Abstract
Background and Aims
Acute liver failure (ALF) is a rare disease with potentially high mortality rates. We aimed to study the recent epidemiology of ALF in one of the Portuguese liver transplant (LT) regions.
Methods
We assessed a retrospective cohort including 34 consecutive patients with ALF admitted to the intensive care unit (ICU) of Curry Cabral Hospital (Lisbon, Portugal) between October 2013 and December 2016.
Results
The median age (IQR) was 49 (31–67) years, and 21 (62%) of the cohort were female. Non-paracetamol etiologies were found in 29 patients (85%). On ICU admission, grade 3–4 hepatic encephalopathy developed in 10 patients (29%); invasive mechanical ventilation, vasopressors, and renal replacement therapy were required for 8 (24%), 7 (21%), and 5 (15%) of the patients, respectively; the King's College criteria (KCC) were fulfilled by 16 patients (47%). Of the 15 (44%) nontransplanted patients, 11 (73%) died during their hospital stay. Of the 19 (56%) transplanted patients, 4 (21%) died during their hospital stay. KCC were not associated with hospital mortality (p = 0.97), but they were significantly associated with LT (p = 0.008).
Conclusions
In a Portuguese cohort of patients with ALF, non-paracetamol etiologies were predominant. Hospital mortality was much lower amongst transplanted patients.
Key Words: Liver failure, Etiology, Transplantation
Resumo
Introdução:
A falência hepática aguda (ALF) é uma doença rara com potenciais altas taxas de mortalidade. Pretendeu-se estudar a epidemiologia recente da ALF numa das regiões portuguesas de transplante hepático (LT).
Mate- riais e Métodos
Coorte retrospectivo incluindo 34 doentes com ALF consecutivamente admitidos na Unidade de Cuidados Intensivos (ICU) do Hospital Curry Cabral (Lisboa, Portugal) entre Outubro de 2013 e Dezembro de 2016.
Resultados
A idade mediana foi 49 (31–67) anos e 21 (62%) doentes eram do sexo feminino. As etiologias não-paracetamol ocorreram em 29 (85%) doentes. Na admissão na ICU, a encefalopatia hepática grau 3–4 desenvolveu-se em 10 (29%) doentes; a ventilação mecânica invasiva, os vasopressores e a técnica de substituição da função renal foram usados em 8 (24%), 7 (21%) e 5 (15%) doentes, respectivamente; os critérios King's College Hospital (KCC) foram cumpridos por 16 (47%) doentes. Entre os 15 (44%) doentes não transplantados, 11 (73%) morreram durante a estadia hospitalar. Entre os 19 (56%) doentes transplantados, 4 (21%) morreram durante a estadia hospitalar. Os KCC não se associaram com a mortalidade hospitalar (p = 0.97), mas associaram-se significativamente com o LT (p = 0.008).
Conclusões
Num coorte de doentes com ALF português, as etiologias nãoparacetamol predominaram. A mortalidade hospitalar foi significativamente menor nos doentes transplantados.
Palavras Chave: Falência hepática, Etiologia, Transplantação
Introduction
Acute liver failure (ALF) is a rare life-threatening disease characterized by recent deterioration of liver function associated with coagulopathy and hepatic encephalopathy (HE) without evidence of cirrhosis [1, 2]. In the absence of HE, this type of liver dysfunction is often defined as acute liver injury (ALI).
The incidence of ALF in Europe has been reported as fewer than 10 cases per million people per year in developed countries [1]. Based on the European Liver Transplant Registry, ALF has been the primary indication in 8% of all liver transplant (LT) procedures undergone between 1988 and 2009: 21% of them were due to viral infection, 21% due to drug-induced liver injury (11% by paracetamol and 10% by other drugs), and 58% due to other causes or unknown etiology [2].
