Abstract
OBJECTIVE
Hepatic insulin clearance is a significant regulator of glucose homestasis. We hypothesized that the improvement in insulin clearance rates (ICRs) under fasting conditions and in response to oral and intravenous (IV) glucose would improve similarly after Roux-en-Y gastric bypass (RYGB) and adjustable gastric banding (AGB) as a function of weight loss; the difference in ICR after oral and IV glucose stimulation will be enhanced after RYGB compared with AGB, an effect mediated by glucagon-like peptide 1 (GLP-1).
RESEARCH DESIGN AND METHODS
In study 1, the ICR was calculated under fasting condition (F-ICR), after oral glucose (O-ICR), and after an isoglycemic IV glucose clamp (IV-ICR) in individuals from an established cohort with type 2 diabetes mellitus (T2DM) before, after 10% matched weight loss, and 1 year after either RYGB (n = 22) or AGB (n = 12). In study 2, O-ICR was studied in a separate cohort of individuals with T2DM (n = 22), before and 3 months after RYGB, with and without exendin(9-39) infusion.
RESULTS
In study 1, age, BMI, T2DM duration and control, and ICR did not differ between RYGB and AGB preintervention. Weight loss at 1 year was two times greater after RYGB than after AGB (31.6 ± 5.9% vs. 16.6 ± 9.8%; P < 0.05). RYGB and AGB both significantly increased F-ICR, O-ICR, and IV-ICR at 1 year. ICR was inversely associated with insulinemia. The difference between IV-ICR and O-ICR was significantly greater after RYGB versus AGB. GLP-1 antagonism with exendin(9-39) led to an increase in O-ICR in subjects post-RYGB.
CONCLUSIONS
Weight loss increased ICR, an effect more pronounced after RYGB compared with AGB. Our data support a potential role for endogenous GLP-1 in the control of postprandial ICR after RYGB.
Introduction
Hyperinsulinemia is a risk factor for cardiovascular disease (1). Circulating concentrations of insulin are dependent on β-cell insulin secretion capacity and insulin clearance from the blood (2). About 70% of secreted insulin is cleared by the liver prior to entering the systemic circulation (3). Hepatic insulin clearance rate (ICR) is decreased in many aspects of the metabolic syndrome, such as obesity (4), hypertension, hypertriglyceridemia, and glucose intolerance (5). The clearance of insulin differs according to the route of glucose administration. ICR is lower after an oral compared with intravenous (IV) glucose load (6). The mechanisms explaining that difference may include gastrointestinal factors, such as the incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP).
ICR is a key regulator of glucose homeostasis; it increases with weight loss by diet (7) or bariatric surgery (8) along with improved glucose control. Roux-en-Y gastric bypass (RYGB) results in rapid amelioration of glucose homeostasis, with improvement of ICR preceeding that of insulin sensitivity (9). Bariatric surgical procedures that involve rerouting of nutrients away from the upper part of the gastrointestinal track, such as RYGB, or accelerated nutrient transit, such as RYGB or vertical sleeve gastrectomy (VSG), are more successful at mitigating type 2 diabetes mellitus (T2DM) than adjustable gastric banding (AGB). The greater effect of RYGB on metabolic improvements is often confounded by greater weight loss post-RYGB (10). Some of the superior effects of RYGB and VSG compared with diet or AGB on postprandial glucose are thought to be mediated by increased postprandial levels of GLP-1 (11,12). It remains unclear what role incretins may play in hepatic ICR, particularly after RYGB in subjects with T2DM.
The primary goal of this study was to assess the role of surgical weight loss by either RYGB or AGB on ICR. We hypothesized that 1) fasting ICR and ICR in response to an IV glucose stimulus would improve similarly after RYGB and AGB, as a function of weight loss, and 2) that the difference in ICR after oral and IV glucose stimuli would be exaggerated after RYGB compared with AGB, an effect mediated by endogenous GLP-1.
Research Design and Methods
Data for this work were derived from experiments designed to study the incretin effect after RYGB and AGB (13,14) and the effect of endogenous GLP-1 (study 2).
Subjects
Individuals with severe obesity and documented T2DM, scheduled to have either RYGB or AGB (study 1) or RYGB (study 2) at St. Luke’s Roosevelt Hospital, provided written informed consent prior to participating. Being treated with dipeptidyl peptidase 4 inhibitors, thiazolidinediones, or GLP-1 agonists was an exclusionary criteria as were active malignancy, recent (<6 months) cardiovascular disease, kidney impairment, liver dysfunction, pregnancy, and hypertriglyceridemia (>600 ng/dL). Diabetes remission was defined using American Diabetes Association criteria, with HbA1c <6.5%, fasting glucose <126 mg/dL, and 120-min postprandial glucose <200 mg/dL, along with lack of antidiabetic drug use (15).
