Abstract
Purpose/Background
The aim of this study was to report a patient with vitreoretinal lymphoma with clinical features providing hypothesis-generating insights into the pathophysiology of this disease.
Methods
Clinical history and imaging studies (i.e., fundus photography, optical coherence tomography, fundus autofluorescence, and fluorescein angiography) were documented.
Results
A 71-year-old woman presented with a 2-month history of blurred vision in the right eye and bilateral vitreous infiltrates unresponsive to topical and systemic steroids. Vitreous biopsy of the left eye was diagnostic for lymphoma. Bulky subretinal deposits in the right eye responded to systemic therapy. The left fundus showed diffuse hypoautofluorescence and punctate, hyperfluorescent sub-retinal pigment epithelial tumor deposits, which resolved leaving hypoautofluorescent atrophic retinal pigment epithelium (RPE) scars, except inferotemporally, where retinal vasculopathy had occurred.
Conclusions
The clinical features suggest that occlusion of the inferotemporal retinal arteriole prevented sub-RPE lymphomatous deposits and subsequent RPE atrophy in this area of vascular nonperfusion. This suggests that “primary” vitreoretinal lymphoma is secondary to hematogenous spread from systemic loci. This finding, together with the ocular tumor control achieved entirely by systemic therapy, indicates scope for studies investigating systemic treatment protocols, especially those including immune-modulatory agents.
Keywords: Lymphoma, Retinal disease
Established Facts
The term “primary intraocular lymphoma” has largely been replaced by “vitreoretinal lymphoma” because this condition is so different from primary uveal lymphoma.
In most centers, vitreoretinal lymphoma is treated by ocular radiotherapy or intravitreal chemotherapy.
Despite successful local tumor control, there is a high mortality as a result of central nervous system lymphoma.
Novel Insights
Retinal arteriolar occlusion prevents lymphomatous retinal infiltration into ischemic areas, providing circumstantial evidence that ocular involvement occurs by hematogenous spread from a systemic focus.
Early/subtle vitreoretinal lymphoma can manifest as diffuse retinal pigment epithelial (RPE) atrophy, causing hypoautofluorescence.
Focal atrophic RPE lesions develop when the sub-RPE lymphoma deposits resolve.
If vitreous infiltration is minimal, systemic chemotherapy can suppress vitreoretinal lymphoma without the need for focal therapy.
The term “vitreoretinal lymphoma” is preferable to “primary vitreoretinal lymphoma” because it is not known whether the disease actually originates intraocularly or whether it is secondary to a subclinical focus in another part of the body.
Introduction
Vitreoretinal lymphoma is characterized by unilateral or bilateral infiltration of malignant lymphocytes into the retina and vitreous and between the retinal pigment epithelium (RPE) and Bruch's membrane [1, 2]. Most patients are more than 50 years old at the time of presentation, except for those with immunosuppression. Vitreoretinal lymphoma is often associated with central nervous system (CNS) lymphoma, which may precede or follow the ocular disease. In most centers, vitreoretinal lymphoma is treated focally, with ocular radiotherapy or intravitreal chemotherapy [3, 4, 5]. About one-third of patients develop CNS disease within 5 years [6]. At the University of California San Francisco (UCSF), we treat vitreoretinal lymphoma with systemic chemotherapy, usually in combination with rituximab and lenalidomide, a second-generation immunomodulatory agent [7]. This treatment induces clinical features that are different from those generally reported. In this article, we describe these features in a case that provides hypothesis-generating insights into the pathology of vitreoretinal lymphoma and which also raises questions about the management of indolent disease progression.
