Abstract
Background
Organ shortage is a growing problem, with a rising number of organs being harvested from extended criteria donors, and this trend will further continue to increase as organ donors are getting older and have more comorbidities. Since this fact is immutable, efforts have been made to reduce the extent of ischemia-reperfusion injury (IRI) as well as of direct and indirect harvest-related graft injury which affects all organs in a more or less distinct way.
Methods
In liver transplantation (LT), the activation of Kupffer cells during organ reperfusion, thus provoking microcirculatory disturbances, hypoxia, and endothelial cell injury, is one of the key mechanisms causing graft dysfunction. Multiple approaches have been taken in order to find efficient preconditioning methods by pharmacological pretreatment, controlled induction of ischemia, controlled denervation of donor organs, and reconditioning with machine perfusion to prevent IRI, whereas marginal organs (i.e. steatotic grafts) are especially vulnerable.
Results
The above-mentioned approaches have been pursued in experimental and clinical settings. At this time point, however, there is not yet enough clinical evidence available to recommend any particular drug pretreatment or any other intervention for organ preconditioning prior to transplantation.
Conclusion
The multifactorial pathophysiology in the setting of IRI in LT requires a multimodal therapeutic approach with the integration of pharmacological and technical means being applied to the donor, the organ per se, and the recipient. Currently, there is no consensus on standardized pretreatment of donor organs in order to improve the transplant outcome.
Keywords: Ischemia-reperfusion injury, Liver, Transplantation, Organ donor
Introduction
Liver transplantation (LT) has become an established approach for the management of end-stage liver diseases [1, 2, 3], with very good survival rates that have continuously improved with growing experience over time [4]. Whereas in the beginning of the LT era, technical aspects of LT and rejection were the main challenges, problems like the growing shortage of donor organs and early graft dysfunction after LT currently are in the focus. Initial graft function after LT is a major determinant of morbidity and mortality after LT. Ploeg et al. [5] distinguished between primary non-function (PNF) and primary poor function (PPF). The rate of PNF is low (about 2%) [6], but this is a severe condition after LT. The rate of PPF of transplanted livers is higher (>15% in the literature [7]), but these livers can recover. The development of PNF and PPF is dependent on many influencing factors [4, 8, 9]. Facing the growing shortage of donor organs, it is of upmost importance to focus on these various influencing factors and to develop new therapeutic concepts in order to ensure preservation of the function of each graft. Currently about 27,000 organs are transplanted in the United Network [10] each year, with 120,000 patients on the waiting list, and the average organ discard rate in this precious situation is 17.27% [11]. Donor organ quality will further decrease because donors are getting older, entailing more comorbidities, and there is an increasing number of grafts used from extended criteria donors (ECD), amounting to more than 60%. ECD organs can imply a suboptimal outcome after transplantation [4]. These extended criteria include donor factors as well as recipient and logistic factors [12], while no unique definition of ECD does exist. Generally, those organs fall into two risk categories, i.e. causing either poor graft function (PNF, PPF, more complication risk in the long term) or possibility of disease transmission (infections, malignancy). According to the Eurotransplant definition, the following criteria define an ECD liver: donor age > 65 years, intensive care unit stay with ventilation > 7 days, BMI > 30 kg/m2, liver steatosis > 40%, serum sodium > 165 U/l, AST > 90 U/l, serum bilirubin > 3 mg/dl. Apart from that, liver grafts can be either Non-DCD (donation after cardiac death) or DCD with extended warm ischemia times, whereas DCD organs are associated with more severe ischemia-reperfusion injury (IRI), PNF, PPF, and biliary ischemia [13]. The risk of disease transmission has to be outlined separately. Scores like the donor risk index (DRI) [14] and the balance of risk score have been developed to quantify the risk of graft dysfunction or failure in recipients of marginal liver grafts. However, all of these scores have their limitations [15], such as the missing consideration of liver steatosis when using the DRI.