ALF etiologies vary worldwide: while in Eastern developing countries, viruses (mainly hepatitis A, B, or E) may account for up to 95% of all ALF cases, the Western developed countries have reported more heterogeneous causes of ALF [3, 4]. In the UK and North America, paracetamol overdosage has been the leading cause of ALF [5, 6]. In parts of Europe (e.g., Germany and Portugal), the most common causes of ALF have been non-paracetamol drug-induced liver injury, seronegative (indeterminate) liver injury, and viruses (mostly hepatitis B) [7, 8].
ALF still portends high mortality rates, but these have been decreasing over time owing to improvements in critical care management and in emergent LT. Overall hospital survival increased from 17% in 1973–1978 to 62% in 2004–2008 at a single center in the UK [3]. In a multicenter registry from the USA, in 1998–2010 ALF 2-year survival has been reported as 92% for LT recipients, 90% for paracetamol-related spontaneous survivors, and 76% for non-paracetamol-related spontaneous survivors [9].
Taking into account the variations in ALF epidemiology with geography worldwide, we sought to study the current ALF epidemiology in Lisbon, one of the LT regions in Portugal.
Subjects and Methods
Design, Setting, and Participants
This was a retrospective cohort study including all consecutive adult patients (≥18 years) diagnosed with ALF admitted to a specialized intensive care unit (ICU) at Curry Cabral Hospital (Lisbon, Portugal) between October 2013 and December 2016. We started patient inclusion at this time point because the quality of the granular data recorded from then on was much better than before.
The inclusion criteria were the following: age ≥18 years, ALF, and first admission to the ICU in the follow-up period (last date available before June 2016). The exclusion criteria were the following: alcoholic hepatitis or previous LT.
Given the observational character of the study, the local ethics committee waived the need for individual informed consent. The principles of the Declaration of Helsinki were respected throughout the study development [10].
Definitions, Data Collection, and Endpoints
ALF was defined as: onset of liver dysfunction < 26 weeks with an INR ≥1.5 and any degree of HE (West Haven criteria) or NH3 ≥100 µmol/L in the absence of cirrhosis. ALI was defined as: onset of liver dysfunction < 26 weeks with an INR ≥1.5, no HE (West Haven criteria), and NH3 < 100 µmol/L in the absence of cirrhosis. Given that the West Haven criteria are often difficult to apply, especially to subclinical HE, we decided to use the arterial NH3 level (at least 2 times the normal level) to help define ALF-related HE [11].
The selection criteria for LT for patients with ALF at our center have been based on the King's College criteria (KCC) [12]: for paracetamol-related ALF, (1) the single criterion of a serum pH < 7.30 or lactate > 3.0 mmol/L or (2) three of the following criteria: grade III–IV HE, an INR > 6.5, and serum creatinine > 3.4 mg/dL; for non-paracetamol-related ALF, (1) the single criterion of an INR > 6.5 or (2) three of the following criteria: age < 10 or > 40 years, time from jaundice to coma > 7 days, an INR > 3.5, serum bilirubin > 17 mg/dL, and/or an unfavorable etiology (drug-induced liver injury, Wilson disease, or seronegative disease).
Data on the patients' characteristics were retrieved by the four investigators from a computerized database concerning the ICU stay. The following data were collected on ICU admission: demographics (age and sex); etiology; arterial blood gas (pH, bicarbonate, and lactate) and arterial laboratory (INR, factor V, alanine transferase, bilirubin, ammonia, creatinine, phosphorus, and lactate) results; grade of HE (West Haven criteria); requirement for organ support (invasive mechanical ventilation [IMV], vasopressors, renal replacement therapy [RRT]); and fulfilment of the KCC.
The primary outcome was all-cause hospital mortality. The secondary outcomes were all-cause 90-day mortality following ICU admission, LT during hospital stay, and ICU and hospital length of stay.
Statistical Analysis
Baseline characteristics are described as number (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. There were 4.8% missing values across variables, thus no imputation was done.
