Recently, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6) suggested that semaglutide may increase the risk for diabetic retinopathy (DR) adverse events (AEs) compared with placebo. Other trials of glucagon-like peptide 1 receptor agonists (GLP-1RA) showed a numerically higher incidence of DR AEs for liraglutide but not exenatide. However, these trials did not systematically assess DR. Our population-based cohort study of older U.S. adults suggested that GLP-1RA use for approximately 1 year does not increase DR risk (1). As current evidence on GLP-1RA–associated DR risk is still limited, we conducted a disproportionality analysis of the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database to examine the association between GLP-1RA and DR events.
We used generic and brand names to identify GLP-1RA (exenatide, liraglutide, albiglutide, and dulaglutide) and comparator drugs in the FAERS database and Medical Dictionary for Regulatory Activities (MedDRA v21.0) preferred terms to identify DR cases (diabetic retinopathy, retinopathy, macular edema, retinopathy proliferative, retinopathy hemorrhagic, blindness, vitreous hemorrhage) from 28 April 2005 (approval date for the first GLP-1RA, exenatide) to 30 September 2017. We performed a disproportionality analysis using the reporting odds ratio (ROR) to assess whether there is a signal for a potentially increased risk of DR among GLP-1RA users. The ROR is calculated by dividing the odds of a DR event reported for the drug of interest by the odds of a DR event reported for the comparison drugs. A signal was defined as an ROR of ≥2. We analyzed data using SAS 9.4 (SAS Institute Inc., Cary, NC).
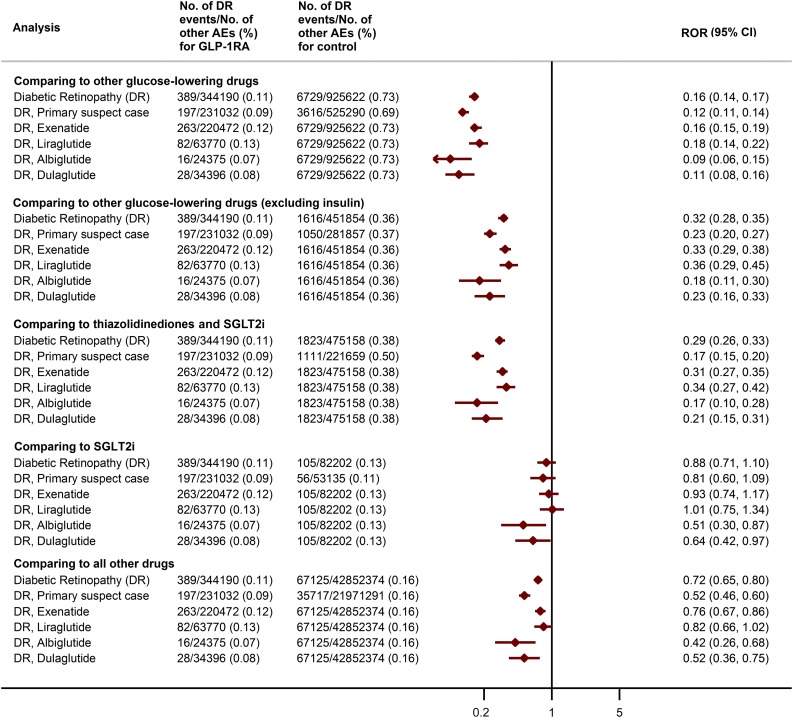
We compared GLP-1RA versus five groups (Fig. 1): 1) other glucose-lowering drugs (GLDs) (metformin, sulfonylureas, thiazolidinediones, sodium–glucose cotransporter 2 inhibitors [SGLT2i], dipeptidyl peptidase 4 inhibitors, insulin, α-glucosidase inhibitors, and glinides); 2) GLDs excluding insulin, as insulin has an “early worsening” effect on DR; 3) two classes of therapeutic alternatives—thiazolidinediones (pioglitazone and rosiglitazone) and SGLT2i (canagliflozin, dapagliflozin, and empagliflozin); 4) SGLT2i, a newer class of GLD, as reporting of AEs is more frequent for newer agents; and 5) all other drugs in the database. We conducted a sensitivity analysis for each comparison, restricting to events where the treatment was reported as “primary suspect” (those drugs directly suspected of causing the AEs), and then performed analyses stratified by each GLP-1RA.
Figure 1.
Number of DR events, other AEs, and ROR in different drug comparisons.