Potentials for Increasing the Donor Pool for Liver Transplantation
In many studies to date, liver steatosis has been identified to be one of the most important risk factors for primary dysfunction of a liver graft [9, 16, 17, 18]. The following recommendations (grade of recommendation level 2b) according to the European Association for the Study of the Liver Clinical Practice Guidelines apply, with the classification of steatosis into a) mild (microsteatosis or 10–30% macrosteatosis), characterizing a donor organ as suitable for transplantation, b) moderate (30–60% macrosteatosis), permitting acceptable outcomes in selected donor-recipient risk combinations, and c) severe (more than 60% macrosteatosis), meaning an unacceptable risk of severe IRI, graft failure, biliary complications, and mortality. Liver grafts of the latter category should be discarded [4]. Discarding organs is troublesome in light of the growing organ shortage; thus, each and every potential for increasing the donor pool will be illuminated in this overview. Improving fatty livers by pretreatment could significantly increase the donor pool as obesity and fatty liver disease are both dramatically rising.
IRI based on ischemia with subsequent reperfusion occurs in all transplanted organs in a more or less severe way. Tissue injury by cold ischemia and subsequent warm reperfusion is a complex process. It is a combination of the injuries triggered by hypoxia and subsequent reoxygenation as well as by hypothermia and subsequent rewarming. In the beginning, organ injury by cold storage is an intracellular process. While some cells directly undergo apoptosis or necrosis even before reperfusion of the graft, complex IRI is triggered during the transplantation, which contributes to varying degrees to further damage of the graft. At least some key factors that trigger this injury have been identified, and the majority of insights has been obtained from cellular models [19]. Cold-induced apoptosis is triggered by an increased cellular chelatable, redox-active iron pool, which converts reactive oxygen species (ROS) of low reactivity into highly reactive species. This iron-dependent ROS generation with subsequent lipid peroxidation plays an important role in the injury to some cell types, such as cultured hepatocytes and liver endothelial cells, during cold incubation. In the later stages of organ injury, inflammatory reactions triggered by the early cell injury play a role, i.e., an acute inflammatory reaction alerts the immune system and contributes to chronic inflammatory processes, vasculopathy, graft dysfunction, and rejection. Studies have shown that structural alterations caused by cold ischemia like hepatocyte swelling [20, 21, 22] are of minor importance for the development of primary graft dysfunction, but the key mechanism is a Kupffer cell-dependent reperfusion injury with endothelial cell death. No predictive parameter does exist for IRI [23].
However, reasons for graft failure are very complex and involve donor factors and organ retrieval, organ preservation with cold and warm ischemic times, and the transplantation per se, encompassing the immune status of the recipient and surgical expertise of the transplant surgeon as well as possible surgical complications.
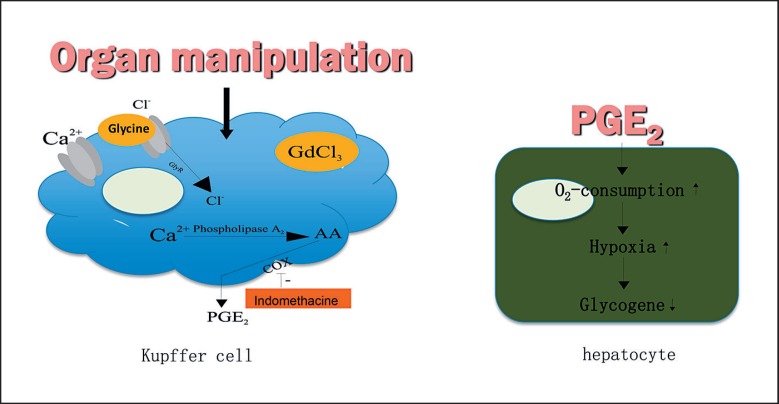
Direct and indirect harvest-related graft injury is another mechanism occurring in all grafts in a more or less pronounced form. Organ manipulation in the setting of the organ donation procedure can adversely affect liver function and microcirculatory processes [24, 25, 26]. It was demonstrated in an experimental model that in contrast to a non-manipulated control group, gentle in situ organ manipulation by gently touching, retracting, and moving liver lobes during harvest significantly reduces survival after LT via Kupffer cell-dependent mechanisms involving disturbances of hepatic microcirculation as well as provoking