Abstract
The sustained expression of the MAFB transcription factor in human islet β-cells represents a distinct difference in mice. Moreover, mRNA expression of closely related and islet β-cell–enriched MAFA does not peak in humans until after 9 years of age. We show that the MAFA protein also is weakly produced within the juvenile human islet β-cell population and that MafB expression is postnatally restricted in mouse β-cells by de novo DNA methylation. To gain insight into how MAFB affects human β-cells, we developed a mouse model to ectopically express MafB in adult mouse β-cells using MafA transcriptional control sequences. Coexpression of MafB with MafA had no overt impact on mouse β-cells, suggesting that the human adult β-cell MAFA/MAFB heterodimer is functionally equivalent to the mouse MafA homodimer. However, MafB alone was unable to rescue the islet β-cell defects in a mouse mutant lacking MafA in β-cells. Of note, transgenic production of MafB in β-cells elevated tryptophan hydroxylase 1 mRNA production during pregnancy, which drives the serotonin biosynthesis critical for adaptive maternal β-cell responses. Together, these studies provide novel insight into the role of MAFB in human islet β-cells.
Introduction
Type 2 diabetes is characterized by peripheral insulin resistance and impaired pancreatic α- and β-cell activity (1). Although many distinct genetic lesions appear to contribute to disease susceptibility, islet-enriched transcription factor mutations commonly are associated with a monogenic form of diabetes termed maturity-onset diabetes of the young (e.g., HNF1α [2], HNF1β [3], PDX1 [4], MAFA [5]). As a consequence of extensive mutational analysis of these and other islet-enriched transcription factors in mice, many were shown to play essential roles in islet cell development and/or function (6). However, striking differences exist in the expression pattern of a few of these key regulators between humans and mice. For example, MAFA mRNA is produced at lower levels in juvenile (<9 years of age) than in adult (>29 years of age) human islet β-cells (7), whereas expression peaks soon after birth in mice (8,9). In addition, the closely related MAFB gene is expressed in primate islet β-cells postnatally (10) but not in rodents (8,9).
Both MafA and MafB are made relatively late during mouse islet cell development compared with other islet-enriched transcription factors (11). Thus, MafB expression begins around embryonic day 10.5 (e10.5) in both insulin-positive (i.e., β-cell) and glucagon-positive (i.e., α-cell) progenitors, whereas MafA is first detected at e13.5 and only in insulin-positive cells (8,9). In contrast, most other islet-enriched transcription factors are produced much earlier during development (e.g., Pdx1 [e8.5 (12)]) and within a larger fraction of the adult islet cell population (e.g., α, β, δ, PP, Pax6 [13], and FoxA1/2 [14]). MafA expression persists in the mouse islet β-cell population postnatally, whereas MafB is restricted to α-cells (8,9). However, MafB is re-expressed in a small fraction of islet β-cells during pregnancy (15). Analysis of mice that lack MafA or MafB during pancreas development has demonstrated that the MafA mutant has the most significant phenotype (MafAΔpancreas [16]), which is manifested as defects in β-cell activity and islet cell architecture after birth. In contrast, there is no overt effect in MafBΔpancreas islet β-cells as a result of postnatal compensation by MafA, although plasma glucagon secretion levels from α-cells are reduced (10).
Of note, human MAFA mRNA is made at low levels in juvenile β-cells in relation to adult islets (7), and MAFB is expressed throughout the lifetime of these cells in nonhuman primates (NHPs) and humans (7,10,17). Here, we first show that the MAFA protein is found in relatively few juvenile and adolescent human islet β-cells in relation to MAFB and that DNA methylation within the 5′ flanking region of mouse MafB correlates with gene silencing in β-cells. The impact of MafB on adult islet β-cells was next examined in βMafBTg transgenic mice that sustain production of this transcription factor postnatally, thus mimicking the expression pattern in humans. Although little impact was observed on coexpression of MafB with endogenous MafA in islet β-cells, production of MafB alone was unable to rescue any of the islet deficiencies of MafAΔpancreas mice. These results suggest that the juvenile MAFB2 homodimer and adult MAFA/MAFB heterodimer regulators could be involved in controlling age-dependent differences in human β-cell gene expression (7). Of note, maternal βMafBTg mice displayed increased β-cell proliferation and function compared with wild-type mice. These data illustrate the many distinct ways MAFB may regulate human islet β-cells.
Research Design and Methods
Tissue Collection and Immunofluorescence
Human pancreata from healthy juvenile (<10 years of age) donors were received in partnership with the International Institute for the Advancement of Medicine and National Disease Research Interchange for histological analysis (Supplementary Table 1). The Vanderbilt University institutional review board declared that studies on deidentified human pancreatic specimens do not qualify as human subject research.
Human and mouse pancreata were fixed in 4% paraformaldehyde in PBS and embedded in either Tissue-Plus O.C.T. (Thermo Fisher Scientific) or paraffin wax. Immunofluorescent images were obtained using the Zeiss Axio Imager M2 widefield microscope with ApoTome. Antibodies are listed in Supplementary Data.
Islet Isolation, Cell Sorting, and DNA Methylation
Mouse islets were isolated using standard islet isolation conditions (18). Islet α- and β-cells were isolated as previously described (19). α-Cells were sorted by FACS to an average purity of 80–85%. For isolation of β-cells from neonatal (postnatal day 5) and adult (age 2.5 months) mice, islets from mouse INS-1 gene promoter-green fluorescent protein transgenic mice were sorted for green fluorescent protein by FACS to an average purity of 85–95%. Cadaveric human islets and mouse islets were processed for DNA extraction and bisulfite treatment as detailed previously (19). Primers and additional details are available upon request.
siRNA-Based Knockdown, RNA Isolation, cDNA Synthesis, and Real-time PCR
Knockdown of Dnmt3a in Min6 cells was performed by transfection with a pool of specific targeting siRNAs or scrambled controls (Dharmacon) using Lipofectamine 2000 (Invitrogen) every 2 days (average transfection efficiency ∼80%). RNA was isolated 4 days posttransfection using the RNeasy Micro Kit (QIAGEN). cDNA was generated using Superscript III Reverse Transcriptase (Invitrogen) by the oligo(dT) priming method. Real-time PCR assays were performed using the LightCycler FastStart DNA Master PLUS SYBR Green kit (Roche) and a LightCycler PCR instrument (Roche). The expression levels were normalized to cyclophilin with previously published primers (19).
Mouse islet RNA was collected using the TRIzol reagent (Life Technologies) and a DNA-free RNA kit (Zymo Research). cDNAs were produced using the iScript cDNA Synthesis Kit (Bio-Rad), and real-time PCR was performed on a LightCycler 480 system (Roche) with primers listed in Supplementary Table 2. Real-time PCR results were analyzed using the ΔΔCt method; Gapdh was used to normalize the mouse islet data.
