Type 1 diabetes is well recognized to be immune mediated, resulting in destruction of β-cells. That this eradication is not always complete was evident in an early report from the Diabetes Control and Complications Trial (DCCT) showing that 11% of adult participants with type 1 diabetes for more than 5 years had measurable C-peptide in the fasting state and following mixed-meal stimulation, while no adolescents had evidence of such (1). Further, in participants with measurable C-peptide responses, C-peptide declined in all after a year of randomized therapy, with this decline being less in those who received intensive insulin therapy. Subsequently, it was shown in adolescents that 2 weeks of intensive insulin therapy, compared with conventional therapy, resulted in improved β-cell function a year later along with superior glucose control (2). In addition to C-peptide, proinsulin has also been shown to be circulating in individuals with type 1 diabetes at the time of diagnosis and to be still measurable 30 months later (3,4). Thus, there is long-standing evidence that at the time of diagnosis and subsequently, individuals with type 1 diabetes have β-cells still capable of synthesizing proinsulin, and many can process it to C-peptide and insulin.
In the current issue of Diabetes Care, Sims et al. (5) report longitudinal findings on proinsulin secretion in type 1 diabetes using a large number of samples from the T1D Exchange. They divided their subjects into three groups based on stimulated C-peptide responses to a mixed meal. We have categorized these groups as having absent, intermediate, and high C-peptide responses, although the latter are a great deal lower than what is observed in healthy people. Individuals in the absent group had C-peptide responses that were below the reliable lower detection limit of the assay, and they were not studied further. These patients had been diagnosed at age 15 years, and at the time of their initial mixed meal they reported having the disease for an average of 19 years. Those in the intermediate and high groups had measurable C-peptide responses and were examined again with mixed-meal tests at 1, 2, and 4 years after their initial test. The high-response group had fasting and stimulated C-peptide values that were nearly sevenfold greater than the intermediate-response group, and they also had better glycemic control. Although both groups had been diagnosed on average 10–12 years earlier, those in the high-response group were markedly older (mean of 29 years of age) at the time of diagnosis than were those with intermediate responses (19 years of age). In addition to C-peptide, proinsulin concentrations were quantified at this initial visit, and all three groups had fasting levels in the reliable measurement range of the assay. The levels of proinsulin relative to C-peptide—long used as a marker of β-cell dysfunction in type 2 diabetes (6)—were disproportionately increased in both groups with detectable C-peptide compared with a group of healthy control subjects. Further, the proinsulin–to–C-peptide ratio was greater in those with the lowest C-peptide responses, compatible with them having poorer β-cell function.
There is renewed interest of late in the idea of residual β-cell function (7–10) and mass (11–13) in type 1 diabetes. What do we know, what have we learned, and what do we need to probe? We know that type 1 diabetes is heterogeneous. Contributors to this heterogeneity could include HLA genotype (14), the number of autoantibodies (15), age of onset (1), and variability in β-cell mass and survival (11). Further, some people with a typical type 2 diabetes phenotype have an underlying immune diathesis comprising antibodies and/or T-cell activation (16). We have now learned that despite long-standing type 1 diabetes, the vast majority of individuals have detectable circulating proinsulin, although not all have measurable C-peptide. Thus, lingering β-cells must be the norm, but whether they have escaped the immune attack or are newly formed remains unanswered. The efficiency of these cells in processing proinsulin varies, with it being poorest in those diagnosed when younger and best in those diagnosed when older. Although one might expect that duration of disease may be a critical factor in determining progression of β-cell dysfunction and thus processing efficiency, this does not appear to be the case. Participants in the current study (5) who were older had the best secretory function and were most efficient in processing proinsulin. Further, this older group had diabetes for somewhat longer than the group with marginal C-peptide responses. As proinsulin is less bioactive, it is possible that in those with residual β-cell function, impaired proinsulin processing contributes to the observed differences in glucose control (as in type 2 diabetes). In those with the best β-cell function, information on the quantity of insulin required to achieve a lower hemoglobin A1c would have been informative. With all this information there is new opportunity to probe deeper in order to better understand what may be happening with the β-cell in individuals with type 1 diabetes.