a hypermetabolic state of the liver, hypoxia, and almost complete denudation of endothelial lining cells [27]. It was shown that both the autonomic nervous system and the gut-derived endotoxin are involved in the activation of Kupffer cells via organ manipulation [28]. Kupffer cells from manipulated livers were shown to produce more tumor necrosis factor-alpha and prostaglandin E2 (PGE2) as well as an increase in hypoxia and of intracellular calcium concentration in response to higher levels of lipopolysaccharide (fig. 1). PGE2 depleted from Kupffer cells by organ manipulation can cause more oxygen consumption, hypoxia, and glycogene depletion in hepatocytes. Proinflammatory cytokines (TGFβ, IL(interleukin)-1, IL-6, IL-12), chemokines, and adhesion molecules are further upregulated and create a vicious circle of microcirculatory disturbances as oxidized proteins, heat shock proteins, ROS, fibrinogen, defensins, cathelicidin, high-mobility group protein B1, and heparan sulfate are released and can result in further tissue damage. Involvement of T-cell receptor contributes to the development of acute and chronic rejection.
Fig. 1.
Harvest-related graft injury: Kupffer cell-dependent reperfusion injury (modified from [34]).
Approaches for Preconditioning Organs in Order to Improve Transplant Outcome
Various approaches for donor preconditioning targeting the different mechanisms of IRI have been described in experimental models and also in clinical studies.
Experimental Studies
One possible method is pharmacological donor preconditioning. To date, numerous agents were described to favor outcome. A systemic meta-analysis on pharmacological donor preconditioning in experimental LT was performed by Yamanaka et al. [29]. In this review, a categorization of pharmacological agents with regard to IRI was performed in i) Kupffer cell inactivators, ii) complement inhibitors, iii) antioxidants, iv) neutrophil inactivators, v) anti-apoptosis agents, vi) heat shock protein and nuclear factor kappa B inducers, vii) metabolic agents, viii) traditional Chinese medicines, which were also investigated, and ix) adenosine agonists, nitric oxide agonists, endothelin agonists, and prostaglandins. These were administered to either the donor or the recipient, or to both donor and recipient in order to decrease the destructive effects of IRI such as Kupffer cell activation, inflammation, apoptosis, microcirculatory disturbances, accumulation of white blood cells, and oxidative stress. The authors found that Kupffer cell-inactivating agents may have a beneficial effect on short-term survival.
Modulation of Kupffer cells before organ harvesting can be targeted by application of gadolinium chloride and glycine that both were shown to prevent activation of Kupffer cells as well as adverse effects of organ manipulation in experimental models in rats [26, 30, 31]. A prospective randomized clinical trial on recipient preconditioning with glycine is currently ongoing [32]. Glycine and taurine preconditioning in steatotic rat livers were shown to be equally effective in the prevention of IRI, most likely via Kupffer cell-dependent mechanisms by decreasing interactions between leukocytes and platelets with endothelial cells and phagocytosis [33].
Indomethacin given prior to organ harvesting was shown to prevent hypermetabolism, hypoxia, glycogen depletion, and PGE2 release from Kupffer cells [34].
It was shown that the early cell injury by iron-dependent ROS generation during ischemia/reperfusion with subsequent lipid peroxidation can strongly be inhibited by both hypoxia and a number of antioxidants. The cold-induced increase of cytosolic iron as the main trigger of this cold-induced injury was addressed by the addition of iron chelators in modified organ preservation solutions [19].
A second category of therapeutic approaches is the induction of ischemia, which is further described in the ‘Clinical Studies’ section below.