Associations of baseline characteristics with the primary endpoint were assessed using univariate logistic regression. The discriminative ability of the KCC for LT was studied using the AUC (area under the receiver operating curve).
Statistical significance was set at p < 0.05. The statistical analysis was performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline Characteristics and Outcomes
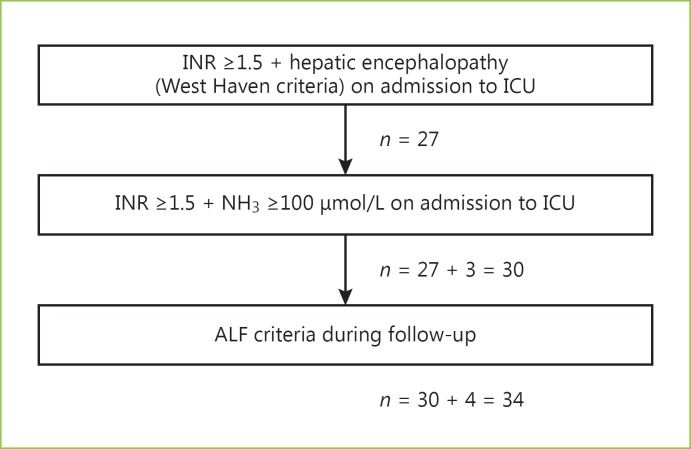
During the ICU stay, 34 patients were diagnosed with ALF (30 on ICU admission) and 15 patients had ALI (Fig. 1).
Fig. 1.
Selection of patients with acute liver failure (ALF) admitted to the intensive care unit (ICU).
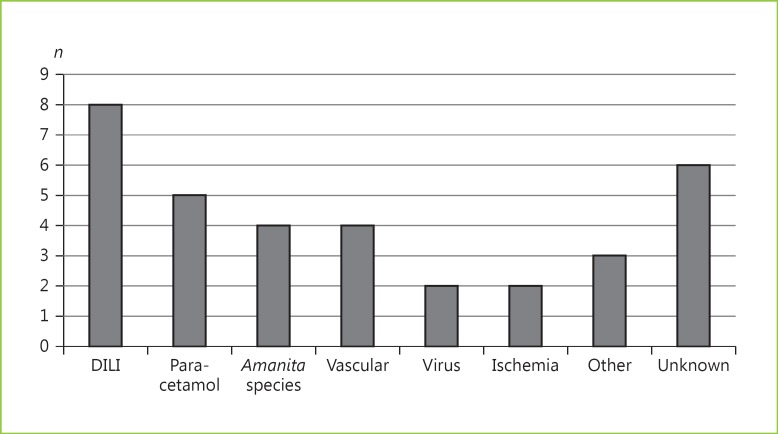
Amongst all patients with ALF (n = 34), the median age (IQR) was 49 (31–67) years, and 21 (62%) were females. Non-paracetamol etiologies were found in 29 patients (85%). The ALF etiologies are specified in Figure 2.
Fig. 2.
Etiologies of acute liver failure amongst patients admitted to the intensive care unit (n = 34). DILI, drug-induced liver injury.
On ICU admission, grade 3–4 HE developed in 10 patients (29%), and the median arterial ammonia level was 152 (93–197) μmol/L. At the same time point, the median arterial INR and bilirubin, creatinine, bicarbonate, and lactate levels were 3.0 (2.2–6.3), 9.8 (3.4–19.7) mg/dL, 1.4 (0.6–2.0) mg/dL, 20 (16–24) mmol/L, and 3.3 (1.9–8.0) mmol/L, respectively. Also on ICU admission, IMV, vasopressors, and RRT were required in 8 (24%), 7 (21%), and 5 patients (15%), respectively. At the same time point, the KCC were fulfilled by 16 patients (47%). All patients' characteristics on ICU admission are shown in Table 1.
Table 1.