Study Design
Study 1 was a longitudinal, prospective, nonrandomized study, with an oral glucose tolerance test (OGTT, 50 g) followed by an isoglycemic IV glucose clamp (iso-IVGT) presurgery, after 10% weight loss and 1 year after surgery. Study 2 was a longitudinal prospective study with OGTT (75 g) before surgery and 3 months after RYGB. The OGTT was performed twice after surgery, under infusion of saline or with the GLP-1 receptor antagonist exendin(9-39) (EX9).
Interventions
All surgical procedures for studies 1 and 2 were performed by the same bariatric team. For RYGB, the jejunum was divided 30 cm from the ligament of Treitz and anastomosed to a 30-mL promimal pouch, and the jejunum was reanastomosed 150 cm distal to the gastrojejunostomy as described previously (13). For AGB, a silicone adjustable band (∼10–12 mm diameter) was placed around the proximal portion of the stomach, creating a 30-mL pouch. Adjustment of the band with saline was performed as needed. Subjects were free-living and followed the recommended postoperative bariatric diet of clear liquids during week 1, pureed diet during weeks 1–3, and solid foods starting at week 4. The diet was not otherwise controlled.
Experimental Procedures for Study 1
OGTT
Participants underwent a 3-h OGTT (50 g of glucose in 200 mL) after a 12-h overnight fast at each study time point. Antidiabetic medications were stopped 2 days prior to each experimental procedure. Glucose was given in the sitting position (about 45° angle) and consumed over 15 min. Blood samples were collected into chilled EDTA tubes over 3 h from an antecubital IV catheter placed in an arterialized arm vein that was kept warm with a heating pad. Samples, collected at 0, 15, 30, 45, 60, 90, 120, and 180 min, were centrifuged at 4°C and stored at −80°C for measurement of glucose, insulin, and C-peptide.
iso-IVGT
To compare differences in ICR after oral and matched IV glucose, an iso-IVGT was performed as described previously and was done within 1 week of the corresponding OGTT (13). Glucose (sterile 20% dextrose solution) was infused via a pump (Gemini; CareFusion, San Diego, CA) over a 3-h time period. Blood glucose levels were monitored using a contralateral antecubital IV access every 5 min, and the glucose infusion rate was adjusted to mimic the glucose concentration profiles achieved for each patient during the preceding OGTT. Blood samples were collected at the same time points as during the OGTT for insulin and C-peptide.
Experimental Procedures for Study 2
OGTT With and Without EX9 Infusion
Prior to RYGB, all participants underwent one OGTT (75 g glucose in 222 mL) with a procedure and timing of blood sampling identical to study 1. Three months after RYGB, participants underwent two OGTTs, separated by at least 3 days, one with infusion of saline and the other with infusion of EX9, administered in random order. At 30 min before the administration of glucose, subjects received either a continuous IV EX9 infusion (Bachem, Weil am Rhein, Germany) of 600 pmol/kg/min for the duration of the experiment (−30 to 180 min) or saline as a control. The dose of EX9 was chosen to ensure complete inhibition of the GLP-1 receptor (16).
Assays
Plasma glucose was determined by the glucose oxidase method (Glucose Analyzer; Analox Instruments, Lunenburg, MA). Plasma insulin and C-peptide were measured by RIA (EMD Millipore, St. Charles, MO) by the Columbia University Diabetes Research Center Translational Biomarkers Analytical Core. Intra- and interassay coefficients of variance ranged from 3.4–7.4% to 4.4–7.4%, respectively.
Calculations
Total areas under the curves (AUCs) during the OGTT and iso-IVGT were calculated using the trapezoidal method (17). HOMA of insulin resistance (HOMA-IR) was calculated as (fasting insulinµU/mL × fasting glucosemg/dL)/405 (18). Insulin sensitivity index (ISI) was derived from OGTT values using the Matsuda Index (19). Insulin secretion rates (ISRs) were calculated by C-peptide deconvolution using a two-compartment model for the OGTT (O-ISR) and iso-IVGT (IV-ISR) (20). ICR during OGTT (O-ICR) and iso-IVGT (IV-ICR) was calculated as ICR = (ISRAUC/insulinAUC) − (V*[(insulinend time − insulinstart time)/insulinAUC]), where V is the volume of distribution of insulin and estimated as 0.14 L/kg (21). The difference between O-ICR and IV-ICR at a given study time point was termed ΔICR. Fasting ICR (F-ICR) was calculated as the ratio of fasting ISR to fasting insulin levels.
Statistical Analysis
Normality was tested, and nonparametric Mann-Whitney and Wilcoxon signed-rank tests were used if variables were not normally distributed. Independent and paired Student t tests were used for RYGB versus AGB and within group comparisons, respectively. Kendall rank test was used to test for correlations among ICR values and other physiologic parameters. Multiple linear regression analysis was used to test the effect of surgery type and percent weight loss on outcome variables; B values, or unstandardized β coefficient from the regression model, are reported. Data are expressed as mean ± SD, except in figures where mean ± SEM are reported. Statistical significance was set at P < 0.05 (two tailed). SPSS 24.0 (IBM, Armonk, NY) was used for all analyses. This study was a post hoc analysis of established cohorts from studies designed and powered to study surgical weight loss on GLP-1 and glucose. Based on prior literature that showed increased O-ICR after RYGB but not AGB, we determined our sample sizes in order achieve at least 90% power to detect significant increases in ICR after RYGB (22).