A third approach was described by Schemmer et al. [35], consisting of denervation of the manipulated graft prior to harvesting. In a rat LT model, it could be shown that organ manipulation during organ harvesting disturbs the hepatic microcirculation and oxygen supply of the liver by innervation-dependent mechanisms. Denervation of the liver prior to harvesting or treatment with hexamethonium was shown to be able to prevent these adverse innervation-dependent effects (hypoxia, impaired microcirculation) caused by organ manipulation.
A fourth category of efforts influencing organ quality prior to transplantation is reconditioning with machine perfusion (MP). Ex vivo MP is one possibility to expand the donor pool by allowing the use of organs that otherwise would have been discarded. MP can not only provide additional diagnostic information but can also improve organ quality by means of therapeutic interventions during the preservation period [36]. The method chosen is dependent on the target organ. The main potential of MP is not only simple resuscitation of donor organs but also the possibility to administer organ-specific therapies with the avoidance of systemic side effects, which is in contrast to all previously described direct donor and recipient preconditioning methods. In LT, efforts were reported in experimental settings to inactivate miRNA-122 utilizing normothermic MP (NMP) in order to prevent hepatitis C virus (HCV) infection after transplantation of HCV-positive organs [37], whereas these are of minor importance since the advent of the new, highly effective anti-HCV treatment options.
The issue of size matching in LT was also addressed by simulating in vivo liver resection for size reduction during application of NMP, showing better post-transplant outcomes in comparison to resection with simple cold storage (SCS) [38]. In order to assess the ability of a small liver to grow and regenerate, hepatocyte replication and biliary epithelial cell regeneration were also reported during NMP [39, 40]. Steatotic livers with their extraordinary sensitivity to IRI seem to improve on hypothermic MP and oxygenated subnormothermic MP in comparison with SCS. First results of treatment of steatotic livers with diverse defatting cocktails while on MP are promising [41, 42].
Clinical Studies
A systematic review on deceased-donor treatment in humans versus placebo or no treatment was performed recently.
Methylprednisolone treatment in liver donors (two studies, 183 participants [43, 44]) showed no effect on acute rejection rates within the first 6 months after LT. Antidiuretic hormone treatment in kidney donors (two studies, 222 participants [45, 46]) showed no benefit in the prevention of delayed graft function. The effect of ischemic preconditioning for 10 min on outcomes after LT was analyzed in 7 studies with a total of 334 participants [47, 48, 49, 50, 51, 52, 53], and showed improved short-term liver function by enhancement of AST and INR levels within the first days post-transplantation but had no effect on long-term transplant outcomes.
According to a recent meta-analysis, currently there is not enough evidence for any particular drug treatment or any intervention in deceased donors that can improve long-term graft or patient survival after transplantation in the clinical setting [54]. The antioxidative treatment approach with the aim of scavenging ROS, which increase during brain death and IRI, was also addressed in the clinical setting; however, they could not show an improvement of survival in LT and kidney transplantation [55, 56, 57, 58], though short-term liver function was shown to be improved by the application of ascorbic acid and donor ventilation with sevoflurane. Sevoflurane was also reported by Minou et al. [58] to be protective in steatotic livers. Currently, a number of ongoing trials are investigating the effect of remote ischemic preconditioning with repetitively administered cycles of ischemia to donor organs [59, 60]. Further ongoing studies are investigating the potential benefits of preconditioning donor organs with melatonin, immunosuppressive agents, levothyroxine, simvastatin, sevoflurane, and also intensive insulin treatment, just to mention some of the current officially registered clinical trials.
At this time point, however, there is not yet enough clinical evidence for the recommendation of any particular pretreatment of donor organs (evidence-based medicine max. level 3).
