Abstract
The World Cancer Research Fund International has released 32 anticancer effects (ACEs) that targeted every stage of cancer processes. Thus, we designed two formulas of natural food combination Diet I and Diet II, mainly produced by elite crop varieties rich in ACEs with different mixture ratios, and evaluated their cancer preventive effects on N‐nitrosodiethylamine (NDEA)‐induced hepatocarcinogenesis. After 20 weeks of dietary intervention, Diet I and Diet II reduced incidence, size, and number of hepatic nodules (p < 0.01) and prevented hepatic tumor formation in NDEA‐induced hepatocarcinogenesis rats. Low‐grade hepatic dysplasia incidence was 20% for Diet II and 40% for Diet I, and apparent hepatocellular carcinomas (HCC) rates were both 0, while 90% HCC in control diet treatment group (p < 0.01). Diet I and Diet II ameliorated abnormal liver function enzymes, reduced serum alpha fetal protein, tumor‐specific growth factor, dickkopf‐related protein 1, tumor necrosis factor‐alpha and interleukin‐6 levels, regulated hepatic phase I and II xenobiotic‐metabolizing enzymes, enhanced antioxidant capacity, suppressed NDEA‐initiated oxidative DNA damage, and induced apoptosis coupled to down‐regulation of proinflammatory, invasion, and angiogenesis markers. Daily intake of combination diet produced from ACEs‐rich elite crop varieties can effectively prevent or delay occurrence and development of NDEA‐induced hepatocarcinogenesis in rats.
Keywords: anticancer effects, cancer prevention, combinative natural foods, elite crop variety, hepatocellular carcinoma, N‐nitrosodiethylamine
1. INTRODUCTION
Cancer prevention has been drawing more and more attention in modern society. At the current stage of research, products like synthetic chemical compounds, single natural plant chemicals, and single natural plants are frequently used for cancer prevention. However, most of these chemopreventive agents only target cancer at certain stages of its occurrence and development to suppress tumor development (Kweon, Adhami, Lee, & Mukhtar, 2006; Liu et al., 2017; Stagos et al., 2012; Zhou et al., 2016). This often renders their preventive effects unsatisfactory. For example, for the breast cancer patients who were positive for estrogen receptor, the postoperative recurrence rate decreased by 47% among those who took tamoxifen continuously for 5 years, but the likelihood of those patients developing early endometrial cancer seemed to increase (Buzdar et al., 2006), suggesting that tamoxifen has a serious side effect. According to a report published by Chen, Wallig, and Jeffery (2016), feeding 10% more broccoli (Brassica oleracea L. var. Green Magic) significantly decreased the levels of hepatic triacylglycerols and tumor necrosis factor, and the rates of nonalcoholic fatty liver disease induced by diethylnitrosamine and diet rich in refined carbohydrate in B6C3F1 mice, but carcinogenesis was not blocked. Yoxall et al. (2005) showed that sulforaphane (SFN) at a typical dietary dose stimulated NAD (P) H quinone dehydrogenase 1 in a dose‐dependent fashion but did not influence glutathione‐S‐transferase (GST), epoxide hydrolase, or uridine diphosphate—glucuronosyl transferase activities in rat livers exposed to SFN in their drinking water for 10 days at equivalent daily doses of 3 and 12 mg/kg.
The 2007 Second Expert Report of the World Cancer Research Fund International (WCRF) has shown that the occurrence and development of cancer are multi‐step processes, and each step can be interrupted by a number of anticancer effects (ACEs) according to Figure 2.5, Chapter 2, such as organic sulfur compounds, epigallocatechin gallate (EGCG), vitamin A, and resveratrol, in total of 32 ACEs (WCRF, 2007) (Supporting Information Table S1).
The concentration of ACEs is crucial to the cancer prevention (Misaka, Miyazaki, Fukushima, Yamada, & Kimura, 2013). In many cases, the effects of chemopreventive agents in cultured cells or tissues are only achievable at supraphysiological concentrations; such concentrations might not be reached when the phytochemicals are administered as part of an organism's diet (Tan, Shi, Tang, Han, & Spivack, 2010). For most people, eating the right foods and drinks is more likely to prevent cancer than dietary supplements, according to the summary of the Third Expert Report of World Cancer Research Fund/American institute for Cancer Research (2018). In our previous work, we have bred and excavated elite crop varieties with high contents of ACEs (Cheng, Xu, Yang, Chen, & Zheng, 2016; Cheng et al., 2014; Zheng, 2004, 2014; Zheng et al., 2012) and the elite crop varieties used in this study as showed in Table 1. For example, broccoli in the market contains a low concentration of SFN, only 0.104 mg/g. We bred a new variety of broccoli, Brassica oleracea var. italica FU‐1, which contains a high concentration of SFN, 0.984 mg/g (Zheng et al., 2012).
Table 1.
Content of ACEs in elite crop varieties
| Compounds | Crop | Elite crop variety | ACEs contents | References | |
|---|---|---|---|---|---|
| Elite crop variety (mg/g) | Common variety (mg/g) | ||||
| EGCG | Tea | E‐101 | 141.3 | 52.8 | Cheng et al. (2016) |
| Total catechins | Tea | E‐101 | 224.9 | 120.1 | Cheng et al. (2016) |
| Sulforaphane | Broccoli | FU‐1 | 0.984 | 0.104 | Zheng et al. (2012) |
| Anthocyanins | Black rice | Fuzi No.2 | 3.096 | 1.390 | Cheng et al. (2016) |
| Mulberry | PR‐01 | 1.93 | 0.19 | Liao et al. (2018) | |
| Procyanidin | Mulberry | PR‐01 | 155.41 | 53.53 | Cheng et al. (2016) |
| Selenium | Black rice | Fuzi No.2 | 7.190 × 10−5 | 1.581 × 10−5 | Unpublished data |
| Coixenolide | Job's‐tears | Strain 22 | 100.8 | 58.9 | Cheng et al. (2016) |
| Total polyphenol | Job's‐tears | Strain 22 | 0.762 | 0.593 | Unpublished data |
| Flavonoid | Job's‐tears | Strain 22 | 46.85 | 24.88 | Unpublished data |
| Mulberry | PR‐01 | 1.090 | 0.494 | Unpublished data | |
| β‐carotenea | Sweet potato | YS‐5 | 0.090 | 0.038 | Zheng et al. (2012) |
| Carrot | Y‐NS | 0.021 | 0.080 | Unpublished data | |
| α‐carotenea | Carrot | Y‐NS | 0.072 | 0.028 | Unpublished data |
| Lycopenea | Carrot | Y‐NS | 0.005 | 0.003 | Unpublished data |
| α‐linolenic acid | Alfalfa | Y‐M551 | 21.63 | 6.11 | Unpublished data |
| Resveratrol | Mulberry | PR‐01 | 0.439 | 0.017 | Cheng et al.(2016) |
| Polysaccharide | Reishi mushroom | G‐8 | 72.0 | 7.40 | Cheng et al.(2016) |
The contents of these substances were determined by fresh sample.
In this study, we designed two formulas of natural food combination Diet I and Diet II, which were mainly produced by ACEs‐rich elite crop varieties with different mixture ratios (Supporting Information Table S2) and evaluated the cancer prevention of the two formulas in N‐nitrosodiethylamine (NEDA)‐induced hepatocellular carcinoma (HCC) rats. The modulatory effects of Diet I and Diet II on xenobiotic‐metabolizing enzymes, antioxidant activity, and markers of cell proliferation, inflammation, apoptosis, invasion, and angiogenesis during NDEA‐induced rat hepatocarcinogenesis are also explored.
2. EXPERIMENTAL SECTION
2.1. Chemicals and materials
All chemicals were ordered from Sigma‐Aldrich (St. Louis, MO, USA). TRIzol reagent, HiScript II Q RT SuperMix for qPCR (+cDNA wiper) and AceQTM qPCR SYBRR Green Master Mix were purchased from Takara Co., Ltd. (Dalian, China). Rabbit polyclonal antibodies B‐cell leukemia‐2 (Bcl‐2), Bcl‐2‐associated X protein (Bax), p53, Caspase‐3, and Caspase‐8 were purchased from Santa Cruz Biotechnology; rabbit polyclonal antibodies proliferating cell nuclear antigen (PCNA), nuclear factor‐kappa B (NF‐Κb), vascular endothelial growth factor (VEGF), matrix metalloproteinases 2 (MMP‐2), cyclooxygenase‐2 (COX‐2), tumor necrosis factor‐alpha (TNF‐α), 8‐hydroxydeoxyguanosine (8‐OH‐dG), and matrix metalloproteinases 9 (MMP‐ 9) were purchased from Cell Signaling (Danvers, MA, USA). All other chemicals used were of analytical grade.
2.2. Animals, diets, and water
The study was performed in accordance with the Chinese national guidelines for the care of laboratory animals and was approved by the Animal Ethics Committee of the Institute of Laboratory Animal Sciences at the Chinese Academy of Medical Sciences. Five‐week‐old SPF‐grade male Wistar rats were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. with animal production license No.: SCXK (Beijing) 2012‐0001, animal certificate number: 11400700135387. All animals were housed in an SPF barrier system of the New Drug Safety Evaluation and Research Center, Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences. Breeding conditions were as follows: temperature 22 ± 2°C, relative humidity 50 ± 10%, 12 hr day/night alternation, and access to food and drinking water ad libitum.
The control diet (ConD) was purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Diet I and Diet II were formulated with energy restriction, and we used natural foods produced by the elite crop varieties (Supporting Information Table S2), which were provided by the Agricultural Product Quality Institute, Fujian Agriculture and Forestry University. The others were purchased from the common market. Diet I is a food combination of nine pure natural foods of plant origin from the elite crop varieties rich in ACEs. 100 g Diet I contains 12 g black rice, 28 g job's‐tears, 10 g sweet potato, 5 g broccoli, 2 g carrot, 1 g alfalfa, 5 g mulberry, and 1.0 g reishi mushroom and is supplemented with 7 g corn, 24 g bean cake, 1 g dried yeast, 2 g bran, 1.0 g oleum morrhuae and then blended to mixture. The mixture was then grain shaped, dried at 60°C and sterilized by Co60. Diet II is composed of primarily by foods of nine pure natural foods of plant origin from the elite crop varieties rich in ACEs and subsidiary by three light meat. 100 g Diet II contains 11.5 g black rice, 26.9 g job's‐tears, 7.7 g sweet potato, 4.8 g broccoli, 1.9 g carrot, 0.5 g alfalfa, 4.8 g mulberry, and 1.4 g reishi mushroom, 3.8 g trepang, 1.0 g abalone, 1.4 g clam and is supplemented with 5.3 g corn, 16.3 g bean cake, 1.4 g dried yeast, 1.9 g bran, 1.0 oleum morrhuae, 1.9 g grifola frondosa, 1.4 g kiwi fruit, 1.4 g pollen, and 1.4 g fig and then blended to mixture. The mixture was then grain shaped, dried at 60°C and sterilized by Co60.
