Abstract
Background
Chronic neutrophilic leukemia (CNL) is an extremely rare myeloproliferative neoplasm (MPN). Due to the difficulty in its diagnosis, the diagnostic criterion was just recently revised in 2016. CNL is defined as: A clonal disorder with sustained primary neutrophilia, with normal neutrophil maturation, that does not meet other MPN criteria, as well as no identifiable mutations of the PDGFRA, PDGFRB or FGFR1 or PCM1-JAK2 genes, and, either, the presence of a CSF3R mutation, or if absent, the presence of sustained neutrophilia (> 3 months), splenomegaly and no other identifiable cause of reactive neutrophilia including the absence of a plasma cell neoplasm, or, if present, demonstration of myeloid cell clonality by cytogenetics. Only about 200 cases have been reported.
Case Presentation
We report a 61-year-old Caucasian male patient who initially presented with unexplained leukocytosis. An outpatient work-up was planned to rule out a myeloproliferative disorder but the patient was acutely admitted for MRSA septic shock. The patient was stabilized prior bone marrow work-up and was then diagnosed with an atypical type of CNL (JAK2 positive, CSF3R negative). The patient refused further treatment due to social circumstances and requested palliative care instead.
Conclusion
This case aims to present atypical findings of an extremely rare MPN. Even though a recent revision has been made to help in its diagnosis, atypical findings must still be considered. This, in turn, will help to further improve the current CNL diagnostic criteria.
Keywords: Chronic neutrophilic leukemia, Atypical, JAK2 positive
Introduction
Chronic neutrophilic leukemia (CNL) is an extremely rare MPN with an unknown true incidence to date [9]. About 200 cases have been reported, with a possible lower true incidence if the present World Health Organization (WHO) diagnostic criteria are taken into consideration [1, 6, 13]. CNL is characterized by the recently revised WHO criteria with its main points being the following: First, the presence of a proliferation of mature neutrophils with leukocytosis of > 25×109/L (> 80% segmented neutrophils plus bands), < 10% immature granulocytes and rarely observed blasts with no identified dysgranulopoiesis in the peripheral blood. Second, bone marrow findings show hypercellularity with normal granulocytic maturation. Third, the presentation does not meet criteria for other BCR-ABL negative MPNs. Fourth, there are no identified rearrangements of PDGFRA, PDGFRB or FGFR1 or PCM1-JAK2 protein lines. Finally, the presence of a mutation in the CSF3R line is observed, or if absent, the presence of sustained neutrophilia (at least 3 months), splenomegaly and no other identifiable cause of reactive neutrophilia including the absence of a plasma cell neoplasm, or, if present, the demonstration of myeloid cell clonality by cytogenetics [1, 13] (Table 1). The identification of the presence of the CSF3R line mutation which has been highly linked to CNL, based on landmark studies, served as a first strong biomolecular marker that allowed for the elucidation of CNL from other BCR-ABL negative MPNs resulting to the updated WHO criteria from its 2008 counterpart [4, 6, 12].
Table 1.
WHO revised diagnostic criteria for chronic neutrophilic leukemia
| 1 | Peripheral blood white blood cell count ≥25×109/L Segmented neutrophils plus banded neutrophils constitute ≥80% of WBCs Neutrophils precursors (promyelocytes, myelocytes, and metamyelocytes) constitute <10% of the WBCs Myeloblasts rarely observed Monocyte count <1×109/L No dysgranulopoeisis |
| 2 | Hypercellular bone marrow Neutrophil granulocytes increased in percentage and number Neutrophil maturation appears normal Myeloblasts constitute <5% of the nucleated cells |
| 3 | Not meeting WHO criteria for BCR-ABL1-positive chronic myeloid leukemia, polycythemia vera, essential thrombocythaemia, or primary myelofibrosis |
| 4 | No rearrangement of PDGFRA, PDGFRB, or FGFR1, and no PCM1-JAK2 fusion |
| 5 | CSF3R T618I or another activating CSF3R mutation Or Persistent neutrophilia (≥3 months), splenomegaly, and no identifiable cause of reactive neutrophilia including absence of a plasma cell neoplasm or, if a plasma cell neoplasm is present, demonstration off clonality of myeloid cells by cytogenetics or molecular studies |
In terms of its epidemiology, besides it being extremely rare, with an unknown true incidence, CNL generally presents in people above the age of 50, with a median age of 66.5 years at the time of diagnosis [2, 5].