Case Report
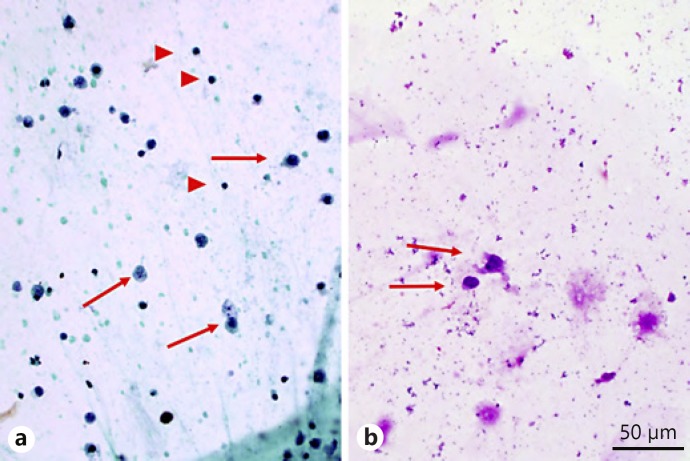
A 71-year-old woman was referred to the ocular oncology service at UCSF in June 2014 with a 2-month history of blurred vision in the right eye and bilateral vitreous infiltrates unresponsive to topical and systemic steroids. She also reported fatigue, amnesia, and loss of balance. Her history included diabetes mellitus and systemic hypertension. Uveitis workup, systemic investigation (including bone marrow biopsy with flow cytometry), full body PET/CT, brain MRI, and lumbar puncture were negative. Vitreous biopsy in the right eye had failed to provide a diagnosis. Left vitreous biopsy in August 2014 showed rare malignant lymphoid cells, described as being “large with scant cytoplasm and enlarged hyperchromatic nuclei with subtle nuclear contour irregularities and coarse granular chromatin” (Fig. 1a, b). Flow cytometry was unsuccessful.
Fig. 1.
Cytology from vitreous biopsy of the left eye with arrows showing large atypical cells and arrowheads showing small mature lymphocytes for size comparison. a Aspirate smear, alcohol-fixed Papanicolau stain. Magnification ×400. b Aspirate smear, air-dried May-Grünwald-Giemsa stain. Magnification ×400. Courtesy of Melike Pekmezci.
In September 2014, the visual acuity with pseudophakic correction was 20/40 OD and 20/20 OS. Both eyes showed multiple, tiny, subtle, yellow RPE lesions (“microflecks”), macular epiretinal membranes, and cystoid macular edema. In addition, the right eye showed large, confluent, sub-RPE tumors, multiple atrophic RPE lesions, and sheathing of a macular arteriole (Fig. 2a, b, c). The left fundus showed diffuse hypoautofluorescence, except inferotemporally (Fig. 3a, b).
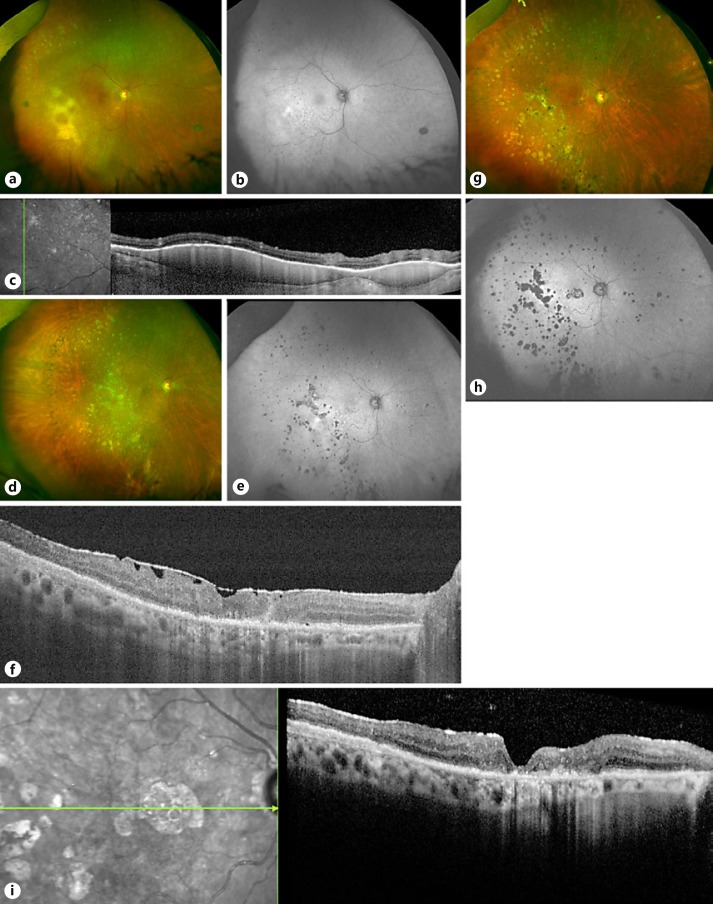
Fig. 2.