Results
Study 1
Subject Characteristics
Thirty-four participants (22 RYGB and 12 AGB) were studied at three time points: before surgery, after 10% weight loss, and 1 year postsurgery. Sex distribution, age, known diabetes duration, presurgery HbA1c, weight, BMI, fasting and postprandial glucose during the OGTT, HOMA-IR, F-ICR, O-ICR and IV-ICR, and glucose infused during the iso-IVGT did not differ between surgery groups prior to intervention (Table 1).
Table 1.
Comparison of AGB and RYGB at 10% weight loss and 1 year post-RYGB
| AGB (n = 12) |
RYGB (n = 22) |
|||||
|---|---|---|---|---|---|---|
| Pre-AGB | 10% weight loss | 1 Year | Pre-RYGB | 10% weight loss | 1 Year | |
| Oral T2DM medication (no. of subjects) | 8/12 | 1/12 | 1/12 | 16/22 | 1/22 | 1/22 |
| Insulin use (no. of subjects) | 1/12 | 0/12 | 0/12 | 1/22 | 0/22 | 0/22 |
| HbA1c (%) | 6.5 ± 1.0 | — | 5.8 ± 0.7* | 6.7 ± 0.6 | — | 5.6 ± 0.4* |
| HbA1c (mmol/mol) | 48 ± 0.09 | — | 40 ± 0.06* | 49.7 ± 0.05 | — | 37.6 ± 0.04* |
| Weight (kg) | 117 ± 11.1 | 106 ± 11.2* | 97.3 ± 15.6* | 120 ± 15.9 | 108 ± 15.6* | 82.2 ± 13.5*$# |
| BMI (kg/m2) | 43.4 ± 4.9 | 39.1 ± 4.6* | 36.1 ± 5.7* | 44.7 ± 3.6 | 40.3 ± 3.6* | 30.6 ± 3.1*$# |
| Weight loss (%) | — | 9.6 ± 2.1 | 16.6 ± 9.8$ | — | 10.0 ± 2.0 | 31.6 ± 5.9$# |
| Weight loss duration (weeks) | — | 9.0 ± 9.4 | 56.1 ± 17.5 | — | 4.2 ± 0.9# | 53.6 ± 8.1 |
| Glucose infused (g) | 41.8 ± 20.2 | 40.5 ± 15.0 | 30.2 ± 12.5# | 39.4 ± 8.9 | 34.1 ± 13.2 | 34.6 ± 13.8 |
| Fasting glucose (mmol/L) | 7.8 ± 1.9 | 6.3 ± 1.2* | 5.6 ± 1.0* | 7.3 ± 1.6 | 5.6 ± 0.9*# | 5.1 ± 1.0* |
| Fasting insulin (pmol/L) | 209 ± 129 | 147 ± 88.6 | 107 ± 73.9* | 213 ± 78.1 | 106 ± 37.2* | 57.3 ± 34.2*$ |
| 120-min glucose (mmol/L) | 10.7 ± 3.2 | 10.3 ± 3.7 | 8.5 ± 2.5*$ | 10.6 ± 3.0 | 6.3 ± 1.9*# | 5.3 ± 1.7*# |
| HOMA-IR | 10.5 ± 7.6 | 5.8 ± 3.7* | 3.8 ± 2.8* | 9.7 ± 4.0 | 3.8 ± 1.6* | 1.8 ± 1.2*$# |
| O-ISR AUC (pmol/kg) | 824 ± 455 | 911 ± 505 | 883 ± 527 | 875 ± 383 | 932 ± 386 | 860 ± 494 |
| IV-ISR AUC (pmol/kg) | 728 ± 496 | 754 ± 387 | 559 ± 257$ | 773 ± 305 | 666 ± 239 | 587 ± 276* |
| ISI | 2.47 ± 0.52 | 3.39 ± 0.70 | 5.36 ± 1.0*$ | 1.87 ± 0.13 | 3.37 ± 0.38* | 6.51 ± 1.04*$ |
| F-ICR (mL/kg/min) | 13.7 ± 9.25 | 14.9 ± 4.64 | 18.5 ± 5.72* | 14.0 ± 5.77 | 21.1 ± 6.60*# | 27.8 ± 30.24* |
| O-ICR (mL/kg/min) | 13.7 ± 6.35 | 13.4 ± 3.81† | 20.6 ± 9.41*$ | 11.6 ± 4.41 | 14.4 ± 4.86*† | 19.7 ± 6.06*$† |
| IV-ICR (mL/kg/min) | 14.8 ± 7.16 | 18.4 ± 4.57* | 21.1 ± 5.44* | 13.2 ± 4.73 | 21.3 ± 5.55* | 32.0 ± 11.4*$# |
| ΔICR (mL/kg/min) | 1.08 ± 5.02 | 5.01 ± 4.18* | 0.48 ± 7.36$ | 1.56 ± 3.05 | 6.87 ± 4.08* | 12.3 ± 10.0*$# |
Data for glucose infused are during the isoglycemic glucose clamp. Data are mean ± SD unless otherwise indicated.