Clinical History
A 61-year-old Caucasian male patient was referred to our oncology clinic due to episodes of syncope, night sweats, non-productive cough and a 20 lb weight loss for about 4 months. He had diffused cachectic features on exam as well as hepatosplenomegaly. The patient's presenting complete blood count (CBC) showed the following: white blood cell (WBC) count of 36.18 × 103/μL, hemoglobin of 8.2 g/dL and platelets of 117 × 103/μL.
His peripheral smear revealed normocytic and normochromic red blood cells and his WBCs were found to be increased with absolute neutrophilia, monocytosis, eosinophilia and basophilia. About 3% circulating blasts were noted. The neutrophils had normal morphology and the lymphocytes were small and mature. The platelets were slightly decreased in number but of normal morphology.
The manual differential count showed 65% segmented neutrophils, 10% bands, 4% lymphocytes, 2% monocytes, 3% eosinophils, 6% basophils, 6% myelocytes, 1% promyelocytes and 3% blasts. The absolute neutrophilic count (ANC) was at 27.50/μL and basophilic strippling, polychromasia (2+) and schistocytes (1+) were noted. The lactate dehydrogenase (LDH) was elevated at 2,025 U/L (reference range: 300–600 U/L). RT-PCR analysis was done and this did not detect any BCR/ABL translocation fusion. A flow cytometry also only identified 3% blasts with no monoclonal B-cell or abnormal T cell populations. A CT abdomen and pelvis was then ordered which showed splenomegaly (26cm) and hepatomegaly (22cm) with no focal lesions.
The patient was to return to clinic for a discussion of the results as above, but about 1 month after his visit, the patient was admitted to the ICU and had to be intubated secondary to MRSA septic shock with acute hypoxic, hypercapnia respiratory failure. CBC on admission showed leukocytosis, anemia and thrombocytopenia: WBC of 19.45 × 103/μL, hemoglobin of 8.2 g/dL and platelets of 74 × 103/μL. The automated differential count showed an elevation of segmented neutrophils and an ANC of 15.06/μL with basophilic stippling, 77% segmented neutrophils, 3.6% lymphocytes, 1.9% monocytes, 0.2% basophils. The patient was immediately started on fluids, broad spectrum antibiotics and pressors. No evidence of endocarditis was identified. After a few weeks, all repeat blood cultures came back negative and the patient became afebrile and improved in terms of his mentation.
However, even after the patient was stabilized, it was noted that his WBC count remained very elevated with his LDH constantly remaining in the 1,000–2,000 s U/L even when his entire hemolysis work-up was negative. The patient's CBC still showed a WBC count of 34.71 × 103/μL. The manual differential count at this time was as follows: 60% segmented neutrophils, 22% bands, 4% lymphocytes (with 0 atypical and reactive lymphocytes), 7% monocytes, 1% eosinophils, 1% basophils, 4% metamyelocytes and 1% myelocytes. The red cells were decreased in number but still showed normocytic and normochromic morphology (Fig. 1, 2). There was still neutrophilia noted, and these neutrophils were mainly bands and segmented, which altogether consisted of more than 80% of the WBC differential (Fig. 2). These neutrophils also showed very minimal dysplastic changes (Fig 1, 2). Even though there was slight left-shift noted, there was no increase in blasts. In addition, no abnormal lymphoid cells were seen. No evident monocytosis or basophilia were noted as well. Platelets were also decreased, but had normal morphology (Fig. 1).
Fig. 1.