Imaging of the right eye over time. a September 2014: color photograph showing pale, yellow sub-RPE deposits inferotemporally and sheathing of an inferior macular arteriole. b Corresponding autofluorescence image, showing hyperautofluorescence in the area of infiltration, with hyperfluorescent spots elsewhere indicating active lesions and hypofluorescent spots indicating atrophic RPE scars. c OCT performed on the same date showing sub-RPE infiltrates in the temporal macula. d Color photograph of the right fundus from March 2015 showing resolution of the large sub-RPE deposits with only systemic therapy. e Corresponding fundus autofluorescence image showing hyper- and hypofluorescent spots indicating active lesions and atrophic RPE scars, respectively. f OCT of the macula on the same date showing an ERM. g Col or photograph of the right fundus, taken in May 2017, showing atrophic RPE lesions, mostly temporally. h Corresponding fundus autofluorescence image showing hypoautofluorescence scars. i OCT of the macula on the same date showing RPE atrophy and a mild ERM. RPE, retinal pigment epithelium; OCT, optical coherence tomography; ERM, epiretinal membrane.
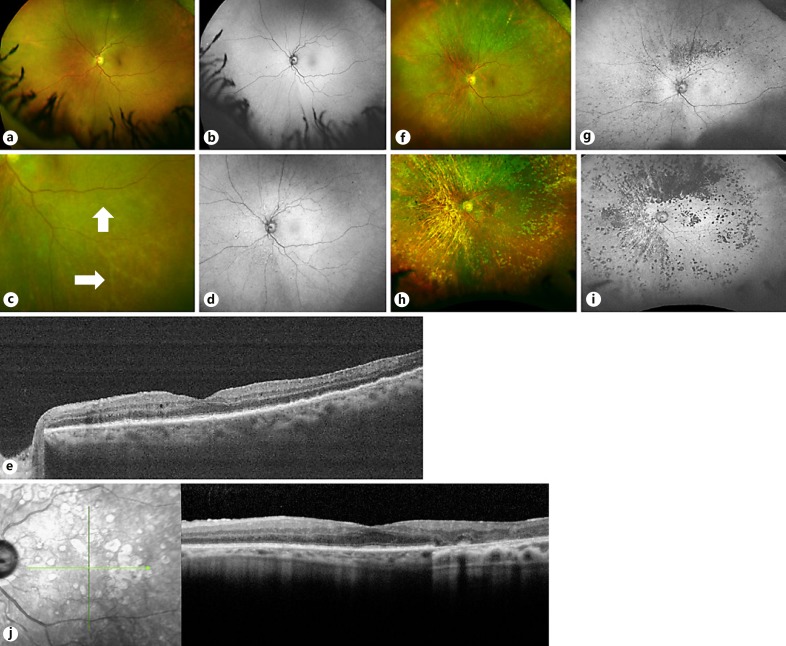
Fig. 3.