Baseline characteristics of the patients with acute liver failure admitted to the intensive care unit (n = 34)
| Age, years | 49 (31–67)1 |
| Female sex | 21 (62%) |
| Non-paracetamol etiology | 29 (85%) |
| Grade 3–4 hepatic encephalopathy | 10 (29%) |
| Ammonia, µmol/L | 152 (93–197) |
| INR | 3.0 (2.2–6.3) |
| Factor V, % | 22 (14–46) |
| Bilirubin, mg/dL | 9.8 (3.4–19.7) |
| ALT, U/L | 1,957 (341–5,884) |
| Invasive mechanical ventilation | 8 (24%) |
| PF ratio, mm Hg | 340 (288–433) |
| Vasopressor support | 7 (21%) |
| Mean arterial pressure, mm Hg | 77 (66–88) |
| Renal replacement therapy | 5 (15%) |
| Creatinine, mg/dL (n = 33) | 1.4 (0.6–2.0)2 |
| pH (n = 31) | 7.40 (7.33–7.47) |
| Bicarbonate, mmol/L (n = 31) | 20 (16–24)3 |
| Potassium, mmol/L | 4.1 (3.8–4.7) |
| Phosphorus, mg/dL (n = 31) | 3.2 (2.6–4.7) |
| Lactate, mmol/L (n = 31) | 3.3 (1.9–8.0) |
| KCC | 16 (47%) |
Values are presented as n (%) or median (interquartile range). ALT, alanine aminotransferase; PF ratio, oxygen arterial pressure/oxygen inspiration fraction; KCC, King's College criteria; OR, odds ratio; 95% CI, 95% confidence interval.
OR (95% CI) for hospital mortality: 1.04 (1.00–1.09); p = 0.039.
OR (95% CI) for hospital mortality: 3.36 (1.14–9.92); p = 0.028.
OR (95% CI) for hospital mortality: 0.80 (0.67–0.85); p = 0.012.
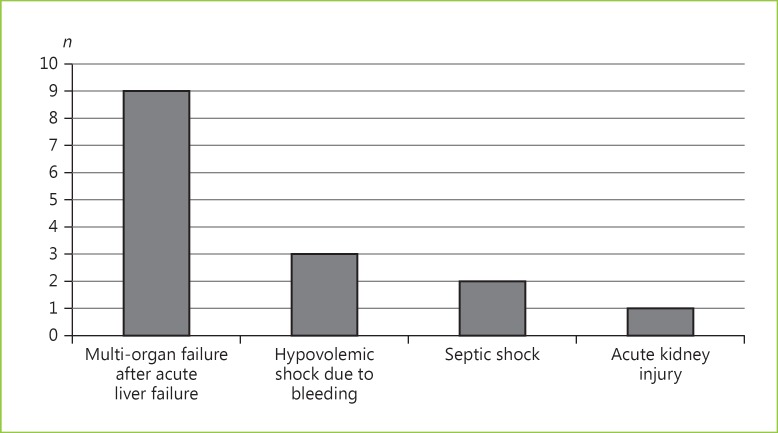
Of all patients with ALF (n = 34), 15 (44%) died during their hospital stay (11 without LT). Following exclusion of 3 foreign patients lost to follow-up (n = 31), 15 (48%) died within 90 days following ICU admission. During their hospital stay, 19 patients (56%) underwent transplantation. LT was significantly associated with lower hospital mortality (21 vs. 73%; p = 0.005). The outcomes and causes of death are detailed in Table 2 and Figure 3, respectively.
Table 2.
Outcomes of the patients with acute liver failure admitted to the intensive care unit (n = 34)
| Intensive care unit stay, days | 3 (6–11) |
| Hospital stay, days | 24 (10–36) |
| Liver transplant during hospital stay | 19 (56%) |
| Time to liver transplant, days | 3 (1–6) |
| Mortality during hospital stay | 15 (44%) |
| Mortality within 90 days1 | 15 (48%) |
| Time to death, days | 4 (2–7) |
| Follow-up1, days | 229 (4–405) |
Values are presented as n (%) or median (interquartile range).