The EGCG‐rich tea [Camellia sinensis (L.) O. Kuntze] variety E‐101 was processed as following: tea powder (30 g) was extracted with 1 L distilled water at 80°C, steeped for 5 min, ultrasonic extraction for 10 min with Power of 200 W, two rounds of extraction, combined filtration, then collection of the E‐101 tea water.
2.3. Experimental design
After 1‐week adaptive feeding on control diet, forty rats were randomly divided into four groups (n = 10/group): normal control group (ConD group), model group (ConD+NDEA group), Diet I+NDEA group, and Diet II+NDEA group. From the first week of experiment, rats in ConD and ConD+NDEA groups were fed with the control diet and offered water ad libitum. The rats in Diet I+NDEA and Diet II+NDEA group were fed with Diet I or Diet II, and both drank E‐101 tea water instead of drinking water for 20 weeks. In the 2–14th week of the experiment, rats in the model group, Diet I+NDEA group, and Diet II+NDEA group were with 25 mg/kg NDEA (twice a week) as described earlier (Liao, Liu, Xu, & Zheng, 2018). Rats in the ConD group received intraperitoneal injection of the same volume of saline. Body weights, feed and tea, or water intake were measured weekly. After 20 weeks, the rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg) for collection of blood samples. Immediately after that, animals were sacrificed by bleeding from the abdominal aorta, and their organs were collected. The size and number of liver nodules were measured as described earlier (Chen et al., 2016). Liver tissue was collected for biochemical, histopathological, and ultrastructural analyses.
2.4. Histological analysis of the liver tissue
Small blocks of liver from median lobe were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned (4 μm), and stained with hematoxylin and eosin (H&E) for histopathological examination. The stained slides were analyzed by assessing the morphological changes under the OPTIPHOT‐2 light microscope by an experienced investigator that was unaware of the experimental conditions. Five microscopy‐stained slides per animal were examined. The extent of cancerization was assessed according to Edmondson classification method (Edmondson & Steiner, 1954).
For liver electron microscopy, pinhead‐sized blocks of liver (1 mm × 1 mm × 1 mm) was fixed in 2.5% glutaraldehyde in 0.1 M Sodium cacodylate buffer (pH 7.4) for 1 hr at 4°C and postfixed in 1% osmium tetroxide. Ultrathin sections (90 nm) were cut on MT‐500 ultramicrotome (DuPont, USA), mounted on 300 mesh copper grids, stained with uranyl acetate and lead citrate, and examined under JEM‐1400 transmission electron microscope (JEOL, Japan).
2.5. Biochemical assays
The levels of serum aspartate transaminase (AST), Alanine aminotransferase (ALT), GST, γ‐glutamyl transpeptidase (GGT), total bilirubin (TBIL), alkaline phosphatase (ALP), total protein (TP), and albumin (ALB) were detected according to the instructions of commercial kits (Leadman Biochemistry Co., Ltd., Beijing, China) on Hitachi 7100 automatic biochemical analyzer (Hitachi, Ltd., Tokyo, Japan). The levels of alpha fetal protein (AFP), tumor‐specific growth factor (TSGF), dickkopf‐related protein 1 (DKK1), tumor necrosis factor‐alpha (TNF‐α), carcinoembryonic antigen (CEA), and interleukin‐6 (IL‐6) were detected using ELISA kit (CUSABIO Biotech Co., Ltd., Wuhan, China) according to manufacturer's instructions. The inflammatory parameters of white blood cell, neutrophil, and lymphocyte counts were measured on the Pentra DX 120 automatic blood analyzer (ABX, France).
Microsomal fractions were prepared from liver tissues as described previously (Velayutham et al., 2007). The concentration of microsomal protein was determined using the BCA kit. The contents of cytochrome b5 (Cyt b5) and cytochrome P450 (Cyt P450) were assayed using the method published by Omura and Sato (1964). The activity of NADPH‐cytochrome b5 reductase (Cyt b5R) was assayed using the methods published by Philips and Langdon (1962). Cytochrome C (Cyt c) was used as the substrate to determine NADH‐cytochrome P450 reductase (Cyt P450R) and DT‐diaphorase (DTD) activity using the methods reported by Cummings, Parker, and Lash (2000) and Smitskamp‐Wilms, Giaccone, Pinedo, vander, and Peters (1995), respectively. Aryl hydrocarbon hydroxylase (AHH) and aniline hydroxylase CYP2E1 (ANH) activity were determined using double‐beam ultraviolet spectrophotometry (Nebert & Gelboin, 1968). The activities of GST and UDP‐glucuronosyltransferase (UGT) in the liver homogenates were detected by specific ELISA kits (Shanghai Jiang Lai Biotechnology Co., Shanghai, China). The epoxide hydrolyzyme (EPT) activity in liver microsome was determined using the Fabian method (Fabian et al., 2016).
The malondialdehyde (MDA) content and the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH‐Px) were assayed within 12 hr using standard commercially available kits according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Engineering Institute, Nanjing, China).
2.6. Immunohistochemical analysis
Paraffin section (4 μm) was dewaxed and rehydrated through a gradual decrease in ethanol concentration. The slides were incubated in sodium citrate buffer for two cycles of 5 min at 37°C for antigen retrieval. After washing with phosphate buffer solution (PBS), the slides were then treated with 3% hydrogen peroxide to remove any endogenous peroxidases. Then the sample was washed with PBS for three cycles of 5 min and blocked with normal goat serum in a humidified chamber for several hours at 4°C. The sections were then incubated overnight at 4°C in a humidified chamber using the appropriate primary antibodies: 8‐OH‐dG, NF‐κB, COX‐2, TNF‐α, p53, Bcl‐2, PCNA, and VEGF with recommended dilution. The slides were washed with TBS and then incubated with HRP‐labeled sheep antirabbit secondary antibody at room temperature for 1 hr followed by streptavidin‐biotin‐peroxidase at room temperature for 30 min. The slides were washed with PBS, and the immunoprecipitation was visualized by treating with 3, 3′‐diaminobenzidine for color development for 25 min. Then slides were counterstained with hematoxylin, and the brown color signifying the presence of antigen bound to antibody was detected by light microscopy. For the negative control, tris‐buffered saline (TBS) was used instead of a primary antibody. From ten randomly selected sections of each slide, 500 cells were counted. The percentage of positive cells for each group was calculated using the Image‐Pro Plus 6.0 image analysis system.
2.7. Quantitative real‐time PCR assay
Total RNA was isolated from liver tissue using TRIzol (Invitrogen). The cDNA was synthesized from the RNA samples. The real‐time quantitative polymerase chain reaction (qRT‐PCR) reaction was carried out using the SYBR® Fast qPCR Mix (Code no. RR430A, TaKaRa, Japan) via the Real‐time PCR System (ABI Prism 7500, Life Technology, MA, USA). The qPCR reaction conditions were as follows: 95°C for 15 s, annealing at 60°C for 10 s, and extension at 72°C for 34 s for a total of 40 cycles. The primer sequences were shown in Supporting Information Table S3. GAPDH served as an internal control for all measurements. Each sample was amplified in triplicate. Relative gene expression data were analyzed using the 2−ΔΔCT method (Sur et al., 2016).
2.8. Western blot analysis
The protein was extracted from liver tissue with RIPA buffer containing protease inhibitor cocktail. The protein extraction was separated using centrifugation (15,000 × g; 15 min; 4°C), and the supernatant was collected and then quantified using the BCA kit. Equal amounts of proteins were separated through 10%–12% SDS‐polyacrylamide electrophoresis gels and transferred to polyvinylidene fluoride membranes. After blocked with 5% skim milk, the membranes were incubated with the appropriate primary antibodies respectively, and β‐actin (CUSABIO Biotech Co., Ltd., Wuhan, China) with recommended dilution at 4°C overnight, and then incubated with goat antirabbit IgG horse radish peroxidase (HRP) secondary antibodies at room temperature for 1 hr. The films were developed using an ECL Plus chemiluminescence reagent kit (Millipore, MA, USA) and visualized using ChemiDocXRS (Bio‐Rad Laboratory, CA, USA). Densitometries were analyzed using Quantity One software and normalized to β‐actin.
2.9. Statistical analysis
Statistical analysis was performed using SPSS19.0 software. All results are expressed as mean ± standard deviation (SD) and were analyzed using a one‐way, 2‐ or 3‐factor ANOVA, followed by LSD test or Tukey's test when the differences were indicated. A p value of <0.05 was here considered significant in this study.
3. RESULTS
3.1. Body weight, relative liver weight, feed and tea/water intake were not changed by long‐term Diet I and Diet II consumption
In NDEA administered rats, the food intake, water consumption, and body weight gain were significantly (p < 0.05) decreased (Supporting Information Table S4) and the relative liver weight was significantly (p < 0.01) increased in NDEA+ConD group rats relative to ConD group rats (Supporting Information Table S4). All the changes induced by NDEA intoxication were significantly (p < 0.01 or p < 0.05) reduced except body weight when rats treated with Diet I and Diet II (Supporting Information Table S4), and there were no significant changes in hematological indicators (Supporting Information Table S5).
3.2. Diet I and Diet II exerted a powerful protective or delaying effect on hepatic tumorigenesis
When treated with NDEA (ConD+NDEA group), 100% rats developed nodules in the liver (for the two rats died of severe hepatic tumor pathogenesis at week 16 and 19 respectively, and the remaining eight rats, the nodule formation was observed. While administration of Diet I (Diet I+NDEA group) and Diet II (Diet II+NDEA group) was found to be associated with marked decreases in the number and multiplicity of the nodules relative to ConD +NDEA group rats (p < 0.01, Table 2). The incidence of nodule growth was reduced to 30% in the Diet I+NDEA group and 50% in the Diet II+NDEA group, respectively (Table 2). No hepatic nodules were observed in the ConD group animals.
Table 2.