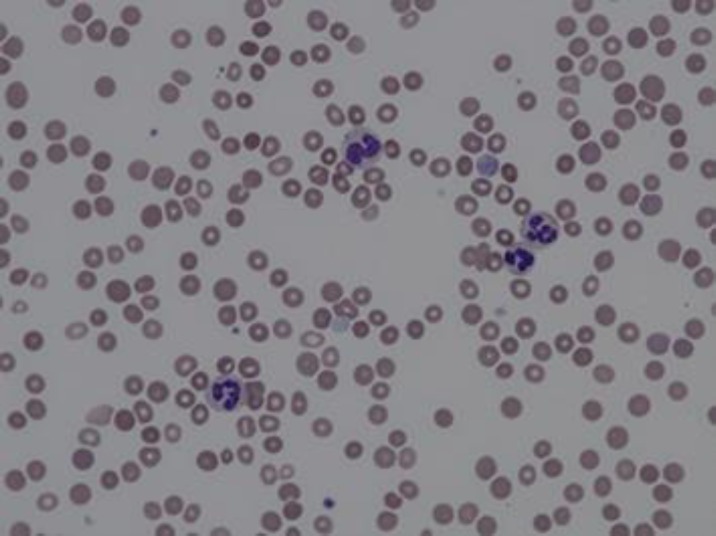
Peripheral blood 50× - Normocytic normochromic red cells that are decreased in number with moderate anisocytosis with target and burr cells, and basophilic stippling. Minimal dysplastic changes are noted on neutrophils. Platelets are decreased in number but with normal morphology.
Fig. 2.
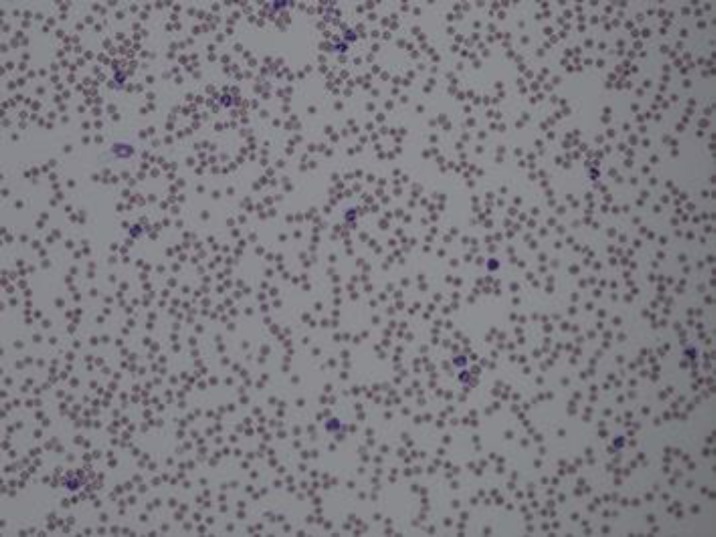
Peripheral blood 20× - Neutrophilia noted and most neutrophils were segmented and bands that altogether consisted of more than 80% of the white blood cell differential.
Bone marrow studies were undertaken again, and the aspirate manual differential showed 68% segmented neutrophils, 7% bands, 6% lymphocytes, 3% eosinophils, 6% metamyelocytes, 1% myelocytes, and 0% blasts, plasma and mast cells. Markedly increased granulopoiesis was noted and the granulocytes morphology showed complete maturation with insignificant dysgranulopoeisis. The megakaryocytes were also markedly decreased, and the lymphocytes were small and mature. Plasma cells were also minimal and blasts were also not increased (Fig. 3).
Fig. 3.
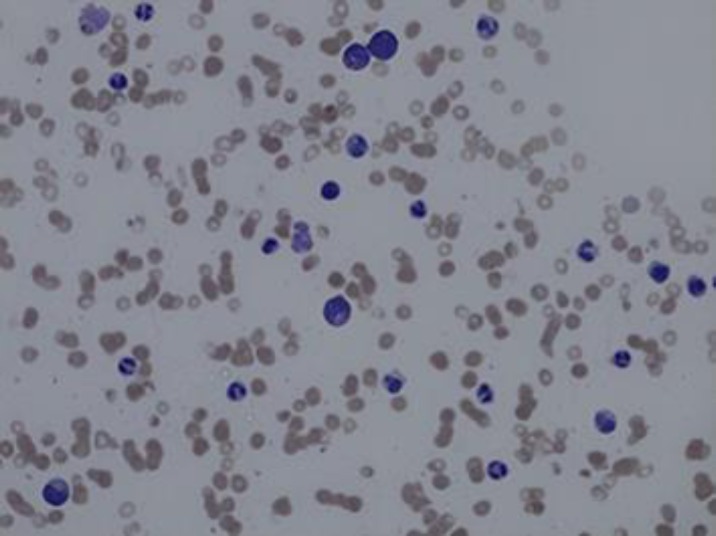
Bone marrow aspirate 40× - Markedly decreased erythropoiesis, increased granulopoiesis with complete maturation, insignificant dysgranulopoiesis are noted along with markedly decreased megakaryocytes, small and mature lymphocytes and rare plasma cells. Blasts are not increased on review.