Chromatin Immunoprecipitation Assays
Chromatin immunoprecipitation (ChIP) experiments with purified α- and β-cells were carried out using the micro-ChIP protocol (20). Islets from 2-month-old mice were used for MafB (Bethyl Laboratories) and Dnmt3a (Novus Biologicals) ChIP. Approximately 500 islets per data point were cross-linked using 1% formaldehyde for 10 min at 37°C and quenched using glycine. Sonication was performed for 3 × 5 min using a Diagenode Bioruptor (Diagenode Diagnostics). Approximately 4 μg antibody was incubated at 4°C overnight. Protein A Dynabeads (Thermo Fisher Scientific) were treated overnight with BSA, herring sperm, and protease inhibitors before being added to the chromatin-antibody mixture for 4 h at 4°C. After washes, chromatin elution was performed with 1% SDS and 0.1 mol/L sodium bicarbonate for 15 min followed by a 5 mol/L NaCl overnight incubation at 65°C and then proteinase K digestion (1 μg) for 1 h at 45°C. The eluted DNA was purified using the QIAGEN PCR Purification Kit. Real-time PCR enrichment of MafB binding was calculated using, as controls, IgG and the albumin gene, whereas IgG and the actin gene were used in the Dnmt3a ChIP (21,22).
Generation of βMafBTg Transgenic Mice
Bacterial artificial chromosome (BAC) recombineering (recombination-mediated genetic engineering) was used to exchange the MafA coding sequences in bMQ-159k16, a BAC clone of ∼120 kb spanning the single exon MafA gene as well as the 5′ and 3′ flanking sequences, with MafB single exon sequences (Fig. 3A). Purified linear MafABAC-MafB DNA was injected into pronuclei from B6D2 donors and then implanted into pseudopregnant recipient mice. Thirty-one pups derived from microinjection were screened for the BAC transgene, with six MafABAC-MafB. βMafBTg founders bred with C57BL/67 females, and three independent mouse lines were characterized in depth. MafB was expressed in ∼87% of islet β-cells in the D line, 70% in the A line, and 36% in the E line (Supplementary Fig. 1). The analysis presented here principally used the D-expressing line, although similar results were obtained with the A line. Mouse studies were performed in compliance with protocols approved by the Vanderbilt institutional animal care and use committee.
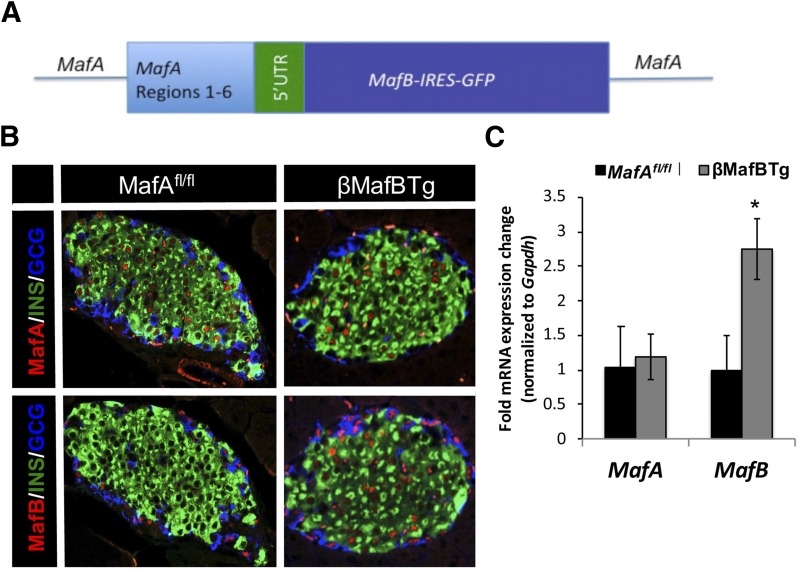
Figure 3.
MafB is produced in a large fraction of adult islet β-cells in βMafBTg mice. A: The MafB transgene is driven by a mouse BAC spanning the region 1–6 control sequences required for β-cell type–specific expression in vivo (31). B: MafB is present in ∼85% of 2-month-old βMafBTg islet β-cells (insulin [INS]) and essentially only α-cells (glucagon [GCG]) in age-matched MafAfl/fl islets. C: MafB mRNA was increased by 2.5-fold, whereas MafA mRNA levels were unchanged in βMafBTg islets. qPCR signals were normalized to Gapdh expression (n = 5). *P < 0.05. UTR, untranslated region.
MafAΔβ Generation, Intraperitoneal Glucose Tolerance Testing, Blood Serum, and Islet Hormone Measurements
MafAΔβ mice were generated by crossing floxed MafA (MafAfl/fl) (8) with rat Ins2 enhancer/promoter-driven Cre (RIP-Cre) mice (23). MafAΔβ mice were subsequently crossed with either the βMafBTg D or the βMafBTg A lines. Intraperitoneal glucose tolerance testing (IPGTT) was performed on adult nonpregnant and pregnant mice fasted for 6 h; tail blood glucose was measured before (0 min) and at 15, 30, 60, and 120 min after intraperitoneal injection of glucose (2 mg/g body weight) prepared in sterile PBS (20% w/v). Blood serum was collected through the tail vein after a 6-h fast. Insulin measurements were conducted in the Vanderbilt Hormone Assay & Analytical Services Core. Serotonin measurements were determined in the Vanderbilt University Neurochemistry Core.
Statistics
Significance was evaluated with the Student two-tailed t test for the quantitative PCR (qPCR), area under the curve (AUC), and β-cell proliferation tests. Statistical significance is denoted in the figure legends.