Effects of Diet I and Diet II on hepatic neoplasm‐related lesions in NDEA‐induced hepatocarcinogenesis rats at 20th week
| Parameters | ConD | ConD+NDEA | Diet I+NDEA | Diet II+NDEA |
|---|---|---|---|---|
| Mortality (%) | 0/10 (0.0) | 2/10 (20.0)* | 0/10 (0.0)# | 0/10 (0.0)# |
| Relative liver weighta | 2.49 ± 0.20 | 3.59 ± 0.34** | 3.23 ± 0.31* | 2.86 ± 0.27## |
| Macroscopic lesions | ||||
| Nodule incidence (%) | 0/10 (0.0) | 8/8 (100.0)** | 3/10 (30.0)## | 5/10 (50.0)# |
| Total number of nodules (n) | 0 | 834** | 3## | 6## |
| Nodulemultiplicity (n)b | 0 | 83.4 ± 61.9** | 0.3 ± 0.5## | 0.6 ± 0.7## |
| <1 mm | — | 281 (42.1) | 2 (66.7) | 3 (60.0) |
| >1 mm <3 mm | — | 238 (35.7) | 1 (33.3) | 2 (40.0) |
| >3 mm | — | 148 (22.2) | 0 (0.0) | 0 (0.0) |
| Max nodule diameter(mm) | — | 23.38 ± 14.52 | 1.00 ± 0.50## | 1.40 ± 0.89## |
| Tumor volume (mm3)/rat | — | 99.70 ± 90.51 | 0.10 ± 0.30## | 6.80 ± 1.60## |
| Microscopic lesions | ||||
| Low‐grade dysplasia incidence (%) | 0/10 (0.0) | 0/10 (0.0) | 4/10 (40.0)# | 2/10 (20.0) |
| Hepatic adenoma incidence (%) | 0/10 (0.0) | 1/10 (10.0) | 0/10 (0.0) | 0/10 (0.0) |
| HCC incidence (%) | 0/10 (0.0) | 9/10 (90.0)** | 0/10 (0.0)## | 0/10 (0.0)## |
| Liver metastases (%) | 0/10 (0.0) | 2/10 (20.0) | 0/10 (0.0) | 0/10 (0.0) |
aRelative liver weight = liver weight/body weight. bAverage number of nodules/nodules bearing liver. Data are means ± SD or n (%), n = 8–10. Comparisons: compared with ConD group, *p < 0.05; **p < 0.01; compared with ConD+NDEA group, # p < 0.05; ## p < 0.01 (Fisher's exact test). ConD: normal control group; ConD+NDEA: model group, NDEA plus control diet‐treated group; Diet I+NDEA: NDEA plus Diet I‐treated group; Diet II+NDEA: NDEA plus Diet II‐treated group.
The results of H&E staining (Figure 1B and Table 2) showed normal hepatocyte structure in the ConD group rats and clustered distribution of tumor cells in the liver tissues of ConD+NDEA group rats. The oval cells and bile duct hyperplasia were readily visible with 90% incidence of apparent HCC in the model group. Administration of Diet I and Diet II associated with a marked decrease in the incidence of hepatic adenoma or HCC formation (with 0 incidence of apparent HCC in the Diet I+NDEA and Diet II+NDEA groups compared to the 90% incidence of apparent HCC in the model group, p < 0.01), and only a few inflammatory cells were observed during histopathological analysis. The morphological structure of the rat hepatocytes in the Diet II+NDEA group was similar to that in normal liver tissues (Figure 1b), and the low‐grade hepatic dysplasia incidence was 20% and 40% in the Diet I+NDEA group (Table 2). Further electron microscopy (Figure 1c) showed that the hepatocytes in the model group were not of uniform size, had increased nuclear volume with mega or double nuclei, swollen mitochondria with large vacuoles and significant reductions in the number of mitochondria relative to the normal control group. Compared with the ConD+NDEA group, the number and size of hepatocyte lesions were significantly lower in the Diet I+NDEA group, and the hepatocyte structure of the Diet II+NDEA group was similar to that of normal cells. These results indicated that Diet I and Diet II could prevent or delay the progress of NDEA‐induced hepatocarcinogenesis and significantly alleviated the hepatocellular precancerous lesions in rats.
Figure 1.
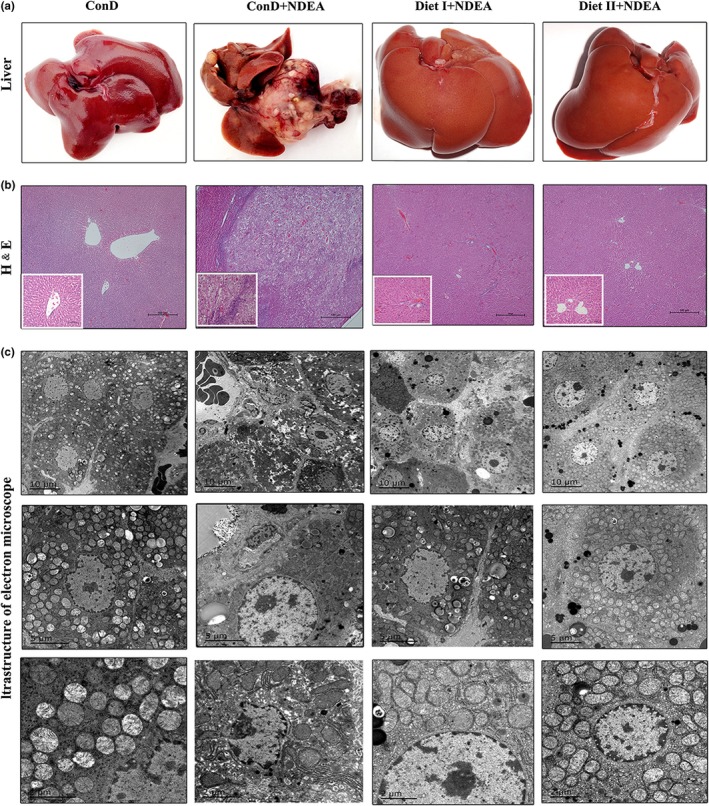
Effects of Diet I and Diet II on liver tissue pathological changes in NDEA‐induced hepatocarcinogenesis rats. (a) Representative images of the livers from each group rats. (b) Representative liver histopathological changes using hematoxylin and eosin (H&E) staining of rats are shown as ×40 magnifications (big figure) and ×200 magnifications (small figure). (c) Representative transmission electron microscope (EM 2000) images of changes in the ultrastructure of hepatic cells in rats. ConD: normal control group; ConD+NDEA: model group, NDEA plus control diet‐treated group; Diet I+NDEA: NDEA plus Diet I‐treated group; Diet II+NDEA: NDEA plus Diet II‐treated group. ConD: control diet; NDEA: N‐nitrosodiethylamine
3.3. Diet I and Diet II ameliorated hepatocyte damage and decreased serum tumor markers
Detection of blood biochemical indicators showed that the levels of liver function markers, such as AST, ALT, GGT, TBIL, ALP, and albumin in serum of ConD+NDEA group rats were significantly higher than in the ConD group (Table 3), indicating that the stimulation of NDEA and its metabolites in vivo on hepatocytes caused severe injury and liver dysfunction in the ConD+NDEA group. In the presence of Diet I and Diet II administration, the activities of the above serum marker enzymes in the serum of Diet I+NDEA and Diet II+NDEA group rats were significantly lower than in the ConD+NDEA group (Table 3), which was similar to the normal control group. The levels of AFP, TSGF, CEA, and DKK1 were much higher in the ConD+NDEA group than in the normal control group (p < 0.01); and Diet I and Diet II significantly inhibited the elevation of the serum tumor markers, AFP, TSGF, and DKK1 (p < 0.01, Table 3).
Table 3.
Effects of Diet I and Diet II on blood plasma biochemistry in NDEA‐induced hepatocarcinogenesis rats at 20th week
| Parameter/Groups | ConD | ConD+NDEA | Diet I+NDEA | Diet II+NDEA |
|---|---|---|---|---|
| AST(U/L) | 65.00 ± 36.33 | 185.63 ± 61.70** | 137.10 ± 4.52**## | 99.20 ± 13.50*## |
| ALT(U/L) | 40.70 ± 8.84 | 107.25 ± 42.48** | 40.30 ± 11.76## | 35.10 ± 9.48## |
| GGT(U/L) | 1.61 ± 0.24 | 2.57 ± 0.21** | 1.57 ± 0.62## | 1.52 ± 0.12## |
| TBIL(μmol/L) | 2.32 ± 0.26 | 4.20 ± 0.94** | 2.24 ± 0.40## | 2.30 ± 0.17## |
| ALP(U/L) | 56.60 ± 10.43 | 85.63 ± 17.11** | 62.20 ± 20.82## | 53.80 ± 9.37## |
| Total protein (g/L) | 65.75 ± 9.78 | 65.60 ± 12.25 | 59.37 ± 9.46 | 60.43 ± 10.83 |
| Albumin (g/L) | 27.04 ± 0.83 | 29.26 ± 2.11* | 25.38 ± 2.50## | 26.76 ± 2.12## |
| Globulin (g/L) | 38.71 ± 10.12 | 36.34 ± 11.43 | 29.99 ± 9.94 | 33.67 ± 10.83 |
| AFP(ng/ml) | 3.73 ± 0.50 | 5.84 ± 1.26** | 3.25 ± 0.54## | 3.53 ± 1.67## |
| CEA(ng/ml) | 1.83 ± 0.34 | 2.25 ± 0.34** | 2.07 ± 0.30 | 2.08 ± 0.38 |
| TSGF(μg/ml) | 23.70 ± 1.34 | 36.31 ± 7.99** | 28.39 ± 5.14## | 26.09 ± 4.79## |
| DKK1(ng/ml) | 1.47 ± 0.23 | 7.83 ± 1.85** | 3.61 ± 0.90## | 3.20 ± 1.20## |
Values are given as mean ± SD, n = 8. Comparisons: compared with ConD group, *p < 0.05; **p < 0.01; compared with ConD+NDEA group,## p < 0.01 (Student's t test). AFP: alpha fetal protein; ALB: albumin; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate transaminase; CEA: carcinoembryonic antigen; DKK1: dickkopf‐related protein 1; GGT: γ‐glutamyl transpeptidase; GST: glutathione‐S‐transferase; TBIL: total bilirubin; TP: total protein; TSGF: tumor‐specific growth factor.
3.4. Diet I and Diet II suppressed the activity of phase I enzymes and enhanced that of phase II enzymes
Carcinogens are primarily activated by phase I enzymes. The body can be protected from carcinogens by induction of phase II enzymes that lead to detoxification and accelerated excretion of carcinogens (Chakraborty et al., 2007; Na & Surh, 2008). As shown in Table 4, NDEA treatment significantly increased the concentrations of Cyt b5, Cyt P450 and the activities of Cyt b5R, Cyt P450R, DTD, and AHH in livers of ConD+NDEA group rats compared to the normal control group rats (all p < 0.01) and significantly reduced the activities of liver phase II enzymes such as GST, UGT, and EPT (all p < 0.01). Administration of Diet I or Diet II to NDEA‐treated animals significantly reduced the concentrations of Cyt b5 and Cyt P450 and suppressed the activities of Cyt b5R, Cyt P450R, DTD, and AHH in the livers (all p < 0.01), significantly increased the activities of GST, UGT, and EPT in livers over rats treated with NDEA alone (all p < 0.01) (Table 4). These results demonstrated that Diet I and Diet II act as dual‐acting agent by suppressing phase I enzymes and enhancing phase II enzyme activity, thereby promoting detoxification and excretion.