*P < 0.05 for paired Student t test vs. preintervention.
†P < 0.05 for paired Student t test vs. IV.
$P < 0.05 for paired Student t test vs. 10% weight loss.
#P < 0.05 for unpaired Student t test vs. AGB.
Effect of Surgery on Weight, Diabetes Remission, and Insulin Sensitivity
One year after surgery, three participants after RYGB and two after AGB were not in diabetes remission; all were treated by diet, except one in each surgery group who were also on an oral diabetes medication. It took twice as long for patients after AGB to lose 10% of their presurgical weight (RYGB 4.2 ± 0.9 weeks, AGB 9.0 ± 9.4 weeks; P = 0.022). As expected, RYGB resulted in about twice the amount of weight loss at 1 year compared with AGB (31.6 ± 5.9 vs. 16.6 ± 9.8%; P < 0.001). HOMA-IR and ISI improved significantly in both cohorts, with no difference between groups 1 year after surgery (Table 1). Absolute weight correlated significantly with HOMA-IR (r = 0.417, P < 0.001) and ISI (r = −0.308, P = 0.002) across all subjects and study time points. Glucose, insulin, and ISR time profiles during the OGTT and iso-IVGT were previously published (13).
Effect of Surgery on F-ICR, O-ICR, and IV-ICR
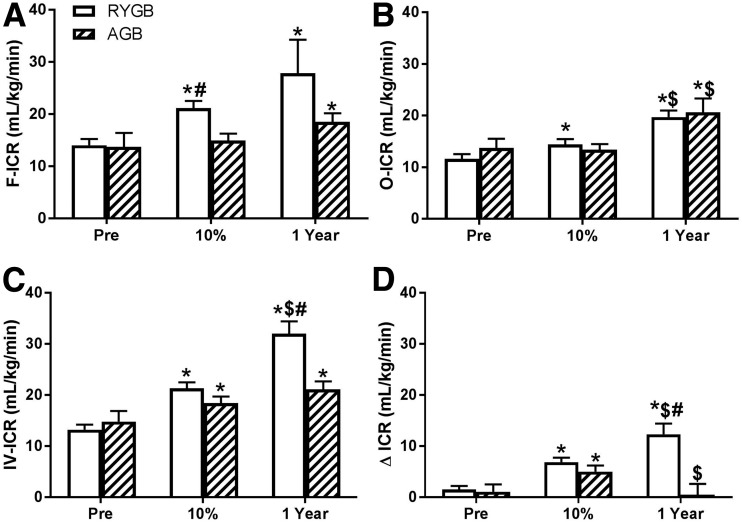
One year after surgical weight loss, by either AGB or RYGB, F-ICR, O-ICR, and IV-ICR (Table 1) all increased, and this increase correlated with weight (r = −0.203, P = 0.002; r = −0.308, P < 0.001; and r = −0.416, P < 0.001, respectively). The pattern of change differed between surgery types and occurred earlier and tended to be more pronounced with RYGB. F-ICR, O-ICR, and IV-ICR increased by 35.0%, 50.3%, and 42.6%, respectively, 1 year after AGB and by 98.6%, 69.8%, and 142.4% after RYGB. After 10% weight loss, the only difference between surgeries was higher absolute F-ICR levels after RYGB compared with AGB, and higher delta change from presurgery to 10% weight loss (RYGB 7.10 ± 5.90 vs. AGB 1.24 ± 7.44 mL/kg/min; P = 0.044). At 1 year, with a greater amount of weight loss after RYGB compared with AGB, IV-ICR levels were significantly different between the two surgical cohorts (Table 1 and Fig. 1) with a greater change from presurgery values after RYGB (RYGB 21.3 ± 5.55 vs. AGB 18.4 ± 4.57 mL/kg/min; P = 0.009). F-ICR and O-ICR (absolute value and delta change from presurgery levels) did not differ between the two surgical cohorts at 1 year (Table 1 and Fig. 1).
Figure 1.
Change in fasting (A), oral (B), IV (C), and Δ (D) ICR before and after AGB or RYGB at presurgery (Pre), 10% matched weight loss, and 1 year postintervention. Mean ± SE. *P < 0.05 for paired Student t test vs. preintervention; $P < 0.05 for paired Student t test vs. 10% matched weight loss; #P < 0.05 for unpaired Student t test vs. AGB.