Results
The MAFA Protein Is Expressed at Very Low Levels in Islet β-Cells From Juvenile Human Donors
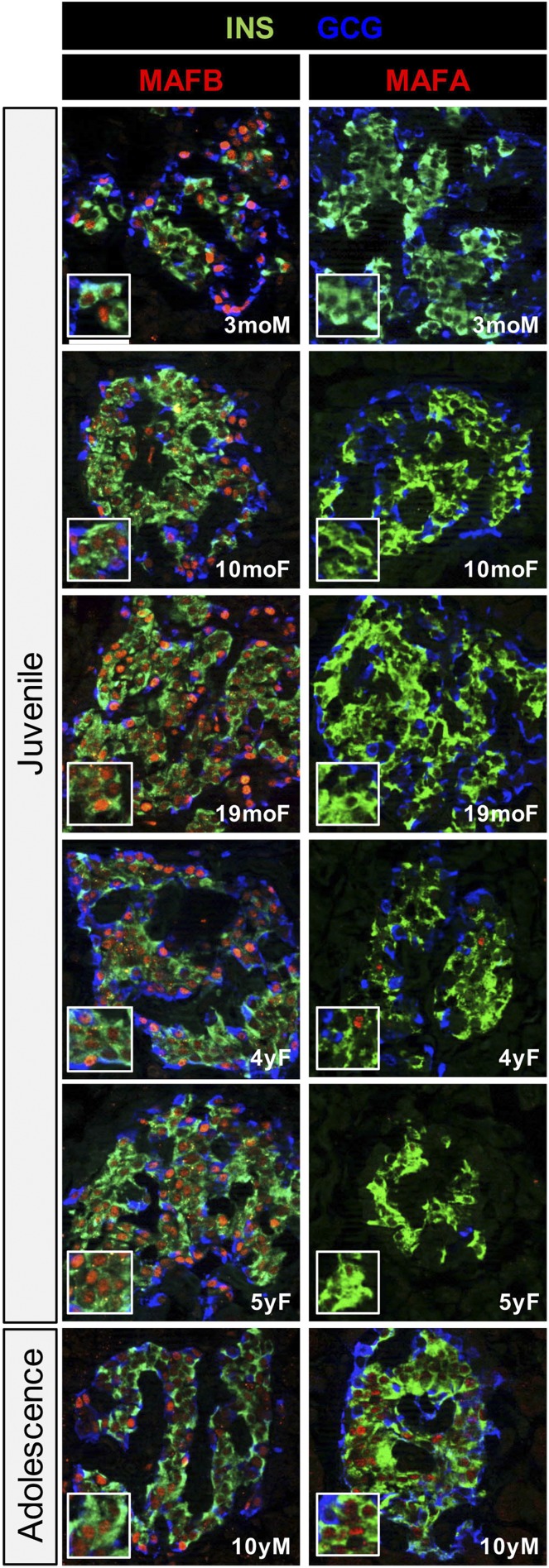
Human and NHP islets are distinct from rodents in many ways, including glucose-stimulated insulin secretion (i.e., basal [5.6 mmol/L] insulin secretion is higher in primates, and stimulated secretion is lower [17,24,25]), islet cell composition (i.e., α-cells are a much larger fraction of the islet cell population in humans [26–28]), and islet cell architecture (27,28). In addition, MAFB mRNA and protein are only produced postnatally in NHP and human islet β-cells, and MAFA mRNA expression is very low in adult NHP (10) and human juvenile islets (7) but produced throughout the lifetime of a rodent β-cell (8,9). We now show that the MAFA protein is rarely detected in juvenile human β-cells (i.e., 3 months, 10 months, 19 months, 4 years, 5 years) in relation to MAFB as well as in a smaller fraction of cells in a 10-year-old donor (Fig. 1). This represents a clear distinction from the penetrant pattern of MAFA in rodent (∼80% from birth [16]) and adult human (∼60% [29]) islet β-cells. Of note, most other islet-enriched transcription factors appear to be at similar levels and cellular distribution within rodents and primates (10). These results not only highlight the possible importance of MAFB in regulating human β-cells but also suggest that coexpression of MAFA with MAFB imparts unique regulatory properties.
Figure 1.
MAFA is expressed in a very small fraction of juvenile human islet β-cells relative to MAFB. Immunodetection of insulin (INS), glucagon (GCG), MAFB, and MAFA in 3-month-old male (3moM), 10-month-old female (10moF), 19-month-old female (19moF), 4-year-old female (4yF), 5-year-old female (5yF), and 10-year-old male (10yM) pancreatic tissue. Insets show nuclear expression of MAFB in early human pancreatic β-cells with MAFA expression principally absent. MAFA is expressed in a much greater fraction of adult human islet β-cells (∼60% [17,29]). Scale bar = 50 μm.
DNA Methylation Drives the Postnatal Silencing of MafB Expression in Mouse β-Cells
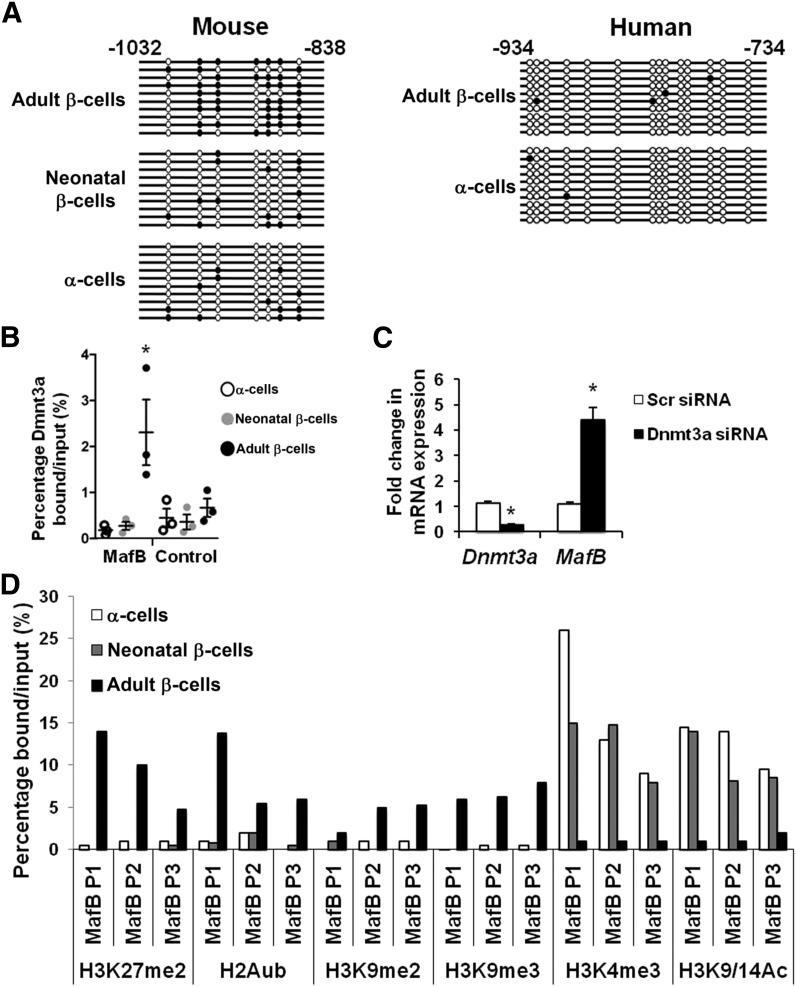
DNA methylation at CpG sites within gene promoters and enhancers is a fundamental epigenetic mark associated with transcriptional repression (30). Because mouse MafB expression in β-cells is no longer detectable 2 weeks after birth (8,9), we analyzed the MafB gene methylation status by bisulfite sequencing in FACS-purified cells in which the gene was either transcriptionally active (i.e., adult α- and neonatal β-cells) or inactive (adult β-cells). Of note, the region between −1,032 and −838 base pairs (bp) upstream from the MafB transcriptional start site was differentially methylated, with much lower CpG methylation in MafB-expressing than -nonexpressing cells (Fig. 2A). Thus, the methylation pattern within the MafB −1,032 to −838 bp region in expressing neonatal mouse β- and α-cells is more similar than to nonexpressing adult β-cells. However, these sequences, as well as those surrounding them, were hypomethylated in MAFB-expressing human islet α- and β-cells (i.e., −934 to −734 bp) (Fig. 2A and Supplementary Fig. 2), indicating that this region is involved in regulating species-specific expression.
Figure 2.