Table 4.
Effect of Diet I and Diet II on Phase I and II enzymes in the livers of NDEA‐induced hepatocarcinogenesis rats
| Parameters | ConD | ConD+NDEA | Diet I+NDEA | Diet II+NDEA |
|---|---|---|---|---|
| Phase I enzymes | ||||
| Cyt b5(nmoles/mg of protein) | 0.26 ± 0.03 | 0.50 ± 0.06** | 0.32 ± 0.05## | 0.28 ± 0.04## |
| Cyt P450(nmoles/mg of protein) | 0.34 ± 0.03 | 0.99 ± 0.11** | 0.52 ± 0.07**## | 0.42 ± 0.06## |
| Cyt b5R (nmoles/min/mg of protein) | 1.12 ± 0.07 | 1.91 ± 0.16** | 1.45 ± 0.19*## | 1.36 ± 0.20## |
| Cyt P450R(cyt c reduced/min/mg of protein) | 1.13 ± 0.10 | 2.04 ± 0.37** | 1.49 ± 0.19## | 1.40 ± 0.17## |
| AHH(nmoles of 3‐OHBaP formed/min/mg of protein) | 4.84 ± 0.16 | 8.79 ± 1.44** | 5.23 ± 1.39## | 5.40 ± 1.23## |
| DTD(cyt c reduced/min/mg of protein) | 1.57 ± 0.11 | 2.36 ± 0.42** | 1.72 ± 0.35## | 1.63 ± 0.27## |
| ANH(nMp‐aminophenol/min/mg protein) | 1.39 ± 0.12 | 1.28 ± 0.22 | 1.55 ± 0.19 | 1.53 ± 0.20 |
| Phase II enzymes | ||||
| GST(μmol/min/mg protein) | 7.14 ± 1.16 | 4.14 ± 0.57** | 6.24 ± 1.25## | 6.30 ± 1.21## |
| UGT(μmol/min/mg protein) | 15.38 ± 0.79 | 6.48 ± 2.14** | 12.75 ± 1.95## | 11.13 ± 3.26*## |
| EPT(μmol/min/mg protein) | 4.34 ± 0.23 | 2.61 ± 0.33** | 4.80 ± 0.47*## | 4.49 ± 0.45## |
Values are expressed as mean ± SD (n = 8). Comparisons: compared with ConD group, *p < 0.05; **p < 0.01; compared with ConD+NDEA group, ## p < 0.01. 3‐OHBaP: 3‐ hydroxybenzo (a) pyrene; AHH: Aryl hydrocarbon hydroxylase; ANH: aniline hydroxylase CYP2E1; Cyt b5: cytochrome b5; Cyt b5R: NADPH‐cytochrome b5 reductase; Cyt c: Cytochrome C; Cyt P450: cytochrome P450; Cyt P450R: NADH‐cytochrome P450 reductase; DTD: DT‐diaphorase; EPT: epoxide hydrolyzyme; GST: glutathione‐S‐transferase; UGT: UDP‐glucuronosyltransferase.
3.5. Diet I and Diet II reduced NDEA‐induced oxidative stress
Oxidative stress is one of the major instigators of the pathogenesis of environmental cancer (Grace et al., 2016). NDEA is converted to reactive oxygen species in liver cells through phase I enzyme metabolism, causing oxidation and inducing oxidative damage to DNA, ultimately leading to pathological changes and initiation of hepatogenesis (Marra et al., 2011). Many chemical carcinogens are associated with free radicals or reactive oxygen species, such as O2−, H2O2, and ROOH, and scavenging this free radical or reactive oxygen is mainly dependent on SOD, CAT, GSH‐Px, and GSH (Bishayee et al., 2011). As shown in Table 5, single intraperitoneal administration of NDEA significantly elevated serum and liver MDA content (p < 0.01) and inhibited the activities of SOD, GSH‐Px, and CAT in serum and livers of ConD+NDEA group as compared to the normal controls (all p < 0.01). However, when fed with Diet I or Diet II for 20 weeks, the activities of SOD, GSH‐Px, and CAT in serum and livers of the Diet I+NDEA or Diet II+NDEA group rats were significantly higher (p < 0.01 or p < 0.05) than those of ConD+NDEA group. MDA content was also significantly lower as compared to ConD+NDEA group (p < 0.01).
Table 5.
Effects of Diet I and Diet II on antioxidant activity in the plasma and livers of rats with NDEA‐induced hepatocarcinogenesis
| Parameters | ConD | ConD+NDEA | Diet I+NDEA | Diet II+NDEA |
|---|---|---|---|---|
| Serum | ||||
| SOD (U/ml) | 40.92 ± 3.64 | 26.36 ± 5.19** | 46.41 ± 5.05## | 49.99 ± 5.83## |
| GSH‐Px (U/ml) | 465.53 ± 24.22 | 363.99 ± 43.51** | 437.94 ± 40.80## | 458.47 ± 22.20## |
| CAT (U/ml) | 21.42 ± 1.70 | 12.37 ± 1.89** | 18.95 ± 2.85## | 19.80 ± 2.32## |
| MDA (nmol/ml) | 4.81 ± 1.77 | 9.59 ± 2.16** | 4.98 ± 1.62## | 5.80 ± 1.32## |
| Liver | ||||
| SOD(U/mg) | 10.64 ± 1.56 | 7.43 ± 0.52** | 8.89 ± 2.19# | 9.62 ± 0.99## |
| GSH‐Px (U/mg) | 116.03 ± 7.24 | 89.26 ± 10.94** | 112.39 ± 8.11# | 123.93 ± 7.58## |
| CAT (U/mg) | 2.77 ± 0.77 | 1.46 ± 0.65** | 2.07 ± 1.03# | 2.87 ± 0.46## |
| MDA (nmol/mg) | 1.38 ± 0.36 | 2.68 ± 0.84** | 1.54 ± 0.36## | 1.60 ± 0.31## |
Values are expressed as mean ± SD (n = 8). Comparisons: compared with ConD group, **p < 0.01; compared with ConD+NDEA group, # p < 0.05; ## p < 0.01. CAT: catalase; GSH‐Px: glutathione peroxidase; MDA: malondialdehyde; SOD: superoxide dismutase.
The 8‐OH‐dG, as a biomarker of oxidative DNA damage, was detected using immunohistochemistry to evaluate oxidative DNA damage in the liver. As shown in Figure 2a, the production of 8‐OH‐dG increased significantly in the liver tissue of ConD+NDEA rats (p < 0.01), indicating severe DNA damage in hepatocytes. However, the production of 8‐OH‐dG in liver tissue of rats fed with Diet I or Diet II was significantly lower than the level of production in the ConD+NDEA group (p < 0.01). These results showed that Diet I and Diet II can reduce NDEA‐induced oxidative stress and protect the body from oxidative DNA damage.
Figure 2.
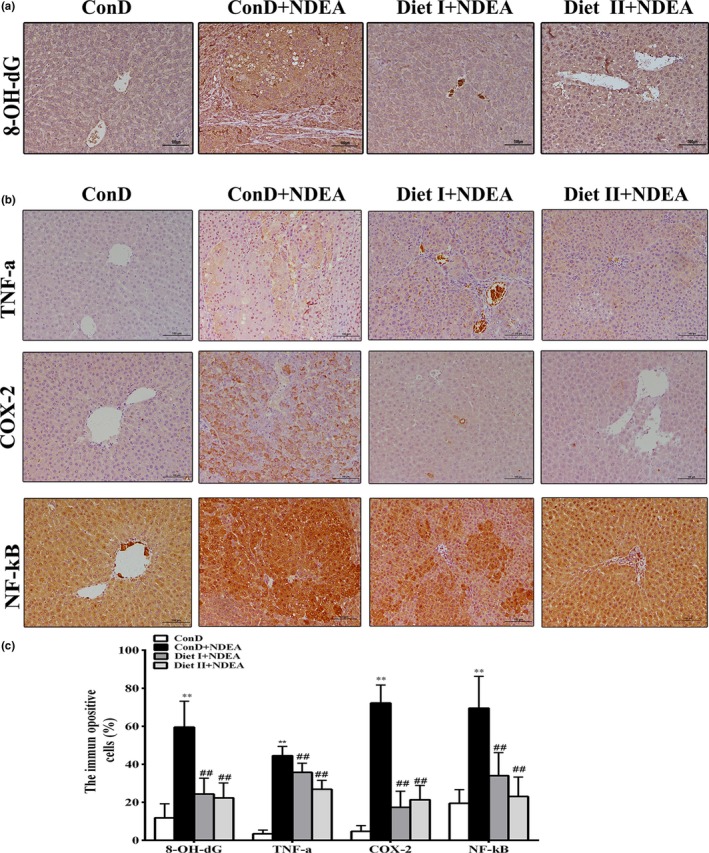
Effects of Diet I and Diet II on biomarker of oxidative DNA damage and inflammation in liver of NDEA‐induced hepatocarcinogenesis rats by immunohistochemical staining. (a) Representative immunohistochemical staining and average percentage of 8‐OH‐dG positive stained cells in the liver of control and experimental animals at ×200 original magnification. (b) Representative immunohistochemical staining of TNF‐α, COX‐2, and NF‐κB positive stained cells in the liver of control and experimental animals at ×200 original magnification. (c) The average percentage of TNF‐α, COX‐2, and NF‐κB positive stained cells in the liver of control and experimental animals. Values are expressed as mean ± SD (n = 6). Comparisons: compared with the ConD group, **p < 0.01, *p < 0.05; compared with the ConD+NDEA group, # p < 0.05; ## p < 0.01. 8‐OH‐dG: 8‐hydroxydeoxyguanosine; NF‐κB: nuclear factor‐kappa B; COX‐2: cyclooxygenase‐2; TNF‐α: tumor necrosis factor‐alpha
3.6. Diet I and Diet II can reduce NDEA‐induced inflammatory response
NDEA may cause severe chronic inflammatory damage to the liver, thus triggering the development and progression of HCC (Raghunandhakumar et al., 2013). Inflammatory factors (TNF‐α, IL‐6, etc.) play important roles in the development of inflammation and tumorigenesis (Sivaramakrishnan & Niranjali, 2009; Sui et al., 2014; Wang et al., 2017; Wojcik et al., 2012). The interaction between TNF‐α, IL‐6, and NF‐κB initiates a vicious cycle in the cytokine network, and TNF‐α and IL‐6 may activate NF‐κB, further amplifying the inflammatory response. Activated NF‐κB may further induce or activate COX‐2 (Wang et al., 2017). The mRNA and protein expression of NF‐κB, COX‐2, and TNF‐α are presented in Figures 2, 3. As shown in Figure 3a, the levels of TNF‐α and IL‐6 in the serum of the model group were significantly higher than in the normal control group (p < 0.01), as well as the number of white blood cells, neutrophils, and lymphocytes (p < 0.01 or p < 0.05). The mRNA and protein expression of NF‐κB, COX‐2, and TNF‐α in the liver of ConD+NDEA group were also significantly increased (p < 0.01 or p < 0.05). These results suggest that NDEA‐induced hepatocarcinogenesis may be associated with the inflammatory response. The Diet I+NDEA and Diet II+NDEA groups showed significantly lower serum TNF‐α and IL‐6 levels than in the ConD+NDEA group (p < 0.01 or p < 0.05), significantly lower number of white blood cells, neutrophils and lymphocytes (p < 0.01 or p < 0.05, except for white blood cell count in the Diet I +NDEA group), and significantly lower mRNA and protein expression of NF‐κB, COX‐2, and TNF‐α in liver (p < 0.01). These results suggest that Diet I and Diet II can inhibit NDEA‐induced systemic inflammatory response, reduce the accumulation of inflammatory factors, thus having an optimal regulatory function against pathological changes in the liver and preventing the occurrence or delaying development of cancer.