Effect of Surgery on Difference Between O-ICR and IV-ICR
Because of the known differences of ICR based on the route of glucose administration and the effect of RYGB on the gastrointestinal track, we assessed differences in ICR after two isoglycemic stimuli, one given orally and one IV. As predicted, prior to surgery, IV-ICR was 10.9% greater than O-ICR (P = 0.011) in both surgical cohorts combined; the magnitude of the difference between O-ICR and IV-ICR was similar within each surgical group (Table 1). ΔICR tripled from presurgery to the 10% weight loss mark (1.08 ± 5.02 to 5.01 ± 4.18 mL/kg/min; P = 0.034) after AGB, only to revert to presurgery levels at 1 year. However, after RYGB, ΔICR, which increased by a factor of four at 10% weight loss, increased further by a factor of seven from presurgery at 1 year, a rise driven by a relative greater increase in IV-ICR to O-ICR (Fig. 1). At 1 year, ΔICR was significantly higher after RYGB compared with AGB (11.7 ± 9.8 vs. 1.83 ± 10.4 mL/kg/min; P = 0.001) as was the magnitude of change in ΔICR (10.7 ± 11.1 vs. −0.61 ± 7.39 mL/kg/min; P = 0.002). Surgery type (B = −12.49, P = 0.011) but not % total weight loss (B = −0.049, P = 0.218) strongly predicted ΔICR at the 1-year mark using multiple linear regression.
Study 2
Effect of GLP-1 Receptor Blockade on ICR During OGTT
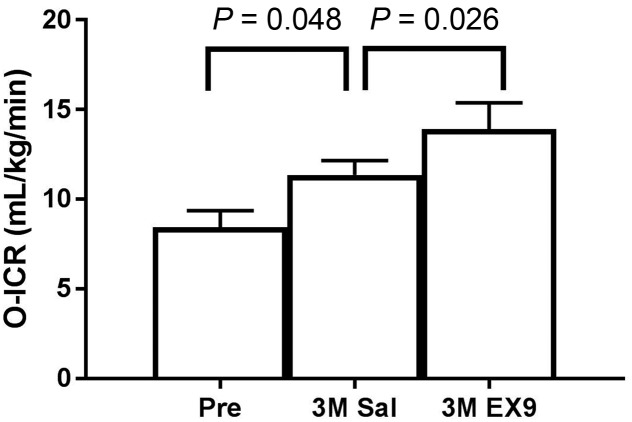
In order to assess the role of endogenous GLP-1 on ICR, we calculated O-ICR during an OGTT with and without the administration of EX9 in a separate cohort (n = 22) (see Supplementary Table 1 for subject characteristics). O-ICR increased by 34% 3 months after RYGB (P = 0.048) (Fig. 2). Blocking endogenous GLP-1 with EX9 resulted in an additional 22% increase of O-ICR (P = 0.026) (Fig. 2). In this same experiment, postprandial insulinemia (AUC) did not change significantly 3 months after surgery but decreased by 50% with the GLP-1 antagonist; ISR increased by 41% at 3 months and was suppressed by 50% by the GLP-1 antagonist (Supplementary Table 1).
Figure 2.
Changes in insulin clearance after 75-g OGTT (O-ICR) before (Pre) and 3 months after (3M) RYGB with saline (Sal) and EX9. Mean ± SE.
Relation of ICR With ISR and Insulinemia
In cohorts 1 and 2, ICR did not correlate with ISR at any time point or under any conditions (data not shown). However, ICR negatively correlated with insulinemia, during both the IV and the oral stimuli in cohort 1 (data not shown) and after RYGB in cohort 2 (presurgery R = −0.393, P = 0.070; 3 months post R = −0.611, P = 0.003; 3 months with EX9 R = −0.387, P = 0.075).
Conclusions
Our main findings are as follows. 1) F-ICR, IV-ICR, and O-ICR increase significantly 1 year after surgical weight loss, regardless of the type of surgical procedure. 2) At 10% weight loss, RYGB caused higher ICR (F-ICR, O-ICR, and IV-ICR), whereas AGB only increased IV-ICR, in spite of a similar amount of weight loss. 3) The difference between O-ICR and IV-ICR is accentuated after RYGB compared with AGB. 4) In spite of a much larger weight loss, O-ICRs are similar 1 year after RYGB and AGB; endogenous GLP-1 may be one of the mechanisms by which O-ICR is relatively suppressed after RYGB.
Our data show a rapid improvement of F-ICR after RYGB in individuals with T2DM; this is apparent after 10% weight loss and sustained up to 1 year after surgery. This is similar to findings from Bojsen-Møller et al. (23) who also show a rapid increase in ICR 1 week and 3 months after RYGB, as well as at 12 months, in 32 subjects with T2DM. Interestingly, our data show no significant change in F-ICR after AGB, in spite of the same amount of weight loss (10%). This suggests either different physiologic adaptations to RYGB compared with AGB in the early postoperative period or perhaps a role for the rate of weight loss, i.e., caloric restriction, which is more pronounced after RYGB, on ICR. Indeed, a very low caloric diet of 500 kcal/day has also been shown to increase F-ICR after 3 weeks (24).