Conclusion
Preconditioning should be performed at the earliest time point possible. The multifactorial pathophysiology in the setting of IRI in transplantation medicine requires a multimodal approach. Pharmacological intervention may be promising, and to date numerous approaches have been described in experimental as well as in clinical settings of LT. Facing the multimodal mechanisms responsible for graft injury prior, during and after transplantation, the most preferable approach is the integration of both pharmacological and technical preconditioning techniques as applied to the donor, the donor organ, and the organ recipient, i.e. pharmacological conditioning in combination with MP. Currently, there is no consensus on the standardized pretreatment of donor organs in order to improve the transplant outcome.
Disclosure Statement
The authors have no conflicts of interest.
References
- 1.Pichlmayer R, Ringe B, Lauchart W, Wonigeit K. Liver transplantation. Transplant Proc. 1987;19:103–112. [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, van Thiel D. Liver transplantation (1) N Engl J Med. 1989;321:1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Demetris AJ, van Thiel D. Liver transplantation (2) N Engl J Med. 1989;321:1092–1099. doi: 10.1056/NEJM198910193211606. [DOI] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver EASL Clinical Practice Guidelines: liver transplantation. J Hepatol. 2016;64:433–485. doi: 10.1016/j.jhep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation - a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Uemura T, Randall HB, Sanchez EQ, Ikegami T, Narasimhan G, McKenna GJ, Chinnakotla S, Levy MF, Goldstein RM, Klintmalm GB. Liver retransplantation for primary nonfunction: analysis of a 20-year single-center experience. Liver Transpl. 2007;13:227–233. doi: 10.1002/lt.20992. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Swain SK, Addala PK, Balasubramaniam R, Gopakumar CV, Zirpe D, Renganathan K, Kollu H, Patel D, Vibhute BB, Rao PS, Krishnan E, Gopasetty M, Khakhar AK, Vaidya A, Ramamurthy A. Initial poor function and primary nonfunction in deceased-donor orthotopic liver transplantation maintaining short cold ischemic time. Prog Transplant. 2016;26:340–347. doi: 10.1177/1526924816663516. [DOI] [PubMed] [Google Scholar]
- 8.Clavien PA, Harvey PRC, Strasberg SM. Preservation and perfusion injury in liver allografts. Transplantation. 1992;53:957–978. doi: 10.1097/00007890-199205000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tullius SG, Volk HD, Neuhaus P. Transplantation of organs from marginal donors. Transplantation. 2001;72:1341–1349. doi: 10.1097/00007890-200110270-00001. [DOI] [PubMed] [Google Scholar]
- 10. https://optn.transplant.hrsa.gv/ (last access 09.08.2018)
- 11. www.aopo.org/ (last access 09.08.2018)
- 12.Nemes B, Gaman G, Polak WG, Gelley F, Hara T, Ono S, Baimakhanov Z, Piros L, Eguchi S. Extended-criteria donors in liver transplantation. Part II: reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Exp Rev Gastroenterol Hepatol. 2016;10:841–859. doi: 10.1586/17474124.2016.1149062. [DOI] [PubMed] [Google Scholar]
- 13.Muesian P, Girlanda R, Jassem W, et al. Single-center experience with liver transplantation from controlled non-heartbeating donors: a viable source of grafts. Ann Surg. 2005;242:732–738. doi: 10.1097/01.sla.0000186177.26112.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blok JJ, Brat AE, Adam R, et al. Validation of donor risk index in orthotopic liver transplantation within the Eurotransplant region. Liver Transpl. 2012;18:112–119. doi: 10.1002/lt.22447. [DOI] [PubMed] [Google Scholar]