Epigenetic modification analysis reveals the inhibited state of the mouse MafB gene in β-cells. A: Bisulfite sequencing results of the −1,032 to −838 bp region of the mouse and analogous −934 to −734 bp region of the human MAFB gene in α- and β-cells. The only methylation site not conserved within this region is located in mouse at −789 CpG −788 bp and human −791 CC −789 bp. B: Dmnt3a binding to the −1,032 to −838 bp region in FACS-sorted mouse adult α-cells, neonatal α-cells, and adult β-cells. Error bars represent the average and SEM (n = 3). C: Fold change in Dnmt3a and MafB mRNA levels after scrambled (Scr) and Dnmt3a siRNA treatment in Min6 cells. D: H3K27me, H2Aub, H3K9me2, H3K9me3, H3K4me3, and H3K9/14Ac signals over the mouse MafB −1,032 to 838 bp region. P1, P2, and P3 represent independent experiments. *P < 0.05.
The de novo DNA methyltransferase Dnmt3a was shown to bind to the −1,032 to −838 bp region in MafB-nonexpressing adult mouse β-cell nuclei (Fig. 2B). Of note, this enzyme also is involved in repression of functionally disallowed genes in maturing islet β-cells (e.g., hexokinase 1 and lactate dehydrogenase A [18]). Suppression of Dnmt3a by siRNA treatment in the mouse Min6 β-cell line also led to increased MafB expression (Fig. 2C), providing further support that this epigenetic regulator represses MafB in mouse islet β-cells in an age- and cell type–specific manner. Furthermore, the dynamic histone protein modification state within the MafB gene added evidence for gene silencing and Dnmt3a regulation, with only repressive chromatin marks enriched in adult β-cells (i.e., H3K27me2, H2Aub, H3K9me2, H3K9me3) and activating (H3K4me3, H3K9/14Ac) in neonatal β- and α-cells (Fig. 2D).
Development of a β-Cell–Specific MafB Overexpression Mouse Model (βMafBTg) to Study the Impact of Sustained MafB Production in β-Cells
A mouse MafA BAC spanning the 5′ flanking regions 1–6 transcription control sequences necessary for directing islet cell–specific expression (31) was used to transgenically drive MafB in ∼86% (SD ±4.91) of adult islet β-cells (Fig. 3A and Supplementary Fig. 1). This is similar to the number of adult islet β-cells that normally express MAFA (8). Of note, the coding sequences for human and mouse MafB are 97% identical. Transgenically produced MafB mRNA was not observed until e15.5 and sustained in adult β-cells (Supplementary Fig. 3), a pattern characteristic of the endogenous MafA gene (8,9). βMafBTg mice were born in normal Mendelian ratios with no overt physical or physiological deficiencies (data not shown). Immunohistochemical analysis revealed that nuclear MafB was produced in a staining pattern similar to that of MafA within adult insulin-positive cells of βMafBTg mice (Fig. 3B). Islet architecture was indistinguishable between MafAfl/fl and βMafBTg mice, with core insulin-positive β-cells surrounded by glucagon-positive α-cells (Fig. 3B). There was a 2.5-fold increase in βMafBTg MafB mRNA levels in 2-month-old pancreatic islets compared with the MafAfl/fl control, with no change in MafA mRNA expression (Fig. 3C) and a staining intensity in β-cells similar to the endogenous MafB of α-cells (Fig. 3B).
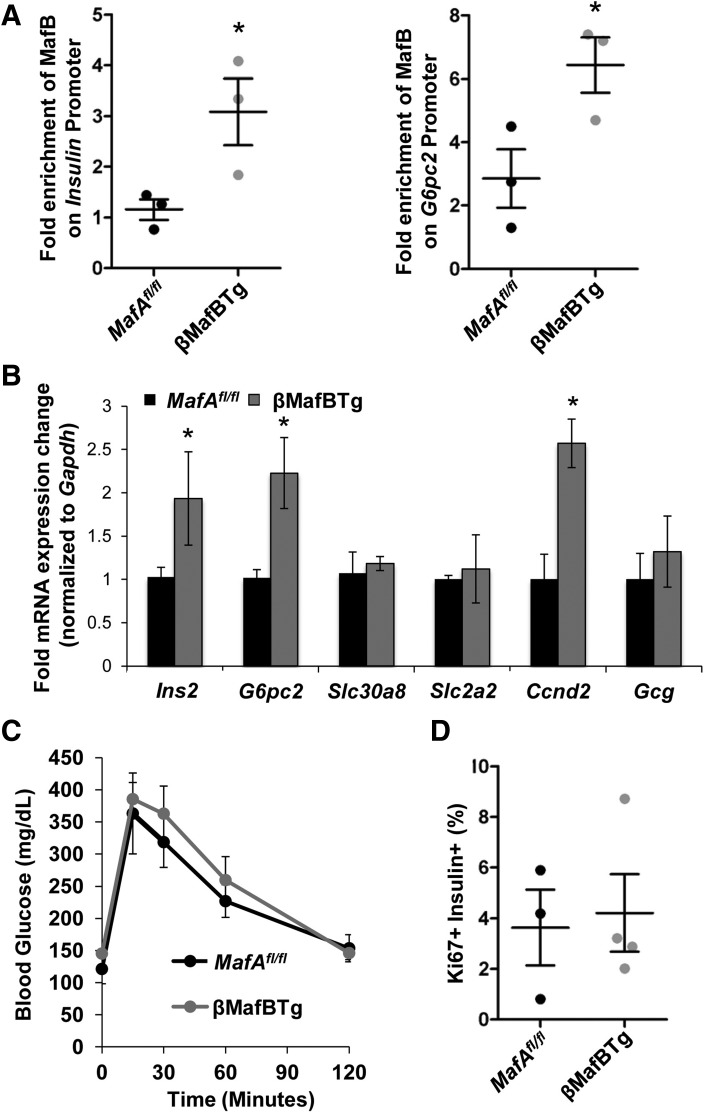
Transcriptional regulation by the MafA and MafB proteins is achieved by homo- or heterodimer binding to large Maf-response elements (32–34). ChIP analysis demonstrated MafB-induced binding to MafA-regulated Insulin and G6pc2 gene transcriptional control sequences in βMafBTg nuclei (Fig. 4A). This resulted in a roughly twofold elevation in Insulin, G6pc2, and Ccnd2 mRNA levels, but not MafA-activated Slc2a2 or Slc30a8 expression (Fig. 4B). However, the induction of these insulin-signaling (i.e., Insulin, G6pc2) or cell proliferation (Ccnd2) genes did not affect the rate of glucose clearance in IPGTTs (Fig. 4C) or β-cell proliferation (Fig. 4D). These results suggest that the heterodimeric MAFA/MAFB regulator of human adult β-cells is essentially functionally equivalent to the mouse MafA2 homodimer.
Figure 4.
The MafA/MafB regulator has activity similar to that of MafA2 in adult islet βMafBTg β-cells. A: ChIP analysis reveals that MafB binds to Insulin and G6pc2 control sequences in 2-month-old βMafBTg islet nuclei. Fold enrichment was calculated relative to the albumin promoter (n = 3). B: MafB enhances the expression of Insulin, G6pc2, and Ccnd2 in 2-month-old βMafBTg islets. qPCR signals were normalized to Gapdh (n = 8–10). C: βMafBTg and MafAfl/fl have the same fasting blood glucose levels and ability to clear a blood glucose bolus in IPGTT assays. D: The number of Ki67+-proliferating islet β-cells between βMafBTg and MafAfl/fl mice were unchanged (n = 3–4). *P < 0.05.