Figure 3.
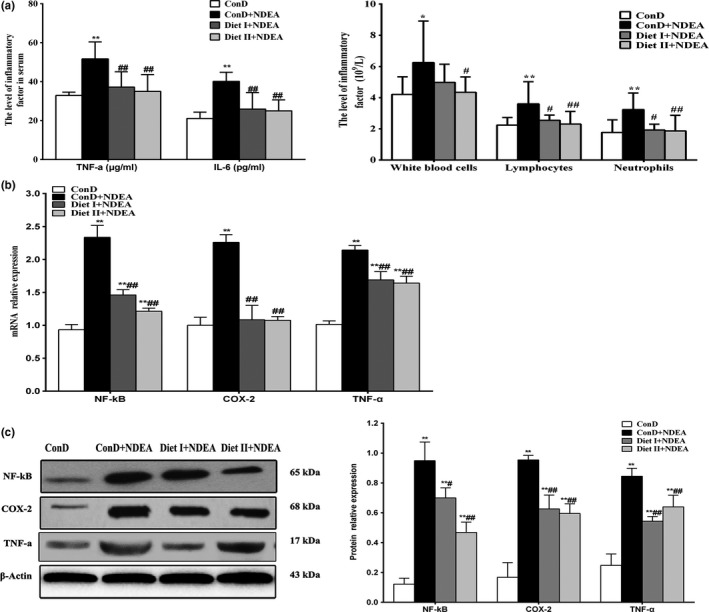
Effects of Diet I and Diet II on markers of inflammation in liver of NDEA‐induced hepatocarcinogenesis rats. (a) The levels of TNF‐α, IL‐6, and leukocyte count, neutrophil and lymphocyte count in blood. (b) The mRNA relative expression levels of NF‐κB, COX‐2, and TNF‐α in control and experimental groups. GAPDH was used as an internal control. (c) Representative immunoblots of NF‐κB, COX‐2, and TNF‐α. β‐actin was used as an internal control. Values are expressed as mean ± SD (n = 3‐6). Comparisons: compared with the ConD group, **p < 0.01, *p < 0.05; compared with the ConD+NDEA group, # p < 0.05; ## p < 0.01. TNF‐α: tumor necrosis factor‐alpha; IL‐6: interleukin‐6; NF‐κB: nuclear factor‐kappa B; COX‐2: cyclooxygenase‐2
3.7. Diet I and Diet II induced cell apoptosis and inhibited tumor cell proliferation, angiogenesis, and invasion
NDEA causes genomic damage in exposed cells. This can trigger the damaged cells to proliferate, leading to the formation of cancerous cells, which showed increased cell proliferation, angiogenesis, and invasion potential (Hanahan & Weinberg, 2011; Yu et al., 2012).
Apoptosis evasion in NDEA‐induced hepatocarcinogenesis is associated with imbalance in proapoptotic and antiapoptotic proteins combined with regulation of caspases (Gupta, Bhatia, Bansal, & Koul, 2016; Subramanian & Arul, 2013). As shown in Figures 4 and 5, Diet I and Diet II significantly reduced the expression of the antiapoptotic gene Bcl‐2 at both mRNA and protein levels in rat liver tissues relative to the ConD+NDEA group (p < 0.01 or p < 0.05), and they increased the expression of Bax, p53, Caspase‐3, and Caspase‐8 at both the mRNA and protein levels (p < 0.01 or p < 0.05). These results suggest that Diet I and Diet II could induce apoptosis of NDEA‐induced HCC at the initial stages.
Figure 4.
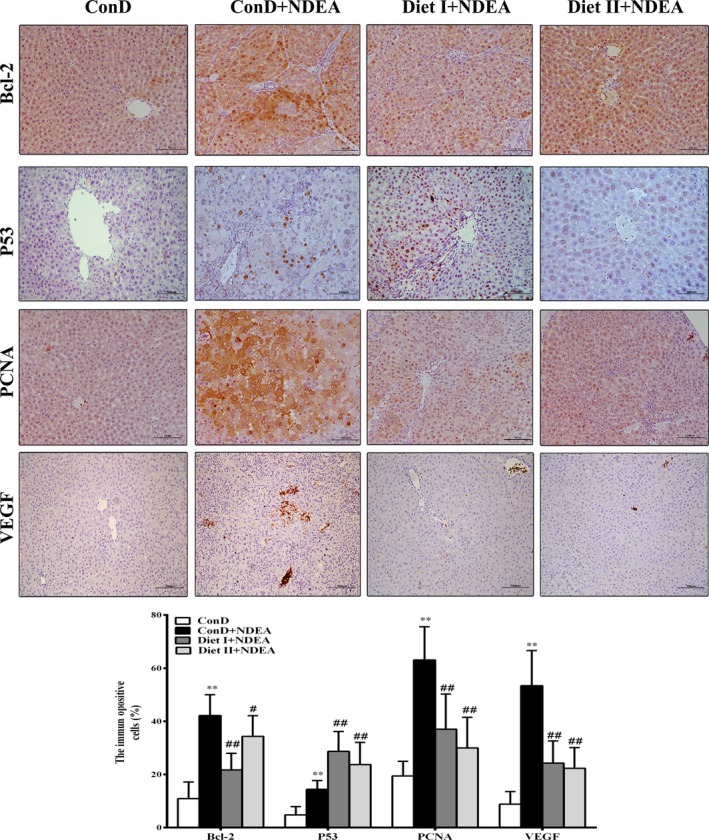
Effect of Diet I and Diet II on the expression of Bcl‐2, P53, PCNA, and VEGF in livers of NDEA‐induced hepatocarcinogenesis rats by immunohistochemical staining. Bar graph represents average percentage of positive stained cells of Bcl‐2, P53, PCNA, and VEGF in the livers of control and experimental groups. Values are expressed as mean ± SD (n = 6). Comparisons: compared with the ConD group, **p < 0.01, *p < 0.05; compared with the ConD+NDEA group, # p < 0.05; ## p < 0.01. Bcl‐2: B‐cell leukemia‐2; Bax: Bcl‐2‐associated X protein; PCNA: proliferating cell nuclear antigen; VEGF: vascular endothelial growth factor; MMP‐2: matrix metalloproteinases 2; MMP‐9: matrix metalloproteinases 9
Figure 5.
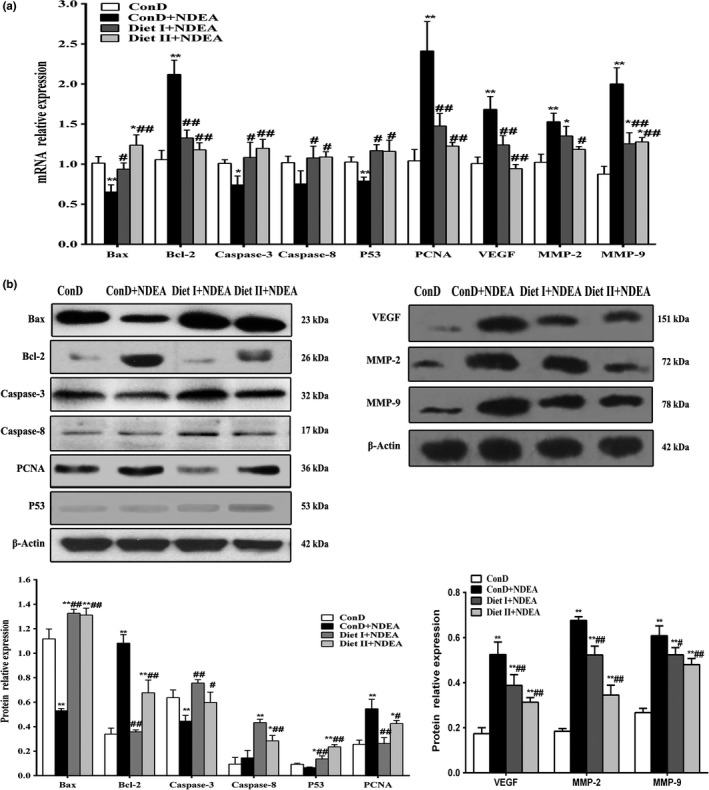
Effect of Diet I and Diet II on the markers of apoptosis, proliferation, angiogenesis, and invasion in livers of NDEA‐induced hepatocarcinogenesis rats by western blot and qRT‐PCR analyses. (a) mRNA relative expression levels of Bax, Bcl‐2, Caspase‐3, Caspase‐8, p53, PCNA, VEGF, MMP‐2, and MMP‐9 in control and treatment groups; GAPDH was used as an internal control. (b) Representative immunoblots of Bax, Bcl‐2, Caspase‐3, Caspase‐8, PCNA, and p53; β‐actin was used as an internal control. Values are expressed as mean ± SD (n = 3–6). Comparisons: compared with the ConD group, **p < 0.01, *p < 0.05; compared with the ConD+NDEA group, # p < 0.05; ## p < 0.01. Bax: Bcl‐2‐associated X protein; Bcl‐2: B‐cell leukemia‐2; PCNA: proliferating cell nuclear antigen; VEGF: vascular endothelial growth factor; MMP‐2: matrix metalloproteinases 2; MMP‐9: matrix metalloproteinases 9
Apoptosis may promote cancer cell proliferation, angiogenesis, invasion, and metastasis[41]. As shown in Figures 4 and 5, the results of immunohistochemistry, qRT‐PCR, and Western blot showed that cell proliferation markers PCNA, angiogenic factor VEGF, and matrix metalloproteinases (MMP‐2, MMP‐9) in the liver tissue of ConD+NDEA group were all significantly higher at both the mRNA and protein levels than in the ConD group (p < 0.01 or p < 0.05). The expression of PCNA, VEGF, MMP‐2, and MMP‐9 in Diet I+NDEA and Diet II+NDEA groups was significantly lower than in the NDEA group (p < 0.01 or p < 0.05), indicating that Diet I and Diet II could inhibit cancer proliferation and affect tumor neovascularization, invasion, and metastasis.