Excluding 3 cases lost to follow-up after hospital discharge (n = 31).
Fig. 3.
Causes of death amongst patients with acute liver failure admitted to the intensive care unit (n = 34).
Of all the baseline characteristics, age (unadjusted odds ratio [uOR] = 1.04 per year increment), creatinine (uOR = 3.36 per unit increment), and bicarbonate (uOR = 0.80 per unit increment) were significantly associated with hospital mortality (Table 1).
Patients Undergoing Transplantation
Amongst the patients with ALF who underwent LT during their hospital stay (n = 19), the median age (IQR) was 44 (32–57) years, and 12 (63%) were female. Non-paracetamol etiologies were found in 18 patients (95%).
On ICU admission, grade 3–4 HE developed in 5 patients (26%), and the median arterial ammonia level was 157 (77–224) μmol/L. At the same time point, the median arterial INR, bilirubin, creatinine, bicarbonate, and lactate levels were 3.4 (2.4–7.0), 15.2 (3.8–21.0) mg/dL, 0.7 (0.5–1.5) mg/dL, 23 (19–25) mmol/L, and 2.8 (1.8–6.8) mmol/L, respectively. Also on ICU admission, IMV, vasopressors, and RRT were required in 3 (15%), 2 (11%), and 2 patients (11%), respectively. At the same time point, the KCC were fulfilled by 13 patients (68%). All transplanted patients' characteristics on ICU admission are shown in Table 3.
Table 3.
Baseline characteristics of the patients with acute liver failure admitted to the intensive care unit who underwent liver transplantation (n = 19)
| Age, years | 44 (32–57) |
| Female sex | 12 (63%) |
| Non-paracetamol etiology | 18 (95%) |
| Grade 3–4 hepatic encephalopathy | 5 (26%) |
| Ammonia, µmol/L | 157 (77–224) |
| INR | 3.4 (2.4–7.0) |
| Factor V, % | 38 (9–50) |
| Bilirubin, mg/dL | 15.2 (3.8–21.0) |
| ALT, U/L | 1,904 (768–5,868) |
| Invasive mechanical ventilation | 3 (15%) |
| PF ratio, mm Hg | 393 (295–433) |
| Vasopressor support | 2 (11%) |
| Mean arterial pressure, mm Hg | 78 (67–87) |
| Renal replacement therapy | 2 (11%) |
| Creatinine, mg/dL (n = 18) | 0.7 (0.5–1.5) |
| pH (n = 16) | 7.42 (7.37–7.48) |
| Bicarbonate, mmol/L (n = 16) | 23 (19–25) |
| Potassium, mmol/L | 4.1 (3.8–4.3) |
| Phosphorus, mg/dL (n = 18) | 2.9 (2.6–4.1) |
| Lactate, mmol/L (n = 16) | 2.8 (1.8–6.8) |
| KCC | 13 (68%) |
Values are presented as n (%) or median (interquartile range). ALT, alanine transferase; PF ratio, oxygen arterial pressure/oxygen inspiration fraction; KCC, King's College criteria.
Of the patients fulfilling the KCC on ICU admission (n = 16), 13 (81%) effectively underwent transplantation during their hospital stay; the 3 remaining patients died during their hospital stay. While the KCC were not associated with hospital mortality (p = 0.97), they were significantly associated with LT during the hospital stay (p = 0.008) (sensitivity 68%, specificity 80%, negative predictive value 67%, and positive predictive value 81%; AUC [95% confidence interval] = 0.74 [0.57–0.91]).
Discussion
Key Findings
In Lisbon, Portugal, the ICU at the local LT center admitted about 10 patients per year with ALF between October 2013 and December 2016. Non-paracetamol etiologies represented 85% of all the ALF cases.
The all-cause mortality rates during the hospital stay and 90 days following ICU admission were 44% (73% without LT and 21% following LT) and 48%, respectively. LT was performed on 56% of the patients.