While proinsulin immunoreactivity was detectable, it is unclear whether it was all intact proinsulin or whether there were changes in the quantity and distribution of the proinsulin conversion intermediates des-31,32- and des-64,65-proinsulin. In type 2 diabetes, the quantity of proinsulin relative to C-peptide is increased, but the relative proportions of intact proinsulin and des-31,32-proinsulin do not differ, in keeping with deficient action of the proprotein convertases in those with hyperglycemia (6,17). Change in expression of the proprotein convertase Pcsk1 as a cause of persistent proinsulin secretion in type 1 diabetes has recently been suggested from studies of samples from the Network for Pancreatic Organ Donors with Diabetes (nPOD) (13). In these pancreas extracts of patients with type 1 diabetes, expression of this protease was decreased and proinsulin–to–C-peptide ratios increased. Thus, the basis for any differences in proinsulin processing in type 1 and type 2 diabetes is an area worth probing.
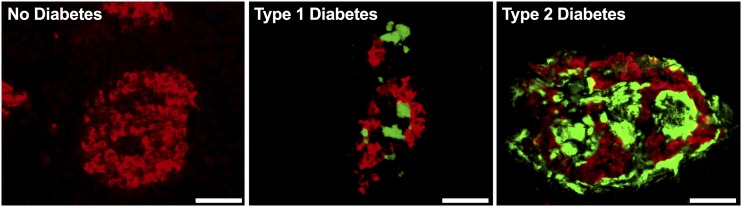
Interestingly, aside from proinsulin, processing of another β-cell proprotein has also recently been described as impaired in type 1 diabetes. We recently reported that an intermediate precursor form of islet amyloid polypeptide (IAPP), called proIAPP1–48, is also disproportionately elevated in persons with type 1 diabetes (18). Together, these findings suggest a common mechanism may exist that leads to persistent propeptide secretion from β-cells in type 1 diabetes. It will be of value to probe whether persistent proIAPP1–48 secretion in type 1 diabetes is derived from the same β-cells as proinsulin, whether in both cases this is due to loss of prohormone convertase expression or action, and whether it occurs in a subset of residual β-cells escaping immune attack. IAPP aggregation leads to formation of amyloid deposits in the islet, a morphological feature thought to be pathognomonic of type 2 diabetes (19). Interestingly, amyloid has now also been described in islet transplants (20) and pancreata of some cases of type 1 diabetes (21). Figure 1 illustrates that the conformation of islet amyloid in type 1 diabetes is indistinguishable from that observed in type 2 diabetes, and the deposits are located in proximity to residual β-cells (S.E.K., A.T.T., and R.L.H., unpublished observation). This location of the amyloid deposits is in keeping with IAPP, their unique peptide component, being a secretory product of the β-cell that typically has to be exocytosed for amyloid to form (22,23). We have proposed that β-cell dysfunction and impaired processing of proIAPP are linked to its aggregation and amyloid formation (24,25), raising the possibility that a common pathway leads to amyloid formation in both type 2 diabetes and in some cases of type 1 diabetes in which residual β-cells remain.
Figure 1.
Representative pancreatic islets from a healthy individual and individuals with type 1 diabetes and type 2 diabetes. Islet amyloid is demonstrated by thioflavin S staining (green) and β-cells by insulin immunostaining (red). Scale bar = 50 µm.
Sims et al. (5) have also provided information regarding the performance of the proinsulin assay in their hands, not simply based on the manufacturer’s information. They are commended for doing so, and we would encourage others to follow their lead. Too frequently, results from purchased assays are reported with blanket acceptance that the assays are well validated. This is clearly not always the case, resulting in inaccurate information making its way into the literature. For this reason, funding agencies such as the National Institutes of Health are requiring applicants to provide information on how they plan to determine the reliability, reproducibility, and authenticity of their observations (26), and some journals now require provision of more than minimal information to allow readers to be better informed (27,28). We believe the broadening of this approach would leave us all better served to investigate and understand not only the pathogenesis of type 1 diabetes but much, much more.
The current study and others, some going back decades, now strongly support the hypothesis that type 1 diabetes is more than just a destructive disease. By probing the meaning of persistent propeptide release, we hope greater insights into β-cell dysfunction in type 1 diabetes will result.
Article Information
Funding. This article was supported in part by U.S. Department of Veterans Affairs grant I01 BX001060 (to S.E.K.); National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants F32 DK107022 (to A.T.T.) and P30 DK017047; JDRF grant 3-SRA-2014-39-Q-R (to C.B.V.); an investigator award from BC Children’s Hospital; and Canadian Institutes of Health Research grant PJT-153156.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 258.