The FAERS database contained 389 DR cases associated with GLP-1RA, 197 of which were “primary suspect” cases. The number of DR events associated with exenatide, liraglutide, albiglutide, and dulaglutide was 263, 82, 16, and 28, respectively. The ROR (95% CI) for DR for the comparisons described above were as follows: 1) 0.16 (0.14, 0.17); 2) 0.32 (0.28, 0.35); 3) 0.29 (0.26, 0.33); 4) 0.88 (0.71, 1.10); and 5) 0.72 (0.65, 0.80). The results were consistent with the primary analyses when restricting to “primary suspect” cases. Similarly, in the analyses stratified by individual GLP-1RA, we saw no signal of increased DR risk (Fig. 1).
Our analysis of the FAERS database indicates that there is no signal for the association between GLP-1RA and DR, which is consistent with the Exenatide Study of Cardiovascular Event Lowering (EXSCEL) and Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trials and our recent cohort study (1). A recent FAERS analysis (2) comparing GLP-1RA with other GLDs and stratified by insulin also suggested no signal. We extend those analyses with a comparison with therapeutic alternatives (thiazolidinediones and SGLT2i), a comparison with a newer agent (SGLT2i), and an analysis restricted to “primary suspect” cases.
GLP-1RA have been reported to protect retinal cells and retinal endothelium from high-glucose–induced damage (3,4); the topical use of GLP-1RA has been proposed as a possible therapy for DR (5). The unexpected increase in DR AEs in SUSTAIN-6 may be explained by rapid lowering of blood glucose or a direct deleterious effect of semaglutide (4). Unfortunately, in our study, there was no available semaglutide data, as the drug was approved by the FDA in December 2017. Furthermore, this database will not be ideal for future analysis because AE reporting is likely to be confounded by greater attention to the potential issue with semaglutide, given the SUSTAIN-6 results. Another possibility is that the increased risk of retinopathy is limited to subpopulations included in SUSTAIN-6 trials but not represented in our study.
Our analyses have limitations. Spontaneous events reporting is subject to reporting bias, lack of denominator data, and confounding. Data on comorbidities, previous treatment, or the duration of treatment for the suspected drugs are often missing. Overall, although the low RORs suggest that GLP-1RA are not associated with DR, further study is needed.
Article Information
Funding and Duality of Interest. Contracted consulting fees for J.B.B. are paid to the University of North Carolina by Adocia, AstraZeneca, Eli Lilly, MannKind, NovaTarg, Novo Nordisk, Senseonics, and vTv Therapeutics. He has grant support from Novo Nordisk, Sanofi, and vTv Therapeutics; is a consultant to Neurimmune AG; holds stock options in Mellitus Health, PhaseBio, and Stability Health; and is supported by a grant from the National Institutes of Health (UL1TR002489). T.S. receives investigator-initiated research funding and support from the National Institute on Aging as a principal investigator (R01AG056479) and from the National Institutes of Health as a co-investigator (R01CA174453, R01HL118255, and R21HD080214). He also receives salary support as codirector of the Biostatistics, Epidemiology, and Research Design program, North Carolina Translational and Clinical Sciences Institute (UL1TR002489), and from the Center for Pharmacoepidemiology (current members are GlaxoSmithKline, UCB BioSciences, Merck, and Shire) and receives research support from pharmaceutical companies (Amgen, AstraZeneca, and Novo Nordisk) to the University of North Carolina Department of Epidemiology. He does not accept personal compensation of any kind from any pharmaceutical company. He owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.W. designed the study and did all statistical analyses. T.W. and W.L. wrote the first draft of the manuscript. H.T., J.B.B., T.S., and E.W.G. were involved in data review and interpretation. T.W., J.B.B., T.S., and E.W.G. contributed to critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript. T.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a poster at the 34th International Conference on Pharmacoepidemiology and Therapeutic Risk Management, Prague, Czech Republic, 22–26 August 2018.
References
- 1.Wang T, Hong J-L, Gower EW, et al. . Incretin-based therapies and diabetic retinopathy: real-world evidence in older U.S. adults. Diabetes Care 2018;41:1998–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fadini GP, Sarangdhar M, Avogaro A. Glucagon-like peptide-1 receptor agonists are not associated with retinal adverse events in the FDA Adverse Event Reporting System. BMJ Open Diabetes Res Care 2018;6:e000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y, Liu K, Wang Q, Ruan Y, Ye W, Zhang Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp Eye Res 2014;127:104–116 [DOI] [PubMed] [Google Scholar]
- 4.Simó R, Hernández C. GLP-1R as a target for the treatment of diabetic retinopathy: friend or foe? Diabetes 2017;66:1453–1460 [DOI] [PubMed] [Google Scholar]
- 5.Hernández C, Bogdanov P, Corraliza L, et al. . Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes 2016;65:172–187 [DOI] [PubMed] [Google Scholar]