Older age, renal dysfunction (creatinine), and metabolic imbalance (bicarbonate) on ICU admission were significantly associated with worse hospital mortality rate. The KCC were not associated with hospital mortality, but they were significantly associated with the decision for LT during the hospital stay.
Comparison with the Previous Literature
The vast majority of ALF cases in our cohort (85%) were not paracetamol related. This finding is in agreement with some reports on ALF epidemiology from continental Europe. In a multicenter study from Germany (2008–2009), non-paracetamol etiologies accounted for 91% of all ALF cases [7]. Conversely, in the UK, paracetamol-related ALF may represent about 65% of all cases [3]. This issue has largely to do with cultural differences regarding the public use of paracetamol; in the UK this has been associated with suicide attempts [13]. In Portugal, this phenomenon does not have the same relevance in terms of public health.
The all-cause mortality rate during the hospital stay in our cohort was 73% without LT and 21% following LT. In the German cohort mentioned above [7], the all-cause mortality rate during the hospital stay was 35% without LT and 20% following LT. While it has been established that patients with non-paracetamol-related ALF have a worse prognosis than those with paracetamol overdosage, both our and the German cohort predominantly had non-paracetamol etiologies; therefore, they likely included more of the subgroup of patients with ALF at the highest risk of death. Nevertheless, the mortality amongst the transplant-free patients in our cohort (73%) was greater than in the German cohort (35%). This difference may to some extent be related to the fact that the following ALF severity markers were more frequently worse in our cohort than in the German one: median age (49 vs. 46 years), INR (3.0 vs. 2.5), creatinine (1.4 vs. 1.0 mg/dL) and lactate (3.3 vs. 3.0 mmol/L) levels, and the proportion of patients fulfilling the KCC on ICU admission (47 vs. 37%) [1]. However, we need to improve the diagnostic and treatment strategies for patients with non-paracetamol-related ALF without LT. Such steps may include the following aspects: earlier referral to the local LT center, N-acetylcysteine use especially for low-grade HE patients, more aggressive organ support in the ICU, and frequent sepsis screening and timely antimicrobial treatment [1].
In our cohort, 56% of the patients with ALF underwent transplantation. This rate is similar to the one found in a Spanish multicenter cohort (56%) [14]. The posttransplant mortality rates were similar in our (21%) and the German (20%) cohort. The selection criteria for LT in this context seem to have affected patient outcomes similarly in these cohorts. Nevertheless, use of the KCC did not show any association with hospital mortality in our cohort. In fact, the KCC have been shown to lack good sensitivity (58%) for predicting hospital mortality from non-paracetamol-related ALF [15, 16]. Furthermore, while these patients benefit more from LT than do patients with paracetamol overdosage, the likelihood of death is much higher if an organ is not timely available or if there are contraindications to LT (e.g., ongoing sepsis, psychiatric disorder, and malignancy).
Limitations
This study has to be considered in the context of the following limitations: (1) its retrospective character carries the risk of selection bias and (2) the low number of patients included may influence the internal and external validity of our findings.
To mitigate the risk of selection bias, we selected consecutive patients with ALF based on specific criteria accepted worldwide. Given that ALF is a rare disease, the number of patients included in a country such as Portugal may in fact be reasonable. To enforce the internal validity of our findings, we used a data set with a low level of missing data (< 5%).
In order to further study the epidemiology of ALF in Portugal, we should aim to combine data from the three LT regions in the country. For that, a fully functioning prospective registry would be of great interest. This kind of measure would surely help to improve the quality of care provided to these patients nationwide.
Conclusions
In a southern Portuguese cohort of patients with ALF, non-paracetamol etiologies were predominant. Hospital mortality was much lower amongst LT patients.
Statement of Ethics
Given the observational character of the study, the local ethics committee waived the need for individual informed consent. The principles of the Declaration of Helsinki were respected throughout study development.