The MafB2 Homodimer Is Not Functionally Equivalent to MafA2 Homodimer in Mouse Islet β-Cells
The MafB2 regulator not only is enriched in NHP (10) and human (7) islet β-cells (Fig. 1), but also is associated with immature β-cell function in mice (35). Therefore, we next analyzed whether production of MafB alone would affect the deficiencies associated with loss of islet β-cell MafA in MafAΔβ mice, which includes abnormalities in islet architecture, impaired glucose tolerance, and reduced expression of factors important for insulin production and secretion (16).
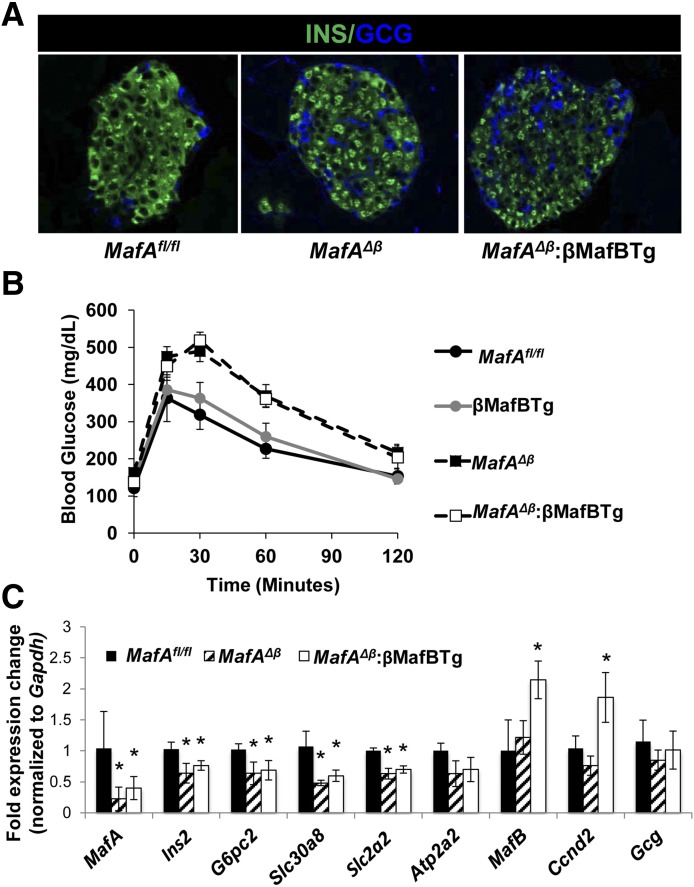
MafAΔβ and βMafBTg mice were crossed to generate islet β-cells only expressing MafB. The resulting MafAΔβ;βMafBTg mice had the same abnormal islet cell architecture of the MafAΔβ mutant marked by the presence of glucagon-positive α-cells throughout the islet core (Fig. 5A). In contrast, α-cells and other non–β-islet cell types were found on the islet periphery in control MafAfl/fl mice (Fig. 5A and data not shown). Moreover, no improvement was observed in the ability of MafAΔβ:βMafBTg mice to clear blood glucose in relation to βMafBTg and MafAfl/fl mice, as indicated by IPGTT (Fig. 5B). Not surprisingly, we found that MafB alone could not rescue the gene expression changes resulting from loss of MafA, including Ins2, G6pc2, Slc30a8, and Slc2a2 (Fig. 5C). The only gene differentially expressed in our candidate gene survey was Ccnd2, which also was elevated in βMafBTg mice (Fig. 4B). Collectively, these results indicate that there will be differences in gene expression, and potentially function, in juvenile β-cells principally expressing MAFB2 compared with adult (mouse) MafA2 or (human) MAFA/MAFB β-cells.
Figure 5.
MafB2 is not functionally equivalent to MafA2 in MafAΔβ:βMafBTg mice. A: In contrast to 2-month-old control MafAfl/fl mice, the islet architectural core contains both α-cells (glucagon [GCG]) and β-cells (insulin [INS]) in MafAΔβ:βMafBTg and MafAΔβ islets. B: Two-month-old MafAΔβ and MafAΔβ:βMafBTg mice are glucose intolerant compared with MafAfl/fl and βMafBTg animals. C: Gene expression changes in MafAΔβ islets are not corrected in MafAΔβ:βMafBTg mice. qPCR signals normalized to Gapdh expression (n = 5–8). *P < 0.05.
MafB Expression Throughout the Islet β-Cell Population Enhances Serotonin Signaling During Pregnancy, Leading to Increased β-Cell Function and Proliferation
MafB is re-expressed in ∼25% of mouse β-cells during pregnancy (15,36), and is presumably present in even a greater percentage in humans. Increased peripheral tissue insulin resistance in pregnancy ensures proper glucose shuttling to the growing fetus and raises insulin demand (36). Thus, β-cells undergo a period of proliferation and increased insulin secretion to adapt to these changes, which occurs in response to serotonin signaling (37–39). We have shown that removal of MafB in maternal β-cells leads to a reduction in serotonin expression and gestational diabetes (36).
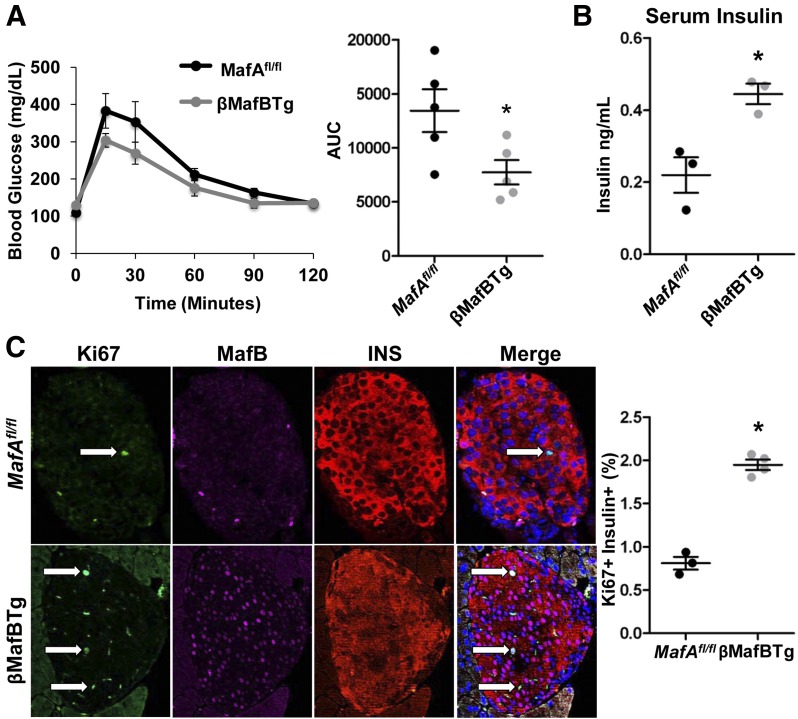
Thus, we asked whether broader MafB production within the islet β-cell population of βMafBTg mice is consequential during pregnancy. Indeed, βMafBTg mice had improved glucose tolerance at gestational day (GD) 14.5 compared with control pregnant MafAfl/fl dams (Fig. 6A). In addition, serum insulin levels in fasted mice were increased in βMafBTg animals (Fig. 6B). There was also a 2.4-fold increase in the number of insulin-positive cells labeled with the Ki67+ proliferation marker in pregnant βMafBTg mice (Fig. 6C), suggesting that MafB directly affects serotonin signaling and, consequently, adaptive maternal β-cell function and mass expansion. Consistent with this proposal, there was also an increase in maternal βMafBTg serotonin staining (Fig. 7A) and islet serotonin levels (Fig. 7B) compared with MafAfl/fl controls.