The bone marrow biopsy was also found to be very hypercellular for age (cell to fat ratio about 90: 10) showing remarkable myeloid hyperplasia. In addition, the myeloid showed mainly mature forms of segmented and band form neutrophils (Fig. 4). There were no increased blasts, the erythroid islands and lymphocytes were also decreased. The megakaryoctyes had dysplastic changes including hypolobulated forms and hyperchromatic nuclei identified by PAS and CD61 staining but no abnormal proliferation was noted. No granuloma was also noted and the bony trabeculae were not thickened. Reticulin staining showed 2+ reticulin fibrosis though.
Fig. 4.
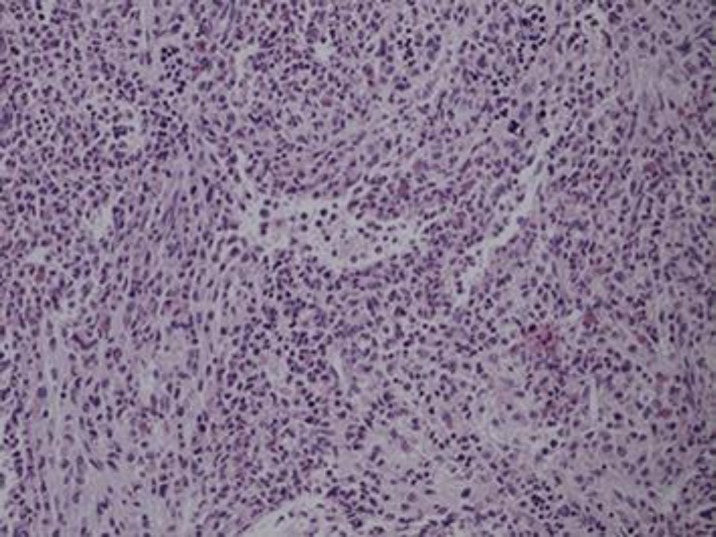
Bone marrow biopsy, hematoxylin and eosin stain, 20× - The bone marrow biopsy was found to be hypercellular for age (cell to fat ratio about 90: 10) showing remarkable myeloid hyperplasia while the myeloid hyperplasia while the myeloid cells were mainly mature forms including segmented and band form neutrophils. +2 mild reticulin fibrosis also identified per staining.
Flow cytometry analysis of the bone marrow revealed no detectable monoclonal B cells, and the T cells were all normal. No increase in blasts was noted. The bone marrow smear showed mostly neutrophils and a few myeloid precursors with no increased blasts or large atypical lymphoid cells.
Immunophenotyping detected a small population of lymphocytes and these were mostly T cells accounting for ∼2.3% of total cells that have no over-all T-cell antigen deletion or aberrant antigen expression identified. The CD4/CD8 ratio was about 4: 1. A very small population of B cells (0.1% of total cells) was identified but this was without demonstrable monoclonality. There was also no immunophenotypic evidence of non-Hodgkin lymphoproliferative disorder/lymphoma or acute leukemia.
Bone marrow pathology studies showed a 50.8% variant frequency of JAK2 c. 1849G>T, p.val617Phe along with a 20% variant frequency of SRSF2 c.284_307del, p.Pro95_Arg102del. No rearrangements were identified on the PDGFRA, PDGFRB or FGFR1 genes. No CSF3R T618I or any other mutations of the CSF3R gene was identified. No Philadelphia (Ph1), translocation or BCR-ABL fusion by Fluorescence in situ Hybridization (FISH) and chromosomal studies were identified.
Due to the over-all presentation of the patient's blood work which was suggestive of a myeloproliferative disorder (before and after septic episode), along with a bone marrow aspirate and biopsy analysis as detailed above (before and after septic episode [when the patient was clinical stabilized on an extended course of antibiotics]), the patient was diagnosed with CNL which presented atypically, due to the absence of a mutation in the CSF3R protein line and the presence of a rearrangement in the JAK2 gene [1, 13] (Table 1).