- 15.Reichert B, Kaltenborn A, Goldis A, Schrem H. Prognostic limitations of the Eurotransplant-donor risk index in liver transplantation. J Negat Results Biomed. 2013;12:18. doi: 10.1186/1477-5751-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koneru B, Dikdan G. Hepatic steatosis and liver transplantation - current clinical and experimental perspectives. Transplantation. 2002;73:325–330. doi: 10.1097/00007890-200202150-00001. [DOI] [PubMed] [Google Scholar]
- 17.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Sem Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 18.Yoong K, Gunson B, Neil D, et al. Impact of donor liver microvesicular steatosis on the outcome of liver retransplantation. Transplant Proc. 1999;31:550–551. doi: 10.1016/s0041-1345(98)01550-4. [DOI] [PubMed] [Google Scholar]
- 19.Rauen U, de Groot H. New insights into the cellular and molecular mechanisms of cold storage injury. J Invest Med. 2004;52:299–309. doi: 10.1136/jim-52-05-29. [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Bentley RC, Madden JF, Clavien PA. Apoptosis of sinusoidal endothelial cells is a critical mechanism of preservation injury in rat liver transplantation. Hepatology. 1998;27:1652–1660. doi: 10.1002/hep.510270626. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 22.Marzi I, Cowper KB, Takei Y, Lindert KA, Lemasters JJ, Thurman RG. Methyl palmitate prevents Kupffer cell activation and improves survival after orthotopic liver transplantation in the rat. Transpl Int. 1991;4:215–220. doi: 10.1007/BF00649106. [DOI] [PubMed] [Google Scholar]
- 23.Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–338. doi: 10.1146/annurev.pharmtox.37.1.327. [DOI] [PubMed] [Google Scholar]
- 24.Schemmer P, Schoonhoven R, Swenberg JA, Bunzendahl H, Raleigh JA, Lemastres JJ, Thurman RG. Gentle organ manipulation during harvest as a key determinant of survival of fatty livers after transplantation in the rat. Transpl Int. 1999;12:351–359. doi: 10.1007/s001470050239. [DOI] [PubMed] [Google Scholar]
- 25.Schemmer P, Bradford BU, Bunzendahl H, Lemasters JJ, Thurman RG. Gentle in situ liver manipulation during organ harvest increases oxygen consumption in liver. Transplant Proc. 2000;32:112. doi: 10.1016/s0041-1345(99)00902-1. [DOI] [PubMed] [Google Scholar]
- 26.Schemmer P, Schoonhoven R, Swenberg JA, Bunzendahl H, Thurman RG. Gentle in situ liver manipulation during organ harvest decreases survival after rat liver transplantation: role of Kupffer cells. Transplantation. 1998;65:1015–1020. doi: 10.1097/00007890-199804270-00001. [DOI] [PubMed] [Google Scholar]
- 27.Schemmer P, Mehrabi A, Kraus T, Sauer P, Gutt C, Uhl W, Büchler MW. New aspects on reperfusion injury to liver - impact of organ harvest. Nehrol Dial Transplant. 2004;19:26–35. doi: 10.1093/ndt/gfh1038. [DOI] [PubMed] [Google Scholar]
- 28.Schemmer P, Enomoto N, Bradford BU, Bunzendahl H, Raleigh JA, Thurman RG. Autonomic nervous system and gut-derived endotoxin: involvement in activation of Kupffer cells after in situ organ manipulation. World J Surg. 2001;25:399–406. doi: 10.1007/s002680020070. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka K, Houben P, Bruns H, Schultze D, Hatano E, Schemmer P. A systematic review of pharmacological treatment options used to reduce ischemia reperfusion injury in rat liver transplantation. PLoS One. 2015;10:e0122214. doi: 10.1371/journal.pone.0122214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schemmer P, Golling M, Kraus T, Herfarth C, Klar E. Glycine reduces reperfusion injury in human liver transplantation: our first patients. Transplant Proc. 2001;33:3750–3752. doi: 10.1016/s0041-1345(01)02588-x. [DOI] [PubMed] [Google Scholar]
- 31.Schemmer P, Golling M, Kraus T, Mehrabi A, Mayatepek E, Herfarth C, Klar E. Extended experience with glycine for prevention of reperfusion injury after human liver transplantation. Transplant Proc. 2002;34:2307–2309. doi: 10.1016/s0041-1345(02)03247-5. [DOI] [PubMed] [Google Scholar]