4. DISCUSSION
NDEA is a potent hepatocarcinogenic nitrosamine present in a variety of foods and also a commonly used chemical carcinogen to induce hepatocellular carcinoma in animal models (Ajiboye et al., 2013). Previous studies have shown that the methods of NDEA‐induced HCC model through administration of NDEA by free drinking water, gavage, or intraperitoneal injection can simulate the real situation of HCC in human liver and represents an ideal in vivo model for evaluating prevention agents of HCC (Bishayee et al., 2011; Gupta et al., 2016). In this study, administration of the low‐dose NDEA (25 mg/kg, 2 times per week) by intraperitoneal injection for 12 weeks had succeeded in inducing liver cancer; the tumor incidence and the apparent HCC incidence were 100%, 90%, respectively, and only a low mortality rate (20%) for 20 weeks in the ConD+NDEA group.
Then we designed natural food formula rich in ACEs according to the 2007 Second Expert Report of WCRF (2007), and containing wholegrains, vegetables, fruit, and beans that fit the recommendations in the 2018 Third Expert Report (“<Summary of Third Expert Report 2018.pdf>,”), which is “similar to” the 2007 Second Expert Report (2007), but shows “a more holistic view” on the explanation of evidence on cancer prevention. Our design of food formulas is in agreement with the guidance of the new edition of WCRF by providing ACEs in the daily foods. The present investigations indicate that the Diet I and Diet II are effective chemopreventive agents against the NDEA‐induced hepatocarcinogenesis. The pathology results showed that treatment of the Diet I and Diet II had a significant effect on reducing the incidence, size, and number of hepatic nodules, and the incidence of hepatic adenoma or HCC formation in the livers of NDEA treatment rats, the low‐grade hepatic dysplasia incidence was 20% for Diet II and 40% for Diet I, the apparent HCC rates were both 0, while the apparent HCC rate of the control diet treatment rats was 90% (p < 0.01). The chemoprevention effects of Diet I and Diet II were superior to those of some single phytochemical or single food studied by previous studies (Bhatia, Gupta, Singh, & Koul, 2015; Gupta et al., 2016; Katayama et al., 2003). Various hepatomas exhibited high levels of AST, ALT, GGT, and AFP, as usually observed in preneoplastic and neoplastic lesions after chemical hepatocarcinogenesis (Santos, Colaco, & Oliveira, 2017). The elevated serum AST, ALT, GGT, TBIL, ALP activities and levels of AFP, TSGF, and DKK1 are indicative of poor hepatic function in the ConD+NDEA group animals compared to the ConD group animals. However, the Diet I+NDEA group and Diet II+NDEA group superior to the ConD+NDEA group showed a significant reduction in these liver function markers and tumor markers, suggesting their ability to inhibit tumor progression. Moreover, long‐term Diet I and Diet II consumption showed no toxicity in this study.
Diet plays a pivotal role in cancers prevention. Foods of plant origin are recommended, such as whole grain, vegetable, fruit, and bean, according to the WCRF (2015, 2018). Numerous epidemiological studies have shown high dietary intake of fruits and vegetables, containing phytochemicals such as resveratrol, EGCG, carotenoids, antioxidative vitamins, phenolic compounds, organosulfur compounds, flavones, isothiocyanates, indoles, and fibers could reduce cancer risk or prevent multistage carcinogenesis (Bishayee, Politis, & Darvesh, 2010; Chinni, Li, Upadhyay, Koppolu, & Sarkar, 2001; Georgia & Catherine, 2014; Gupta et al., 2016; Iriti & Varoni, 2013; Kang, Tsai, & Lee, 1999; Karen‐Ng et al., 2011; Stagos et al., 2012; Sur et al., 2016). However, there has been no report up to now on the cancer prevention effects of applying multiple food components which are derived from elite crop varieties containing high ACEs. In designing combinations, we took into account multiply plant origin foods and rich ACEs in the bred and excavated elite crop varieties. The whole grains of Job's‐tears (Coix lacryma‐jobi L.) variety strain 22, black rice (Oryza sativa L.) variety Fuzi No.2, corn, and wheat bran, etc., were used mainly for energy supply. This was complemented by the nonstarchy vegetables and fruits of various colors including Brassica oleracea var. italica FU‐1, carrot (Daucus carota L.) variety Y‐NS and mulberry (Morus alba L.) variety PR‐01, etc., and by nonsugar tea [Camellia sinensis (L.) O.Kuntze] variety E‐101. This is in accordance with the cancer prevention recommendation of the Third Expert Report of WCRF (2018), which emphasizes the coordination of ACEs for cancer prevention. In this study, the foods of Diet I and Diet II were produced by elite crop varieties rich in specific ACEs (Supporting Information Table S2), thus increasing concentrations of these ACEs. We observed NDEA‐induced hepatocarcinogenesis that displayed enhanced phase I enzymes and decreased phase II enzymes. This was accompanied by apoptosis evasion, enhanced cell proliferation, inflammation, invasion, and angiogenesis. Treatment of Diet I or Diet II effectively suppressed tumor incidence which was associated with modulation of liver phase I and II enzymes, accompanied by amelioration of cell proliferation, inflammation, invasion, angiogenesis, and induction of apoptosis. Thus, Diet I and Diet II appeared to affect multistage carcinogenesis related to hepatocellular carcinoma. The reasons for cancer prevention of Diet I and Diet II may include the following: (a) The food combinations containing 28 ACEs released by WCRF may exert anticancer effect at every stage of cancer processes. These 28 ACEs may also act synergistically in vivo. In particular, the foods of Diet I and Diet II were produced by elite crop varieties rich in specific ACEs (Supporting Information Table S2). (b) Daily intake of Diet I or Diet II may delay or inhibit cancer at every stage during the processes of cancer occurrence and development in a repeated fashion.
In this study, the three formulas, ConD, Diet I, and Diet II, contained the same 19 ACEs. The additional 9 unique ACEs were only included in the Diet I and Diet II. However, long‐term Diet I and Diet II consumption had no apparent HCC in Diet I+NDEA and Diet II+NDEA group, and the low‐grade hepatic dysplasia incidence was different, 20% for Diet II and 40% for Diet I, while the incidence of apparent HCC in the ConD+NDEA group was 90%. This suggests that these 9 unique ACEs are probably crucial to cancer prevention. Previous studies have also shown that the two main decomposers of glucosinolates, indoles, and isothiocyanates can inhibit the role of polyarene and nitrosamines by inhibiting cytochrome P450 isomerase (Herr & Buchler, 2010; Konsue & Ioannides, 2010; Perocco et al., 2006). EGCG, a major green tea polyphenol, has been shown to induce expression of GST, glutathione peroxidase, glutamate cysteine ligase, hemeoxygenase‐1, etc., which are involved in the elimination or inactivation of reactive oxygen species and electrophiles implicated in multistage carcinogenesis (Wang, Wang, Wan, Yang, & Zhang, 2015). Earlier reports have shown that n‐3PUFA, resveratrol, lycopene, and energy restriction may suppress phase I enzyme or enhance phase II enzyme activities to inhibit the chemical carcinogenesis (Ebert, Seidel, & Lampen, 2005; Harvie & Howell, 2016; Johnson et al., 2011; Tan et al., 2010). Numerous studies had suggested the cancer chemoprevention of organosulfur compounds may be related to its ability to inhibit phase I enzymes (such as the cytochrome P450‐dependent monooxygenases); as well as to enhance detoxification processes by inducing expression of phase II enzymes to include GST, quinone reductase, and epoxide hydrolase (Reddy, Rao, Rivenson, & Kelloff, 1993; Georgia & Catherine, 2014; Park, Kweon, & Choi, 2002; Guyonnet, Belloir, Suschetet, Siess, & Le Bon, 2001). This demonstrates that xenobiotic‐metabolizing enzymes, especially phase II enzyme are probably crucial to cancer prevention.
The metabolic activation of NDEA by cytochrome P450 enzymes produces active ethyl radical metabolites that are mainly responsible for initiation of carcinogenesis in the liver (Sadeeshkumar et al., 2017). Subsequently, reactive product of NDEA can be detoxified by phase II enzymes including GST and quinone reductase, etc. (Bishayee et al., 2011; Sindhu, Firdous, Ramnath, & Kuttan, 2013). Increased the concentrations of cytochrome P450, b5, etc. accompanied by decreased activities of GST, UGT, and EPT observed in the present study provides evidence for the initiation of carcinogenesis in ConD+NDEA group rats. Previous studies have reported that dual‐acting agents are ideal chemopreventive agents with high efficacy (De Flora et al., 2001). Our results suggest that Diet I and Diet II act as potential dual‐acting agents by suppressing phase I and enhancing phase II enzyme activity, thereby promoting detoxification and excretion. Thus, we believed that the stage “carcinogens and other environmental exposures” in Supporting Information Table S1 is the most important step responsible for these effects. During this stage, the metabolic activation of NDEA is inhibited, and its reactive products are detoxified and excreted.
In conclusion, the combinative natural food formulas Diet I and Diet II, which were produced by elite crop varieties rich in ACEs according to WCRF, both exhibited cancer preventive or delaying effects on NDEA‐induced hepatocarcinogenesis in rats, with a 0 incidence of apparent HCC (the low‐grade hepatic dysplasia incidence was 20% for Diet II and 40% for Diet I) relative to the 90% incidence of apparent HCC in the model group (p < 0.01). Diet I and Diet II also can ameliorate the abnormal changes in liver function enzymes and reduce the increase in tumor markers. The results of highly significant cancer prevention or delay of Diet I and Diet II indicated excellent prospects of daily dietary for cancer prevention and the importance of the discovery and creation of ACEs‐rich crop varieties.
CONFLICT OF INTEREST
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
ETHICAL STATEMENT
This study was approved by the Animal Ethics Committee of the Institute of Laboratory Animal Sciences at the Chinese Academy of Medical Sciences.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Key Technology R&D Program of China (2013BAD01B05), the National Key Research and Development Program of China (2017YFD0100100), the National Natural Science Foundation of China (30600415), and the Science and Technology Innovation Platform Development Program of Fujian Agriculture and Forestry University of China (PTJH13001, PTJH12015). The authors’ responsibilities were as follows—Zheng, J.G., He, J., and Liao, S.F. conceived and designed experiments, interpreted the data, and wrote the manuscript; Zheng, J.G., He, J., Liao, S.F., Chen, T.S., and Li, X.C. participated in experiments and analyzed the data; Zheng, J.G., Liao, S.F., Cheng, Z.X., Lin, J.K., Huang, K., Zheng, K.B., Chen, X.Y., Li, L.H., Xia, F.G., Liu, J.H., Xu, M., Huang, X.Y., Cao, X.H., and Yang, Z.J. contributed to ACE‐rich elite crop varieties, reagents, and analysis tools. All authors discussed the contents of the manuscript and approved the submission.