In agreement with our hypothesis, IV-ICR improves similarly after RYGB and AGB, perhaps as a function of weight loss; this increase is significant in both groups after 10% weight loss and at 1 year when IV-ICR is significantly higher in the RYGB group, in relation to the greater weight loss after RYGB compared with AGB (31.6% vs. 16.6%). The greater improvement in F-ICR or IV-ICR after RYGB compared with AGB could be related to a greater reduction in liver fat after RYGB (25). A 5-year prospective longitudinal study shows a superior reduction in fatty liver disease with RYGB compared with AGB, driven primarily by greater weight loss (26). It is also possible that the caloric restriction is greater 1 month after RYGB compared with the early months after AGB. Diet was uncontrolled in our surgical cohorts; other studies, however, show that caloric restriction of 500–600 kcal/day for 8 weeks led to an 11% increase in ICR during an OGTT (7).
O-ICR increases only after RYGB after 10% weight loss. Our data are similar to that of Bunt et al. (22), who recently reported significant increases in ICR during a mixed-meal test after RYGB, but not after AGB, 2 months after intervention. Bunt et al. (22) did not adjust for weight loss differences, and their subjects lost twice more weight after RYGB than after AGB. The increase in O-ICR in the early postoperative RYGB period may be related to the altered nutrient delivery, prior to significant weight loss. This was illustrated in a study in subjects with and without T2DM who had greater O-ICR when nutrients were delivered directly into the jejunum, compared with the duodenum (27).
Weight loss was twice the amount of AGB after RYGB, yet O-ICR increased by the same magnitude in the two surgeries at 1 year. Therefore, whereas IV-ICR and O-ICR increased by the same magnitude after AGB, IV-ICR increased more than O-ICR after RYGB, suggesting that weight loss is not the only determinant of O-ICR after RYGB and that factors specific to RYGB may exert relative suppression of OCR. The greater increase in ΔICR after RYGB compared with AGB may be due to enhanced postprandial incretin concentrations after RYGB (11). Indeed, blocking the GLP-1 receptor with EX9 leads to an additional 23% rise in O-ICR after RYGB, suggesting that GLP-1 exerts a relative suppressive effect on O-ICR after this surgery.
These data, however, have to be interpreted with caution. Our data and others confirm that ICR is negatively correlated with insulin concentrations, but not with ISR (28). In addition, the relationship between ICR and insulinemia may be a saturable, nonlinear process. Circulating insulin concentrations change with manipulation of GLP-1 after RYGB, as shown in this study. The effect of GLP-1 inhibition on ICR, independent of insulinemia, therefore cannot be established. A better experimental design with an insulin clamp at steady-state conditions would better address this question.
The effect of the administration of GLP-1 and/or GIP on ICR is mixed, with some studies showing an increase and others no change. The administration of liraglutide, a GLP-1 receptor agonist, in subjects with pre-T2DM leads to a decrease in insulin clearance during a mixed-meal test compared with placebo administration (29), as does GLP-1 injection in rodents (30,31). GLP-1 and/or GIP infusions were shown to have no effect on ICR in metabolically young healthy humans (32) or in dogs (33). Other studies, however, show that infusion of GIP decreases hepatic ICR in metabolically healthy volunteers (34), first-degree relatives of subjects with T2DM (35), and subjects with T2DM (36).
It is difficult to explain why at 10% matched weight loss, when GLP-1 levels are only increased after RYGB, O-ICR and IV-ICR do not differ between surgery groups. This seems in contradiction with the rest of the data, suggesting a possible “gut factor” effect on O-ICR, independent of weight loss, after RYGB. The data collected at 10% matched weight loss controlled for weight loss amount, as per study design, but did not account for duration of weight loss. Whereas RYGB subjects lost 10% of their weight in 4 weeks, it took AGB subjects 9 weeks to achieve the same weight loss. A relative suppression of O-ICR mediated in part by GLP-1 may not be seen acutely after RYGB, at a time of severe calorie restriction, and may become apparent at 1 year, in a new state of energy balance. This would need further investigation. The discrepancy between the data at 1 month and at 1 year after RYGB also points out the importance of longitudinal studies after bariatric surgery. Our group has shown temporal changes of various variables after RYGB, such as the bile acid pool (37) and the variance of GLP-1 release, which increases over time after surgery (38).
In all, our data suggest that ICR is an important component of insulin concentration and glucose homeostasis after surgical weight loss. We confirm an increase in ICR after RYGB and AGB (8,9,23). Our data also suggest that potential mechanisms, independent of weight loss, may be responsible for a relative suppression of postprandial ICR after RYGB. Of note, Salehi et al. (39) show that subjects with postprandial neuroglycopenia after RYGB had reduced ICR after a mixed-meal test compared with asymptomatic controls.