- 32.Luntz SP, Unnebrink K, Seibert-Grafe M, Bunzendahl H, Kraus TW, Büchler MW, Klar E, Schemmer P. HEGPOL: randomized, placebo controlled, multicenter, double-blind clinical trial to investigate hepatoprotective effects of glycine in the postoperative phase of liver transplantation [ISRCTN69350312] BMC Surg. 2005;5:18. doi: 10.1186/1471-2482-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruns H, Watanpour I, Gebhard MM, Flechtenmacher C, Galli U, Schulze-Bergkamen H, Zorn M, Büchler MW, Schemmer P. Glycine and taurine equally prevent fatty livers from Kupffer cell-dependent injury: an in vivo microscopy study. Microcirculation. 2011;18:205–213. doi: 10.1111/j.1549-8719.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 34.Schemmer P, Enomoto N, Bradford BU, Bunzendahl H, Raleigh JA, Lemasters JJ, Thurman RG. Activated Kupffer cells cause a hypermetabolic state after gentle in situ manipulation of liver in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1076–1082. doi: 10.1152/ajpgi.2001.280.6.G1076. [DOI] [PubMed] [Google Scholar]
- 35.Schemmer P, Bunzendahl H, Raleigh JA, Thurman RG. Graft survival is improved by hepatic denervation before organ harvesting. Transplantation. 1999;67:1301–1307. doi: 10.1097/00007890-199905270-00002. [DOI] [PubMed] [Google Scholar]
- 36.Karimian N, Yeh H. Opportunities for therapeutic intervention during machine perfusion. Curr Transplant Rep. 2017;4:141–148. doi: 10.1007/s40472-017-0144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldaracena N, Spetzler VN, Echeverri J, et al. Inducing hepatitis C virus resistance after pig liver transplantation - ‘A proof of concept of liver graft modification using warm ex vivo perfusion’. Am J Transplant. 2017;17:970–978. doi: 10.1111/ajt.14100. [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZB, Gao W, Shi Y, et al. Protective role of normothermic machine perfusion during reduced-size liver transplantation in pigs. Liver Transpl. 2016;22:968–978. doi: 10.1002/lt.24453. [DOI] [PubMed] [Google Scholar]
- 39.Dong J, Xia L, Shen H, et al. Growing a whole porcine liver organ ex situ for six hours without red blood cells or hemoglobin. Am J Transl Res. 2016;8:2562–2574. [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Q, Nassar A, Farias K, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014;20:987–999. doi: 10.1002/lt.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nativ NI, Yarmush G, So A, et al. Elevated sensitivity of macrosteatotic hepatocytes to hypoxia/reoxygenation stress is reversed by a novel defatting protocol. Liver Transpl. 2014;20:1000–1011. doi: 10.1002/lt.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q, Berendsen T, Izamis ML, Uygun B, Yarmush ML, Uygun K. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant Proc. 2013;45:3209–3213. doi: 10.1016/j.transproceed.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amatschek S, Wilfingseder J, Pones M, et al. The effect of steroid pretreatment of deceased organ donors on liver allograft function: a blinded randomized placebo-controlled trial. J Hepatol. 2012;56:1305–1309. doi: 10.1016/j.jhep.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kotsch K, Ulrich F, Reutzel-Selke A, et al. Methylprednisolone therapy in deceased donors reduces inflammation in the donor liver and improves outcome after liver transplantation. Ann Surg. 2008;248:1042–1050. doi: 10.1097/SLA.0b013e318190e70c. [DOI] [PubMed] [Google Scholar]
- 45.Pennefather SH, Bullock RE, Mantle D, Dark JH. Use of low dose arginine vasopressin to support brain-dead organ donors. Transplantation. 1995;59:58–62. doi: 10.1097/00007890-199501150-00011. [DOI] [PubMed] [Google Scholar]