Figure 6.
Glucose tolerance and β-cell proliferation levels are enhanced during pregnancy in βMafBTg mice. A: MafAfl/fl and βMafBTg pregnant dams (GD15.5) were subjected to IPGTT, and βMafBTg mice had improved glucose tolerance. AUC is shown for statistical analysis (n = 8–10). B: GD15.5 βMafBTg mice have increased serum insulin levels after a 6-h fast. C: βMafBTg islets have an increased number of Ki67+ β-cell nuclei (arrows) compared with MafAfl/fl pregnant mice. Graph indicates the percentage of proliferating (Ki67+) insulin-positive (INS+) cells (n = 4). *P < 0.05.
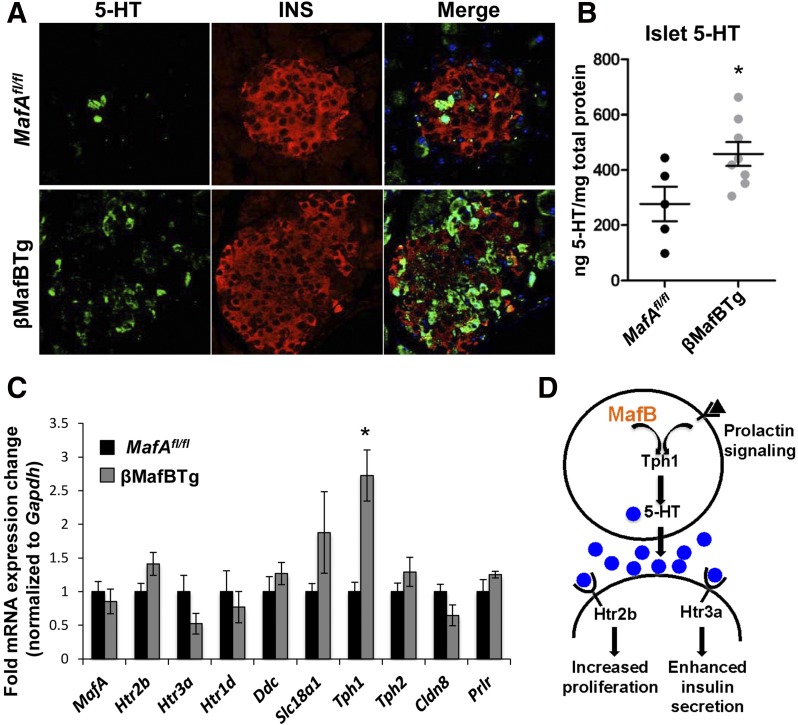
Figure 7.
Increased Tph1 elevates serotonin signaling in maternal βMafBTg β-cells. A and B: βMafBTg islets have increased cellular serotonin (5-HT) staining (n = 3) and total islet 5-HT protein levels compared with MafAfl/fl islets during pregnancy (n = 5–8). C: Only Tph1 transcript levels were increased at GD15.5 and not MafA or a variety of analyzed mediators of 5-HT signaling in βMafBTg islets (n = 4). D: Model illustrating that MafB activation of Tph1 elevates the 5-HT–mediated increase in β-cell mass and function. *P < 0.05.
To obtain mechanistic insight into how MafB influenced serotonin production, the expression of multiple genes involved in this signaling pathway was examined in GD15.5 βMafBTg and MafAfl/fl islets (Fig. 7C). No difference was observed in expression of either the prolactin receptor (Prlr), which lies upstream of MafB and serotonin production (36), or the serotonin-responsive receptors that mediate β-cell proliferation (i.e., Htr2b [39]) or function (Htr3a [39]). In fact, only Tph1 (and not Tph2 [38]), the rate-limiting enzyme in serotonin production from tryptophan in maternal β-cells (38), was significantly increased in βMafBTg islets (Fig. 7C). We propose that the high level of MafB expression within the primate β-cell population also affects the adaptive response of these cells during pregnancy (Fig. 7D).
Discussion
Human pancreas tissue and cellular samples recently have become much more accessible for analyses because of the establishment of a variety of collaborative human tissue repositories, including, for example, the Network of Pancreatic Organ Donors With Diabetes, Integrated Islet Distribution Program, and Human Pancreas Analysis Program. Consequently, we now recognize similarities and differences in pancreatic biology between our extensively analyzed rodent models and humans, with distinctions in islets ranging from aspects of architecture/cell composition to physiological function (17,24,25,27,28). However, very few experimental systems currently exist to study human islet cell biology, so rodent models are still necessary for revealing the potential significance of these differences to physiology and disease. Here, we focus on the MAFA and MAFB transcription factors, essential regulators of postnatal mouse islet β-cell (i.e., MafA) and α-cell (MafB) activity whose expression pattern is distinctly different between rodents and primates. The current study leverages mouse genetics to understand the mechanistic basis of transcription factor regulation unique to human β-cells.
Distinct age-dependent differences in chromatin landscape, gene expression, and proliferative capacity between juvenile and adult human islet α- and β-cells have been shown (7). This includes epigenetic regulatory modifications of both the N-terminal tails of histone proteins and CpG dinucleotides in DNA. In addition, only adult human islets have enhanced basal, low (5.6 mmol/L) glucose-stimulated insulin secretion in perfusion assays (7), a property not observed in either juvenile human or rodent islets. Yet, what factors control this novel feature of human islets is still unclear. Of note, misexpression of the adult-induced SIX2 and SIX3 transcription factors was found to enhance human β-cell maturation (7). Islet β-cell–specific MAFA mRNA levels also are induced in adult human cells, and we now show that little to no cellular protein is produced within juvenile β-cells compared with MAFB (Fig. 1). A caveat to this observation is limitations in human donor availability, which precluded possible distinguishing variabilities among individuals, sex, and ethnicity.