Strengths of this study include the following: the study of ICR after two different types of surgeries, with vastly different mechanisms of action on the gut; a comparison at matched weight loss and again, a year later, after different amounts of weight loss; and the comparison of ICR after two types of stimuli, oral and IV glucose, demonstrating that the RYGB-related phenomenon of increased ΔICR could be mediated in part by endogenous GLP-1. However, this study has some limitations. We did not measure whole-body and hepatic insulin sensitivity by hyperinsulinemic-euglycemic clamp and/or hepatic glucose production, methods that would have been necessary to address the possible role of GLP-1 on ICR at steady-state conditions; the number of subjects in the AGB group was smaller than in the RYGB group; as some subjects were not in full T2DM remission after surgical weight loss, this may have biased some of our findings; and ICR was determined indirectly, but a direct measurement of ICR would have required invasive blood sampling of the portal and hepatic veins (40). Further, our work presents ICR data from two studies that were primarily directed to look at GLP-1 and glucose metabolism, and not ICR, after bariatric surgery. Unfortunately, we did not test the effect of EX9 on ICR in subjects with obesity and T2DM prior to RYGB to serve as a prebariatric control.
In conclusion, surgical weight loss by RYGB and AGB leads to increases in F-ICR, O-ICR, and IV-ICR; however, the increases in F-ICR appear earlier after RYGB. The increased difference in IV-ICR and O-ICR after RYGB suggests postprandial factors, independent of weight loss, that may induce a relative suppression of O-ICR. Although insulin clearance is the least-studied aspect of insulin metabolism, its contributions to insulin concentration and to glucose homeostasis are significant. This is particularly apparent after RYGB, when increased ICR must be considered, as peripheral insulin levels alone may underestimate β-cell function. Further studies are needed to determine the exact determinants of ICR after RYGB, VSG, and AGB, including the role of GIP and changes over time after intervention. This would allow for a better understanding into the rapid improvement in T2DM after RYGB and the underlying pathophysiology of T2DM.
Supplementary Material
Article Information
Acknowledgments. The authors thank the bariatric surgeons from St. Luke’s Roosevelt Hospital who referred participants; Carolina Espinosa, Daniel Baron-Brenner, and Blaine Huss from the New York Obesity Nutrition Research Center who assisted in some of the experiments; and our participants.
Funding. The study was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (R01-DK-067561, R01-DK-098056, P30-DK-26687, and P30-DK-063608), and the American Diabetes Association (7-08-CR 34). A.S. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant F32-DK-113747. R.D. was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant 5T32-DK-007559-22. This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1-TR-000040.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.S. helped with data analysis and manuscript writing. M.M.H., F.R., V.M., R.D., and J.M. collected and analyzed some of the data. B.Le. assisted with statistical analysis. B.La. designed the study, collected and analyzed the data, and wrote the manuscript. B.La. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of the data were presented as a poster at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
Clinical trial reg. no. NCT02287285, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-1036/-/DC1.
References
- 1.Després JP, Lamarche B, Mauriège P, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996;334:952–957 [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Reaven GM. Insulin clearance: an underappreciated modulator of plasma insulin concentration. J Investig Med 2016;64:1162–1165 [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol 1983;244:E517–E527 [DOI] [PubMed] [Google Scholar]
- 4.Meistas MT, Margolis S, Kowarski AA. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am J Physiol 1983;245:E155–E159 [DOI] [PubMed] [Google Scholar]
- 5.Pivovarova O, Bernigau W, Bobbert T, et al. Hepatic insulin clearance is closely related to metabolic syndrome components. Diabetes Care 2013;36:3779–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986;63:492–498 [DOI] [PubMed] [Google Scholar]
- 7.Svendsen PF, Jensen FK, Holst JJ, Haugaard SB, Nilas L, Madsbad S. The effect of a very low calorie diet on insulin sensitivity, beta cell function, insulin clearance, incretin hormone secretion, androgen levels and body composition in obese young women. Scand J Clin Lab Invest 2012;72:410–419 [DOI] [PubMed] [Google Scholar]
- 8.Paszkiewicz RL, Bergman RN. Mechanisms of improved glucose handling after metabolic surgery: the big 6. Surg Obes Relat Dis 2016;12:1192–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Weijer BA, Aarts E, Janssen IM, et al. Hepatic and peripheral insulin sensitivity do not improve 2 weeks after bariatric surgery. Obesity (Silver Spring) 2013;21:1143–1147 [DOI] [PubMed] [Google Scholar]
- 10.Mingrone G, Castagneto M. Bariatric surgery: unstressing or boosting the β-cell? Diabetes Obes Metab 2009;11(Suppl. 4):130–142 [DOI] [PubMed] [Google Scholar]