Imaging of the left eye over time. a Color photograph in September 2014, prior to treatment, showing only faint microflecks. b Corresponding fundus autofluorescence image showing diffuse hypoautofluorescence inferonasally and perhaps superiorly and temporally. c Color photograph from November 2015, showing sheathing of inferotemporal arterioles (white arrows). d Corresponding fundus autofluorescence image showing diffuse hypoautofluorescence, except inferotemporally. e OCT of the left eye in November 2015 showing bumpy appearance of the RPE layer. f Color photograph from March 2016 showing increasing RPE atrophy, especially superiorly. g Corresponding fundus autofluorescence image showing active hyperautofluorescent infiltrates and hypoautofluorescent atrophic RPE scars. h Fundus photograph from May 2017 showing extensive RPE atrophy that spares the inferotemporal retina. i Corresponding autofluorescence image, which shows sparing of the inferotemporal retina from atrophic RPE lesions. This is the area of prior vascular occlusion. j OCT on the same date showing a mild ERM as well as extensive loss of the RPE layer corresponding to atrophy seen in color and autofluorescence imaging. RPE, retinal pigment epithelium; OCT, optical coherence tomography; ERM, epiretinal membrane.
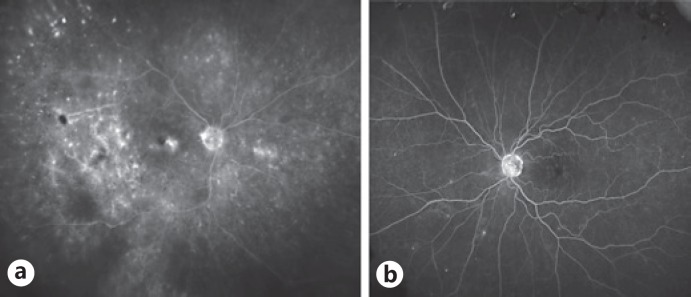
Fluorescein angiography of the right eye showed slow arteriolar blood flow inferiorly in the macula and hypofluorescence temporal to the macula at the site of the largest lymphomatous accumulation (Fig. 4a). The left eye was largely unremarkable except for a few areas of hyperfluorescence nasally (Fig. 4b).
Fig. 4.
Fluorescein angiography prior to treatment in September 2014. a Late-frame image of the right fundus showing hypofluorescence temporal to the macula at the site of the largest sub-RPE lymphomatous infiltrate, with macular edema as well as focal lesions elsewhere, with fuzzy spots indicating active lesions and discrete spots indicating RPE scars. b Late-frame image of the left fundus showing only a few areas of hyperfluorescence nasally, with no obvious retinal vascular abnormalities. RPE, retinal pigment epithelium.
The clinical features were consistent with vitreoretinal lymphoma. The patient was treated with systemic methotrexate, temozolomide, and rituximab.
Six months later, in March 2015, the tumors in the right eye had regressed completely (Fig. 2d, e, f). The vision had deteriorated to counting fingers because of atrophic scars involving the fovea. The left eye was unchanged, with Snellen visual acuity of 20/30 and microflecks as before.
In May 2015, the status of the right eye was unchanged. The left eye had a corrected Snellen visual acuity of 20/50, mild anterior uveitis, and cystoid macular edema. The systemic chemotherapy was stopped, because it was poorly tolerated. Prednisolone drops were prescribed for the left eye as treatment for anterior uveitis and macular edema.
In July 2015, the corrected Snellen visual acuity in the left eye was 20/40 with no evidence of uveitis and slight improvement of the macular edema.
In September 2015, the left eye had a visual acuity of 20/30+ with no uveitis or macular edema, but ophthalmoscopy showed subtle, widespread microflecks, which were hyperautofluorescent.
In November 2015, the right eye had visual acuity of counting fingers as before with no active lymphoma. The left eye had Snellen visual acuity of 20/30, sheathing of the left inferotemporal retinal arterioles, multiple hyperfluorescent active lesions on autofluorescence imaging, and tiny subretinal deposits temporal to the left fovea on optical coherence tomography (OCT) (Fig. 3c, d, e). The patient was advised to continue the prednisolone drops, which she had stopped taking.
In March 2016, the right eye was unchanged. The left eye had a visual acuity of 20/40. There were active hyperautofluorescent infiltrates and more extensive hypoautofluorescent RPE atrophic lesions (Fig. 3f, g).
In June 2016, the right eye was stable. The left eye had a visual acuity of 20/80+2 and more extensive atrophic RPE lesions. Treatment with systemic lenalidomide was started.