Zheng J, He J, Liao S, et al. Preventive effects of combinative natural foods produced by elite crop varieties rich in anticancer effects on N‐nitrosodiethylamine‐induced hepatocellular carcinoma in rats. Food Sci Nutr. 2019;7:339–355. 10.1002/fsn3.896
Contributor Information
Jingui Zheng, Email: jinguizheng@126.com.
Jun He, Email: jun_he92@163.com.
REFERENCES
- Ajiboye, T. O. , Komolafe, Y. O. , Oloyede, O. B. , Ogunbode, S. M. , Adeoye, M. D. , Abdulsalami, I. O. , & Nurudeen, Q. O. (2013). Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxification in N‐nitrosodiethylamine‐treated rats. Food Science and Human Wellness, 2, 39–45. 10.1016/j.fshw.2013.02.001 [DOI] [Google Scholar]
- Bhatia, N. , Gupta, P. , Singh, B. , & Koul, A. (2015). Lycopene Enriched Tomato Extract Inhibits Hypoxia, Angiogenesis, and Metastatic Markers in early Stage N‐Nitrosodiethylamine Induced Hepatocellular Carcinoma. Nutrition Cancer, 67(8), 1268–1275. 10.1080/01635581.2015.1087040 [DOI] [PubMed] [Google Scholar]
- Bishayee, A. , Bhatia, D. , Thoppil, R. J. , Darvesh, A. S. , Nevo, E. , & Lansky, E. P. (2011). Pomegranate‐mediated chemoprevention of experimental hepatocarcinogenesis involves Nrf2‐regulated antioxidant mechanisms. Carcinogenesis, 32(6), 888–896. 10.1093/carcin/bgr045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee, A. , Politis, T. , & Darvesh, A. S. (2010). Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treatment Reviews, 36(1), 43–53. 10.1016/j.ctrv.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Buzdar, A. , Howell, A. , Cuzick, J. , Wale, C. , Distler, W. , Hoctin‐Boes, G. , … Nabholtz, J. M. (2006). Comprehensive side‐effect profile of anastrozole and tamoxifen as adjuvant treatment for early‐stage breast cancer: Long‐term safety analysis of the ATAC trial. Lancet Oncology, 7, 633–643. 10.1016/s1470-2045(06)70767-7 [DOI] [PubMed] [Google Scholar]
- Chakraborty, T. , Chatterjee, A. , Rana, A. , Dhachinamoorthi, D. , Kumar, P. A. , & Chatterjee, M. (2007). Carcinogen‐induced early molecular events and its implication in the initiation of chemical hepatocarcinogenesis in rats: Chemopreventive role of vanadium on this process. Biochimica et Biophysica Acta, 1772(1), 48–59. 10.1016/j.bbadis.2006.10.019 [DOI] [PubMed] [Google Scholar]
- Chen, Y. J. , Wallig, M. A. , & Jeffery, E. H. (2016). Dietary broccoli lessens development of fatty liver and liver cancer in mice given diethylnitrosamine and fed a western or control diet. Journal of Nutrition, 146(3), 542–550. 10.3945/jn.115.228148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. X. , Lin, J. C. , Lin, T. X. , Xu, M. , Huang, Z. W. , Yang, Z. J. , … Zheng, J. G. (2014). Genome‐wide analysis of radiation‐induced mutations in rice (Oryza sativa L. ssp. indica). Molecular BioSystems, 10(4), 795–805. 10.1039/c3mb70349e [DOI] [PubMed] [Google Scholar]
- Cheng, Z. X. , Xu, M. , Yang, Z. J. , Chen, X. W. , & Zheng, J. G. (2016). A preliminary report of excellent crop germplasm with rich functional ingredients. Fujian Agricultural Science and Technology, 7, 83–84. [Google Scholar]
- Chinni, S. R. , Li, Y. , Upadhyay, S. , Koppolu, P. K. , & Sarkar, F. H. (2001). Indole‐3‐carbinol (I3C) induced cell growth inhibition G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene, 20, 2927–2936. 10.1038/sj.onc.1204365 [DOI] [PubMed] [Google Scholar]
- Cummings, B. S. , Parker, J. C. , & Lash, L. H. (2000). Role of cytochrome P450 and glutathione S‐transferase alpha in the metabolism and cytotoxicity of trichloroethylene in rat kidney. Biochemical. Pharmacology, 59(5), 531–543. 10.1016/S0006-2952(99)00374-3 [DOI] [PubMed] [Google Scholar]
- De Flora, S. , Izzotti, A. , D'Agostini, F. , Balansky, R. M. , Noonan, D. , & Albini, A. (2001). Multiple points of intervention in the prevention of cancer and other mutation‐related diseases. Mutation Research, 9(22), 480–481. [DOI] [PubMed] [Google Scholar]
- Ebert, B. , Seidel, A. , & Lampen, A. (2005). Induction of phase‐1 metabolizing enzymes by oltipraz, flavone and indole‐3‐carbinol enhance the formation and transport of benzo[a]pyrene sulfate conjugates in intestinal Caco‐2 cells. Toxicology Letters, 158(2), 140–151. 10.1016/j.toxlet.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Edmondson, H. A. , & Steiner, P. E. (1954). Primary carcinoma of the liver: A study of 100 cases among 48900 necropsies. Cancer, 7, 462–503. [DOI] [PubMed] [Google Scholar]
- Fabian, E. , Wang, X. , Engel, F. , Li, H. , Landsiedel, R. , & van Ravenzwaay, B. (2016). Activities of xenobiotic metabolizing enzymes in rat placenta and liver in vitro. Toxicology In Vitro, 33, 174–179. 10.1016/j.tiv.2016.02.024 [DOI] [PubMed] [Google Scholar]
- Georgia, S. , & Catherine, H. K. (2014). The immunomodulation and anti‐inflammatory effects of garlic organosulfur compound in cancer chemoprevention. Anticancer Agents in Medicinal Chemistry, 14(2), 233–240. 10.2174/18715206113136660370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace, M. H. , Esposito, D. , Timmers, M. A. , Xiong, J. , Yousef, G. , Komarnytsky, S. , & Lila, M. A. (2016). Chemical composition, antioxidant and anti‐inflammatory properties of pistachio hull extracts. Food Chemistry, 210, 85–95. 10.1016/j.foodchem.2016.04.088 [DOI] [PubMed] [Google Scholar]
- Gupta, P. , Bhatia, N. , Bansal, M. P. , & Koul, A. (2016). Lycopene modulates cellular proliferation, glycolysis and hepatic ultrastructure during hepatocellular carcinoma. World Journal of Hepatology, 8(29), 1222–1233. https://doi.org/doi:10.4254/wjh.v8.i29.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyonnet, D. , Belloir, C. , Suschetet, M. , Siess, M. H. , & Le Bon, A. M. (2001). Antimutagenic activity of organosulfur compounds from allium is associated with phase II enzyme induction. Mutation Research, 495(1–2), 135–145. 10.1016/S1383-5718(01)00205-4 [DOI] [PubMed] [Google Scholar]
- Hanahan, D. , & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Harvie, M. N. , & Howell, T. (2016). Could intermittent energy restriction and intermittent fasting reduce rates of cancer in obese, overweight, and normal‐weight subjects? A Summary of Evidence Advanced Nutritional, 7(4), 690–705. 10.3945/an.115.011767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr, I. , & Buchler, M.W. (2010). Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treatment Reviews, 36(5), 377–383. doi:10.1016/ j.ctrv.2010.01.002 [DOI] [PubMed] [Google Scholar]
- Iriti, M. , & Varoni, E.M. (2013). Chemopreventive potential of flavonoids in oral squamous cell carcinoma in human studies. Nutrients, 5(7), 2564–2576. 10.3390/nu5072564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, W.D. , Morrissey, R.L. , Usborne, A.L. , Kapetanovic, I. , Crowell, J.A. , Muzzio, M. , & McCormick, D.L. (2011). Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chemistry Toxicology, 49(12), 3319–3327. 10.1016/j.fct.2011.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Z.C. , Tsai, S.J. , & Lee, H. (1999). Quercetin inhibits benzo[a]pyrene‐induced DNA adducts in human Hep G2 cells by altering cytochrome P‐450 1A1 gene expression. Nutrition Cancer, 35(2), 175–179. 10.1207/s15327914nc352_12 [DOI] [PubMed] [Google Scholar]
- Karen‐Ng, L. P. , Marhazlinda, J. , Rahman, Z. A. , Abraham, M. T. , Cheong, S. C. , & Zain, R. B. (2011). O45. combined effects of isothiocyanates (itcs) intake, glutathione s‐transferases (gsts) polymorphism and risk habits on oral squamous cell carcinoma (oscc) associated with earlier age of disease onset. Oral Oncology, 47(3), S43. [Google Scholar]
- Katayama, M. , Sugie, S. , Yoshimi, N. , Yamada, Y. , Sakata, K. , Qiao, Z. , … Mori, H. (2003). Preventive effect of fermented brown rice and rice bran on diethylnitrosoamine and phenobarbital‐induced hepatocarcinogenesis in male F344 rats. Oncology Reports, 10(4), 875–880. 10.3892/or.10.4.875 [DOI] [PubMed] [Google Scholar]
- Konsue, N. , & Ioannides, C. (2010). Modulation of carcinogen‐metabolising cytochromes P450 in human liver by the chemopreventive phytochemical phenethyl isothiocyanate, a constituent of cruciferous vegetables. Toxicology, 268(3), 184–190. 10.1016/j.tox.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Kweon, M. H. , Adhami, V. M. , Lee, J. S. , & Mukhtar, H. (2006). Constitutive overexpression of Nrf2‐dependent heme oxygenase‐1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3‐gallate. Journal of Biological Chemistry, 281(44), 33761–33772. 10.1074/jbc.M604748200 [DOI] [PubMed] [Google Scholar]
- Liao, S. F. , Liu, J. H. , Xu, M. , & Zheng, J. G. (2018). Evaluation of the liver cancer prevention of anthocyanin extracts from Mulberry (Morus alba L.) variety PR‐01. Advances in Bioscience and Biotechnology, 09(09), 423–442. 10.4236/abb.2018.99030 [DOI] [Google Scholar]
- Liu, H. , Yang, J. , Huang, S. , Liu, R. , He, Y. , Zheng, D. , & Liu, C. (2017). Mulberry crude extracts induce Nrf2 activation and expression of detoxifying enzymes in rat liver: implication for its protection against NP‐induced toxic effects. Journal of Functional Foods, 32, 367–374. 10.1016/j.jff.2017.03.024 [DOI] [Google Scholar]