- 11.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007;30:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiménez A, Casamitjana R, Flores L, et al. Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg 2012;256:1023–1029 [DOI] [PubMed] [Google Scholar]
- 13.Holter MM, Dutia R, Stano SM, et al. Glucose metabolism after gastric banding and gastric bypass in individuals with type 2 diabetes: weight loss effect. Diabetes Care 2017;40:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutia R, Brakoniecki K, Bunker P, et al. Limited recovery of β-cell function after gastric bypass despite clinical diabetes remission. Diabetes 2014;63:1214–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Standards of Medical Care in Diabetes—2016: summary of revisions. Diabetes Care 2016;39(Suppl. 1):S4–S5 [DOI] [PubMed] [Google Scholar]
- 16.Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 1998;101:1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care 1995;18:245–250 [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 19.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 20.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 21.Jung SH, Jung CH, Reaven GM, Kim SH. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia 2018;61:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunt JC, Blackstone R, Thearle MS, Vinales KL, Votruba S, Krakoff J. Changes in glycemia, insulin and gut hormone responses to a slowly ingested solid low-carbohydrate mixed meal after laparoscopic gastric bypass or band surgery. Int J Obes 2017;41:706–713 [DOI] [PubMed] [Google Scholar]
- 23.Bojsen-Møller KN, Dirksen C, Jørgensen NB, et al. Increased hepatic insulin clearance after Roux-en-Y gastric bypass. J Clin Endocrinol Metab 2013;98:E1066–E1071 [DOI] [PubMed] [Google Scholar]
- 24.Jackness C, Karmally W, Febres G, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes 2013;62:3027–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab 2007;293:E1709–E1715 [DOI] [PubMed] [Google Scholar]
- 26.Caiazzo R, Lassailly G, Leteurtre E, et al. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg 2014;260:893–898; discussion 898–899 [DOI] [PubMed] [Google Scholar]
- 27.Salinari S, Carr RD, Guidone C, et al. Nutrient infusion bypassing duodenum-jejunum improves insulin sensitivity in glucose-tolerant and diabetic obese subjects. Am J Physiol Endocrinol Metab 2013;305:E59–E66 [DOI] [PubMed] [Google Scholar]
- 28.Bril F, Lomonaco R, Orsak B, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014;59:2178–2187 [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Liu A, Ariel D, et al. Pancreatic beta cell function following liraglutide-augmented weight loss in individuals with prediabetes: analysis of a randomised, placebo-controlled study. Diabetologia 2014;57:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahrén B, Thomaseth K, Pacini G. Reduced insulin clearance contributes to the increased insulin levels after administration of glucagon-like peptide 1 in mice. Diabetologia 2005;48:2140–2146 [DOI] [PubMed] [Google Scholar]
- 31.Pacini G, Thomaseth K, Ahrén B. Dissociated effects of glucose-dependent insulinotropic polypeptide vs glucagon-like peptide-1 on beta-cell secretion and insulin clearance in mice. Metabolism 2010;59:988–992 [DOI] [PubMed] [Google Scholar]
- 32.Meier JJ, Holst JJ, Schmidt WE, Nauck MA. Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab 2007;293:E849–E856 [DOI] [PubMed] [Google Scholar]
- 33.Hanks JB, Andersen DK, Wise JE, Putnam WS, Meyers WC, Jones RS. The hepatic extraction of gastric inhibitory polypeptide and insulin. Endocrinology 1984;115:1011–1018 [DOI] [PubMed] [Google Scholar]
- 34.Kindmark H, Pigon J, Efendic S. Glucose-dependent insulinotropic hormone potentiates the hypoglycemic effect of glibenclamide in healthy volunteers: evidence for an effect on insulin extraction. J Clin Endocrinol Metab 2001;86:2015–2019 [DOI] [PubMed] [Google Scholar]
- 35.Rudovich NN, Rochlitz HJ, Pfeiffer AF. Reduced hepatic insulin extraction in response to gastric inhibitory polypeptide compensates for reduced insulin secretion in normal-weight and normal glucose tolerant first-degree relatives of type 2 diabetic patients. Diabetes 2004;53:2359–2365 [DOI] [PubMed] [Google Scholar]
- 36.Groop PH, Groop L, Tötterman KJ, Fyhrquist F. Relationship between changes in GIP concentrations and changes in insulin and C-peptide concentrations after guar gum therapy. Scand J Clin Lab Invest 1986;46:505–510 [DOI] [PubMed] [Google Scholar]
- 37.Dutia R, Embrey M, O’Brien S, et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes 2016;40:554. [DOI] [PubMed] [Google Scholar]
- 38.Van der Schueren BJ, Homel P, Alam M, et al. Magnitude and variability of the glucagon-like peptide-1 response in patients with type 2 diabetes up to 2 years following gastric bypass surgery. Diabetes Care 2012;35:42–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab 2014;99:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaden M, Harding P, Field JB. Effect of intraduodenal glucose administration on hepatic extraction of insulin in the anesthetized dog. J Clin Invest 1973;52:2016–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.