In September 2016, the left eye had Snellen visual acuity of 20/50 with further atrophic RPE lesions, especially temporally, and regression of the subretinal deposits on OCT.
In February 2017, the ocular status was unchanged in both eyes.
In May 2017, the right eye showed atrophic RPE scars that were most dense temporally. There were no active sub-RPE lymphomatous deposits (Fig. 2g–i). The left eye showed multiple, small areas of RPE atrophy that previously showed hyperautofluorescence, with relative sparing inferotemporally, where the arteriolar sheathing had occurred (Fig. 3h–j).
In February 2018, her ocular condition was unchanged and quiescent, with vision of counting fingers OD and 20/20 OS. There was no sign of CNS disease.
The vitreous infiltrates were initially moderate in both eyes (3+ cells on slit-lamp examination with obscuration of the retinal capillaries on photography) becoming minimal or absent after July 2014 in the left eye, when vitrectomy was performed, and after July 2015 in the right eye.
Discussion
This case is instructive because it (1) demonstrates the efficacy of systemic therapy, particularly that of lenalidomide, in suppressing vitreoretinal lymphoma; (2) provides circumstantial evidence that the lymphoma cells can reach the retina hematogenously, at least in some cases; (3) illustrates the features of RPE disease on fundus autofluorescence imaging; and (4) raises questions about the management of indolent vitreoretinal lymphoma.
Interestingly, at an early stage, the left eye showed diffuse RPE atrophy, evident as semi-transparency on color photography and hypoautofluorescence. This was absent inferotemporally, in the area where the retinal sheathing subsequently developed (Fig. 3i). This suggests that subclinical vasculopathy may have been present before the ophthalmic manifestations appeared.
In patients with lymphoma apparently confined to the retina and vitreous and no evident systemic disease, it is not known whether the disease originates intraocularly or whether it disseminates to the eyes and/or CNS from a subclinical source. The relative absence of sub-RPE lymphoma deposits in the area of arteriolar sheathing and occlusion provides circumstantial evidence that the lymphoma cells reach the retina via the retinal artery. This is in accordance with the hypothesis that the lymphoma cells originate in a neoplastic focus extraocularly, spreading hematogenously to the retina and CNS [8]. Vitreoretinal lymphoma has been reported after overt systemic lymphoma [1]. It is possible that the primary lymphoma is undetectable, as often happens with metastases to the eye. This hypothesis is in keeping with the observations that both eyes tend to be affected simultaneously and that eradication of the vitreoretinal disease by focal ocular treatment does not prevent subsequent CNS disease. Hematogenous spread to the eye, even if confirmed, would not preclude the possibility of tumor spread along the optic nerve from the brain to the retina in some patients.
We hypothesize that the lymphoma cells spread through the neurosensory retina and RPE until they reach Bruch's membrane, where they accumulate to form sub-RPE tumors. When such tumors become large enough, the cells furthest from the choriocapillaris undergo necrosis, probably as a result of ischemia. Similar migration of tumor cells through the RPE, with accumulation at Bruch's membrane, has been observed in eyes with retinoblastoma.
If vitreoretinal lymphoma is indeed secondary to extraocular disease, as suggested by the present case, this would indicate scope for further studies investigating systemic therapy, which in addition to alleviating ocular symptoms may also extend life. A multicenter study showed no evidence that systemic therapy prolongs survival in patients presenting with vitreoretinal lymphoma [6]; however, this situation may be improving thanks to therapeutic advances. Whether the systemic therapy has prevented overt CNS disease in our patient is uncertain. Longer follow-up is needed.