Unfortunately, the patient did not qualify for bone marrow transplant or experimental therapies such as the use of Ruxolitinib, a JAK1/2 kinase inhibitor that has been explored to treat CNL and CML, due to social circumstances [7, 8]. Cytoreductive therapies such as oral hydroxyurea which has been shown to demonstrate some efficacy, though transient [7, 8, 11] was offered but patient, after discussion about his prognosis and any associated adverse effects and benefits preferred hospice and comfort measures instead.
Discussion
Our patient was diagnosed with CNL mainly because of how our patient presented to us with B symptoms and the finding of mature neutrophilic leukocytosis in very high counts (WBC ≥25 × 109/L with about ∼75% segmented and banded mature neutrophils identified by his primary provider about 4 months prior to his arrival at our clinic. On born marrow and peripheral blood studies, these neutrophils had very minimal dysplastic changes on morphology with no identifiable mutations in the of the PDGFRA, PDGFRB or FGFR1 genes. Hepatosplenomegaly from his follow up imaging was also noted which is common in CNL. During this time, prior to his septic presentation, no other cause of reactive neutrophilia was also identified.
Interestingly, his CNL was labeled atypical due to the following: First, our patient's cytogenetics did not yield a CSF3R line mutation which is a diagnostic components of the revised WHO CNL diagnostic criteria [1, 13] (Table 1). The CSF3R line mutation has been identified as a strong molecular marker for CNL and studies showed up to 90% of CNL patients were carriers versus 40% in those who were diagnosed with aCML [10]. However, the updated criteria also considered those patients without a rearrangement in this particular gene- if certain conditions can be satisfied, like what was seen with our patient, that is, how he has had this neutrophilia for more than 3 months, even before his septic presentation.
Second, another consideration of why our patient has an atypical presentation of CNL is the positive finding of a mutation in his JAK2 gene. A variant in the JAK2 gene is typically under the umbrella of BCR-ABL negative MPNs such as polycythemia vera (PV) and essential thrombocythemia (ET). According to the WHO, the presence of a JAK2 variant is considered as part of PV's and ET's major criteria [1, 13] and its absence is considered a key criteria for CNL and aCML [1, 13]. However, based on our patient's blood work, polycythemia vera and essential thrombocythemia as diagnosis were very less likely due to how our patient did not have an abnormally elevated hemoglobin and hematocrit or even a platelet count in the category of true thrombocythemia, respectively [1, 13]. JAK2 mutations are also not found in < 5% of PV and in about 40–50% of ET but have been identified in about 1–5% of CNL patients [3]. In addition, based on findings in our patient's bone marrow aspirate, the erythrocytes were noted to be reduced as well, unlike what is typically found in PV where erythroid proliferation is typically more noted (Fig. 3).
During our discussions about our patient's final diagnosis, aCML was also in the top of our differentials due to our patient's cytogenetics not having a CSF3R mutation, a negative BCR-ABL mutation, along with the presence of neutrophilic leukocytosis in high counts. However, our patient's blood and bone marrow showed insignificant dysgranulopoiesis with minimal left-shift (Fig. 1, 2, 3) which argues against aCML [1, 8, 12, 13]. In addition, about 60% of patients diagnosed with aCML have been found to have a negative CSF3R rearrangement per recent studies [11].
Other differentials besides CNL that we considered, is primary myelofibrosis (primary MF) and pre-fibrotic primary myelofibrosis (pre-PMF) due to the presence of 2+ reticulin fibrosis on reticulin staining found in his bone marrow studies. However, the marrow did not have sufficient megakaryocytic hyperplasia and proliferation to support this diagnosis (Fig. 3, 4). In addition, reticulin staining of 1+ and 2+ showing fibrosis in bone marrow were common in late stages of MPNs, including CNL [14].
In terms of the patient's septic presentation and the need to exclude reactive neutrophilia, when the patient first presented to us, there was no evidence of active infection per labs or presentation. Blood work during that time already showed significant neutrophilia and a negative BCR-ABL fusion gene; hepatosplenomegaly was already noted per imaging as well [1, 13]. In addition, the patient was already having symptoms for about ∼4 months before presenting to our oncology clinic. Although he did have a septic episode afterwards which could have confounded this case presentation; thus, causing expected neutrophilia during this time, the patient was treated and stabilized prior his bone marrow biopsy scheduled weeks after. In addition, manual differential count during that time noted no atypical or reactive lymphocytes. The septic episode could have occurred due to his immunosuppressed state from his myeloproliferative disorder.