The very low human juvenile MAFA protein levels is strikingly different from the high and penetrant expression pattern within the rodent embryonic, postnatal, and adult β-cell population (8,9). In fact, misexpression of MafA within MafB-enriched immature rat perinatal β-cells induces maturation marker expression and glucose-stimulated insulin section (40). These results indicate that elevated MAFA affects adult islet β-cell gene expression and in vitro glucose-dependent insulin secretion. Of note, juvenile islets had a much lower insulin secretion level when exposed to basal 5.6 mmol/L glucose levels than adult human islets in perfusion assays (7). However, these juvenile and adult cell populations must be functionally similar in vivo because there are no overt age-dependent differences in homeostatic glucose levels. Consequently, we propose that juvenile MAFB-enriched human embryonic-derived β-like cells will have therapeutic utility similar to those expressing adult markers, as presently evaluated, for example, by MAFA production (41–44).
In stark contrast to rodents, MAFB is predominantly expressed in primate β-cells, with no apparent induction of MAFA in aging NHP β-cells (10). Methylation by Dnmt3a of CpG sequences between −1,032 and −838 bp was found to facilitate silencing of mouse MafB gene transcription in β-cells postnatally (Fig. 2), whereas this region was unmethylated in MafB-expressing mouse α-cells, newborn mouse β-cells, human β-cells, and human α-cells. It is possible that the absence of the mouse −789 CpG −788 bp site in the human MAFB gene prevents DNMT3A action within this otherwise highly conserved region. Of note, the loss of MafB in MafBΔpancreas mice during development has no consequence to islet β-cells because of compensation by MafA (10). Determining how MAFB influences formation of the β-like cells derived from human embryonic stem cells, a system used by others to analyze how key model system–defined islet-enriched transcription factors act during this important formative period (45,46), would be of great interest. Moreover, because some juvenile human β-cells do not appear to express MAFB, varying levels of this transcription factor may contribute to functional differences within the cell population. Such experimentation is predicted to reveal a unique role for MAFB in human β-cells.
βMafBTg animals were derived to gain insight into how MAFB could affect adult islet β-cells. Ectopic MafB appeared to be expressed first at the onset of embryonic MafA expression (Supplementary Fig. 3) and at levels comparable to islet α-cells (Fig. 3) and neonatal β-cells (data not shown). MafB also was bound to endogenous MafA target gene sequences in islet βMafBTg β-cell nuclei (Fig. 4A). We conclude from these results that MafB was being produced in βMafBTg β-cells at functionally active levels. The lack of any overt physiological or molecular phenotype upon coexpression of MafA and MafB in βMafBTg β-cells likely means that the mouse MafA2 homodimer is functionally equivalent to the adult human MAFA/MAFB heterodimer. However, MAFB2 alone was unable to rescue any of the islet cell deficiencies of MafAΔβ mice (Fig. 5). It is possible that unique and enabling sets of transcriptional coregulators are recruited by human MAFB2 or MAFA/MAFB. If so, this is likely due to binding within the COOH-terminal sequences of these proteins, which have been shown in chimeric analysis to impart defining functional regulatory properties (47). MafA activation is known to be controlled by the histone acetyltransferase p300/CBP-factor (PCAF) coactivator (48), whereas both MAFA and MAFB recruit the Mll3 and Mll4 histone 3 lysine 4 methyltransferase complexes (22). Such MAFB2- and MAFA/MAFB-recruited factors also could influence the functional properties of the four distinct adult human islet β-cell populations termed β1 (most active) to β4 (least active) (49).
MafB expression in βMafBTg islet β-cells led to increased serotonin signaling during pregnancy, which presumably resulted in enhanced cell proliferation and function (Fig. 7). Our results demonstrate that this entails activation of Tph1 mRNA levels, which encodes the rate-limiting enzyme for serotonin synthesis from tryptophan in maternal β-cells. Of note, the mouse Tph1 gene contains three MafB consensus binding sites at −9,184/−9,189, −8,184/−8,189, and −8,147/−8,152 bp that are near a region bound by Stat5 (−8,754/−8,723 bp), which mediates prolactin signaling in maternal β-cells (50). Thus, MafB may directly bind in conjunction with Stat5 to increase Tph1 transcription, thereby increasing serotonin production and leading to greater islet β-cell mass and function that compensates for the insulin resistance induced by pregnancy. Preventing MafB induction in these cells leads to a form of gestational diabetes as a result of an inability to facilitate these processes (36). Although it is unclear whether increased MafB expression in βMafBTg mice simulates the conditions in the human maternal islet β-cell population, these results raise the possibility that such an increase in MAFB levels would buffer the detrimental effects of obesity in the mother and her offspring (51).
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Hail Kim at the Korea Advanced Institute of Science and Technology (Daejeon, Korea) for helpful guidance during the pregnancy studies. The authors apologize for not providing a more comprehensive list of citations, a limitation imposed by journal regulations.
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants (F32-DK102283 to H.A.C.; F32-DK109577 to E.M.W). This research was performed using resources and/or funding provided by the NIDDK-supported Human Islet Research Network (RRID: SCR_014393 [https://hirnetwork.org/]; DK104119 to K.H.K.; DK104211, DK108120, and DK112232 to A.C.P.; DK106755 to A.C.P.; and R01-DK909570 to R.S.) and the Vanderbilt Diabetes Research and Training Center (NIDDK grant DK20593). Funds from the Larry L. Hillbolm Foundation (2012-D-006-SUP) were provided to S.D. The JDRF, Leona M. and Harry B. Helmsley Charitable Trust, and U.S. Department of Veterans Affairs also support A.C.P. Imaging was performed with National Institutes of Health support from the Vanderbilt University Medical Center Cell Imaging Shared Resource (National Cancer Institute grant CA68485; NIDDK grants DK20593, DK58404, and DK59637; Eunice Kennedy Shriver National Institute of Child Health and Human Development [NICHD] grant HD15052; and National Eye Institute grant EY08126), and islet hormone analysis support was by the Vanderbilt University Medical Center Islet Procurement and Analysis Core (NIDDK grant DK20593) and Vanderbilt University Neurochemistry Core (NICHD grant U54-HD-083211). Human pancreatic islets were provided by the NIDDK-funded Integrated Islet Distribution Program at City of Hope (National Institutes of Health grant 2UC4DK098085).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.A.C., E.M.W., Y.H., S.D., R.H., L.B., and D.A. performed experiments. H.A.C., E.M.W., Y.H., S.D., R.H., and D.A. prepared figures. H.A.C., E.M.W., Y.H., S.D., R.H., D.A., M.B., K.H.K., A.B., A.C.P., and R.S. approved the final version of the manuscript. H.A.C., E.M.W., Y.H., S.D., R.H., D.A., K.H.K., A.B., A.C.P., and R.S. analyzed data, interpreted the results of experiments, and edited and revised the manuscript. H.A.C., E.M.W., Y.H., and R.S. contributed to the conception and design of the research and drafted the manuscript. R.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0903/-/DC1.
H.A.C. and E.M.W. are co-first authors.
H.A.C. is currently affiliated with the Department of Biological Sciences, Marshall University, Huntington, WV.
Y.H. is currently affiliated with the Department of Developmental Biology, Stanford University School of Medicine, Stanford, CA.