Disclosure Statement
The authors have no conflicts of interest to report.
Author Contributions
C.S., S.M., and M.V. helped to collect data and draft the manuscript. F.S.C. developed the idea, helped to collect data, performed the statistical analysis, and finalized the manuscript.
Acknowledgments
Thanks are due to the Intensive Care Unit and Liver Transplant Program personnel at Curry Cabral Hospital for their collaboration.
References
- 1.Cardoso FS, Marcelino P, Bagulho L, Karvellas CJ. Acute liver failure: an up-to-date approach. J Crit Care. 2017;39:25–30. doi: 10.1016/j.jcrc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Germani G, Theocharidou E, Adam R, Karam V, Wendon J, O'Grady J, et al. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012;57:288–296. doi: 10.1016/j.jhep.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, et al. Lessons from look-back in acute liver failure? A single centre experience of 3,300 patients. J Hepatol. 2013;59:74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Acharya SK, Batra Y, Hazari S, Choudhury V, Panda SK, Dattagupta S. Etiopathogenesis of acute hepatic failure: Eastern versus Western countries. J Gastroenterol Hepatol. 2002;17((suppl 3)):S268–S273. doi: 10.1046/j.1440-1746.17.s3.12.x. [DOI] [PubMed] [Google Scholar]
- 5.Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 6.Gulmez SE, Larrey D, Pageaux GP, Bernuau J, Bissoli F, Horsmans Y, et al. Liver transplant associated with paracetamol overdose: results from the seven-country SALT study. Br J Clin Pharmacol. 2015;80:599–606. doi: 10.1111/bcp.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadem J, Tacke F, Bruns T, Langgartner J, Strnad P, Denk GU, et al. Etiologies and outcomes of acute liver failure in Germany. Clin Gastroenterol Hepatol. 2012;10:664–669. doi: 10.1016/j.cgh.2012.02.016. e2. [DOI] [PubMed] [Google Scholar]
- 8.Areia M, Romãozinho JM, Ferreira M, Amaro P, Leitão MC. Fulminant hepatic failure: a Portuguese experience. Eur J Gastroenterol Hepatol. 2007;19:665–669. doi: 10.1097/MEG.0b013e3281ac20da. [DOI] [PubMed] [Google Scholar]
- 9.Fontana RJ, Ellerbe C, Durkalski VE, Rangnekar A, Reddy RK, Stravitz T, et al. Two-year outcomes in initial survivors with acute liver failure: results from a prospective, multicentre study. Liver Int. 2015;35:370–380. doi: 10.1111/liv.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 11.Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844–1852. doi: 10.1002/hep.21838. [DOI] [PubMed] [Google Scholar]
- 12.O'Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663–670. doi: 10.1016/j.jhep.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Hawton K, Bergen H, Simkin S, Dodd S, Pocock P, Bernal W, et al. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ. 2013;346:f403. doi: 10.1136/bmj.f403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escorsell A, Mas A, de la Mata M; Spanish Group for the Study of Acute Liver Failure. Acute liver failure in Spain: analysis of 267 cases. Liver Transpl. 2007;13:1389–1395. doi: 10.1002/lt.21119. [DOI] [PubMed] [Google Scholar]
- 15.McPhail MJ, Farne H, Senvar N, Wendon JA, Bernal W. Ability of King's College criteria and model for end-stage liver disease scores to predict mortality of patients with acute liver failure: a meta-analysis. Clin Gastroenterol Hepatol. 2016;14:516–525. doi: 10.1016/j.cgh.2015.10.007. e5; quiz e43–e45. [DOI] [PubMed] [Google Scholar]
- 16.Craig DGN, Ford AC, Hayes PC, Simpson KJ. Systematic review: prognostic tests of paracetamol-induced acute liver failure. Aliment Pharmacol Ther. 2010;31:1064–1076. doi: 10.1111/j.1365-2036.2010.04279.x. [DOI] [PubMed] [Google Scholar]