This case also demonstrates total resolution of sub-RPE lymphoma deposits with systemic therapy alone. The large tumors in the right eye at presentation showed a complete response to systemic chemotherapy (Fig. 2c, f). The disease in the left eye, which progressed only when the systemic therapy was discontinued, resolved with systemic lenalidomide. Regression of vitreoretinal lymphoma with lenalidomide monotherapy has been reported previously [9]. Lenalidomide has been used successfully to treat many lymphoma subtypes, including follicular lymphoma, marginal zone B-cell lymphoma, and mantle-cell lymphoma. The exact mechanism of action for lenalidomide in many contexts is still uncertain, but it is known to be an immunostimulant and may also play a role in disrupting tumor-stromal interactions that promote lymphoma within the microenvironments of the eye and CNS [9].
The attenuated course of this patient's disease is similar to that of several other patients who received systemic therapy at our institution [Damato et al., in preparation]; however, further studies are indicated. It would be worth investigating whether variation in the behavior of vitreoretinal lymphoma reflects therapy or diverse neoplastic subgroups of differing pathogenicity.
We now reserve focal therapy for dense lymphomatous vitreous infiltrates, which seem to be more resistant to systemic therapy [Damato et al., in preparation] as indeed is the case with vitreous seeding in retinoblastoma. We found that vitreous infiltrates diminish following diagnostic vitrectomy, and we have recently started evaluating therapeutic vitrectomy for eyes with dense vitreous infiltrates [10]. A recent case report documents resolution of vitreous infiltrates following vitrectomy [11]. Another case report suggests that vitrectomy may also induce regression of sub-RPE lymphomatous deposits [12]. We hypothesize that by removing the vitreal reservoir of proliferating lymphoma cells, vitrectomy reduces the rate of sub-RPE accumulation of lymphoma cells so that clearance mechanisms are not overwhelmed. Disappearance of sub-RPE deposits following intravitreal chemotherapy or ocular radiotherapy provides evidence for such clearance. There is scope for studies comparing therapeutic vitrectomy with ocular radiotherapy as well as intravitreal chemotherapy with methotrexate or melphalan [13].
The fundus autofluorescence imaging and OCT findings in this patient suggest that the focal atrophic RPE lesions sometimes develop only when the sub-RPE lymphomatous deposits regress, as indeed happens with Best vitelliform dystrophy and choroidal osteomas. Such RPE atrophy is not inevitable, as demonstrated by the RPE preservation in the right eye following regression of the large tumor deposits temporal to the macula.
Whereas vitreoretinal lymphomas are all high-grade neoplasms, uveal lymphomas are low-grade tumors, with an excellent prognosis, which is why the term “primary intraocular lymphoma” is discouraged [14].
If vitreoretinal lymphoma is indeed secondary to systemic/CNS lymphoma, it would seem inappropriate to describe the ocular disease as “primary.” This adjective implies, wrongly in our opinion, that the lymphoma originates in the eye and that focal ocular therapy may, therefore, prevent lymphoma in the brain and elsewhere, which seems not to be the case. It would seem preferable to refer to this subtype of ocular lymphoma simply as “vitreoretinal lymphoma.”
Our impression is that systemic therapy is most effective for sub-RPE lymphoma, whereas vitreous infiltrates are more resistant to such therapy, requiring radiotherapy or intravitreal chemotherapy, as indeed is the case with retinoblastoma. It is, therefore, useful to describe the severity of retinal, vitreal, and sub-RPE lymphomatous infiltration separately in each eye of every patient with vitreoretinal lymphoma.
In conclusion, this case provides food for thought regarding the pathology of vitreoretinal lymphoma. There is a need for further studies comparing systemic with focal ocular therapies, taking account of the distribution of lymphomatous infiltrates within the eye. We also propose the term “vitreoretinal lymphoma” for this condition, thereby avoiding the assumption that the disease is originating intraocularly.
Statement of Ethics
The patient has given consent for publication of this case.
Disclosure Statement
J.L. Rubenstein receives funding from Genetech and Celgene. This funding is not directly related to the submitted paper. The remaining authors have no financial interests to disclose. None of the authors have any conflicts of interest related to this article.