References
- 1.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 2.Yamagata K, Oda N, Kaisaki PJ, et al. . Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3). Nature 1996;384:455–458 [DOI] [PubMed] [Google Scholar]
- 3.Bellanné-Chantelot C, Clauin S, Chauveau D, et al. . Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes 2005;54:3126–3132 [DOI] [PubMed] [Google Scholar]
- 4.Stoffers DA, Ferrer J, Clarke WL, Habener JF. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet 1997;17:138–139 [DOI] [PubMed] [Google Scholar]
- 5.Iacovazzo D, Flanagan SE, Walker E, et al. . MAFA missense mutation causes familial insulinomatosis and diabetes mellitus. Proc Natl Acad Sci U S A 2018;115:1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev 2008;22:1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arda HE, Li L, Tsai J, et al. . Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab 2016;23:909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artner I, Hang Y, Mazur M, et al. . MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 2010;59:2530–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura W, Kondo T, Salameh T, et al. . A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev Biol 2006;293:526–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad E, Dai C, Spaeth J, et al. . The MAFB transcription factor impacts islet α-cell function in rodents and represents a unique signature of primate islet β-cells. Am J Physiol Endocrinol Metab 2016;310:E91–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hang Y, Stein R. MafA and MafB activity in pancreatic β cells. Trends Endocrinol Metab 2011;22:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guz Y, Montminy MR, Stein R, et al. . Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development 1995;121:11–18 [DOI] [PubMed] [Google Scholar]
- 13.Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev 1997;11:1662–1673 [DOI] [PubMed] [Google Scholar]
- 14.Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev 2008;22:3435–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pechhold S, Stouffer M, Walker G, et al. . Transcriptional analysis of intracytoplasmically stained, FACS-purified cells by high-throughput, quantitative nuclease protection. Nat Biotechnol 2009;27:1038–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hang Y, Yamamoto T, Benninger RKP, et al. . The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes 2014;63:1994–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai C, Brissova M, Hang Y, et al. . Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 2012;55:707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhawan S, Tschen S-I, Zeng C, et al. . DNA methylation directs functional maturation of pancreatic β cells. J Clin Invest 2015;125:2851–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhawan S, Georgia S, Tschen S-I, Fan G, Bhushan A. Pancreatic β cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell 2011;20:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahl JA, Collas P. MicroChIP--a rapid micro chromatin immunoprecipitation assay for small cell samples and biopsies. Nucleic Acids Res 2008;36:e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deramaudt TB, Sachdeva MM, Wescott MP, Chen Y, Stoffers DA, Rustgi AK. The PDX1 homeodomain transcription factor negatively regulates the pancreatic ductal cell-specific keratin 19 promoter. J Biol Chem 2006;281:38385–38395 [DOI] [PubMed] [Google Scholar]
- 22.Scoville DW, Cyphert HA, Liao L, et al. . MLL3 and MLL4 methyltransferases bind to the MAFA and MAFB transcription factors to regulate islet β-cell function. Diabetes 2015;64:3772–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray MK, Fagan SP, Moldovan S, DeMayo FJ, Brunicardi FC. Development of a transgenic mouse model using rat insulin promoter to drive the expression of CRE recombinase in a tissue-specific manner. Int J Pancreatol 1999;25:157–163 [DOI] [PubMed] [Google Scholar]
- 24.Henquin J-C, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes 2006;55:3470–3477 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Diaz R, Molano RD, Weitz JR, et al. . Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metab 2018;27:549–558.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 27.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 2006;103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brissova M, Fowler MJ, Nicholson WE, et al. . Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 29.Guo S, Dai C, Guo M, et al. . Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 2013;123:3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golson ML, Kaestner KH. Epigenetics in formation, function, and failure of the endocrine pancreas. Mol Metab 2017;6:1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raum JC, Hunter CS, Artner I, et al. . Islet beta-cell-specific MafA transcription requires the 5′-flanking conserved region 3 control domain. Mol Cell Biol 2010;30:4234–4244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurokawa H, Motohashi H, Sueno S, et al. . Structural basis of alternative DNA recognition by Maf transcription factors. Mol Cell Biol 2009;29:6232–6244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellegrino S, Ronda L, Annoni C, et al. . Molecular insights into dimerization inhibition of c-Maf transcription factor. Biochim Biophys Acta 2014;1844:2108–2115 [DOI] [PubMed] [Google Scholar]
- 34.Lu X, Guanga GP, Wan C, Rose RB. A novel DNA binding mechanism for maf basic region-leucine zipper factors inferred from a MafA-DNA complex structure and binding specificities. Biochemistry 2012;51:9706–9717 [DOI] [PubMed] [Google Scholar]
- 35.Artner I, Blanchi B, Raum JC, et al. . MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A 2007;104:3853–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banerjee RR, Cyphert HA, Walker EM, et al. . Gestational diabetes mellitus from inactivation of prolactin receptor and MafB in islet β-cells. Diabetes 2016;65:2331–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieck S, White P, Schug J, et al. . The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol 2009;23:1702–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Toyofuku Y, Lynn FC, et al. . Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 2010;16:804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohara-Imaizumi M, Kim H, Yoshida M, et al. . Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc Natl Acad Sci U S A 2013;110:19420–19425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aguayo-Mazzucato C, Koh A, El Khattabi I, et al. . Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia 2011;54:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rezania A, Bruin JE, Arora P, et al. . Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014;32:1121–1133 [DOI] [PubMed] [Google Scholar]
- 42.Kroon E, Martinson LA, Kadoya K, et al. . Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 43.Russ HA, Parent AV, Ringler JJ, et al. . Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 2015;34:1759–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pagliuca FW, Millman JR, Gürtler M, et al. . Generation of functional human pancreatic β cells in vitro. Cell 2014;159:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiyaboonchai A, Cardenas-Diaz FL, Ying L, et al. . GATA6 plays an important role in the induction of human definitive endoderm, development of the pancreas, and functionality of pancreatic β cells. Stem Cell Reports 2017;8:589–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Z, Li QV, Lee K, et al. . Genome editing of lineage determinants in human pluripotent stem cells reveals mechanisms of pancreatic development and diabetes. Cell Stem Cell 2016;18:755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Artner I, Hang Y, Guo M, Gu G, Stein R. MafA is a dedicated activator of the insulin gene in vivo. J Endocrinol 2008;198:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rocques N, Abou Zeid N, Sii-Felice K, et al. . GSK-3-mediated phosphorylation enhances Maf-transforming activity. Mol Cell 2007;28:584–597 [DOI] [PubMed] [Google Scholar]
- 49.Dorrell C, Schug J, Canaday PS, et al. . Human islets contain four distinct subtypes of β cells. Nat Commun 2016;7:11756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iida H, Ogihara T, Min MK, et al. . Expression mechanism of tryptophan hydroxylase 1 in mouse islets during pregnancy. J Mol Endocrinol 2015;55:41–53 [DOI] [PubMed] [Google Scholar]
- 51.Elshenawy S, Simmons R. Maternal obesity and prenatal programming. Mol Cell Endocrinol 2016;435:2–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.