information may help to develop more individualized treatment strategies for patients with PTCL with varying prognosis, as well as aid in the design and interpretation of clinical trials in the future.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
N.M.-S. was supported by the National Cancer Institute of the National Institutes of Health under Award Number K12 CA167540.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: N.M.-S., K.I., S.M.H., and H.S. contributed to the design of the project; N.M.-S., K.I., and K.B. contributed to the data collection; and N.M.-S., K.I., K.B., A.J.M., C.S., S.M.H., and H.S. contributed to analysis of the results and to the writing of the manuscript.
Conflict-of-interest disclosure: N.M.-S. has research funding from Celgene, Bristol-Myers Squibb, Genentech, and Verastem; and served as a consultant for Kyowa-Kirin. H.S. has served as a consultant for Aileron Therapeutics. S.M.H. has research support from ADC Therapeutics, Aileron, Celgene, Forty-Seven, Infinity/Verastem, Kyowa-Hakka-Kirin, Millennium/Takeda, Seattle Genetics, and Trillium; and consults for Celgene, Millennium/Takeda, Kyowa-Hakka-Kirin, Seattle Genetics, Forty-Seven, Mundipharma, Verastem, Portola, Beigene, Innate Pharma, Miragen, Aileron, and Corvus. C.S. has research funding from Juno Therapeutics and Sanofi-Genzyme; and has served as a consultant for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Precision Biosciences, Kite, and GlaxoSmithKline. A.J.M. has research funding from Seattle Genetics, Merck, Bristol-Myers Squibb, Incyte, Miragen, and Kyowa-Hakko-Kirin; and has served as a consultant for Kyowa-Hakka-Kirin, Miragen Therapeutics, Takeda Pharmaceuticals, ACD Therapeutics, Seattle Genetics, Cell Medica, Bristol-Myers Squibb, and Erytech Pharma. The remaning authors declare no competing financial interests.
Correspondence: Neha Mehta-Shah, Washington University in St. Louis, 660 S Euclid Ave, Box 8056, St. Louis, MO 63110; e-mail: mehta-n@wustl.edu.
References
- 1.Vose J, Armitage J, Weisenburger D, International TCLP; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130. [DOI] [PubMed] [Google Scholar]
- 2.Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014;124(10):1570-1577. [DOI] [PubMed] [Google Scholar]
- 3.Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15(10):1467-1475. [DOI] [PubMed] [Google Scholar]
- 4.d’Amore F, Relander T, Lauritzsen GF, et al. . Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-3099. [DOI] [PubMed] [Google Scholar]
- 5.Reimer P, Rüdiger T, Geissinger E, et al. . Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106-113. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014;123(17):2636-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta N, Maragulia JC, Moskowitz A, et al. . A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clin Lymphoma Myeloma Leuk. 2013;13(6):664-670. [DOI] [PubMed] [Google Scholar]
- 8.Moskowitz AJ, Schöder H, Gavane S, et al. . Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood. 2017;130(20):2196-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sauter CS, Matasar MJ, Meikle J, et al. . Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015;125(16):2579-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gisselbrecht C, Gaulard P, Lepage E, et al. . Prognostic significance of T-cell phenotype in aggressive non-Hodgkin’s lymphomas. Groupe d’Etudes des Lymphomes de l’Adulte (GELA). Blood. 1998;92(1):76-82. [PubMed] [Google Scholar]
- 11.Gallamini A, Stelitano C, Calvi R, et al. ; Intergruppo Italiano Linfomi. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474-2479. [DOI] [PubMed] [Google Scholar]
- 12.Mak V, Hamm J, Chhanabhai M, et al. . Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31(16):1970-1976. [DOI] [PubMed] [Google Scholar]
- 13.Meignan M, Gallamini A, Haioun C, Polliack A. Report on the Second International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 8-9 April 2010. Leuk Lymphoma. 2010;51(12):2171-2180. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Choi JY, Hyun SH, et al. ; Asia Lymphoma Study Group. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol. 2015;2(2):e66-e74. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz S, Coiffier B, Foss F, et al. . Utility of 18fluoro-deoxyglucose positron emission tomography for prognosis and response assessments in a phase 2 study of romidepsin in patients with relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26(4):774-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casulo C, Schöder H, Feeney J, et al. . 18F-fluorodeoxyglucose positron emission tomography in the staging and prognosis of T cell lymphoma. Leuk Lymphoma. 2013;54(10):2163-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung SH, Ahn JS, Kim YK, et al. . Prognostic significance of interim PET/CT based on visual, SUV-based, and MTV-based assessment in the treatment of peripheral T-cell lymphoma. BMC Cancer. 2015;15(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ham JS, Kim SJ, Choi JY, et al. . The prognostic value of interim and end-of-treatment PET/CT in patients with newly diagnosed peripheral T-cell lymphoma. Blood Cancer J. 2016;6(2):e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomita N, Hattori Y, Fujisawa S, et al. . Post-therapy 18F-fluorodeoxyglucose positron emission tomography for predicting outcome in patients with peripheral T cell lymphoma. Ann Hematol. 2015;94(3):431-436. [DOI] [PubMed] [Google Scholar]
- 20.Cottereau AS, Becker S, Broussais F, et al. . Prognostic value of baseline total metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with peripheral T-cell lymphoma (PTCL). Ann Oncol. 2016;27(4):719-724. [DOI] [PubMed] [Google Scholar]
- 21.Cottereau AS, El-Galaly TC, Becker S, et al. . Predictive value of PET response combined with baseline metabolic tumor volume in peripheral T-cell lymphoma patients. J Nucl Med. 2018;59(4):589-595. [DOI] [PubMed] [Google Scholar]
- 22.El-Galaly TC, Pedersen MB, Hutchings M, et al. . Utility of interim and end-of-treatment PET/CT in peripheral T-cell lymphomas: A review of 124 patients. Am J Hematol. 2015;90(11):975-980. [DOI] [PubMed] [Google Scholar]
- 23.Feeney J, Horwitz S, Gönen M, Schöder H. Characterization of T-cell lymphomas by FDG PET/CT. AJR Am J Roentgenol. 2010;195(2):333-340. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini C, Argnani L, Broccoli A, et al. . Prognostic value of interim positron emission tomography in patients with peripheral T-cell lymphoma. Oncologist. 2014;19(7):746-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moskowitz AJ, Yahalom J, Kewalramani T, et al. . Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116(23):4934-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younes A, Hilden P, Coiffier B, et al. . International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol. 2017;28(7):1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cottereau A, et al. . Prognostic value of baseline quantitative PET metrics for patients with unfavourable early stage Hodgkin lymphoma enrolled in the standard arm of the EORTC/Lysa/FIL H10 Trial. Blood. 2016;128:184. [Google Scholar]
- 29.Cottereau AS, Lanic H, Mareschal S, et al. . Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2016;22(15):3801-3809. [DOI] [PubMed] [Google Scholar]
- 30.Schöder H, Moskowitz C. Metabolic tumor volume in lymphoma: hype or hope? J Clin Oncol. 2016;34(30):3591-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larson SM, Erdi Y, Akhurst T, et al. . Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2(3):159-171. [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, Horning SJ, Coiffier B, et al. ; NCI Sponsored International Working Group. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 33.Cottereau AS, Hapdey S, Chartier L, et al. . Baseline total metabolic tumor volume measured with fixed or different adaptive thresholding methods equally predicts outcome in peripheral T cell lymphoma. J Nucl Med. 2017;58(2):276-281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.