Conclusion
In summary, we believe our patient to more likely have a diagnosis of an atypical type of CNL due to the absence of a CSF3R line mutation and the presence of a positive JAK2 line variant, 50.8% frequency, in his cytogenetics. His over-all presentation based on his initial and post septic episode findings, all argued for a CNL type of presentation.
CNL is already an extremely rare type of myeloproliferative neoplasm. In this report, we present a patient who has CNL with an atypical presentation due to the absence of a CSF3R line mutation and the presence of a positive JAK2 line mutation which typically is not rearranged in this type of neoplasm. Per literature, it has already been noted that about 10% of CNL patients may not have a CSF3R mutation and about 1–5% may have a JAK2 variant [3]. We hope that this case finding can help other clinicians and researchers in further elucidating CNL from other types of BCR-ABL negative neoplasms. Although its diagnostic criteria has just been recently revised, we hope that findings from our patient further adds to the knowledge of other possible (more atypical) presentations of this extremely rare disorder.
Statement of Ethics
Ethics approval and consent to participate: Not applicable.
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Disclosure Statement
The authors declare that they have no competing interests.
Funding Sources
Not applicable.
Author Contributions
VM: primary author; YQ: reviewer, figures provided; BJ: Reviewer.
Acknowledgements
Rohit Venkatesan, MD for helping manage/care for patient. Our patient, who we are thankful to for giving his permission to write this case report.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood Volume. 127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Böhm J, Schaefer HE. Chronic neutrophilic leukaemia: 14 new cases of an uncommon myeloproliferative disease. J Clin Pathol. 2002 Nov;55((11)):862–4. doi: 10.1136/jcp.55.11.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cashen A, Van Tine B. Hematology and Oncology: Subspecialty Consult. 4th Ed. 2016. Myeloproliferative Neoplasms: Polycythemia Vera. (Chapter 9) [Google Scholar]
- 4.Elliott MA. Chronic neutrophilic leukemia: a contemporary review. Curr Hematol Rep. 2004 May;3((3)):210–7. [PubMed] [Google Scholar]
- 5.Elliott MA. Chronic neutrophilic leukemia and chronic myelomonocytic leukemia: WHO defined. Best Pract Res Clin Haematol. 2006;19((3)):571–93. doi: 10.1016/j.beha.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Elliott MA, Hanson CA, Dewald GW, Smoley SA, Lasho TL, Tefferi A. WHO-defined chronic neutrophilic leukemia: a long-term analysis of 12 cases and a critical review of the literature. Leukemia. 2005 Feb;19((2)):313–7. doi: 10.1038/sj.leu.2403562. [DOI] [PubMed] [Google Scholar]
- 7.Elliott MA, Pardanani A, Hanson CA, Lasho TL, Finke CM, Belachew AA, et al. ASXL1 mutations are frequent and prognostically detrimental in CSF3R-mutated chronic neutrophilic leukemia. Am J Hematol. 2015 Jul;90((7)):653–6. doi: 10.1002/ajh.24031. [DOI] [PubMed] [Google Scholar]
- 8.Elliott M, Tefferi A. ACME Information: Chronic Neutrophilic Leukemia 2016: Update on diagnosis, molecular genetics, prognosis. Am J Hematol. 2016 Mar;91((3)):341–349. doi: 10.1002/ajh.24284. [DOI] [PubMed] [Google Scholar]
- 9.Gotlib J, Maxson JE, George TI, Tyner JW. The new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatment. Blood. 2013 Sep;122((10)):1707–1711. doi: 10.1182/blood-2013-05-500959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013 May;368((19)):1781–90. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SEER Hematopoietic and Lymphoid Neoplasm Database; Chronic Neutrophilic Leukemia https://seer.cancer.gov/seertools/hemelymph/
- 12.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon: IARC Press; 2008. [Google Scholar]
- 13.Szuber N, Tefferi A. Chronic neutrophilic leukemia: new science and new diagnostic criteria. Blood Cancer J. 2018 Feb;8((2)):19. doi: 10.1038/s41408-018-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuter D, Bain B, Mufti B. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. British Journal of Haematology. 139:351–362. doi: 10.1111/j.1365-2141.2007.06807.x. [DOI] [PubMed] [Google Scholar]