- 46.Guesde R, Barrou B, Leblanc I, et al. Administration of desmopressin in brain-dead donors and renal function in kidney recipients. Lancet. 1998;352:1187–1181. doi: 10.1016/S0140-6736(98)05456-7. [DOI] [PubMed] [Google Scholar]
- 47.Koneru B, Shareef A, Dikdan G, et al. The ischemic preconditioning paradox in deceased donor liver transplantation - evidence from a prospective randomized single blind clinical trial. Am J Transplant. 2007;7:2788–2796. doi: 10.1111/j.1600-6143.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- 48.Cescon M, Grazi GL, Grassi A, et al. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transpl. 2006;12:628–635. doi: 10.1002/lt.20640. [DOI] [PubMed] [Google Scholar]
- 49.Cescon M, Carini R, Grazi G, et al. Variable activation of phosphoinositide 3-kinase influences the response of liver grafts to ischemic preconditioning. J Hepatol. 2009;50:937–947. doi: 10.1016/j.jhep.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 50.Amador A, Grande L, Marti J, et al. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant. 2007;7:2180–2189. doi: 10.1111/j.1600-6143.2007.01914.x. [DOI] [PubMed] [Google Scholar]
- 51.Jassem W, Fuggle S, Thompson R, et al. Effect of ischemic preconditioning on the genomic response to reperfusion injury in deceased donor liver transplantation. Liver Transpl. 2009;15:1750–1765. doi: 10.1002/lt.21936. [DOI] [PubMed] [Google Scholar]
- 52.Franchello A, Gilbo N, David E, et al. Ischemic preconditioning (IP) of the liver as a safe and protective technique against ischemia/reperfusion injury (IRI) Am J Transplant. 2009;9:1629–1639. doi: 10.1111/j.1600-6143.2009.02680.x. [DOI] [PubMed] [Google Scholar]
- 53.Zapata-Chavira HA, Cordero-Perez P, Casillas-Ramirez A, et al. Is ischemic preconditioning a useful therapeutic strategy in liver transplantation? Results from the first pilot study in Mexico. Arch Med Res. 2015;46:296–302. doi: 10.1016/j.arcmed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 54.van Erp AC, van Dullemen LFA, Ploeg RJ, Leuvenink HGD. Systematic review on the treatment of deceased organ donors. Transplant Rev (Orlando) 2018;32:194–206. doi: 10.1016/j.trre.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Barros MAP, Vasconcelos PRL, Souza CM, et al. L-alanyl-glutamine attenuates oxidative stress in liver transplantation patients. Transplant Proc. 2015;47:2478–2482. doi: 10.1016/j.transproceed.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Kazemi M, Tabei SMB, Najafizadeh K, et al. Evaluation of the effect of ascorbic acid administration on gene expression level of IL-6 and TNF-α cytokines in deceased donors. Iran J Allergy Asthma Immunol. 2015;14:149–157. [PubMed] [Google Scholar]
- 57.Orban J-C, Quintard H, Cassuto E, Jambou P, Samat-Long C, Ichai C. Effect of N-acetylcysteine pretreatment of deceases organ donors on renal allograft function: a randomized controlled trial. Transplantation. 2015;99:746–753. doi: 10.1097/TP.0000000000000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minou AF, Dzyadzko AM, Shcherba AE, Rummo OO. The influence of pharmacological preconditioning with sevoflurane on incidence of early allograft dysfunction in liver transplant recipients. Anaesthesiol Res Pract. 2012;2012:930487. doi: 10.1155/2012/930487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koneru B. Phase III study of efficacy of remote ischemic preconditioning in improving outcomes in organ transplantation (RIPCOT) NCT00975702. https://clinicaltrials.gov/ct2/show/NCT00975702. [Google Scholar]
- 60.Koneru B, Washburn WK. Remote ischemic preconditioning in neurological death organ donors (RIPNOD) NCT01515072. https://clinicaltrials.gov/ct2/show/NCT01515072. [Google Scholar]