References
- 1.The DCCT Research Group Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual β-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab 1987;65:30–36 [DOI] [PubMed] [Google Scholar]
- 2.Shah SC, Malone JI, Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med 1989;320:550–554 [DOI] [PubMed] [Google Scholar]
- 3.Ludvigsson J, Heding L. Abnormal proinsulin/C-peptide ratio in juvenile diabetes. Acta Diabetol Lat 1982;19:351–358 [DOI] [PubMed] [Google Scholar]
- 4.Snorgaard O, Kjems LL, Røder ME, Hartling SG, Dinesen B, Binder C. Proinsulin immunoreactivity in recent-onset IDDM: the significance of insulin antibodies and insulin autoantibodies. Diabetes Care 1996;19:146–150 [DOI] [PubMed] [Google Scholar]
- 5.Sims EK, Bahnson HT, Nyalwidhe J, et al.; T1D Exchange Residual C-peptide Study Group . Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care 2019;42:258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn SE, Halban PA. Release of incompletely processed proinsulin is the cause of the disproportionate proinsulinemia of NIDDM. Diabetes 1997;46:1725–1732 [DOI] [PubMed] [Google Scholar]
- 7.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic β-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes 2010;59:2846–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Lovejoy NF, Faustman DL. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabetes Care 2012;35:465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014;57:187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis AK, DuBose SN, Haller MJ, et al.; T1D Exchange Clinic Network . Prevalence of detectable C-peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015;38:476–481 [DOI] [PubMed] [Google Scholar]
- 11.Klinke DJ., 2nd Extent of beta cell destruction is important but insufficient to predict the onset of type 1 diabetes mellitus. PLoS One 2008;3:e1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasserfall C, Nick HS, Campbell-Thompson M, et al. Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab 2017;26:568–575.e3 [DOI] [PMC free article] [PubMed]
- 14.Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–467 [DOI] [PubMed] [Google Scholar]
- 15.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks-Worrell BM, Boyko EJ, Palmer JP. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care 2014;37:3286–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiner DF. The proprotein convertases. Curr Opin Chem Biol 1998;2:31–39 [DOI] [PubMed] [Google Scholar]
- 18.Courtade JA, Klimek-Abercrombie AM, Chen YC, et al. Measurement of pro-islet amyloid polypeptide (1-48) in diabetes and islet transplants. J Clin Endocrinol Metab 2017;102:2595–2603 [DOI] [PubMed] [Google Scholar]
- 19.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 1999;48:241–253 [DOI] [PubMed] [Google Scholar]
- 20.Westermark GT, Westermark P, Berne C, Korsgren O; Nordic Network for Clinical Islet Transplantation . Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med 2008;359:977–979 [DOI] [PubMed] [Google Scholar]
- 21.Westermark GT, Krogvold L, Dahl-Jørgensen K, Ludvigsson J. Islet amyloid in recent-onset type 1 diabetes—the DiViD study. Ups J Med Sci 2017;122:201–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn SE, D’Alessio DA, Schwartz MW, et al. Evidence of cosecretion of islet amyloid polypeptide and insulin by β-cells. Diabetes 1990;39:634–638 [DOI] [PubMed] [Google Scholar]
- 23.Aston-Mourney K, Hull RL, Zraika S, Udayasankar J, Subramanian SL, Kahn SE. Exendin-4 increases islet amyloid deposition but offsets the resultant beta cell toxicity in human islet amyloid polypeptide transgenic mouse islets. Diabetologia 2011;54:1756–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porte D Jr, Kahn SE. Hyperproinsulinemia and amyloid in NIDDM. Clues to etiology of islet β-cell dysfunction? Diabetes 1989;38:1333–1336 [DOI] [PubMed] [Google Scholar]
- 25.Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB. Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes 2006;55:2192–2201 [DOI] [PubMed] [Google Scholar]
- 26.Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature 2014;505:612–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poitout V, Satin LS, Kahn SE, et al. A call for improved reporting of human islet characteristics in research articles. Diabetologia. In press. DOI: 10.1007/s00125-018-4784-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poitout V, Satin LS, Kahn SE, et al. A call for improved reporting of human islet characteristics in research articles. Diabetes 2019;68:239–240 [DOI] [PubMed] [Google Scholar]