References
- 1.Araujo I, Coupland SE. Primary vitreoretinal lymphoma - a review. Asia Pac J Ophthalmol. 2017;6:283–289. doi: 10.22608/APO.2017150. [DOI] [PubMed] [Google Scholar]
- 2.Reichstein D. Primary vitreoretinal lymphoma: an update on pathogenesis, diagnosis and treatment. Curr Opin Ophthalmol. 2016;27:177–184. doi: 10.1097/ICU.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 3.Witmer MT. Primary vitreoretinal lymphoma: management of isolated ocular disease. Cancer Control. 2016;23:110–116. doi: 10.1177/107327481602300204. [DOI] [PubMed] [Google Scholar]
- 4.Teckie S, Yahalom J. Primary intraocular lymphoma: treatment outcomes with ocular radiation therapy alone. Leuk Lymphoma. 2014;55:795–801. doi: 10.3109/10428194.2013.819576. [DOI] [PubMed] [Google Scholar]
- 5.Frenkel S, Hendler K, Siegal T, Shalom E, Pe'er J. Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. Br J Ophthalmol. 2008;92:383–388. doi: 10.1136/bjo.2007.127928. [DOI] [PubMed] [Google Scholar]
- 6.Riemens A, Bromberg J, Touitou V, Sobolewska B, Missotten T, Baarsma S, Hoyng C, Cordero-Coma M, Tomkins-Netzer O, Rozalski A, Tugal-Tutkun I, Guex-Crosier Y, Los LI, Bollemeijer JG, Nolan A, Pawade J, Willermain F, Bodaghi B, ten Dam-van Loon N, Dick A, Zierhut M, Lightman S, Mackensen F, Moulin A, Erckens R, Wensing B, Ie Hoang P, Lokhorst H, Rothova A. Treatment strategies in primary vitreoretinal lymphoma: a 17-center European collaborative. JAMA Ophthalmol. 2015;133:191–197. doi: 10.1001/jamaophthalmol.2014.4755. [DOI] [PubMed] [Google Scholar]
- 7.Carnevale J, Rubenstein JL. The challenge of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2016;30:1293–1316. doi: 10.1016/j.hoc.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCann KJ, Ashton-Key M, Smith K, Stevenson FK, Ottensmeier CH. Primary central nervous system lymphoma: tumor-related clones exist in the blood and bone marrow with evidence for separate development. Blood. 2009;113:4677–4680. doi: 10.1182/blood-2008-09-179366. [DOI] [PubMed] [Google Scholar]
- 9.Rubenstein JL, Treseler PA, Stewart PJ. Regression of intraocular large B-cell lymphoma with lenalidomide monotherapy. J Clin Oncol. 2011;29:e595–e597. doi: 10.1200/JCO.2011.34.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bever GJ, Kim DJ, Afshar AR, Rubenstein JL, Damato BE. Therapeutic vitrectomy as an adjunct treatment to systemic chemotherapy for intraocular lymphoma. Retin Cases Brief Rep. 2017 doi: 10.1097/ICB.0000000000000668. DOI: 10.1097/ICB.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesh P, Gogia V, Khanduja S, Gupta S, Kumar L, Garg S. Therapeutic vitrectomy for vitreal recurrence of intraocular lymphoma resistant to intravitreal methotrexate post systemic chemotherapy. J Cancer Res Ther. 2015;11:668. doi: 10.4103/0973-1482.140824. [DOI] [PubMed] [Google Scholar]
- 12.Pakdel A, Mammo Z, Hollands H, Forooghian F. Regression of subretinal lymphoma after diagnostic vitrectomy. JAMA Ophthalmol. 2017;135:503–505. doi: 10.1001/jamaophthalmol.2017.0464. [DOI] [PubMed] [Google Scholar]
- 13.Shields CL, Sioufi K, Mashayekhi A, Shields JA. Intravitreal melphalan for treatment of primary vitreoretinal lymphoma: a new indication for an old drug. JAMA Ophthalmol. 2017;135:815–818. doi: 10.1001/jamaophthalmol.2017.1810. [DOI] [PubMed] [Google Scholar]
- 14.Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Exp Ophthalmol. 2008;36:564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]