- Marra, M. , Sordelli, I. M. , Lombardi, A. , Lamberti, M. , Tarantino, L. , Giudice, A. , & Sperlongano, P. (2011). Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: An overview. Journal of Translational Medicine, 9, 171 10.1186/1479-5876-9-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaka, S. , Miyazaki, N. , Fukushima, T. , Yamada, S. , & Kimura, J. (2013). Effects of green tea extract and (‐)‐epigallocatechin‐3‐gallate on pharmacokinetics of nadolol in rats. Phytomedicine, 20(14), 1247–1250. 10.1016/j.phymed.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Na, H. K. , & Surh, Y. J. (2008). Modulation of Nrf2‐mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chemistry Toxicology, 46(4), 1271–1278. 10.1016/j.fct.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Nebert, D. W. , & Gelboin, H. V. (1968). Substrate‐inducible microsomal aryl hydroxylase in mammalian cell culture. l. Assembly and properties of induced enzyme. Journal of Biological Chemistry, 243(23), 6242–6249. [PubMed] [Google Scholar]
- Omura, T. , & Sato, R. (1964). The carbon monoxide binding pigment of liver microsomes. Journal of Biological Chemistry, 239, 2370–2378. [PubMed] [Google Scholar]
- Park, K. A. , Kweon, S. , & Choi, H. (2002). Anticarcinogenic effect and modification of cytochrome P450 2E1 by dietary garlic powder in diethylnitrosamine‐initiated rat hepatocarcinogenesis. Journal of Steroid Biochemistry & Molecular Biology, 35(6), 615–622. [DOI] [PubMed] [Google Scholar]
- Perocco, P. , Bronzetti, G. , Canistro, D. , Valgimigli, L. , Sapone, A. , Affatato, A. , & Abdel‐Rahman, S. Z. (2006). Glucoraphanin, the bioprecursor of the widely extolled chemopreventive agent sulforaphane found in broccoli, induces phase‐I xenobiotic metabolizing enzymes and increases free radical generation in rat liver. Mutation Research, 595(1–2), 125–136. 10.1016/j.mrfmmm.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Philips, A. H. , & Langdon, R. G. (1962). Hepatic triphospho‐pyridine nucleotide cytochrome C reductase. Isolation, characterization and kinetic studies. Journal of Biological Chemistry, 237, 2562–2660. [PubMed] [Google Scholar]
- Raghunandhakumar, S. , Paramasivam, A. , Senthilraja, S. , Naveenkumar, C. , Asokkumar, S. , Binuclara, J. , … Devaki, T. (2013). Thymoquinone inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N‐nitrosodiethylamine‐induced experimental rat hepatocellular carcinoma. Toxicology Letter, 223(1), 60–72. 10.1016/j.toxlet.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Reddy, B. S. , Rao, C. V. , Rivenson, A. , & Kelloff, G. (1993). Chemoprevention of colon carcinogenesis by organosulfur compounds. Cancer Reseach, 53, 3493–3498. [PubMed] [Google Scholar]
- Sadeeshkumar, V. , Duraikannu, A. , Ravichandran, S. , Kodisundaram, P. , Fredrick, W. S. , & Gobalakrishnan, R. (2017). Modulatory efficacy of dieckol on xenobiotic‐metabolizing enzymes, cell proliferation, apoptosis, invasion and angiogenesis during NDEA‐induced rat hepatocarcinogenesis. Molecular and Cellular Biochemistry, 433, 195–204. 10.1007/s11010-017-3027-8 [DOI] [PubMed] [Google Scholar]
- Santos, N. P. , Colaco, A. A. , & Oliveira, P. A. (2017). Animal models as a tool in hepatocellular carcinoma research: A Review. Tumour Biology, 39(3), 1010428317695923 10.1177/1010428317695923 [DOI] [PubMed] [Google Scholar]
- Sindhu, E. R. , Firdous, A. P. , Ramnath, V. , & Kuttan, R. (2013). Effect of carotenoid lutein on N‐nitrosodiethylamine‐ induced hepatocellular carcinoma and its mechanism of action. European Journal of Cancer, 22(4), 320–327. 10.1097/CEJ.0b013e32835b69ff [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan, V. , & Niranjali, D. S. (2009). Morin regulates the expression of NF‐kappaB‐p65, COX‐2 and matrix metalloproteinases in diethylnitrosamine induced rat hepatocellular carcinoma. Chemico‐Biological Interactions, 180, 353–359. 10.1016/j.cbi.2009.02.004 [DOI] [PubMed] [Google Scholar]
- Smitskamp‐Wilms, E. , Giaccone, G. , Pinedo, H.M. , vander, G.J. , & Peters, B.F. (1995). DT‐diaphorase activity in normal and neoplastic human tissues; an indicator for sensitivity to bioreductive agents? British Journal of Cancer., 72(4), 917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagos, D. , Amoutzias, G. D. , Matakos, A. , Spyrou, A. , Tsatsakis, A. M. , & Kouretas, D. (2012). Chemoprevention of liver cancer by plant polyphenols. Food Biochemistry Toxicology, 50(6), 2155–2170. 10.1016/j.fct.2012.04.002 [DOI] [PubMed] [Google Scholar]
- Subramanian, P. , & Arul, D. (2013). Attenuation of NDEA‐induced hepatocarcinogenesis by naringenin in rats. Cell Biochemistry and Function, 31(6), 511–517. 10.1002/cbf.2929 [DOI] [PubMed] [Google Scholar]
- Sui, W. , Zhang, Y. , Wang, Z. , Wang, Z. , Jia, Q. , Wu, L. , & Zhang, W. (2014). Antitumor effect of a selective COX‐2 inhibitor, celecoxib, may be attributed to angiogenesis inhibition through modulating the PTEN/PI3K/Akt/HIF‐1 pathway in an H(2)(2) murine hepatocarcinoma model. Oncology Reports, 31(5), 2252–2260. 10.3892/or.2014.3093 [DOI] [PubMed] [Google Scholar]
- Sur, S. , Pal, D. , Roy, R. , Barua, A. , Roy, A. , Saha, P. , & Panda, C. K. (2016). Tea polyphenols EGCG and TF restrict tongue and liver carcinogenesis simultaneously induced by N‐nitrosodiethylamine in mice. Toxicology and Applied Pharmacology, 300, 34–46. 10.1016/j.taap.2016.03.016 [DOI] [PubMed] [Google Scholar]
- Tan, X. L. , Shi, M. , Tang, H. , Han, W. , & Spivack, S. D. (2010). Candidate dietary phytochemicals modulate expression of phase II enzymes GSTP1 and NQO1 in human lung cells. Journal of Nutrition, 140(8), 1404–1410. 10.3945/jn.110.121905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayutham, M. , Muthukumaran, R. B. , Sostaric, J. Z. , McCraken, J. , Fishbein, J. C. , & Zweier, J. L. (2007). Interactions of the major metabolite of the cancer chemopreventive drug oltipraz with cytochrome c: A novel pathway for cancer chemoprevention. Free Radical Biology & Medicine, 43(7), 1076–1085. 10.1016/j.freeradbiomed.2007.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. S. , Lv, Y. , Wang, Z. , Ma, J. , Mi, C. , Li, X. , … Jin, X. J. (2017). Imperatorin efficiently blocks TNF‐α‐mediated activation of ROS/PI3K/Akt/NF‐κB pathway. Oncology Reports, 6(37), 3397–3404. 10.3892/or.2017.5581 [DOI] [PubMed] [Google Scholar]
- Wang, D. , Wang, Y. , Wan, X. , Yang, C. S. , & Zhang, J. (2015). Green tea polyphenol (‐)‐epigallocatechin‐3‐gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicology and Applied Pharmacology, 283(1), 65–74. 10.1016/j.taap.2014.12.018 [DOI] [PubMed] [Google Scholar]
- Wojcik, M. , Ramadori, P. , Blaschke, M. , Sultan, S. , Khan, S. , Malik, I. A. , … Schultze, F. C. (2012). Immunodetection of cyclooxygenase‐2 (COX‐2) is restricted to tissue macrophages in normal rat liver and to recruited mononuclear phagocytes in liver injury and cholangiocarcinoma. Histochemistry and Cell Biology, 137(2), 217–233. 10.1007/s00418-011-0889-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research . (2007). Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; Retrieved from https://www.wcrf.org/dietandcancer/changes-since-2007-second-expert-report [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research . (2015). Continuous update project report: diet, nutrition, physical activity and liver cancer. Retrieved from http://wcrf.org/sites/default/files/Liver‐ Cancer‐2015‐ Report.pdf.
- World Cancer Research Fund/American institute for Cancer Research . (2018). Diet, Nutrition, Physical Activity and Cancer: a Globle Perspective. Continuous Update Project Expert Report. Retrieved from https://www.wcrf.org/dietandcancer
- Yoxall, V. , Kentish, P. , Coldham, N. , Kuhnert, N. , Sauer, M. J. , & Ioannides, C. (2005). Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. International Journal of Cancer, 117(3), 356–362. 10.1002/ijc.21191 [DOI] [PubMed] [Google Scholar]
- Yu, X. , Wang, L. N. , Du, Q. M. , Ma, L. , Chen, L. , You, R. , … Ji, H. (2012). Akebia Saponin D attenuates amyloid beta‐induced cognitive deficits and inflammatory response in rats: Involvement of Akt/NF‐kappaB pathway. Behavioural Brain Research, 235(2), 200–209. 10.1016/j.bbr.2012.07.045 [DOI] [PubMed] [Google Scholar]
- Zheng, J. G. (2004). Science on Agricultural Product Quality, 1st ed. China: Xiamen University Press. [Google Scholar]
- Zheng, J. G. (2014). Science on Agricultural Product Quality, 2nd ed. China: Xiamen University Press. [Google Scholar]
- Zheng, J. G. , Lin, J. K. , Cheng, Z. X. , Xu, M. , Huang, Z. W. , Yang, Z. J. , … Zheng, B. D. (2012). Study on Breeding for Crop Fine Varieties Rich in Food Factors and their Functions. Journal of Food and Drug Analysis, 20(Suppl. 1), 198–202. [Google Scholar]
- Zhou, Y. , Li, Y. , Zhou, T. , Zheng, J. , Li, S. , & Li, H. B. (2016). Dietary natural products for prevention and treatment of liver cancer. Nutrients, 8(3), 156 10.3390/nu8030156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


